Investigation of the Corrosion Behavior of Electroless Ni-P Coating in Flue Gas Condensate
Abstract
:1. Introduction
2. Materials and Methods
3. Results and Discussions
3.1. Morphology Observations and Microstructures Properties
3.2. OCP Measurements
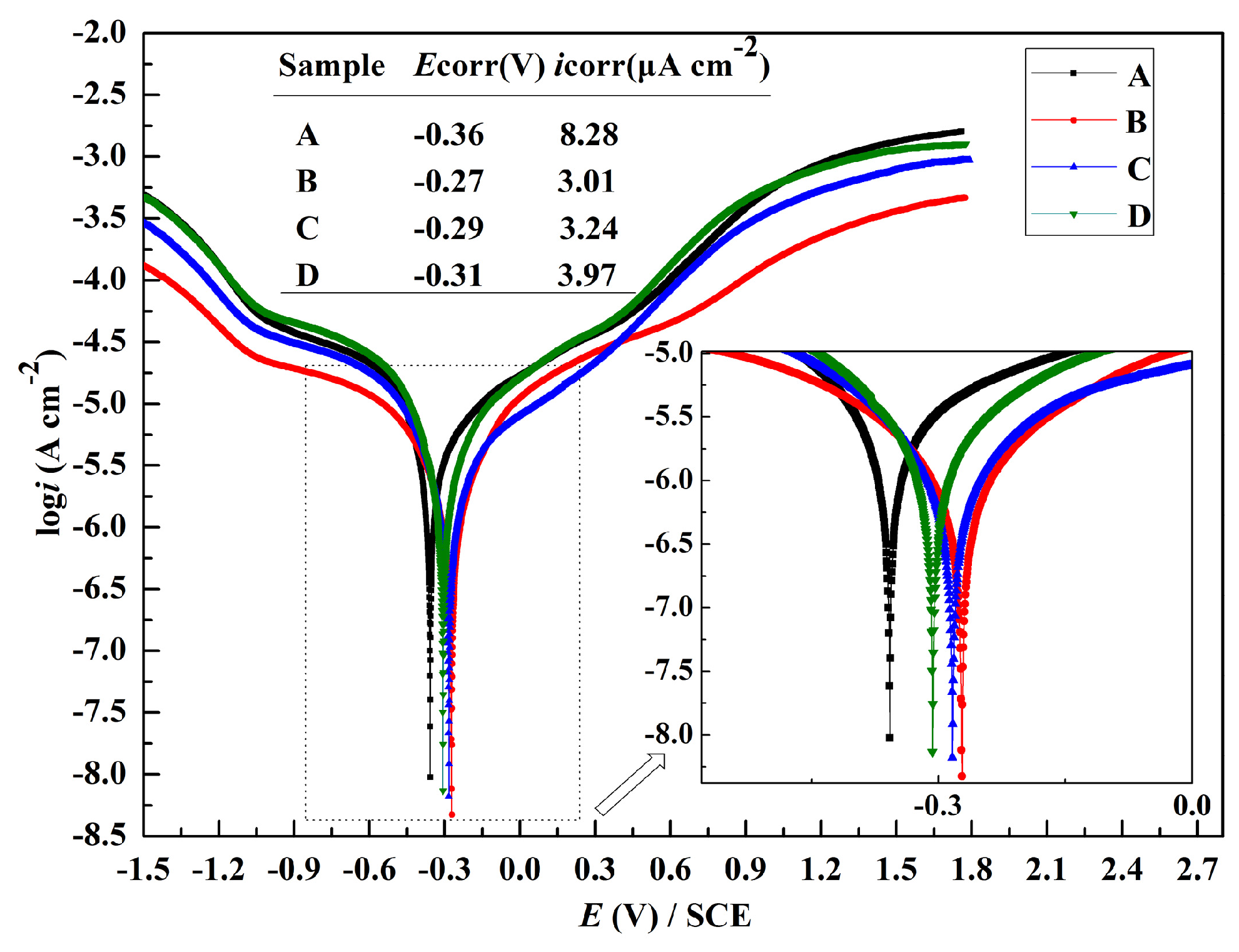
3.3. Potentiodynamic Polarization
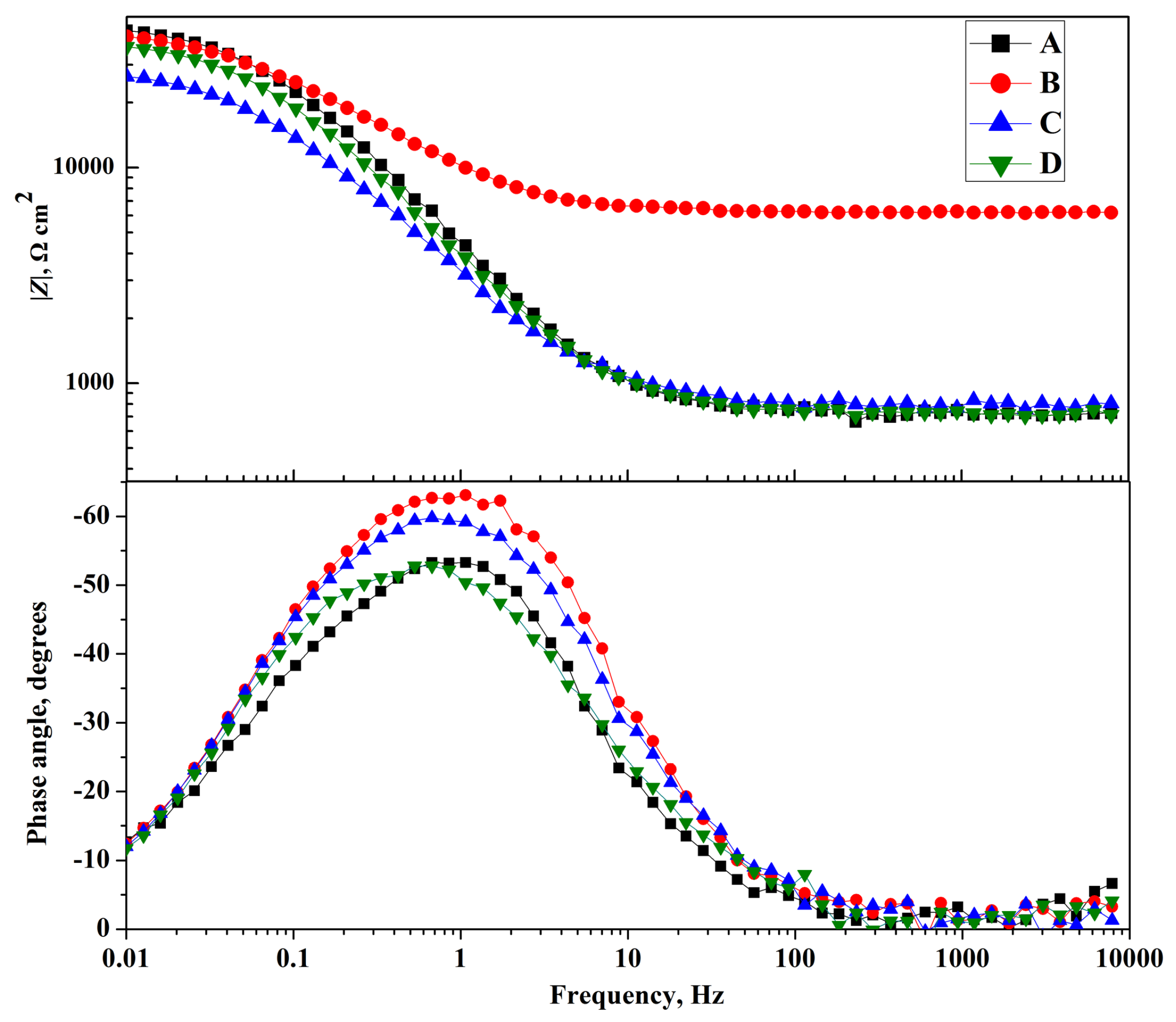
