Mercerization of Agricultural Waste: Sweet Clover, Buckwheat, and Rapeseed Straws
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Fiber Alkali Treatment (Mercerization)
2.3. Fiber Characterization
2.3.1. Optical Microscopy (OM)
2.3.2. Scanning Electron Microscopy (SEM)
2.3.3. X-ray Diffractometer (XRD)
2.3.4. Fourier Transform Infrared Spectroscopy (FTIR)
2.3.5. Thermogravimetric Analysis (TGA)
2.3.6. Chemical Composition Analysis
3. Results and Discussion
3.1. FTIR
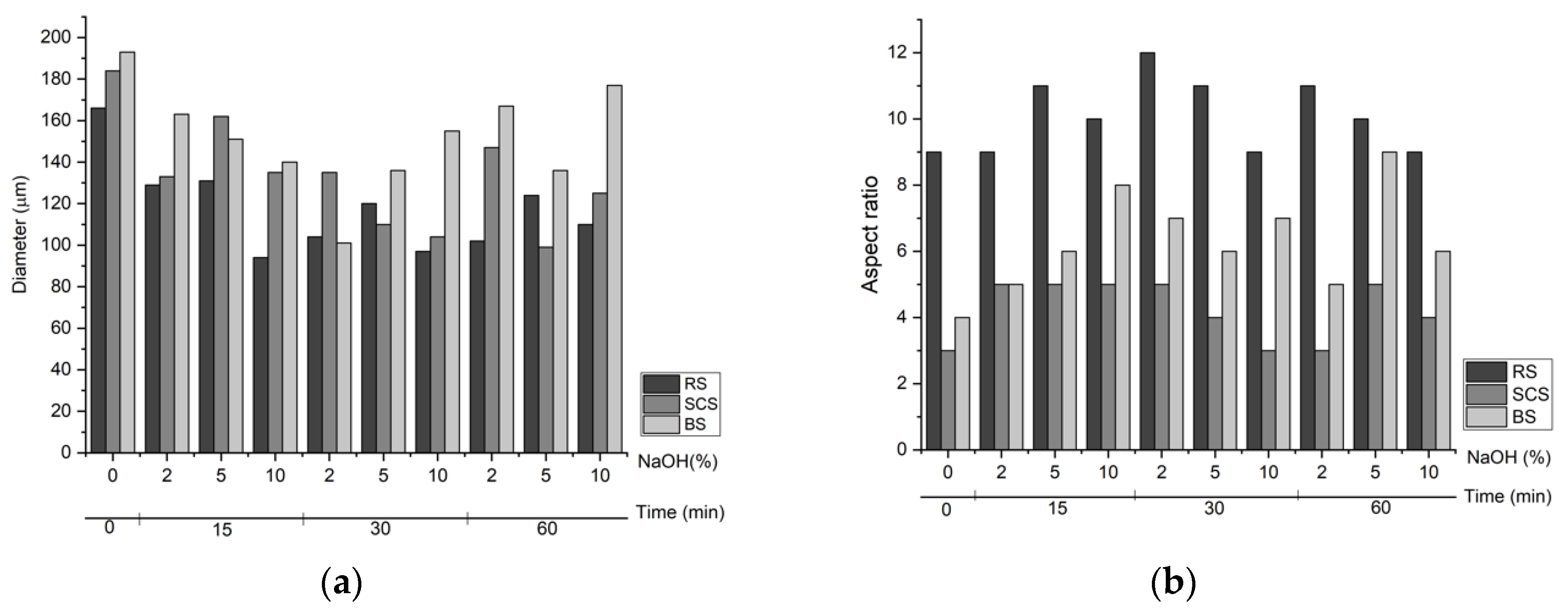
3.2. Optical Microscopy
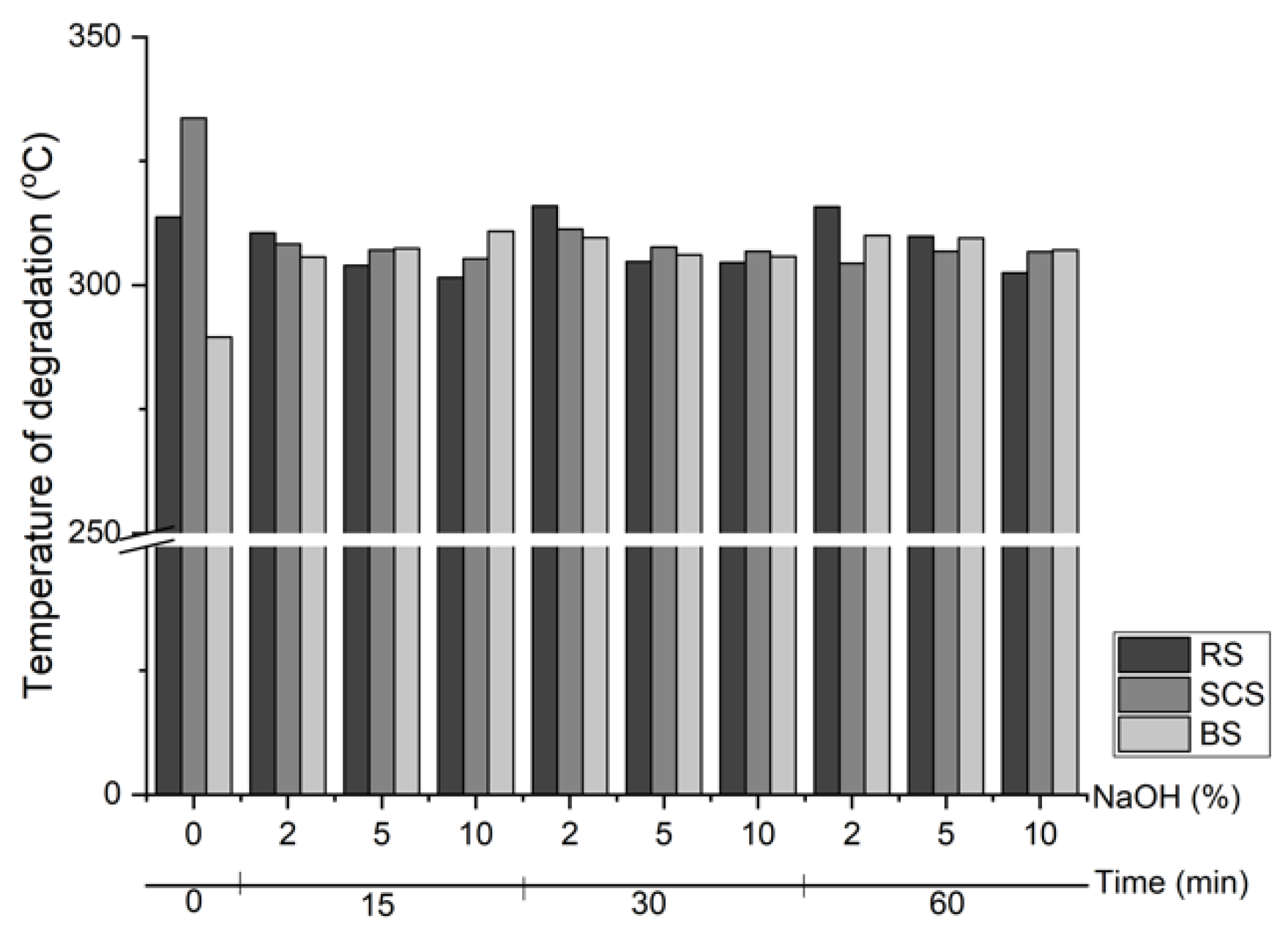
3.3. Thermal Properties
3.4. Chemical Composition
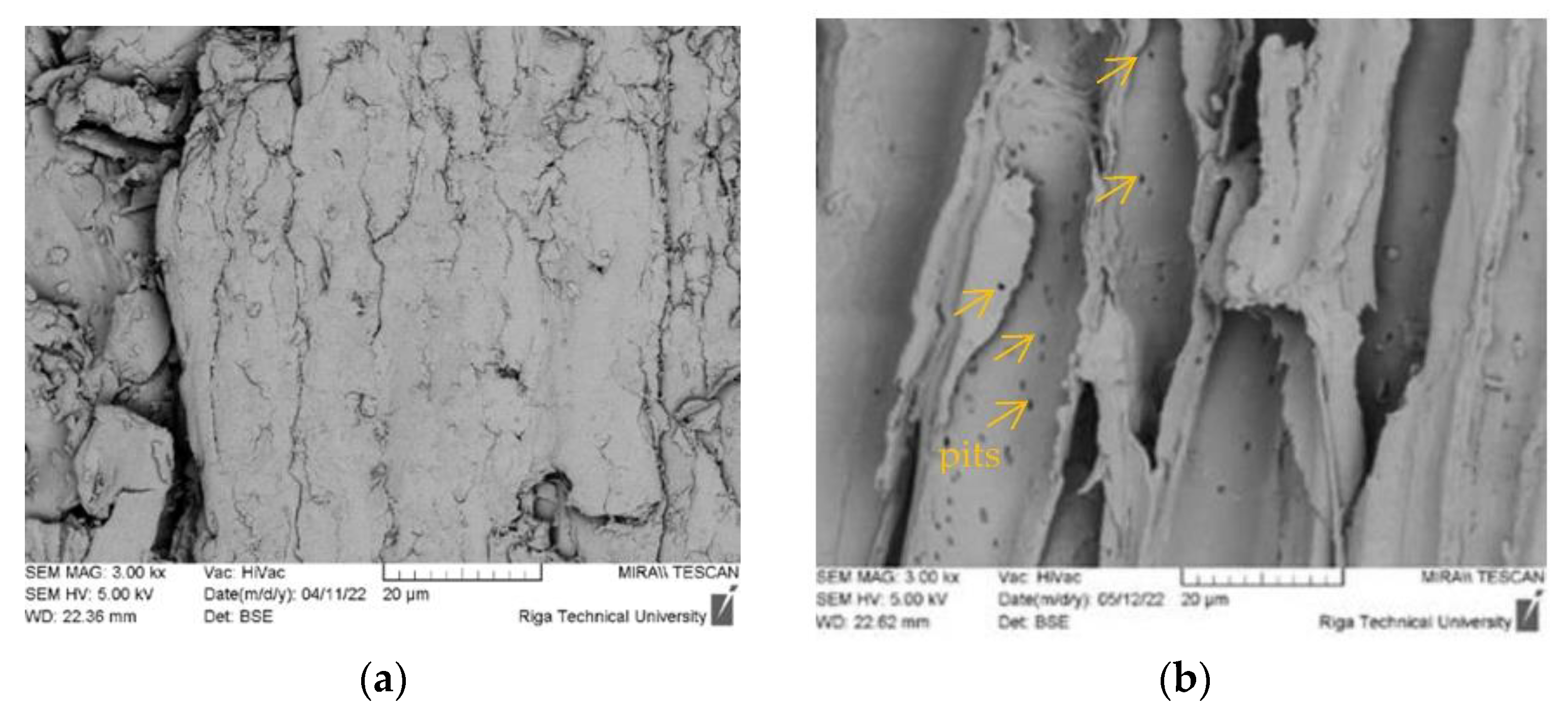
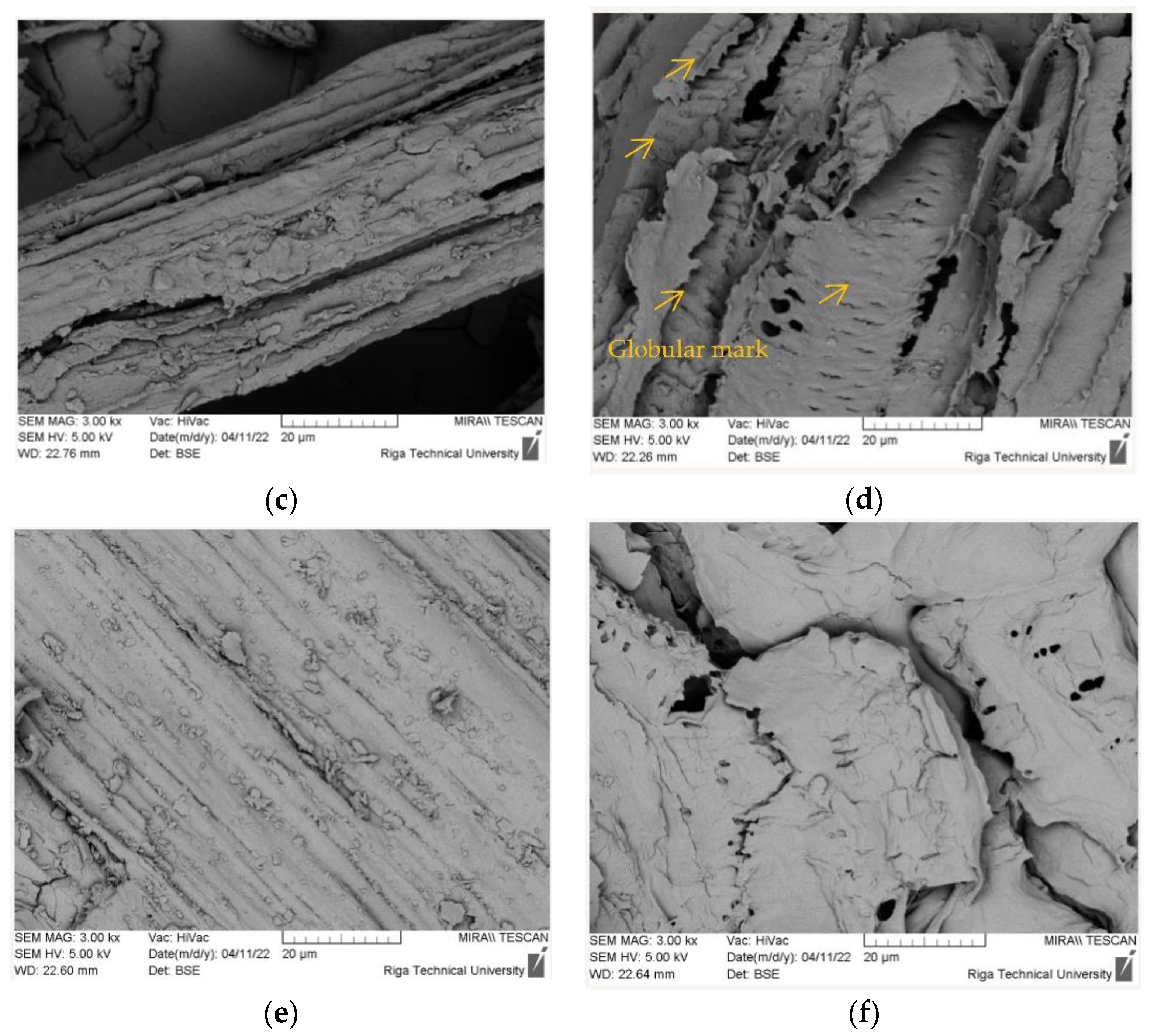
3.5. Surface Morphology
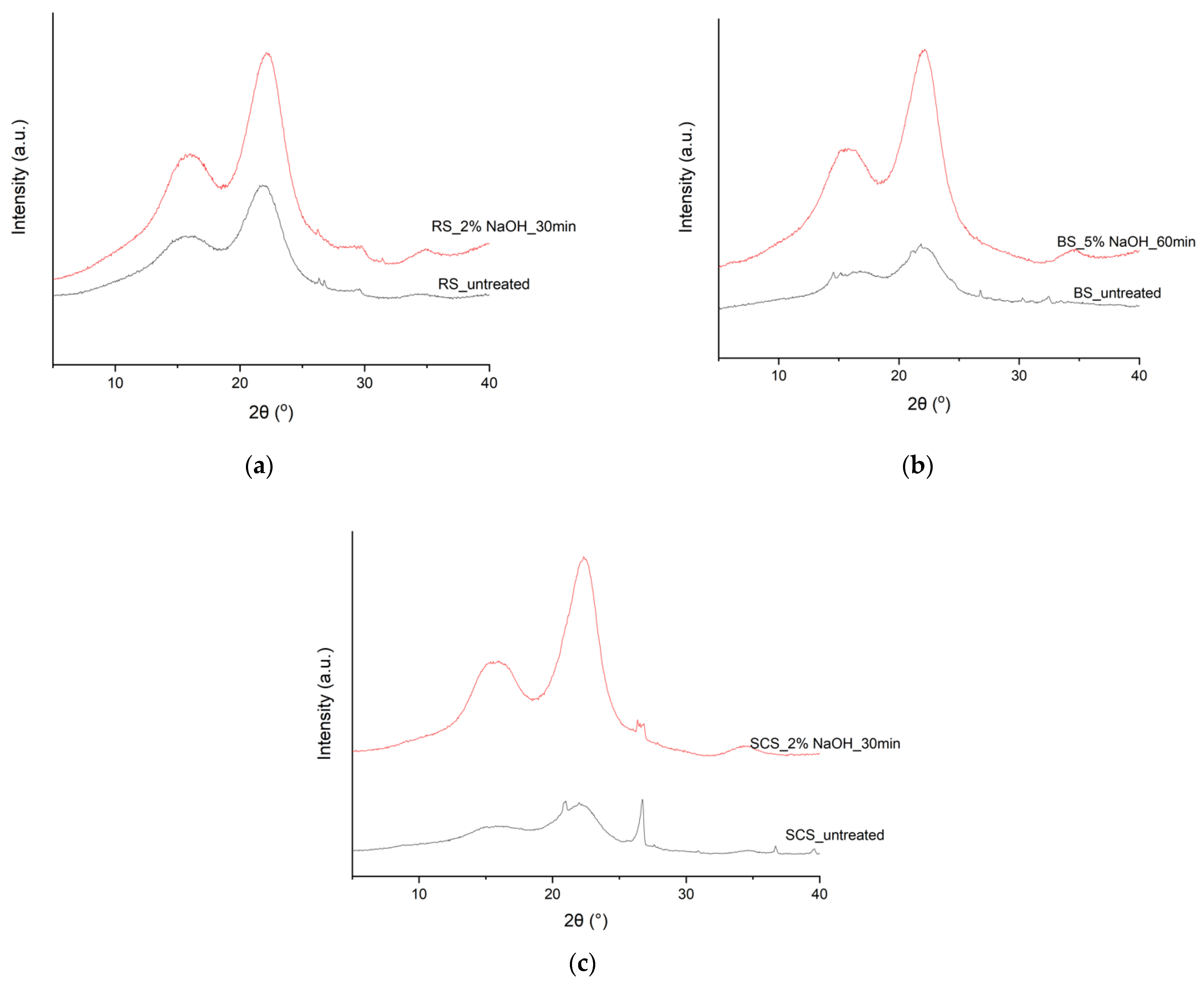
3.6. XRD
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Pilvere, I. Central Statistical Bureau Republic of Latvia. Lauksaimniecības Attīstības Prognozēšana un Politikas Scenāriju Izstrāde Līdz 2050. Gadam Projekta Atskaite. 2021, 21, 1–165. Available online: www.csb.gov.lv/lv/statistika/statistikas-temas/lauksaimnieciba/augkopiba/meklet-tema/2605-lauksaimniecibas-kulturu-sejumu-platibas-un (accessed on 1 August 2022).
