Surface Modification of Sol-Gel Silica Antireflective Coatings by F-PMHS: A Simple Method for Improvement of Amphiphobicity
Abstract
:1. Introduction
2. Materials and Methods
2.1. Mateirals
2.2. Fabrication of Silica Sols
2.3. Preparation of Sol-Gel Silica ARCs
2.4. Surface Modification of Silica ARCs
2.5. Characterization
3. Results and Discussion
3.1. FTIR Characterization
3.2. XPS Analysis
3.3. Morphology of ARCs
3.4. Hydrophobicity and Oleophobicity
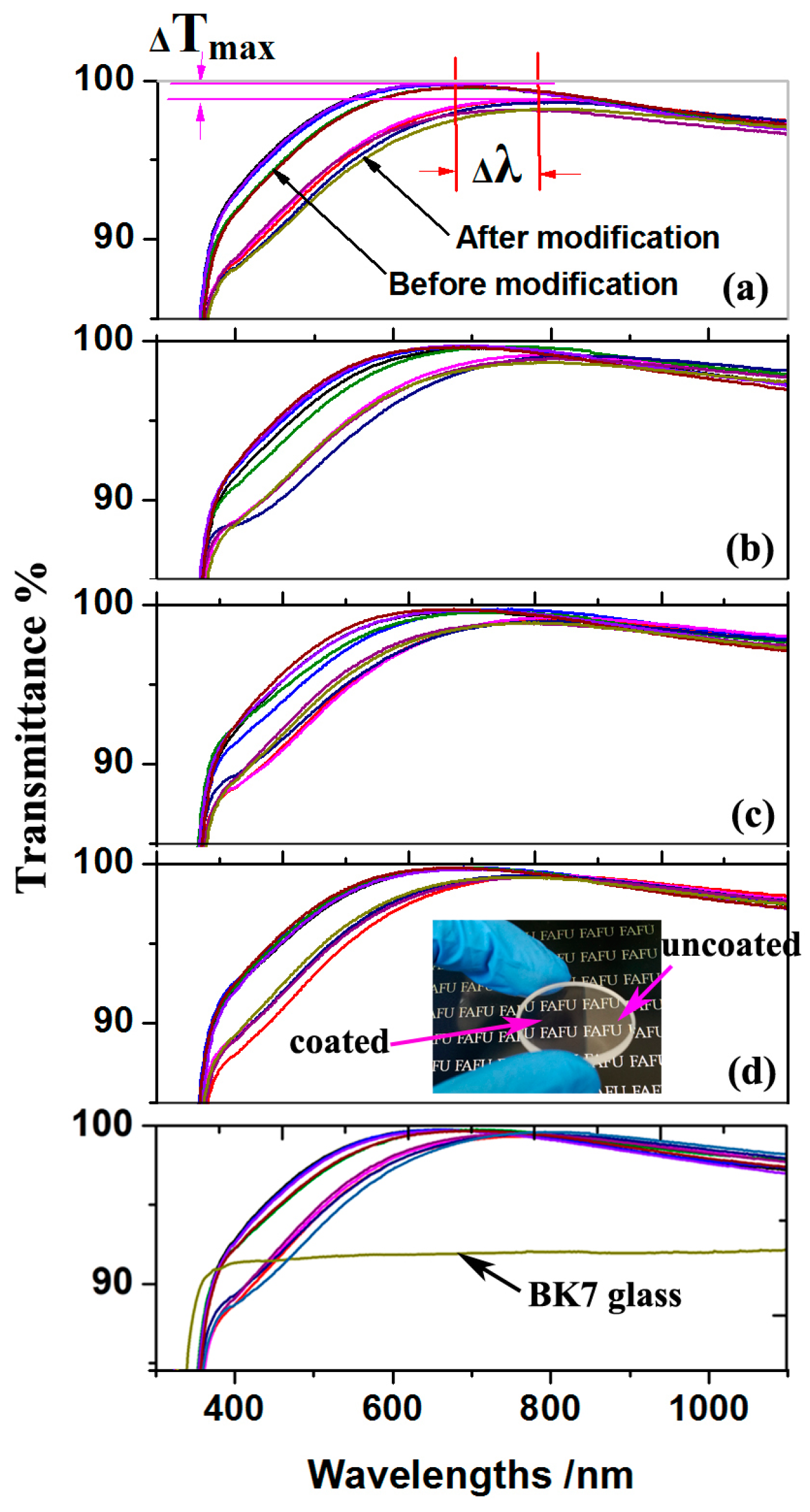
3.5. Optical Property of ARCs
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Hsu, C.C.; Lan, W.L.; Chen, N.P.; Wu, C.C. The hydrophobic and omnidirectional antireflection coating of SiO2 nanospheres with C18-TEOS. Opt. Laser Technol. 2014, 58, 202–206. [Google Scholar] [CrossRef]
- Sim, D.M.; Choi, M.J.; Hur, Y.H.; Nam, B.; Chae, G.; Park, J.H.; Jung, Y.S. Ultra-high optical transparency of robust, graded-index, and anti-fogging silica coating derived from Si-containing block copolymers. Adv. Opt. Mater. 2013, 1, 428–433. [Google Scholar] [CrossRef]
- Guo, Z.L.; Zhao, H.X.; Zhao, W.; Wang, T.; Kong, D.P.; Chen, T.J.; Zhang, X.Y. High-quality hollow closed-pore silica antireflection coatings based on styrene-acrylate emulsion@ organic-inorganic silica precursor. ACS Appl. Mater. Interfaces 2016, 8, 11796–11805. [Google Scholar] [CrossRef] [PubMed]