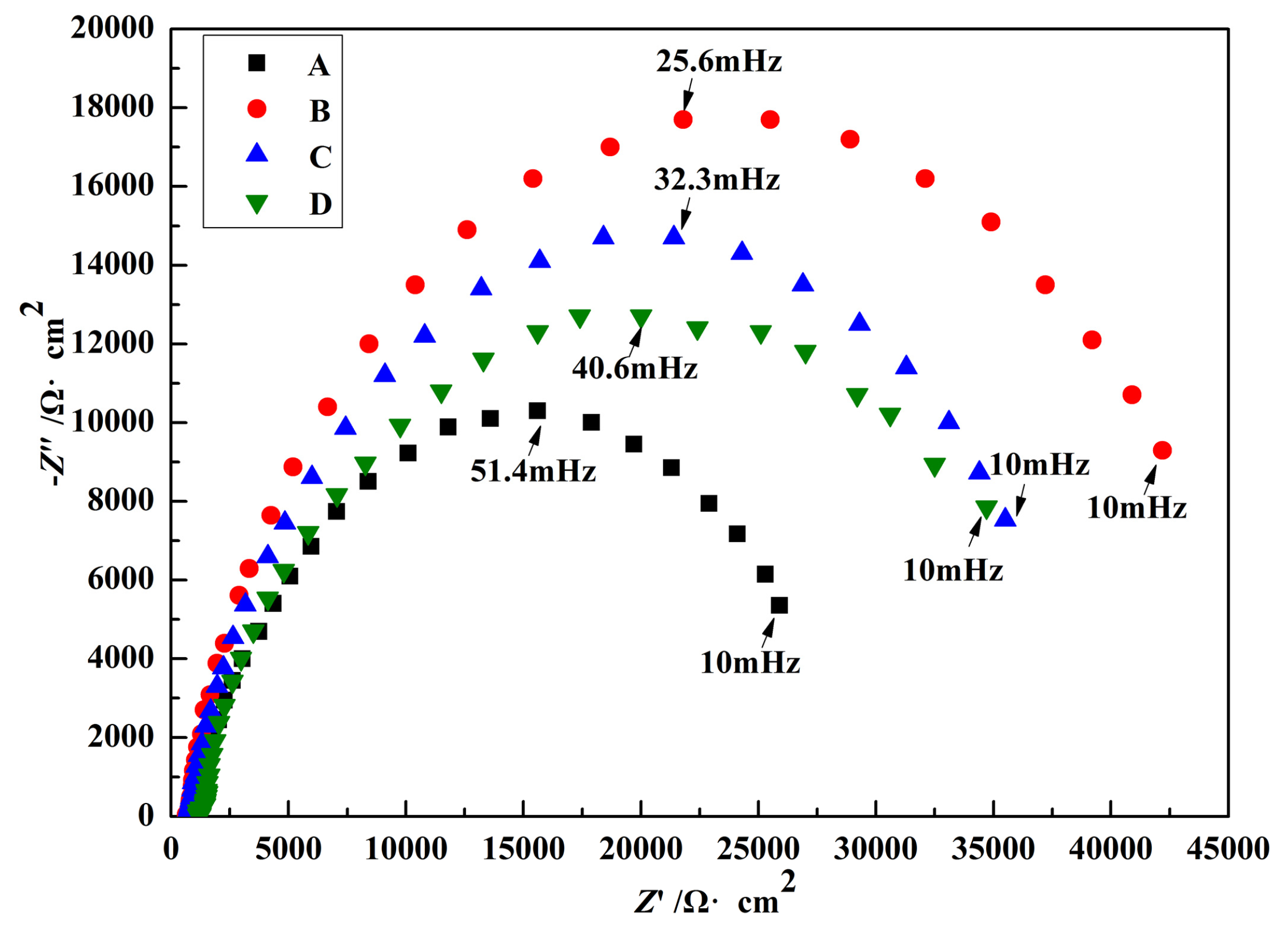
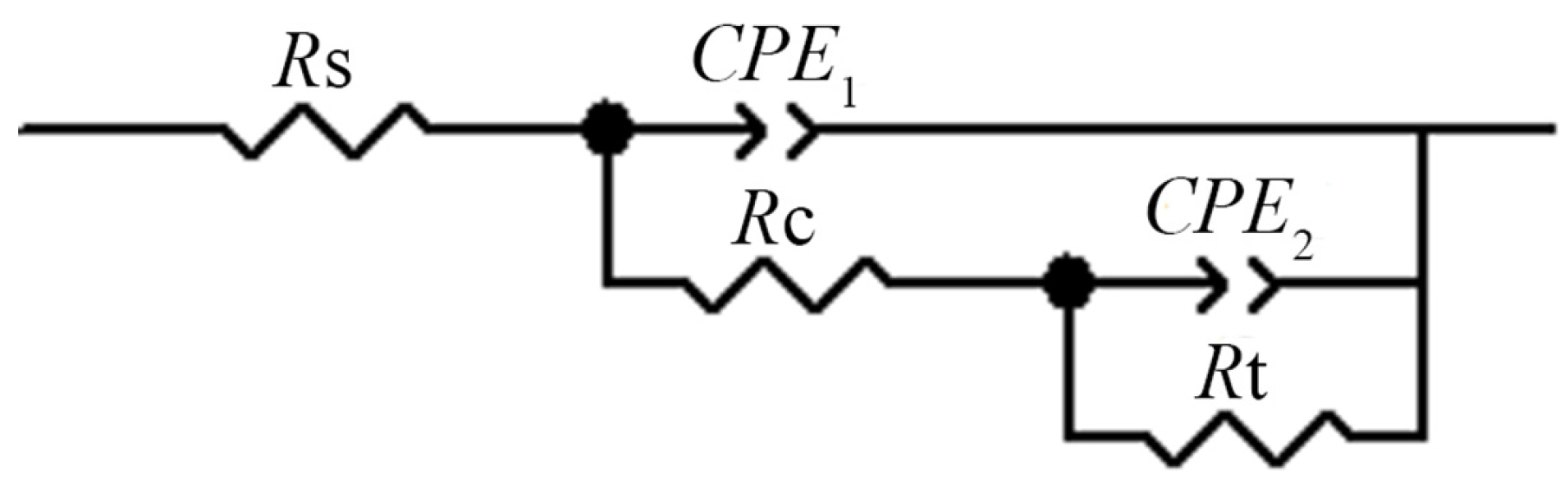
3.4. Electrochemical Impedance
4. Conclusions
- The corrosion resistance of the Ni-P coating is related to the porosity of the coating and the components of the aggressive ions. Reducing the porosity of the Ni-P coating, alleviating the corrosive ions, and developing corrosion inhibitors can decrease the corrosion process.
- Cl− and are favorable for the formation of corrosion pits due to their chaotropic nature and the smaller ionic radius of Cl−.
- accelerates the corrosion process by invading the bottom of the pits in the Ni-P coating and destroying the corrosion product layer.
- acts as an inhibitor by absorbing on the surface of the samples.