- European Parliament. Circular economy: Definition, Importance and Benefits. 2022. Available online: https://www.europarl.europa.eu/news/en/headlines/economy/20151201STO05603/circular-economy-definition-importance-and-benefits (accessed on 23 August 2022).
- Manitoba Government Inquiry, Extending Livestock Feed Supplies Section One. Available online: https://www.gov.mb.ca/agriculture/livestock/production/beef/extending-livestock-feed-supplies-section-one.html#6 (accessed on 23 August 2022).
- Rigal, M.; Rigal, L.; Vilarem, G.; Vandenbossche Maréchal, V. Sweet Clovers, a Source of Fibers Adapted for Growth on Wet and Saline Soils. J. Nat. Fibers 2016, 13, 410–422. [Google Scholar] [CrossRef]
- Brahim, M.; Boussetta, N.; Grimi, N.; Vorobiev, E.; Zieger-Devin, I.; Brosse, N. Pretreatment optimization from rapeseed straw and lignin characterization. Ind. Crops Prod. 2017, 95, 643–650. [Google Scholar] [CrossRef]
- Smuga-Kogut, M.; Bychto, L.; Walendzik, B.; Cielecka-Piontek, J.; Marecik, R.; Kobus-Cisowska, J.; Grajek, K.; Szymanowska-Powałowska, D. Use of Buckwheat Straw to Produce Ethyl Alcohol Using Ionic Liquids. Energies 2019, 12, 2014. [Google Scholar] [CrossRef]