- Moghal, J.; Kobler, J.; Sauer, J.; Best, J.; Gardener, M.; Watt, A.A.; Wakefield, G. High-performance, single-layer antireflective optical coatings comprising mesoporous silica nanoparticles. ACS Appl. Mater. Interfaces 2012, 4, 854–859. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.T.; Lu, N.; Hao, J.Y.; Xu, H.B.; Qi, D.P.; Chi, L.F. Self-assembled monolayer islands masked chemical etching for broad-band antireflective silicon surfaces. J. Phys. Chem. C 2010, 114, 1989–1995. [Google Scholar] [CrossRef]
- Poirié, T.; Schmitt, T.; Bousser, E.; Vernhes, R.; Martinu, L.; Klemberg-Sapieha, J.E. Hybrid organic/inorganic nanolaminate structures with enhanced tribo-mechanical properties for optical applications. Surf. Coat. Technol. 2017, 315, 399–407. [Google Scholar] [CrossRef]
- Thomas, I.M.; Burnham, A.K.; Ertel, J.R. Method for reducing the effect of environmental contamination of sol-gel optical coatings. SPIE 1999, 3492, 220–229. [Google Scholar] [CrossRef]
- Cai, S.; Xue, Q.L.; Xia, B.B.; Yang, J.; Lv, H.B.; Yan, H.W.; Jiang, B. Hydrophobic–oleophobic antireflective film with excellent optical property prepared by a simple sol–gel route. Mater. Lett. 2015, 156, 14–16. [Google Scholar] [CrossRef]