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Finšgar, M.; Jackson, J. Application of corrosion inhibitors for steels in acidic media for the oil and gas industry: A review. Corros. Sci. 2014, 86, 17–41. [Google Scholar] [CrossRef]
- Zheng, Y.G.; Liu, G.Q.; Zhang, Y.M.; Hu, H.X.; Song, Q.N. Corrosion failure analysis of a condenser on the top of benzene tower in styrene unit. J. Fail. Anal. Prev. 2014, 14, 286–295. [Google Scholar] [CrossRef]
- Zeinalov, E.B.; Abbasov, V.M.; Alieva, L.I. Petroleum acids and corrosion. Petrol. Chem. 2009, 49, 185–192. [Google Scholar] [CrossRef]
- Miller, W.S.; Zhuang, L.; Bottema, J.; Wittebrood, A.J.; de Smet, P.; Haszler, A.; Vieregge, A. Recent development in aluminum alloys for the automotive industry. Mater. Sci. Eng. A 2000, 280, 37–49. [Google Scholar] [CrossRef]
- Ahmad, Z. A review of corrosion and pitting resistance of Al 6061 and 6013 silicon carbide composites in neutral salt solution and seawater. Corros. Rev. 2001, 19, 119–156. [Google Scholar] [CrossRef]
- Jafarzadeh, K.; Shahrabi, T.; Oskouei, A. A Novel approach using EIS to study flow accelerated pitting corrosion of AA5083-H321 aluminum-magnesium alloy in NaCl solution. J. Appl. Electrochem. 2009, 39, 1725–1731. [Google Scholar] [CrossRef]
