Influence of Sludge Initial pH on Bioleaching of Excess Sludge to Improve Dewatering Performance
Abstract
:1. Introduction
2. Materials and Methods
2.1. Original Sludge Properties
2.2. Bioleaching Experiments
2.3. Preparation of Inoculum
2.4. Microbial Community Analysis
2.4.1. DNA Extraction and PCR Amplification
2.4.2. Illumina HiSeq Sequencing of PCR Products
2.4.3. Data Analysis
- Raw data filtering: raw data was mass filtered using Trimmomatic software to obtain quality data after quality control.
- Clean data PE reads: raw contigs were obtained by splicing each pair of PE reads using software, including Mothur and Flash.
- Raw contig sequence quality filtering: clean contigs were obtained using Mothur for quality control and filtering of stitched sequences.
- Clean contig sample allocation: according to barcode and primer information, Qiime software was used to assign the spliced sequences to their corresponding samples.
2.4.4. Diversity Calculation
3. Results and Discussion
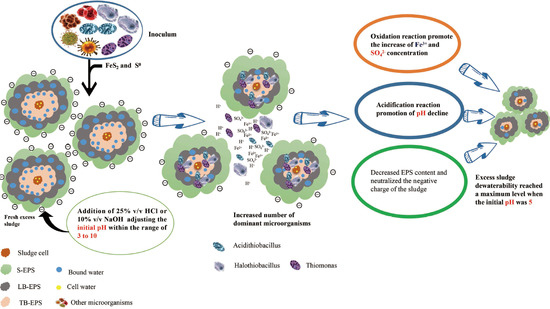