- Manca, M.; Cannavale, A.; De Marco, L.; Arico, A.S.; Cingolani, R.; Gigli, G. Durable superhydrophobic and antireflective surfaces by trimethylsilanized silica nanoparticles-based sol-gel processing. Langmuir 2009, 25, 6357–6362. [Google Scholar] [CrossRef] [PubMed]
- Tao, C.; Yan, H.; Yuan, X.; Yin, Q.; Zhu, J.; Ni, W.; Yan, L.; Zhang, L. Hydrophobic antireflective coatings with ultralow refractive index synthesized by deposition of methylated hollow silica nanoparticles. Mater. Lett. 2016, 183, 374–377. [Google Scholar] [CrossRef]
- Xia, B.B.; Luo, J.H.; Li, Y.Y.; Yang, B.W.; Zhang, S.M.; Jiang, B. Preparation of sponge-like porous SiO2 antireflective coatings with excellent environment-resistance by an acid-catalysed sol-gel method. RSC Adv. 2017, 7, 26834–26838. [Google Scholar] [CrossRef]
- Li, T.; He, J. A facile hybrid approach to high-performance broadband antireflective thin films with humidity resistance as well as mechanical robustness. J. Mater. Chem. C 2016, 4, 5342–5348. [Google Scholar] [CrossRef]
- Arturi, K.R.; Jepsen, H.; Callsen, J.N.; Søgaard, E.G.; Simonsen, M.E. Superhydrophilicity and durability of fluoropolymer-TiO2 coatings. Prog. Organ. Coat. 2016, 90, 132–138. [Google Scholar] [CrossRef]