- Sakairi, M.; Sasaki, R.; Kaneko, A.; Seki, Y.; Nagasawa, D. Evaluation of metal cation effects on galvanic corrosion behavior of the A5052 aluminum alloy in low chloride ion containing solutions by electrochemical noise impedance. Electrochim. Acta 2014, 131, 123–129. [Google Scholar] [CrossRef]
- Shahidi, M.; Gholamhosseinzadeh, M.R. Electrochemical evaluation of AA6061 aluminum alloy corrosion in citric acid solution without and with chloride ions. J. Electroanal. Chem. 2015, 757, 8–17. [Google Scholar] [CrossRef]
- Wang, C.Y.; Wen, G.W.; Wu, G.H. Improving corrosion resistance of aluminum metal matrix composites using cerium sealed electroless Ni-P coatings. Corros. Eng. Sci. Technol. 2011, 46, 471–476. [Google Scholar] [CrossRef]
- Sadreddini, S.; Afshar, A. Corrosion resistance enhancement of Ni-P-nano SiO2 composite coatings on aluminum. Appl. Surf. Sci. 2014, 303, 125–130. [Google Scholar] [CrossRef]
- Qin, W. Microstructure and corrosion behavior of electroless Ni-P coatings on 6061 aluminum alloys. J. Coat. Technol. Res. 2011, 8, 135–139. [Google Scholar] [CrossRef]
- Sridhar, N.; Udaya Bhat, K. Effect of deposition time on the morphological features and corrosion resistance of electroless Ni-high P coatings on aluminum. J. Mater. 2013, 2013, 1–7. [Google Scholar] [CrossRef]