- Hasan, A.; Rabbi, M.S.; Billah, M.M. Making the lignocellulosic fibers chemically compatible for composite: A comprehensive review. Clean. Mater. 2022, 4, 100078. [Google Scholar] [CrossRef]
- Sanjay, M.R.S.; Siengchin, S.; Parameswaranpillai, J.; Jawaid, M.; Pruncu, C.I.; Khan, A. A comprehensive review of techniques for natural fibers as reinforcement in composites: Preparation, processing and characterization. Carbohydr. Polym. 2019, 207, 108–121. [Google Scholar] [CrossRef]
- Puke, M.; Godina, D.; Kirpluks, M.; Rizikovs, J.; Brazdausks, P. Residual Birch Wood Lignocellulose after 2-Furaldehyde Production as a Potential Feedstock for Obtaining Fiber. Polymers 2021, 13, 1816. [Google Scholar] [CrossRef] [PubMed]
- Chen, H. Biotechnology of Lignocellulose: Theory and Practice; Chemical Industry Press: Beijing, China; Springer Science C Business Media: Dordrecht, The Netherlands, 2014. [Google Scholar] [CrossRef]
- Abbass, A.; Paiva, M.C.; Oliveira, D.V.; Lourenço, P.B.; Fangueiro, R. Insight into the effects of solvent treatment of natural fibers prior to structural composite casting: Chemical, physical and mechanical evaluation. Fibers 2021, 9, 54. [Google Scholar] [CrossRef]