- Shabnam, R.; Ahmad, H. Hydrophobic poly(lauryl methacrylate)-coated magnetic nano-composite particles for removal of organic pollutants. Polym. Adv. Technol. 2015, 26, 408–413. [Google Scholar] [CrossRef]
- Zhang, X.X.; Xia, B.B.; Ding, B.; Zhang, Y.L.; Luo, J.H.; Jiang, B. Ultra-fast surface hydrophobic modification of sol–gel silica antireflective coating with enhanced abrasion-resistance. Mater. Lett. 2013, 104, 31–33. [Google Scholar] [CrossRef]
- Ganbavle, V.V.; Bangi, U.K.H.; Latthe, S.S.; Mahadik, S.A.; Rao, A.V. Self-cleaning silica coatings on glass by single step sol–gel route. Surf. Coat. Technol. 2011, 205, 5338–5344. [Google Scholar] [CrossRef]
- Mosquera, M.J.; de los Santos, D.M.; Rivas, T. Surfactant-synthesized ormosils with application to stone restoration. Langmuir 2010, 26, 6737–6745. [Google Scholar] [CrossRef] [PubMed]
- Zhang, G.W.; Lin, S.D.; Wyman, I.; Zou, H.L.; Hu, J.W.; Liu, G.J.; Wang, J.D.; Li, F.; Liu, F.; Hu, M.L. Robust superamphiphobic coatings based on silica particles bearing bifunctional random copolymers. ACS Appl. Mater. Interfaces 2013, 5, 13466–13477. [Google Scholar] [CrossRef] [PubMed]
- Vincent, A.; Babu, S.; Brinley, E.; Karakoti, A.; Deshpande, S. Role of catalyst on refractive index tunability of porous silica antireflective coatings by sol−gel technique. J. Phys. Chem. C 2007, 111, 8291–8298. [Google Scholar] [CrossRef]