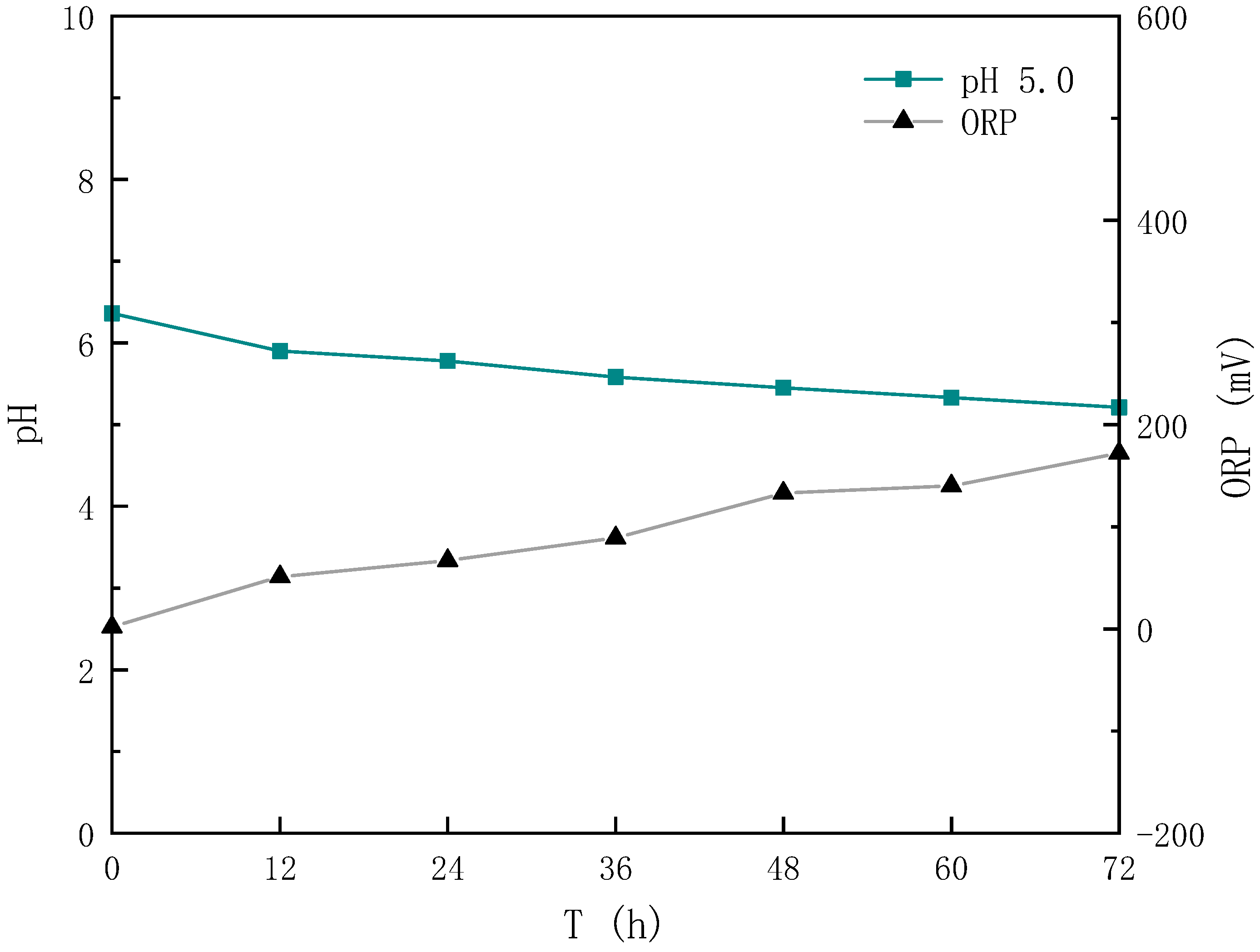
3.1. Effect of Different Sludge Initial pH Values on Acidification and Oxidation during Sludge Bioleaching
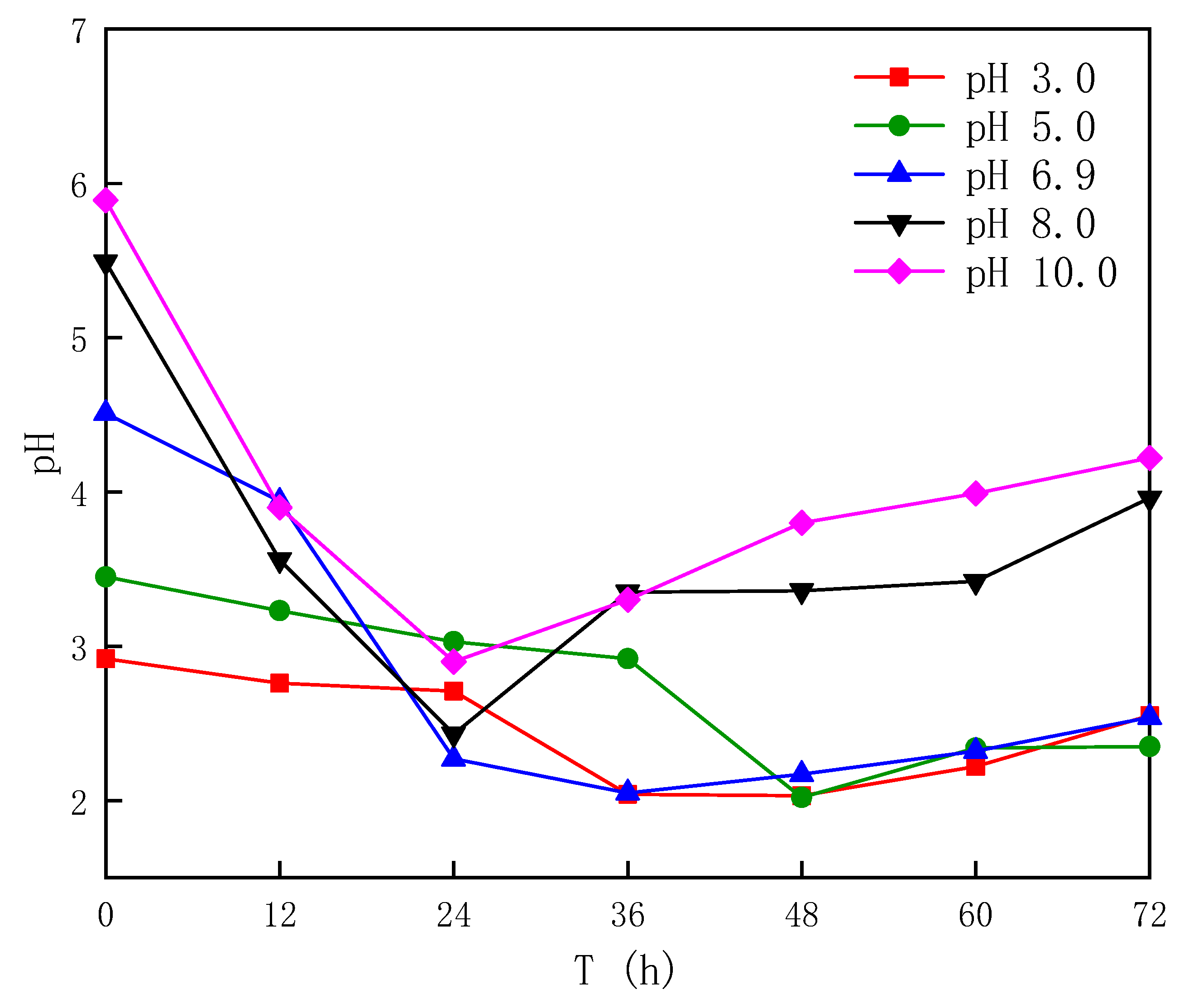
3.1.1. Effect of Different Sludge Initial pH Values on Acidification