- Maheswari, C.U.; Reddy, K.O.; Muzenda, E. Extraction, chemical composition, morphology and characterization of cellulose microfibrils from ficus leaves. J. Biobased Mater. Bioenergy 2014, 8, 409–414. [Google Scholar] [CrossRef]
- Liua, Y.; Lvc, X.; Baoa, J.; Xied, J.; Tanga, X.; Chea, J.; Maa, Y.; Tong, J.Y. Characterization of silane treated and untreated natural cellulosic fibre from corn stalk waste as potential reinforcement in polymer composites. Carbohydr. Polym. 2019, 218, 179–187. [Google Scholar] [CrossRef]
- Gomes, A.; Goda, K.; Ohgi, J. Effects of Alkali Treatment to Reinforcement on Tensile Properties of Curaua Fiber Green Composites. JSME Int. J. Ser. A 2004, 47, 541–546. [Google Scholar] [CrossRef]
- Alvarez, V.A.; Vazquez, A. Influence of fiber chemical modification procedure on the mechanical properties and water ab-sorption of MaterBi-Y/sisal fiber composites. Compos. Part A 2006, 37, 1672–1680. [Google Scholar] [CrossRef]
- De Lemos, A.L. Characterization of Natural Fibers: Wood, Sugarcane and Babassu for Use in Biocomposites. Cellul. Chem. Technol. 2017, 51, 711–718. [Google Scholar]
- Poletto, M.; Ornaghi Júnior, H.L.; Zattera, A.J. Native Cellulose: Structure, Characterization and Thermal Properties. Materials 2014, 7, 6105–6119. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Xiao, S.; Shi, S.Q. The Effect of Hemicellulose Content on Mechanical Strength, Thermal Stability, and Water Resistance of Cellulose-rich Fiber Material from Poplar. Bioresources 2019, 14, 5288–5300. [Google Scholar] [CrossRef]
- Ando, D.; Nakatsubo, F.; Yano, H. Thermal stability of lignin in ground pulp (GP) and the effect oflignin modification on GP’s thermal stability: TGA experiments with dimeric lignin model compounds and milled wood lignins. Holzforschung 2019, 73, 493–499. [Google Scholar] [CrossRef]
- Nurazzi, N.M.; Asyraf, M.R.M.; Rayung, M.; Norrrahim, M.N.F.; Shazleen, S.S.; Rani, M.S.A.; Shafi, A.R.; Aisyah, H.A.; Radzi, M.H.M.; Sabaruddin, F.A.; et al. Thermogravimetric Analysis Properties of Cellulosic Natural Fiber Polymer Compo-sites: A Review on Influence of Chemical Treatments. Polymers 2021, 13, 2710. [Google Scholar] [CrossRef]
- Chandrasekar, M.; Ishak, M.R.; Sapuan, S.M.; Leman, Z.; Jawaid, M. A review on the characterisation of natural fibres and their composites after alkali treatment and water absorption. Plast. Rubber Compos. 2017, 46, 119–136. [Google Scholar] [CrossRef]
- Benini, K.C.; Mulinari, D.; Voorwald, H.; Cioffi, M.O. Chemical modification effect on the mechanical properties of hips/ coconut fiber composites. BioResources 2010, 5, 1143–1155. [Google Scholar]