- Sun, J.; Cui, X.; Zhang, C.; Zhang, C.; Ding, R.; Xu, Y. A broadband antireflective coating based on a double-layer system containing mesoporous silica and nanoporous silica. J. Mater. Chem. C 2015, 3, 7187–7194. [Google Scholar] [CrossRef]
- Latthe, S.; Liu, S.; Terashima, C.; Nakata, K.; Fujishima, A. Transparent, adherent, and photocatalytic SiO2-TiO2 coatings on polycarbonate for self-cleaning applications. Coatings 2014, 4, 497–507. [Google Scholar] [CrossRef]
- Kuo, T.W.; Wang, N.F.; Tsai, Y.Z.; Hung, P.K.; Houng, M.P. Broadband triple-layer SiOx/SiOxNy/SiNx antireflective coatings in textured crystalline silicon solar cells. Mater. Sci. Semicond. Process. 2014, 25, 211–218. [Google Scholar] [CrossRef]
- Brassard, J.D.; Sarkar, D.K.; Perron, J. Synthesis of monodisperse fluorinated silica nanoparticles and their superhydrophobic thin films. ACS Appl. Mater. Interfaces 2011, 3, 3583–3588. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.J.; Quan, Y.W.; Zhang, J.S.; Chen, Q.M. Preparation of super water-repellent membrane by radiation-induced copolymerization. Surf. Coat. Technol. 2006, 200, 5493–5497. [Google Scholar] [CrossRef]