- Fetohi, A.E.; Hameed, R.M.A.; El-Khatib, K.M. Development of electroless Ni-P modified aluminum substrates in a simulated fuel cell environment. J. Ind. Eng. Chem. 2015, 30, 239–248. [Google Scholar] [CrossRef]
- Yang, H.; Gao, Y.; Qin, W.; Li, Y. Microstructure and corrosion behavior of electroless Ni-P on sprayed Al-Ce coating of 3003 aluminum alloy. Surf. Coat. Technol. 2015, 281, 176–183. [Google Scholar] [CrossRef]
- Elsener, B.; Crobu, M.; Scorciapino, M.A.; Rossi, A. Electroless deposited Ni-P alloys: Corrosion resistance mechanism. J. Appl. Electrochem. 2008, 38, 1053–1060. [Google Scholar] [CrossRef]
- Li, X.W.; Chen, Z.L.; Hou, H.B.; Hao, L. Corrosion behaviour of electroless Ni-P coatings in simulated acid rain. Corros. Eng. Sci. Technol. 2010, 45, 277–281. [Google Scholar] [CrossRef]
- Liu, G.; Yang, L.; Wang, L.; Wang, S.; Chongyang, L.; Wang, J. Corrosion behavior of electroless deposited Ni-Cu-P coating in flue gas condensate. Surf. Coat. Technol. 2010, 204, 3382–3386. [Google Scholar] [CrossRef]
- Yang, H.J.; Gao, Y.M.; Qin, W.C.; Ma, S.Q.; Wei, Y.K. Investigation of corrosion behavior of 3003 aluminum alloy in flue gas condensate. Mater. Corros. 2016. [Google Scholar] [CrossRef]
- Standard Specification for Autocatalytic (Electroless) Nickel-Phosphorus Coatings on Metal; ASTM International: West Conshohocken, PA, USA, 2004.