- Lee, C.H.; Khalina, A.; Lee, S.H. Importance of Interfacial Adhesion Condition on Characterization of Plant-Fiber-Reinforced Polymer Composites: A Review. Polymers 2021, 13, 438. [Google Scholar] [CrossRef]
- Liu, C.-F.; Sun, R.-C. Chapter 5-Cellulose. In Cereal Straw as a Resource for Sustainable, Biomaterials and Biofuels; Sun, R.-C., Ed.; Elsevier: Amsterdam, The Netherlands, 2010; pp. 131–167. ISBN 9780444532343. [Google Scholar] [CrossRef]
- Gupta, V.; Ramakanth, D.; Verma, C.; Maji, P.K.; Gaikwad, K.K. Isolation and characterization of cellulose nanocrystals from amla (Phyllanthus emblica) pomace. Biomass Convers. Biorefinery 2021, 1–12. [Google Scholar] [CrossRef]
- Boukir, A.; Fellak, S.; Doumenq, P. Structural characterization of Argania spinosa Moroccan wooden artifacts during natural degradation progress using infrared spectroscopy (ATR-FTIR) and X-Ray diffraction (XRD). Heliyon 2019, 5, e02477. [Google Scholar] [CrossRef] [PubMed] [Green Version]

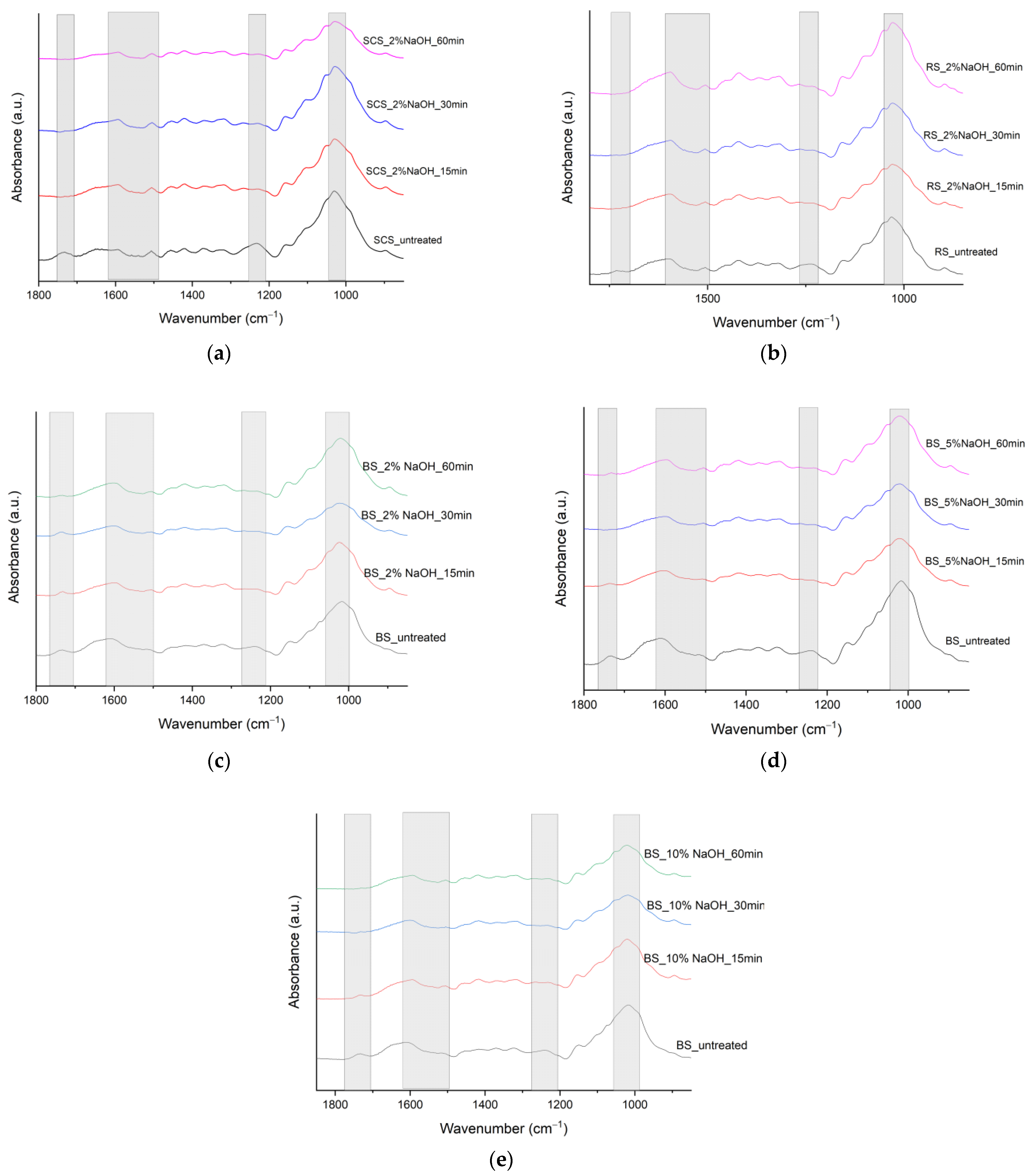

| SCS υ (cm−1) | BS υ (cm−1) | RS υ (cm−1) | Peak Assignment | References |
|---|---|---|---|---|