- Yang, F.C.; Guo, Z.G. Bio-inspired design of a transparent TiO2/SiO2 composite gel coating with adjustable wettability. J. Mater. Sci. 2016, 51, 7545–7553. [Google Scholar] [CrossRef]
- Ren, X.H.; Fan, H.Q.; Ma, J.W.; Wang, C.; Zhao, Y.W.; Lei, S.H. Triboelectric nanogenerators based on fluorinated wasted rubber powder for self-powering application. ACS Sustain. Chem. Eng. 2017, 5, 1957–1964. [Google Scholar] [CrossRef]
- Cui, X.M.; Ding, R.M.; Wang, M.C.; Zhang, C.; Zhang, C.; Zhang, J.; Xu, Y. In situ surface assembly derived ultralow refractive index MgF2-SiO2 hybrid film for tri-layer broadband antireflective coating. Adv. Opt. Mater. 2016, 4, 722–730. [Google Scholar] [CrossRef]
- Goussé, C.; Chanzy, H.; Excoffier, G. Stable suspensions of partially silylated cellulose whiskers dispersed in organic solvents. Polymer 2002, 43, 2645–2651. [Google Scholar] [CrossRef]
- Kim, S.; Cho, A.; Kim, S.; Cho, W.; Chung, M.H.; Kim, F.S.; Kim, J.H. Multi-purpose overcoating layers based on PVA/silane hybrid composites for highly transparent, flexible, and durable AgNW/PEDOT:PSS films. RSC Adv. 2016, 6, 19280–19287. [Google Scholar] [CrossRef]
- Siddique, R.H.; Gomard, G.; Holscher, H. The role of random nanostructures for the omnidirectional anti-reflection properties of the glasswing butterfly. Nat. Commun. 2015, 6, 6909. [Google Scholar] [CrossRef] [PubMed]