- Flis, J.; Duquette, D.J. Effect of Phosphorus on anodic dissolution and passivation of nickel in near-neutral solutions. Corrosion 1985, 41, 700–706. [Google Scholar] [CrossRef]
- Balaraju, J.N.; Selvi, V.E.; Grips, V.K.W.; Rajam, K.S. Electrochemical studies on electroless ternary and quaternary Ni-P based alloys. Electrochim. Acta 2006, 52, 1064–1074. [Google Scholar] [CrossRef]
- Abdel Hameed, R.M.; Fekry, A.M. Electrochemical impedance studies of modified Ni-P and Ni-Cu-P deposits in alkaline medium. Electrochim. Acta 2010, 55, 5922–5929. [Google Scholar] [CrossRef]
- Mishra, A.K.; Balasubramaniam, R. Corrosion inhibition of aluminium by rare earth chlorides. Mater. Chem. Phys. 2007, 103, 385–393. [Google Scholar] [CrossRef]
- Song, Y.W.; Shan, D.Y.; Han, E.H. Corrosion behaviors of electroless plating Ni-P coatings deposited on magnesium alloys in artificial sweat solution. Electrochim. Acta 2007, 53, 2009–2015. [Google Scholar] [CrossRef]
- Leontidis, E. Hofmeister anion effects on surfactant self-assembly and the formation of mesoporous solids. Curr. Opin. Colloid 2002, 7, 81–91. [Google Scholar] [CrossRef]
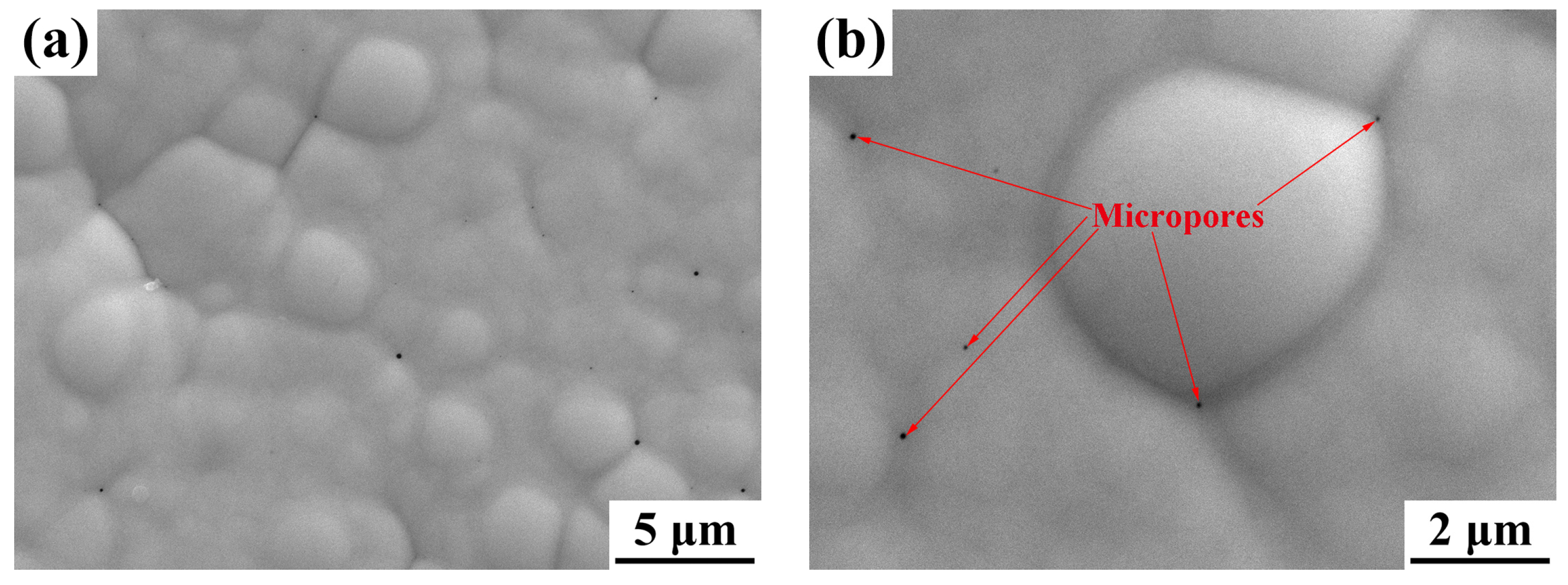
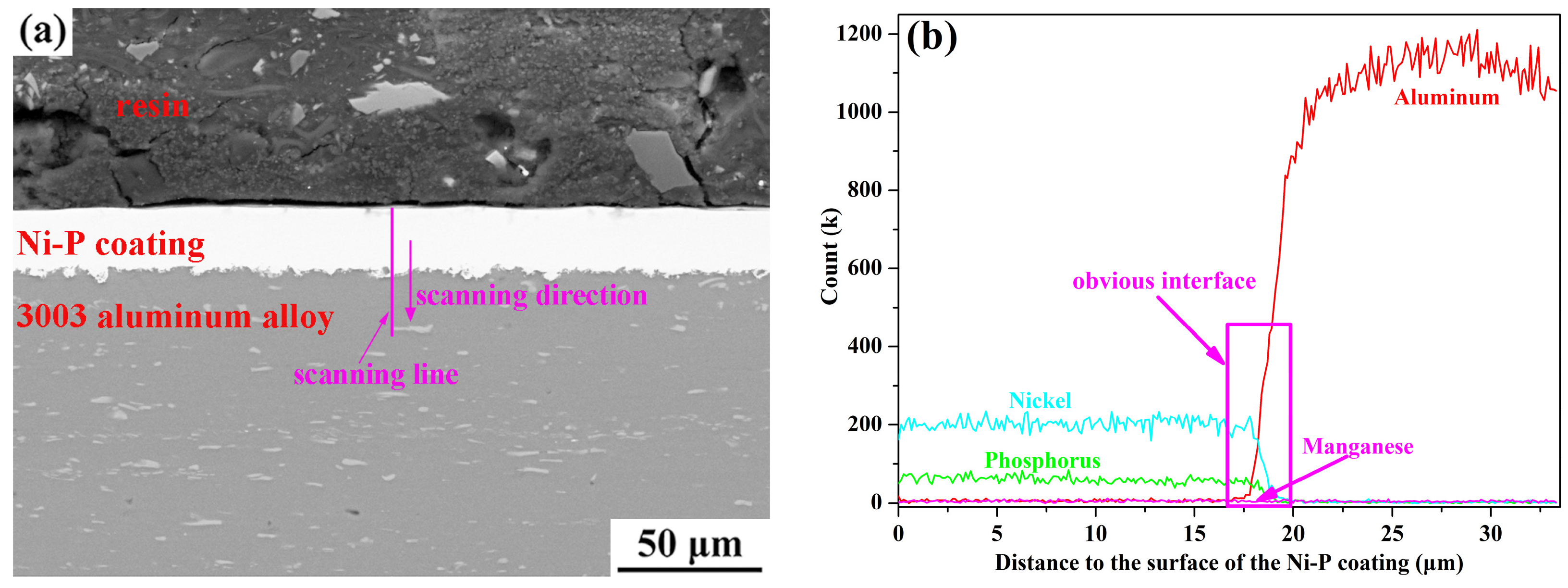
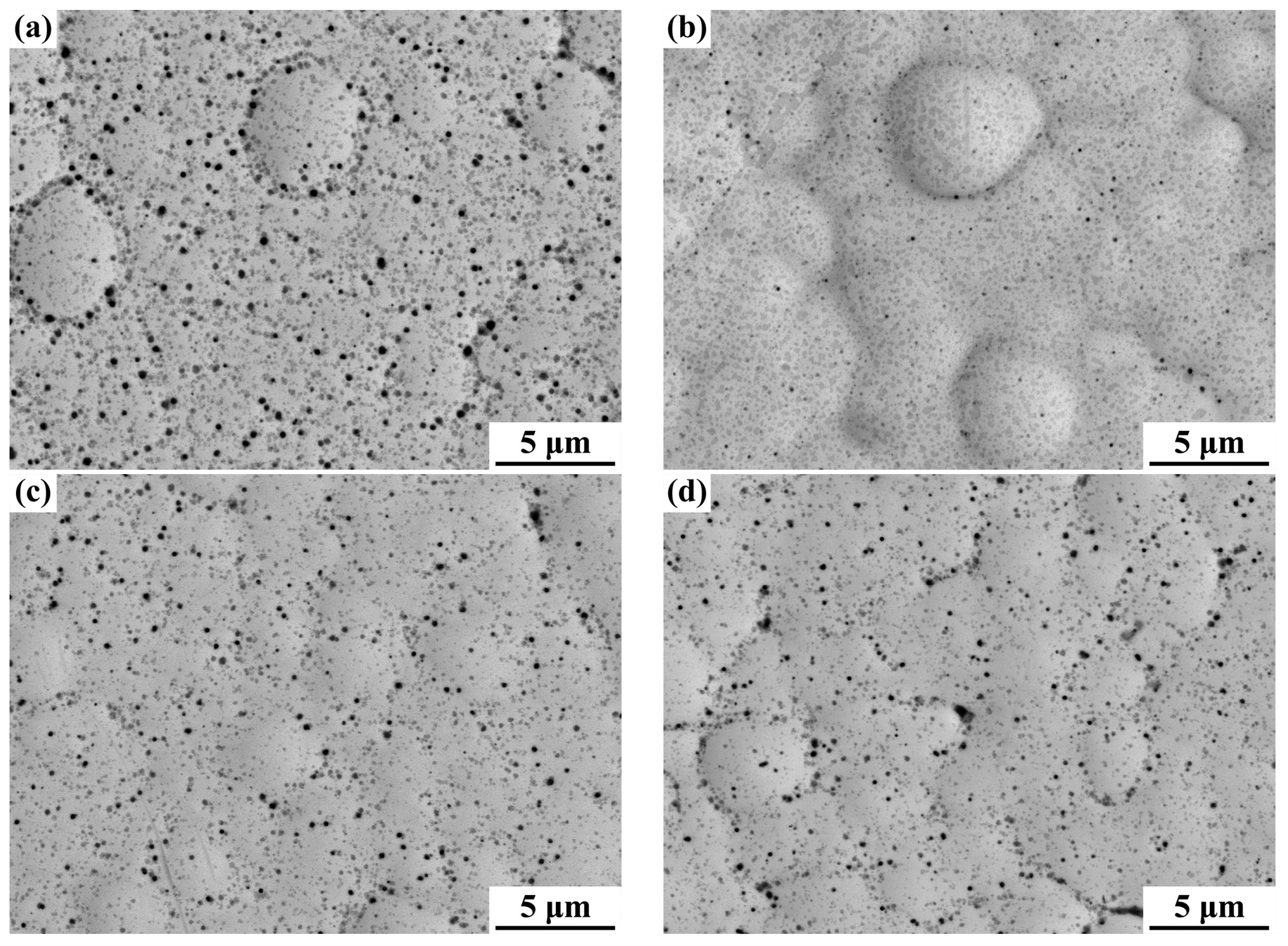
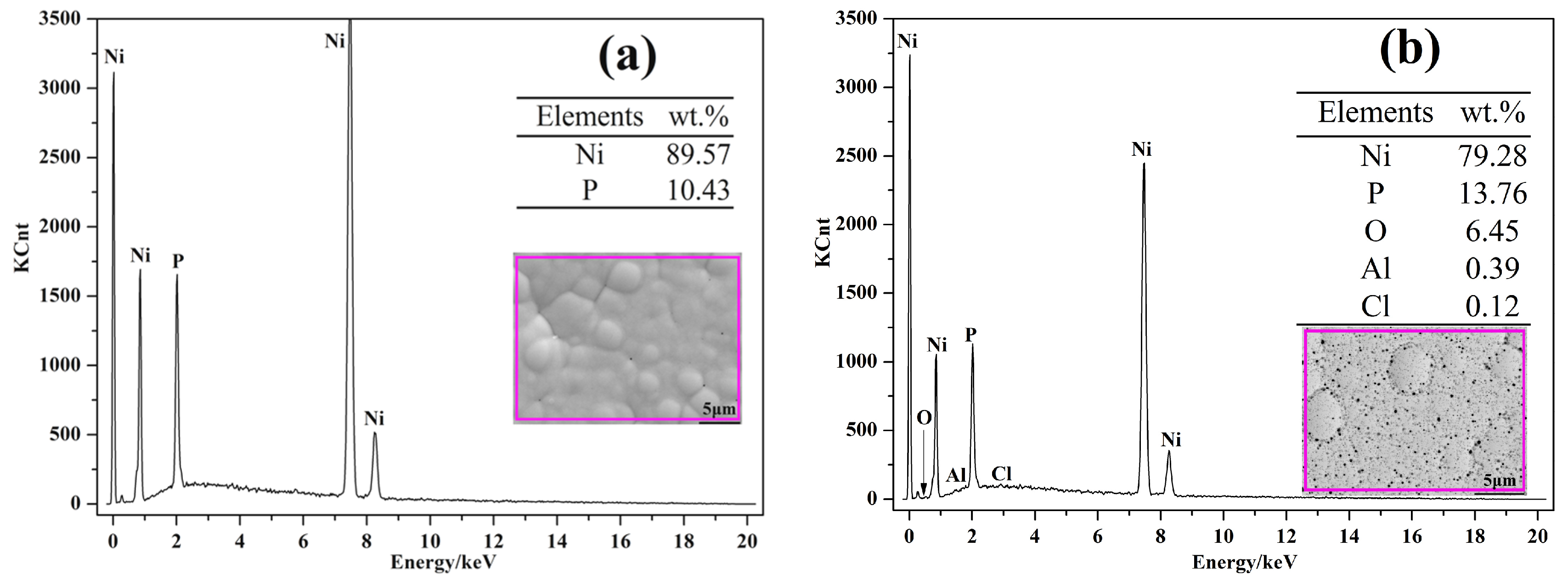
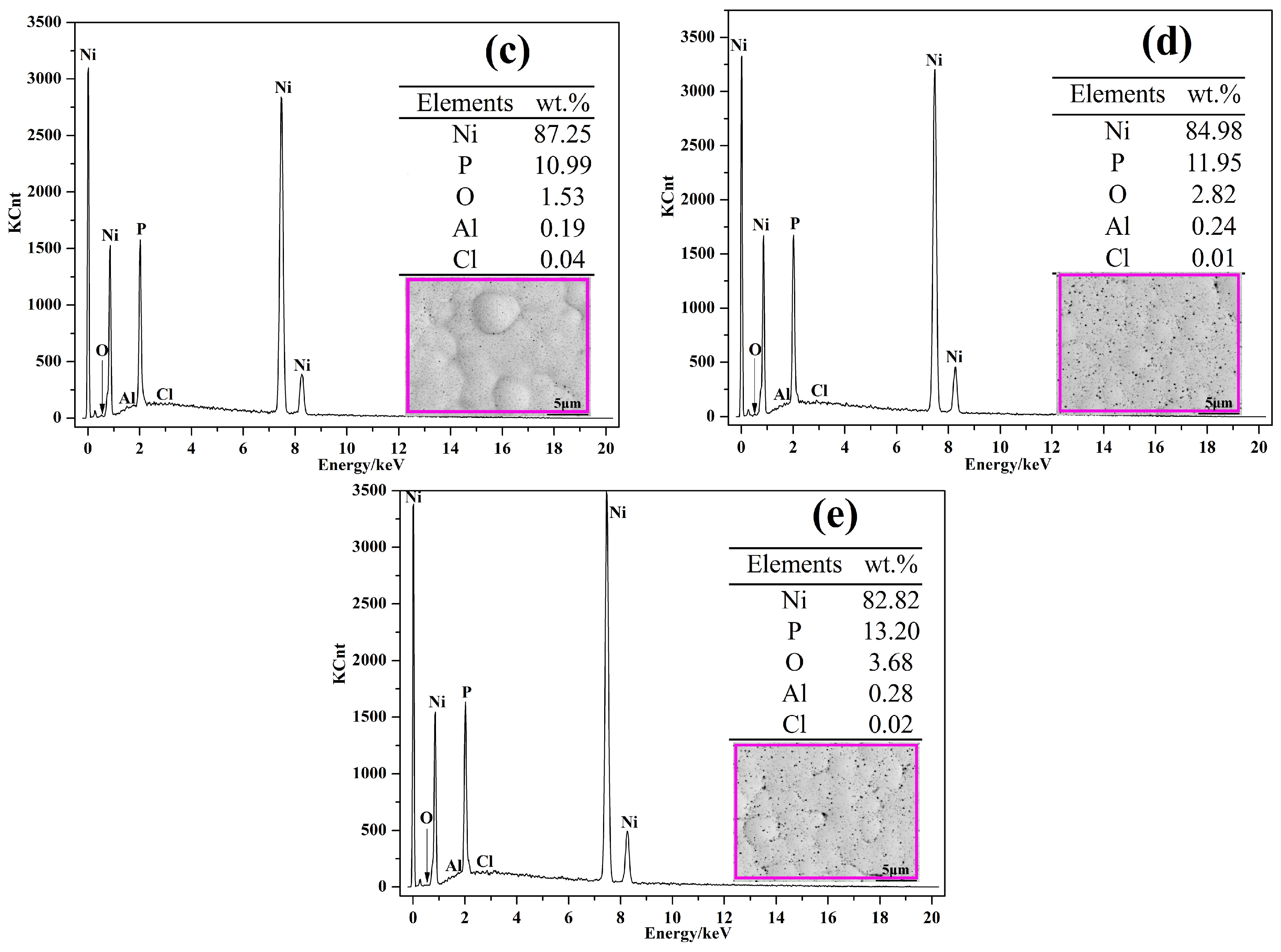
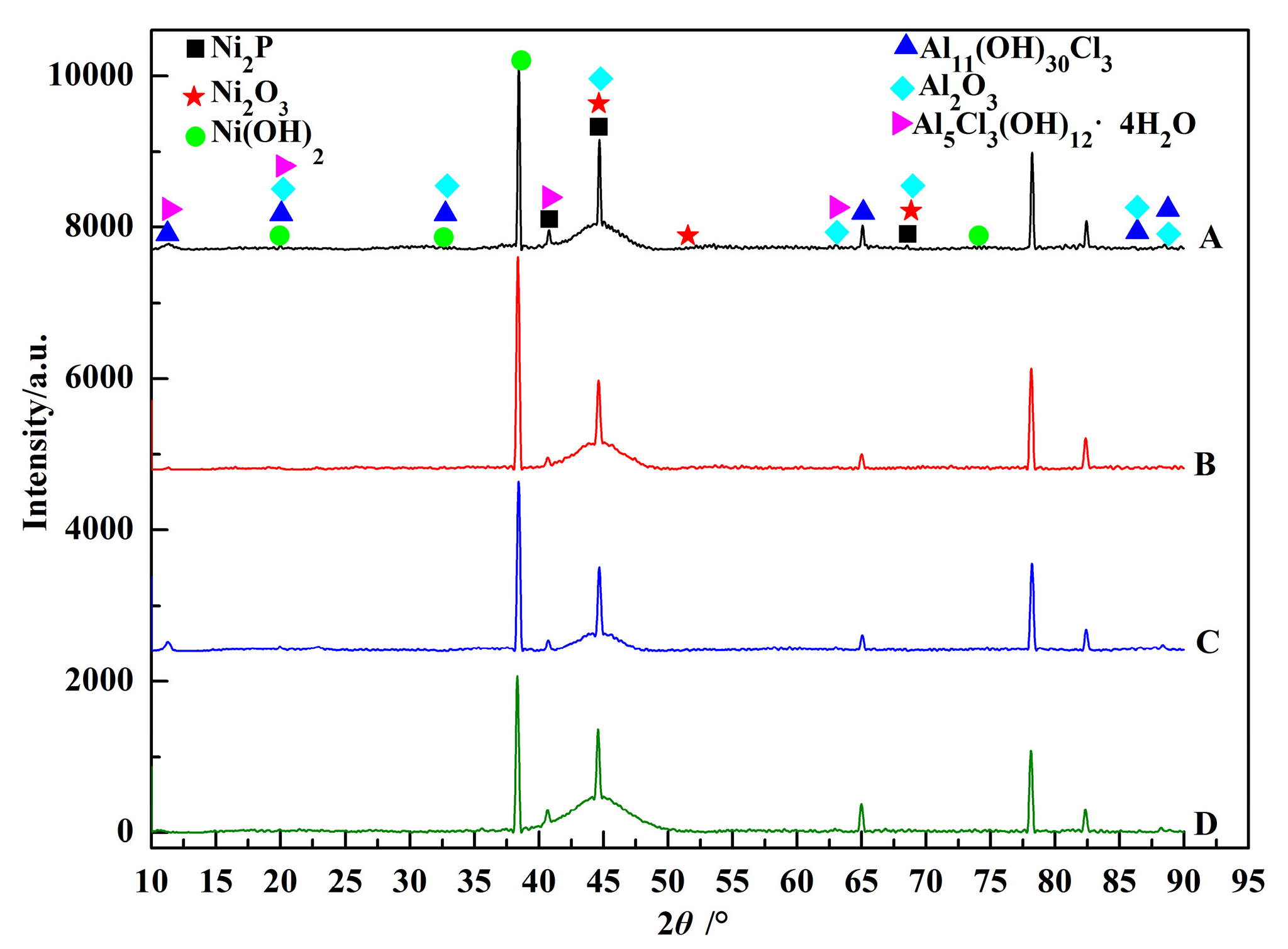