| 3328 | 3283 | 3322 | O–H stretching vibration in cellulose | [11,12,13] |
| 2912 | 2918 and 2855 | 2891 | C–H stretching vibrations of methyl and methylene groups in cellulose, hemicellulose, pectin, and fatty acids and lipids | [11,12,13] |
| 1733 | 1732 | 1732 | Carboxyl/aldehyde group in hemicellulose; carbonyl/carboxyl groups in lignin, pectin, or waxy fraction | [11,12,13] |
| 1652 | 1614 | 1598 | C=C double bonds in aromatic moieties in lignin; may overlap with OH bending of water | [11,12] |
| 1506 | 1506 | 1506 | C=C stretching in lignin | [5] |
| 1418 | 1416 | 1418 | CH2 bending in cellulose | [13] |
| 1373 | 1370 | 1372 | C–H bending in cellulose and hemicellulose | [11] |
| 1319 | 1324 | 1319 | CH2 wagging in crystalline cellulose | [11] |
| 1234 | 1241 | 1235 | C–O stretching of hemicellulose or C–O–C of lignin | [11,12,13] |
| 1030 | 1018 | 1031 | C–O stretching of cellulose and C–H deformation in lignin | [11,12] |
| 898 | – | 896 | C–O–C and C–H out-of-plane bending or stretching in amorphous cellulose; β-glyosidic linkages of cellulose | [11,12,13] |
| Compound | Fiber | Fiber after Optimal Treatment | ||||
|---|---|---|---|---|---|---|
| Amount % from Dry Mass | BS | SCS | RS | BS_5%NaOH _60 Min | SCS_2%NaOH _30 Min | RS_2%NaOH _30 Min |