- Xia, B.B.; Zhang, Q.H.; Yao, S.Y.; Zhang, Y.L.; Xiao, B.; Jiang, B. Sol–gel silica antireflective coating with enhanced abrasion-resistance using polypropylene glycol as porogen. J. Sol-Gel Sci. Technol. 2014, 71, 291–296. [Google Scholar] [CrossRef]
- Yao, L.; He, J.H. Broadband antireflective superhydrophilic thin films with outstanding mechanical stability on glass substrates. Chin. J. Chem. 2014, 32, 507–512. [Google Scholar] [CrossRef]
- Floch, H.G.; Belleville, P.F. A scratch-resistant single-layer antireflective coating by a low temperature sol-gel route. J. Sol-Gel Sci. Technol. 1994, 13, 293–304. [Google Scholar] [CrossRef]
- Sun, Y.Y.; Zheng, J.X.; Huang, R.C.; Zhang, X.X.; Chen, C.X.; Jiang, B.; Chen, H.X.; Yan, L.H.; Yang, W.B. A simple method to control the microstructure and properties of sol–gel silica antireflective coatings. RSC Adv. 2017, 7, 31950–31959. [Google Scholar] [CrossRef]
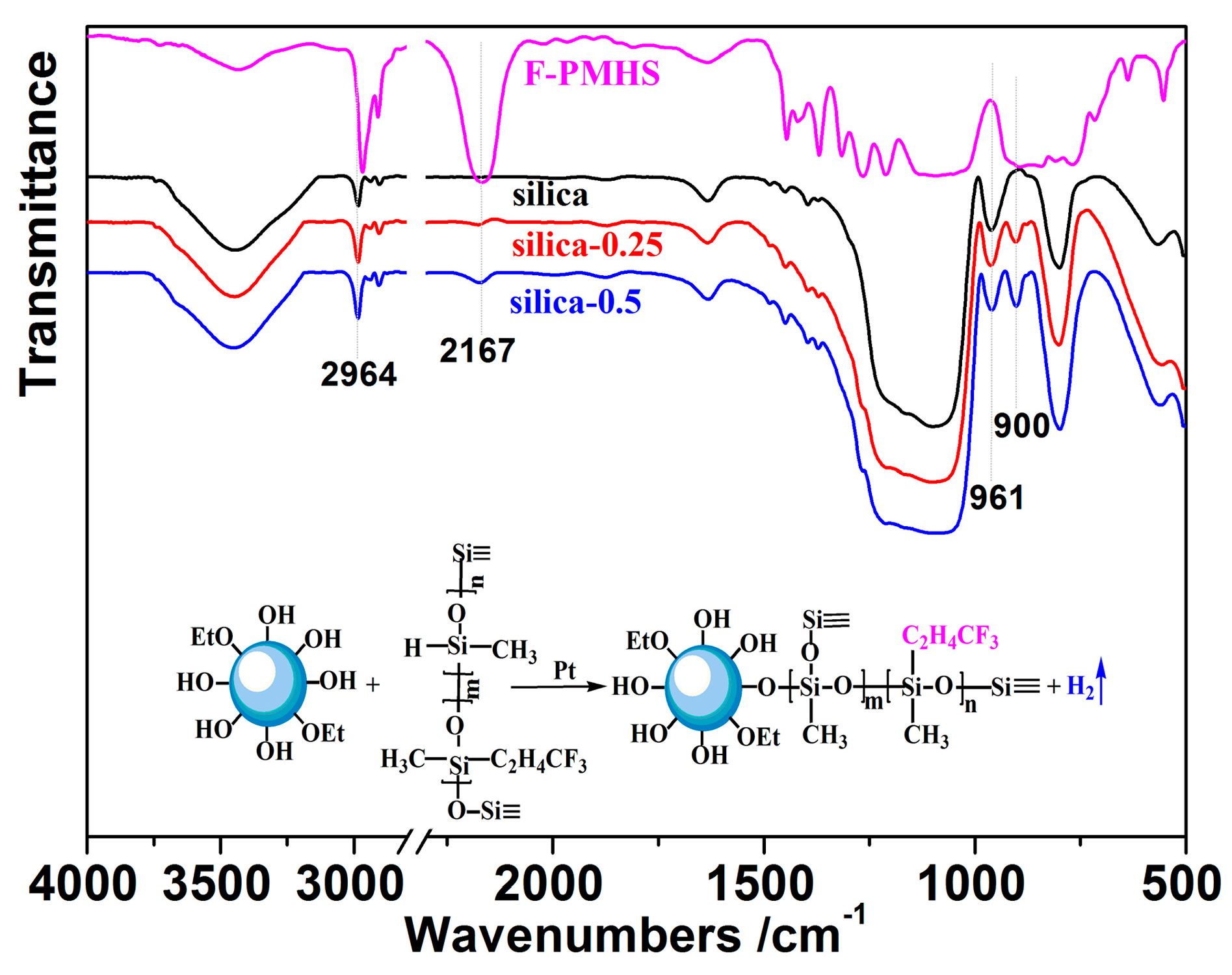
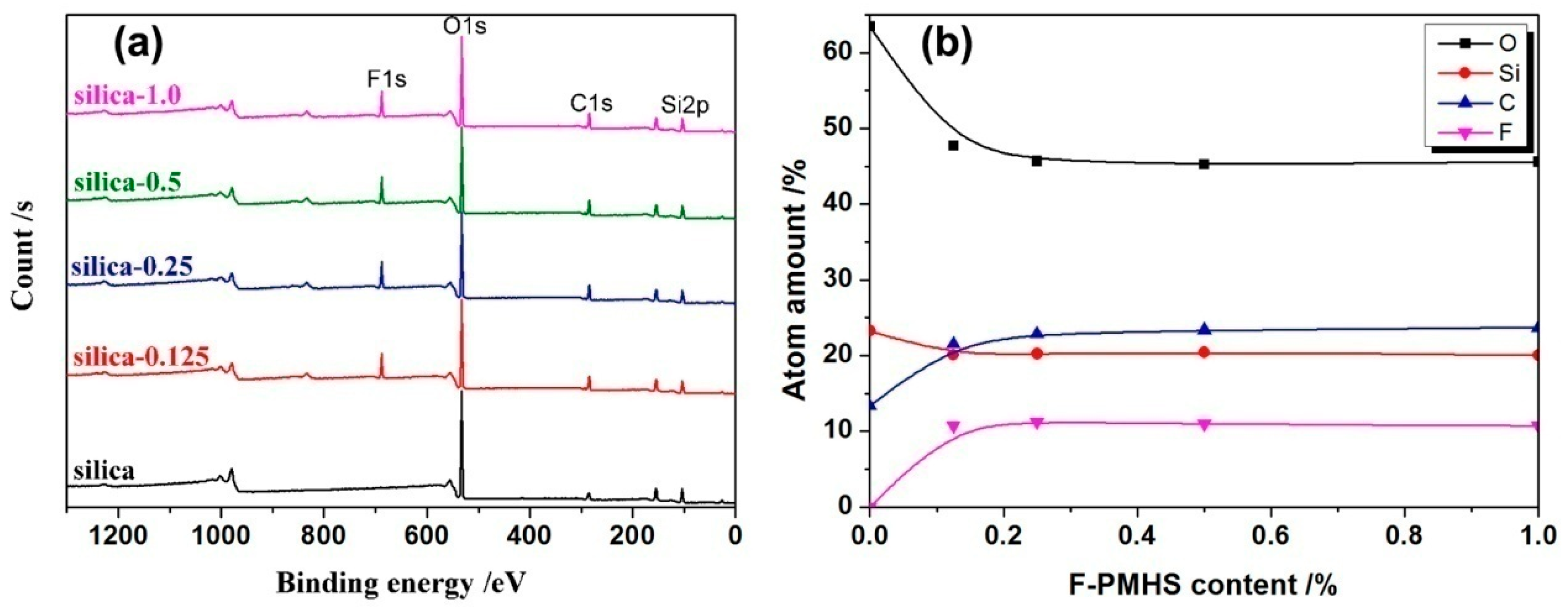
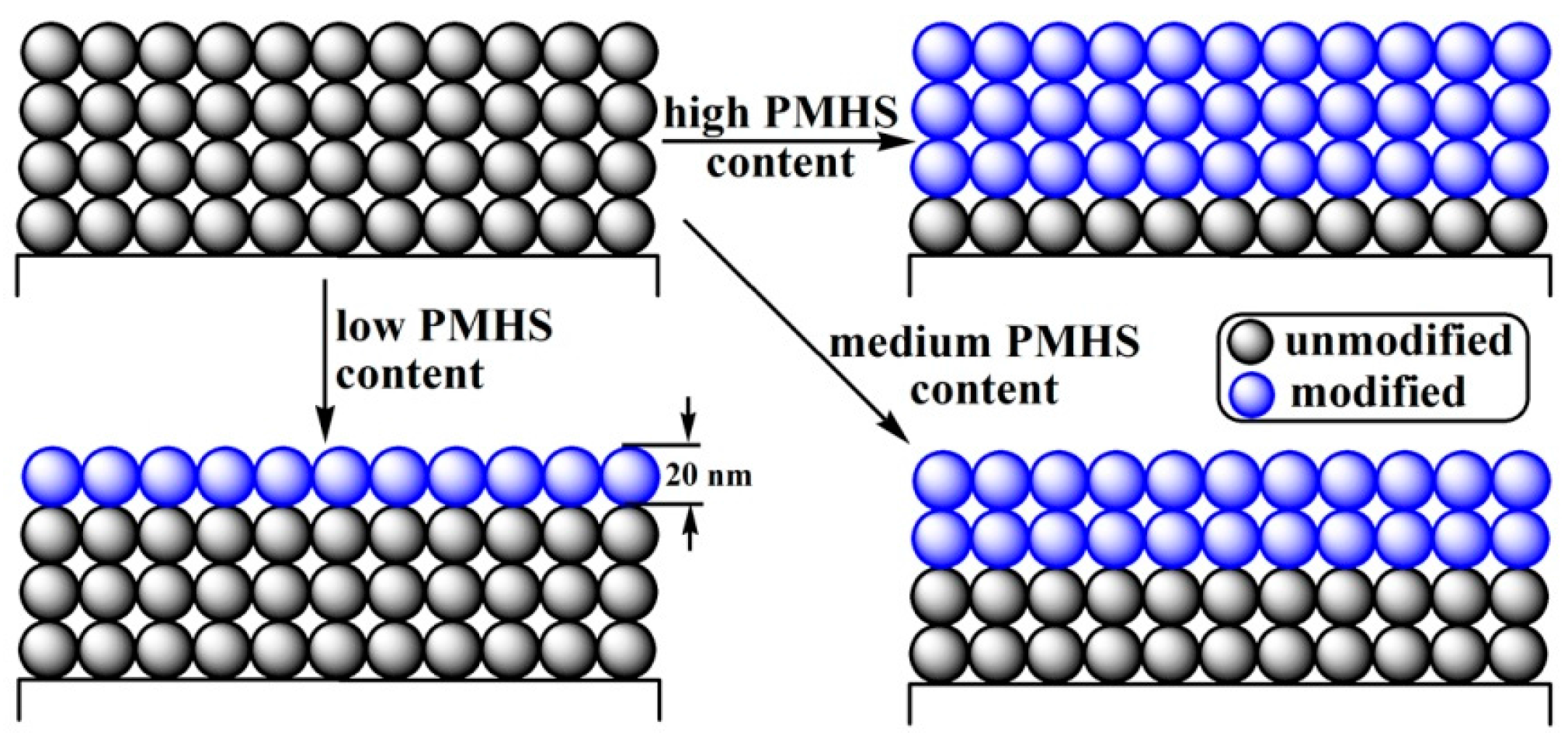
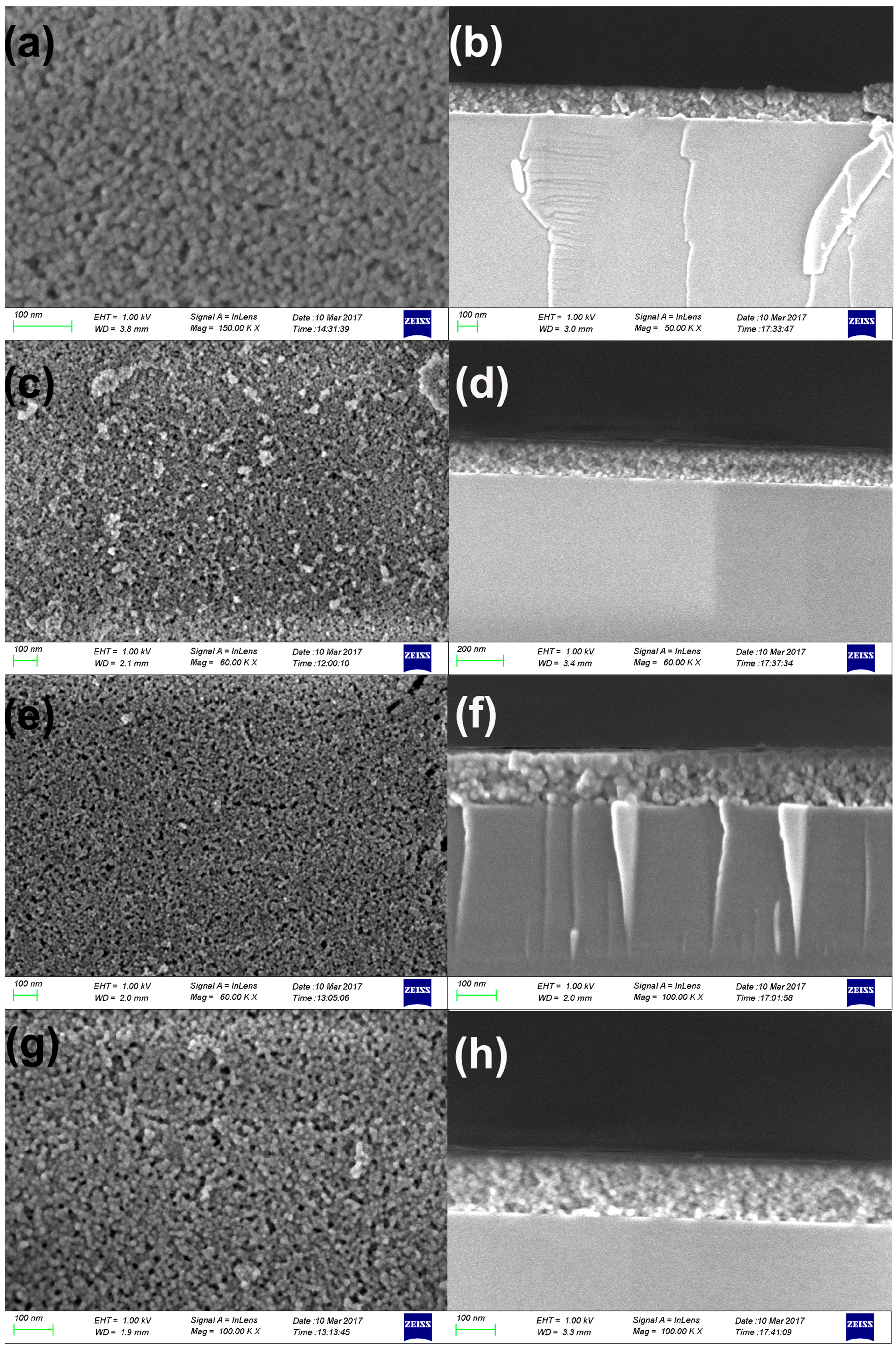

| F-PMHS Concentration/% | Refractive Index | Thickness/nm | Porosity/% |
|---|---|---|---|
| 0 | 1.25 | 136 | 50.3 |
| 0.25 | 1.28 | 140 | 43.6 |
| 0.5 | 1.29 | 142 | 41.3 |
| 0.75 | 1.30 | 141 | 39.0 |