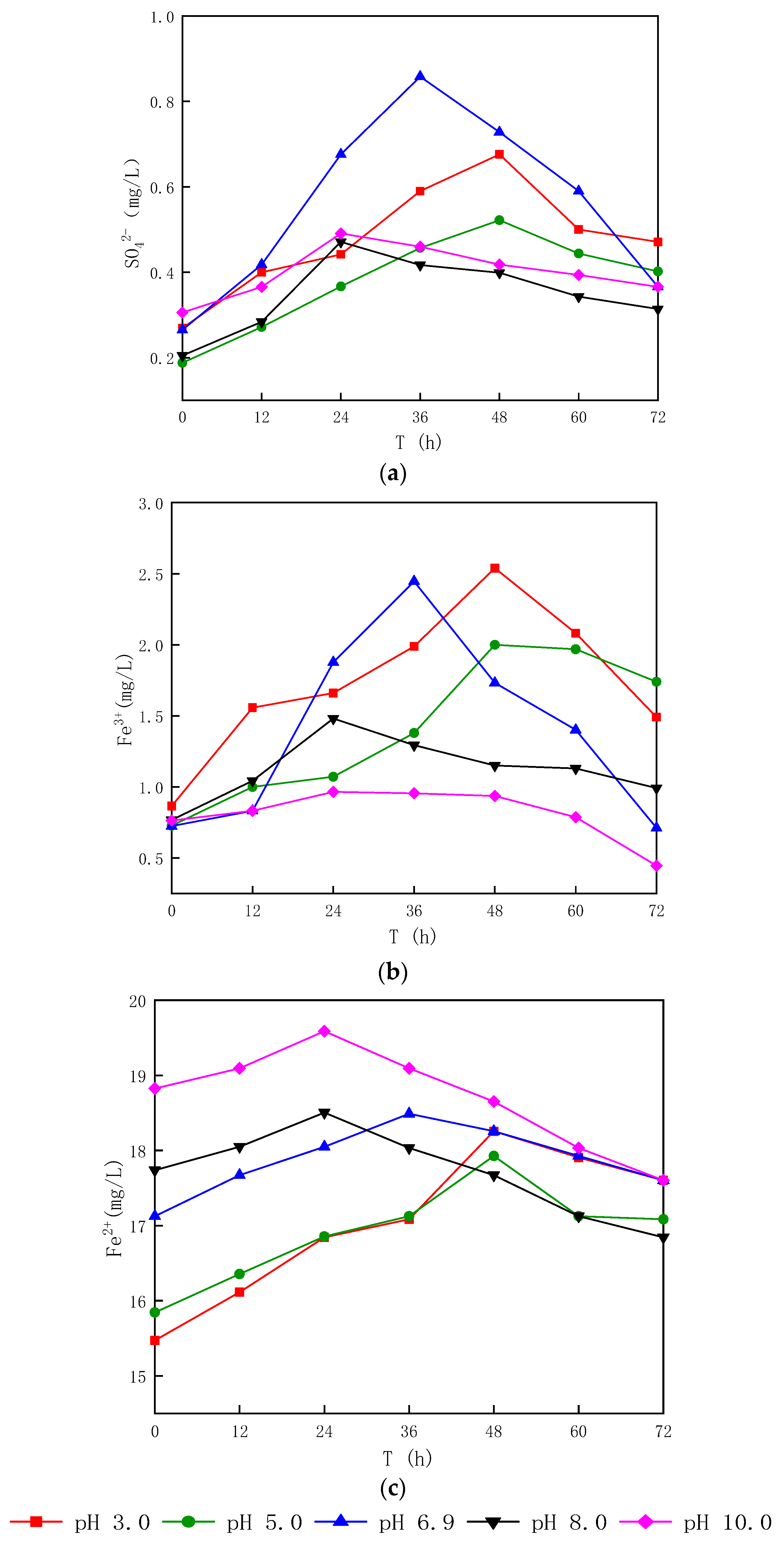
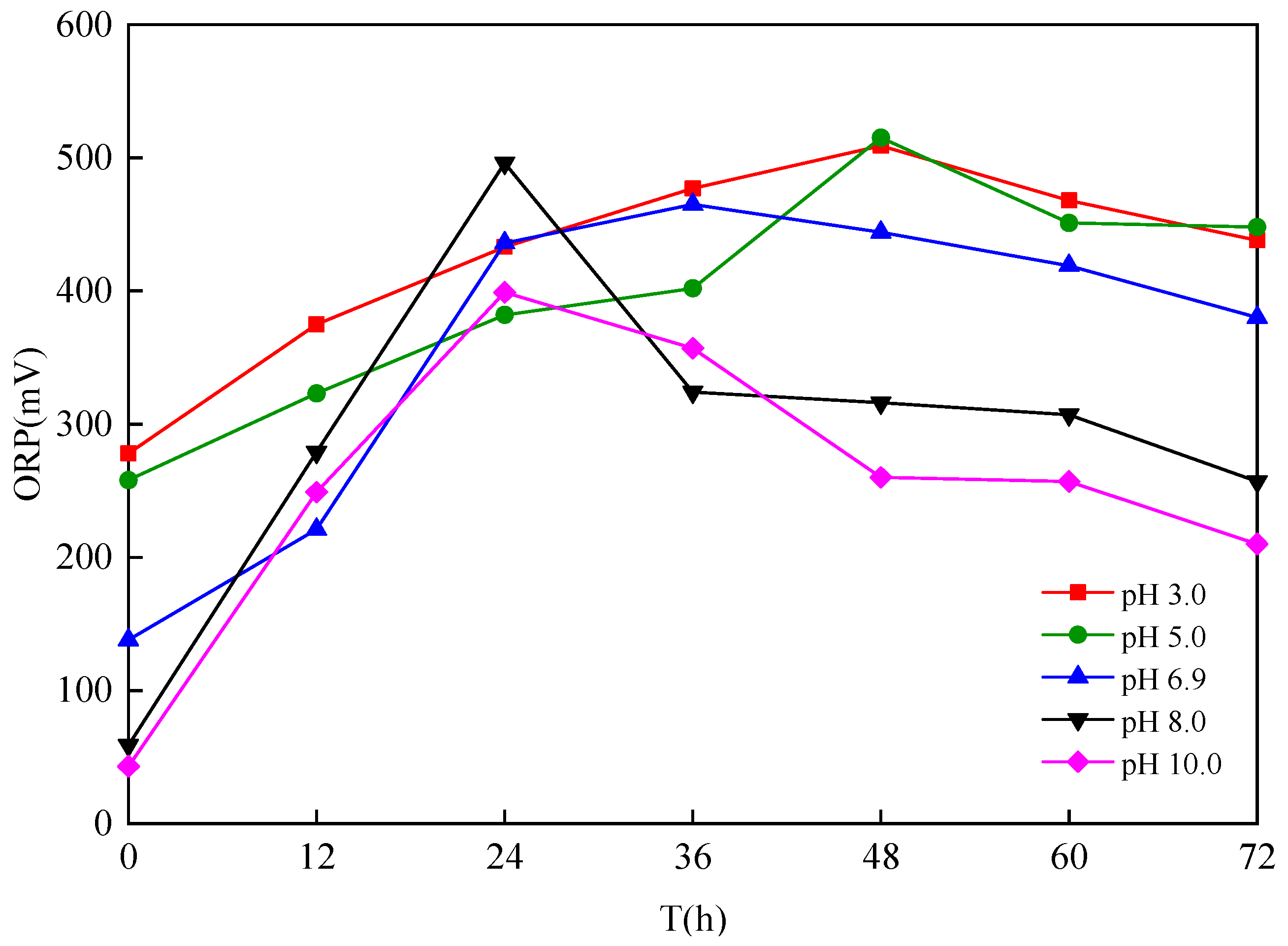
3.1.2. Effect of Different Sludge Initial pH Values on Oxidation
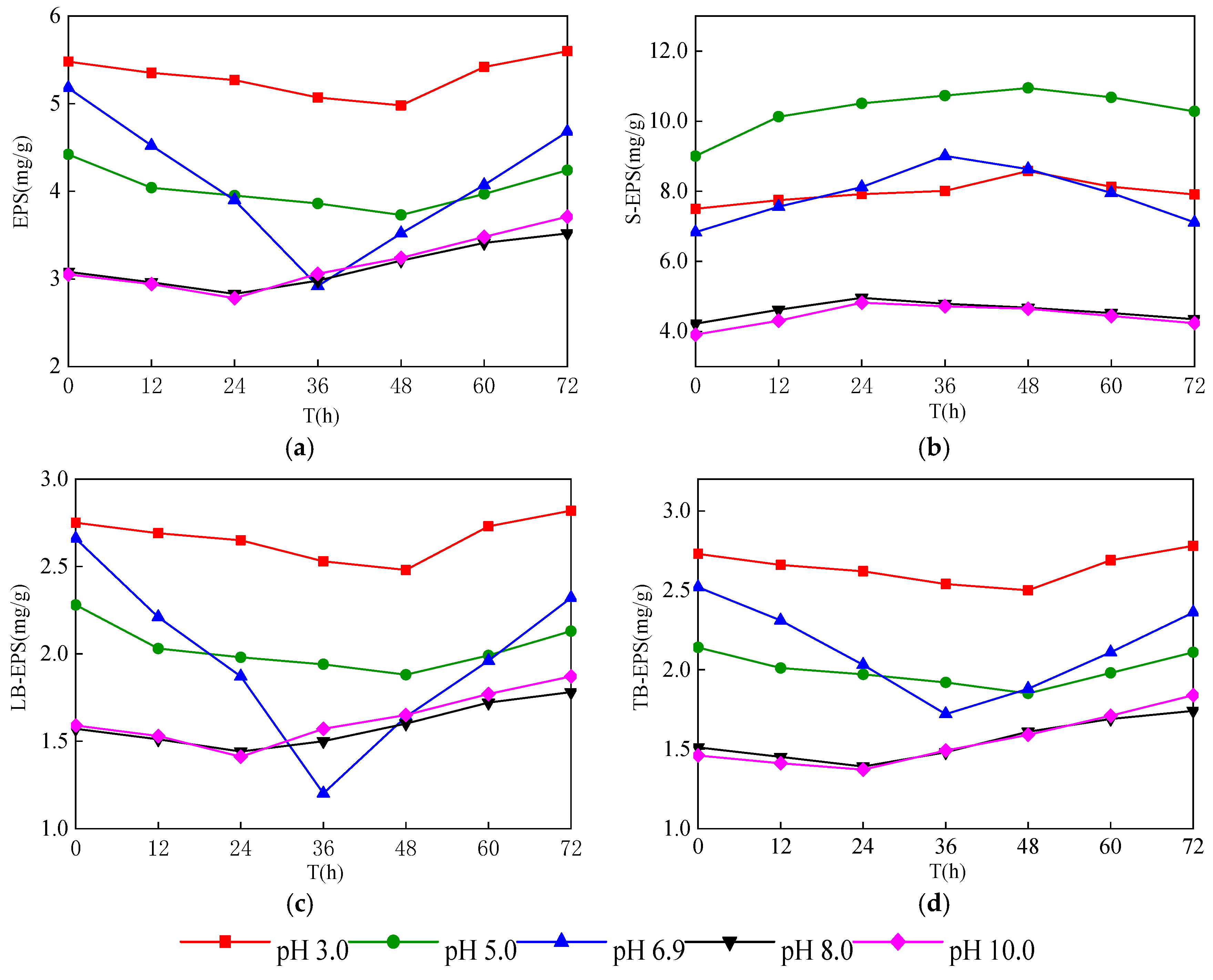
3.2. Change of EPS Content
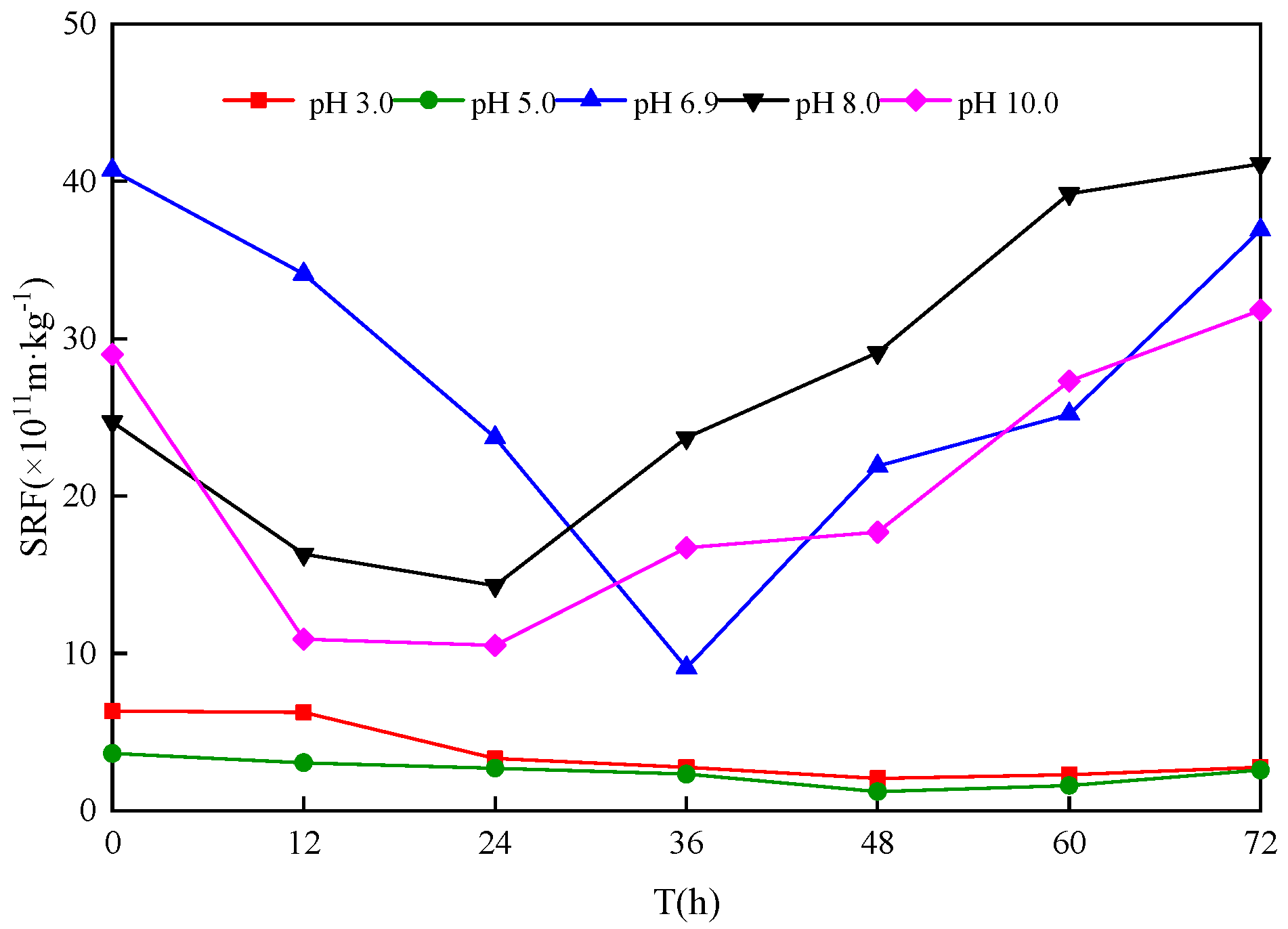