| Glucose | 42.43 | 37.48 | 37.67 | 43.58 | 42.52 | 39.70 |
| Xylose | 9.82 | 14.66 | 17.36 | 8.36 | 13.75 | 14.81 |
| Galactose | 2.42 | 1.34 | 1.68 | 1.26 | 1.18 | 1.30 |
| Arabinose | 0.51 | 0.83 | 1.36 | 0.79 | 0.56 | 0.72 |
| Mannose | 1.18 | 2.04 | 2.95 | 1.49 | 2.10 | 2.30 |
| Acetic acid | 3.94 | 4.18 | 3.94 | 0.00 | 0.28 | 0.18 |
| Levulinic acid, formic acid, 5-HMF | 1.43 | 0.98 | 1.43 | 1.02 | 1.17 | 1 |
| Ash | 0.05 | 3.02 | 0.06 | 0.02 | 3.41 | 0.02 |
| Insoluble lignin | 18.7 | 25.76 | 21.75 | 19.27 | 23.81 | 21.22 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Žiganova, M.; Ābele, A.; Iesalniece, Z.; Meri, R.M. Mercerization of Agricultural Waste: Sweet Clover, Buckwheat, and Rapeseed Straws. Fibers 2022, 10, 83. https://doi.org/10.3390/fib10100083
Žiganova M, Ābele A, Iesalniece Z, Meri RM. Mercerization of Agricultural Waste: Sweet Clover, Buckwheat, and Rapeseed Straws. Fibers. 2022; 10(10):83. https://doi.org/10.3390/fib10100083
Chicago/Turabian StyleŽiganova, Madara, Agnese Ābele, Zanda Iesalniece, and Remo Merijs Meri. 2022. "Mercerization of Agricultural Waste: Sweet Clover, Buckwheat, and Rapeseed Straws" Fibers 10, no. 10: 83. https://doi.org/10.3390/fib10100083