| 1.0% | 1.31 | 140 | 36.7 |
| 1.25 | 1.32 | 141 | 34.4 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lin, W.; Sun, Y.; Zheng, J.; Zheng, Y.; Yan, L.; Jiang, B.; Yang, W.; Chen, H.; Zhang, X. Surface Modification of Sol-Gel Silica Antireflective Coatings by F-PMHS: A Simple Method for Improvement of Amphiphobicity. Coatings 2018, 8, 57. https://doi.org/10.3390/coatings8020057
Lin W, Sun Y, Zheng J, Zheng Y, Yan L, Jiang B, Yang W, Chen H, Zhang X. Surface Modification of Sol-Gel Silica Antireflective Coatings by F-PMHS: A Simple Method for Improvement of Amphiphobicity. Coatings. 2018; 8(2):57. https://doi.org/10.3390/coatings8020057
Chicago/Turabian StyleLin, Wensheng, Yingying Sun, Jiaxian Zheng, Yanmei Zheng, Lianghong Yan, Bo Jiang, Wenbin Yang, Hanxian Chen, and Xinxiang Zhang. 2018. "Surface Modification of Sol-Gel Silica Antireflective Coatings by F-PMHS: A Simple Method for Improvement of Amphiphobicity" Coatings 8, no. 2: 57. https://doi.org/10.3390/coatings8020057
APA StyleLin, W., Sun, Y., Zheng, J., Zheng, Y., Yan, L., Jiang, B., Yang, W., Chen, H., & Zhang, X. (2018). Surface Modification of Sol-Gel Silica Antireflective Coatings by F-PMHS: A Simple Method for Improvement of Amphiphobicity. Coatings, 8(2), 57. https://doi.org/10.3390/coatings8020057