3.3. Effect of Different Sludge Initial pH Values on Sludge Dewatering Performance
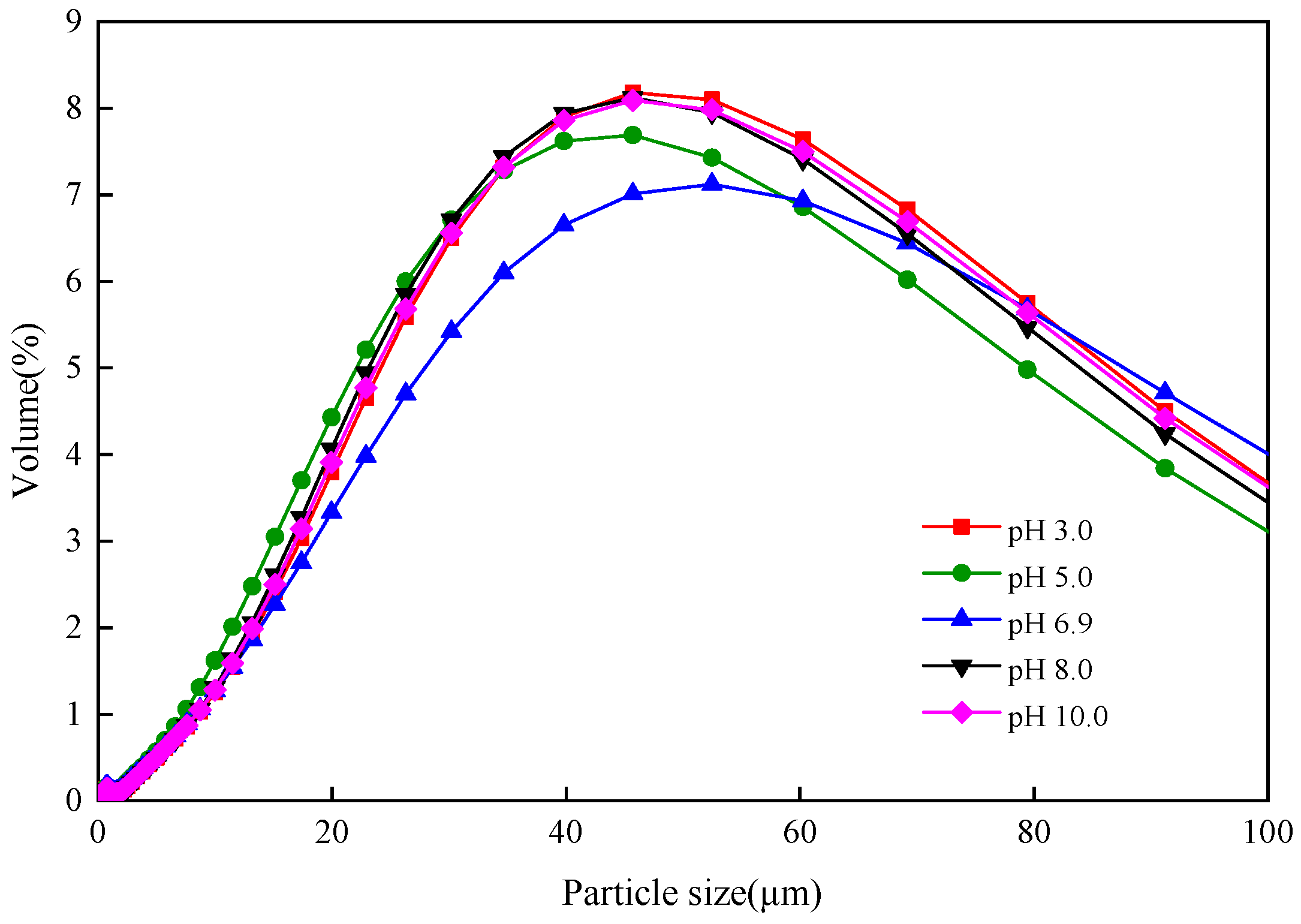
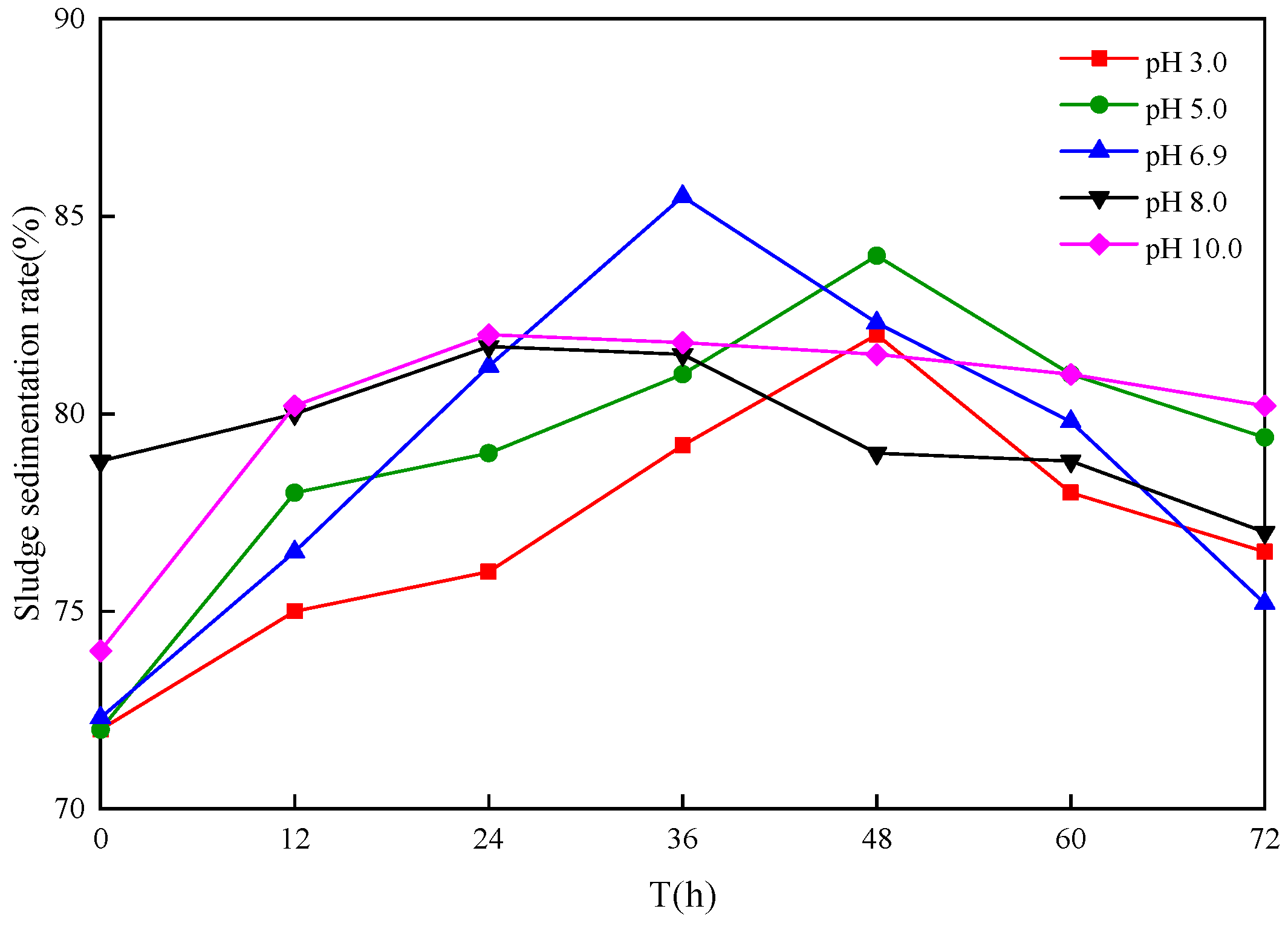
3.4. Influence of Different Sludge Initial pH Values on Settling Sludge Performance