| Elements | wt % | Elements | wt % |
|---|---|---|---|
| Mn | 1.04 | Cu | 0.057 |
| Fe | 0.56 | Zn | 0.0094 |
| Si | 0.042 | Al | Balance |
| Solutions | Cl− | H2O | pH | |||
|---|---|---|---|---|---|---|
| A | 10 | 20 | 4.07 | 2.98 | Balance | 3.12 |
| B | 10 | – | 4.07 | 2.98 | Balance | 3.12 |
| C | 10 | 20 | – | 2.98 | Balance | 3.12 |
| D | 10 | 20 | – | – | Balance | 3.12 |
| Solutions | Rs/Ω·cm2 | CPE1/μF·cm−2 | Rt/Ω·cm2 |
|---|---|---|---|
| A | 720.0 | 57.47 | 3248 |
| B | 601.0 | 45.67 | 10,001 |
| C | 698.1 | 46.77 | 8414 |
| D | 710.3 | 50.20 | 6830 |
| Ions | η1/% | η2/% |
|---|---|---|
| −63.6 | −67.5 | |
| −60.9 | −61.4 | |
| 18.4 | 18.8 |
© 2017 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, H.; Gao, Y.; Qin, W. Investigation of the Corrosion Behavior of Electroless Ni-P Coating in Flue Gas Condensate. Coatings 2017, 7, 16. https://doi.org/10.3390/coatings7010016
Yang H, Gao Y, Qin W. Investigation of the Corrosion Behavior of Electroless Ni-P Coating in Flue Gas Condensate. Coatings. 2017; 7(1):16. https://doi.org/10.3390/coatings7010016
Chicago/Turabian StyleYang, Hejie, Yimin Gao, and Weichao Qin. 2017. "Investigation of the Corrosion Behavior of Electroless Ni-P Coating in Flue Gas Condensate" Coatings 7, no. 1: 16. https://doi.org/10.3390/coatings7010016