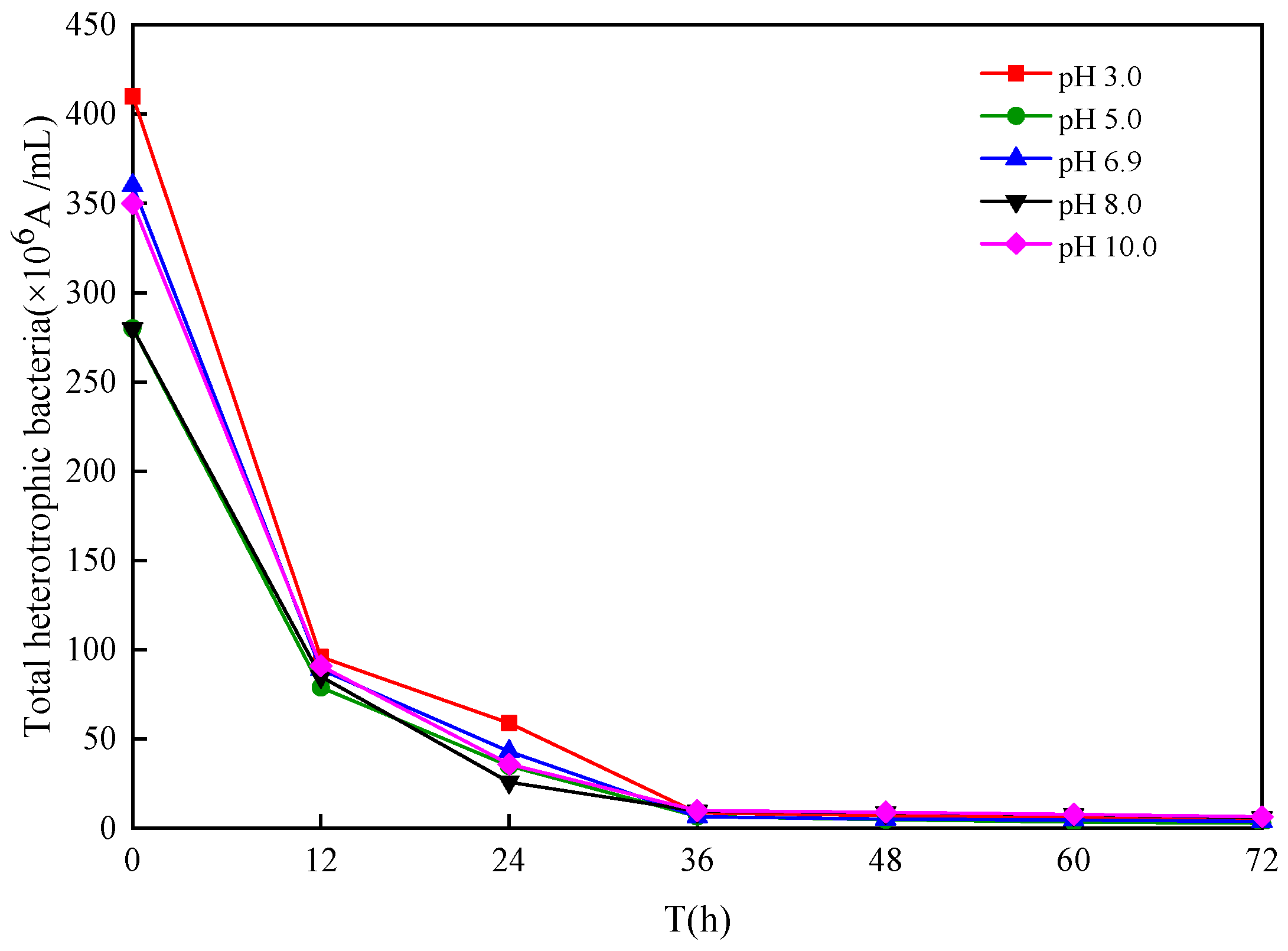
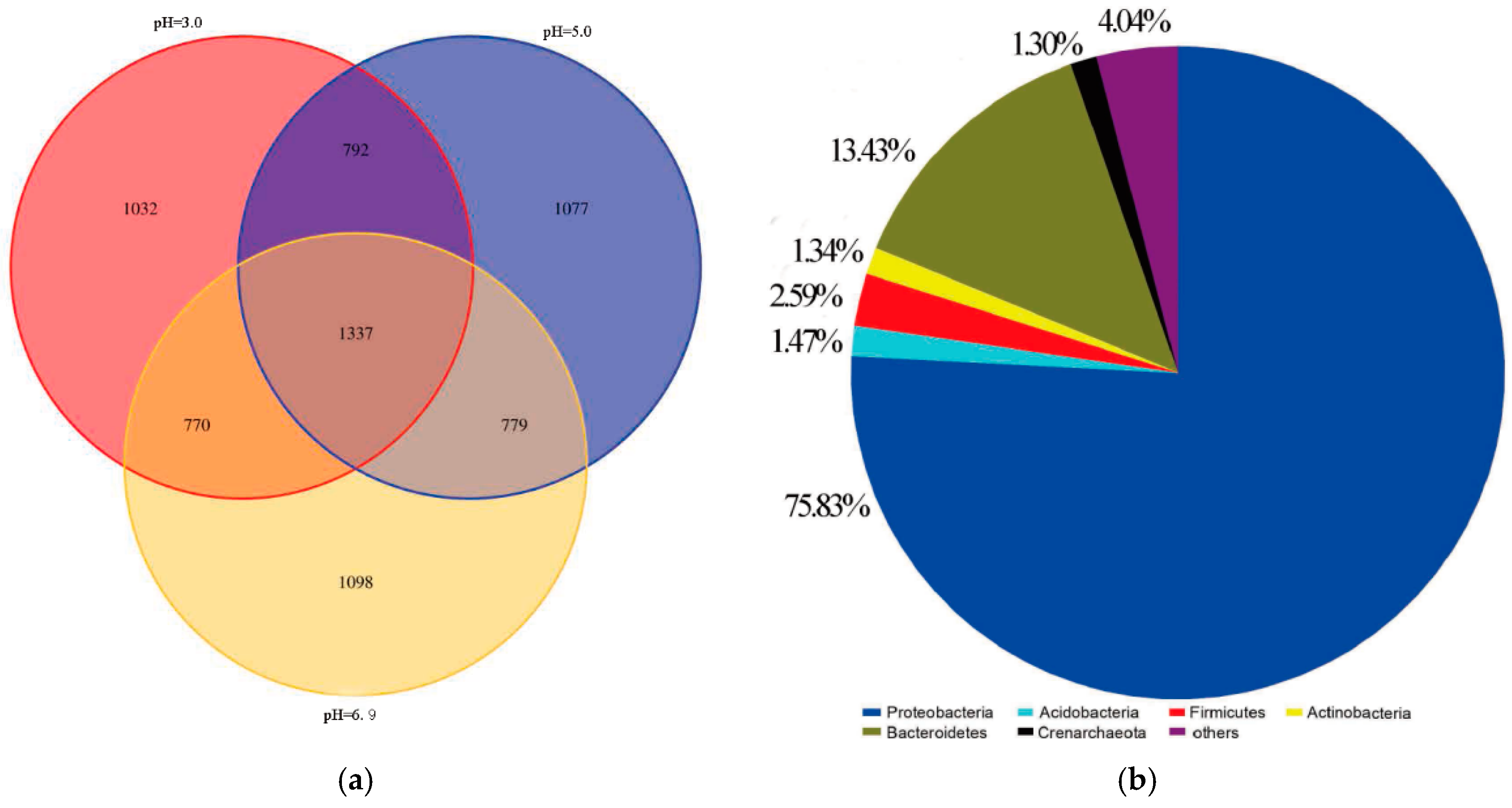
3.5. Microbial Community Structure
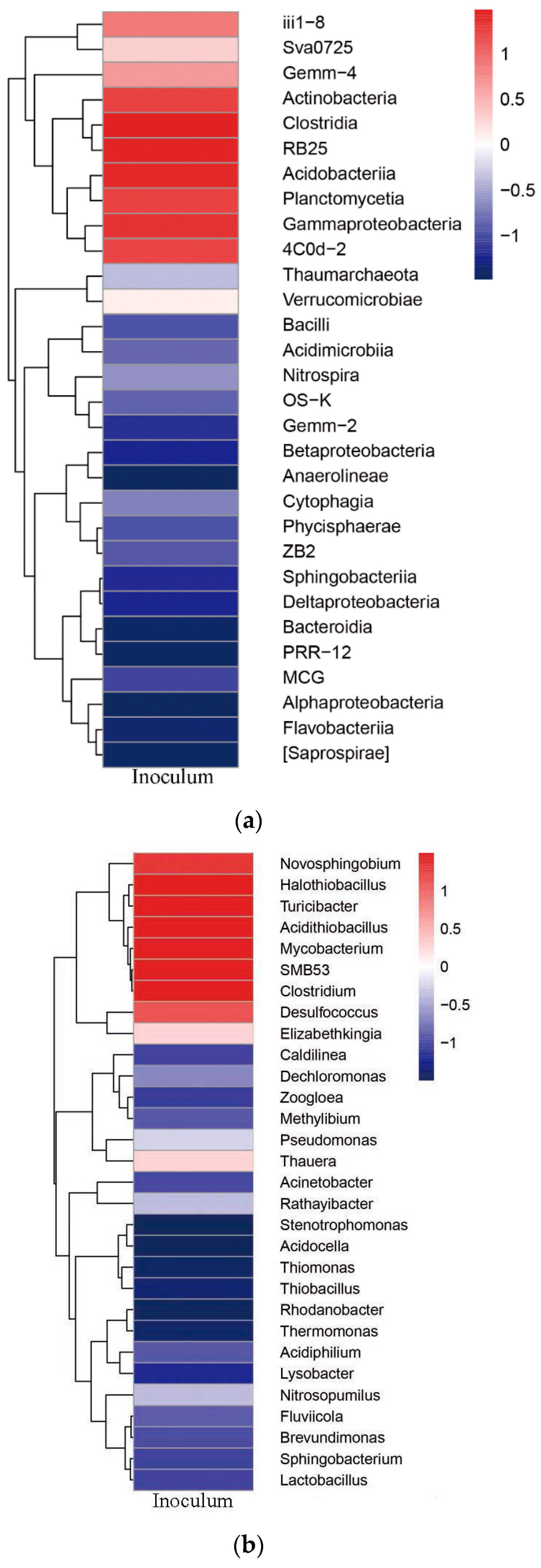
Microbial Community Structure of the Inoculum
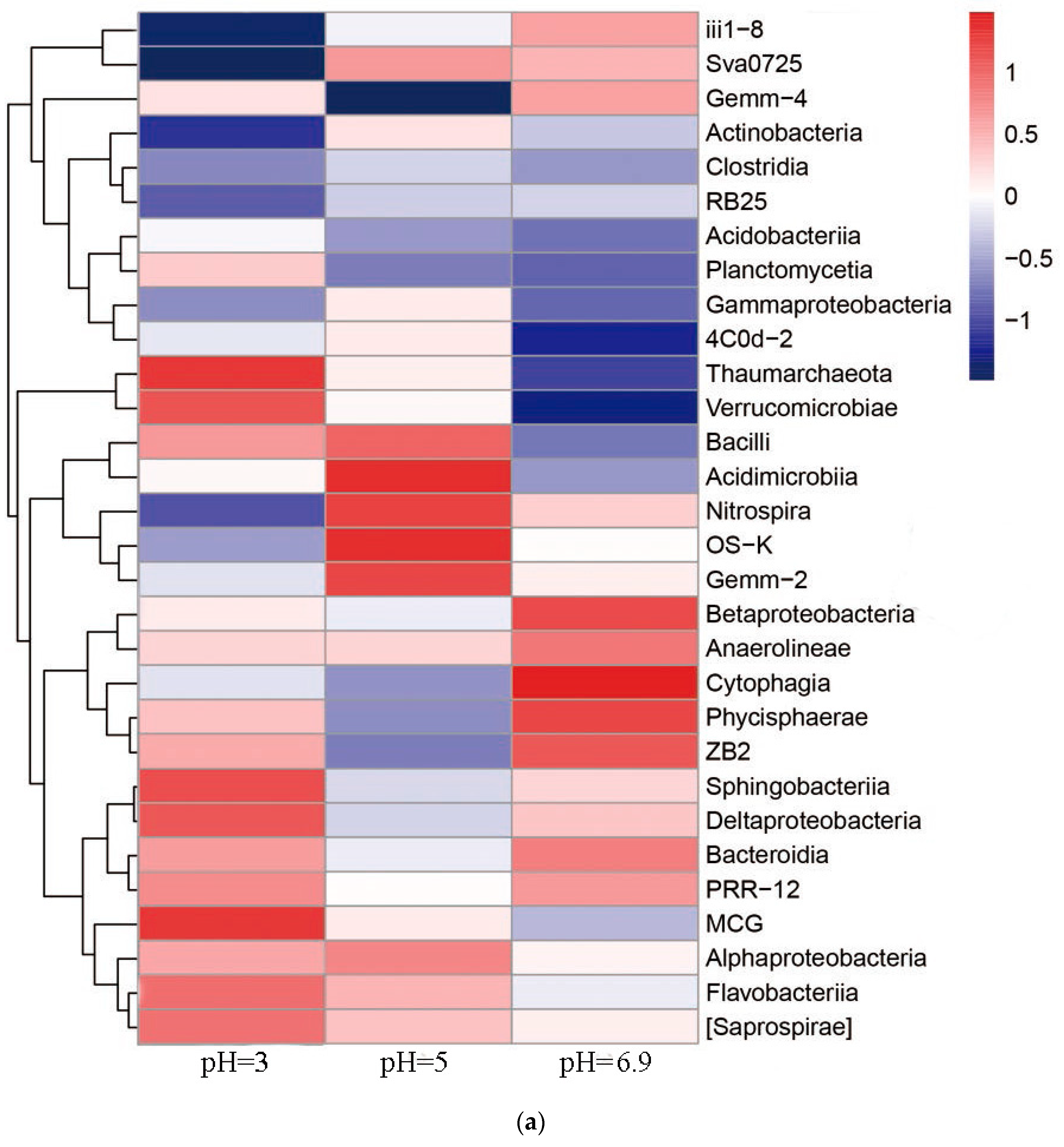
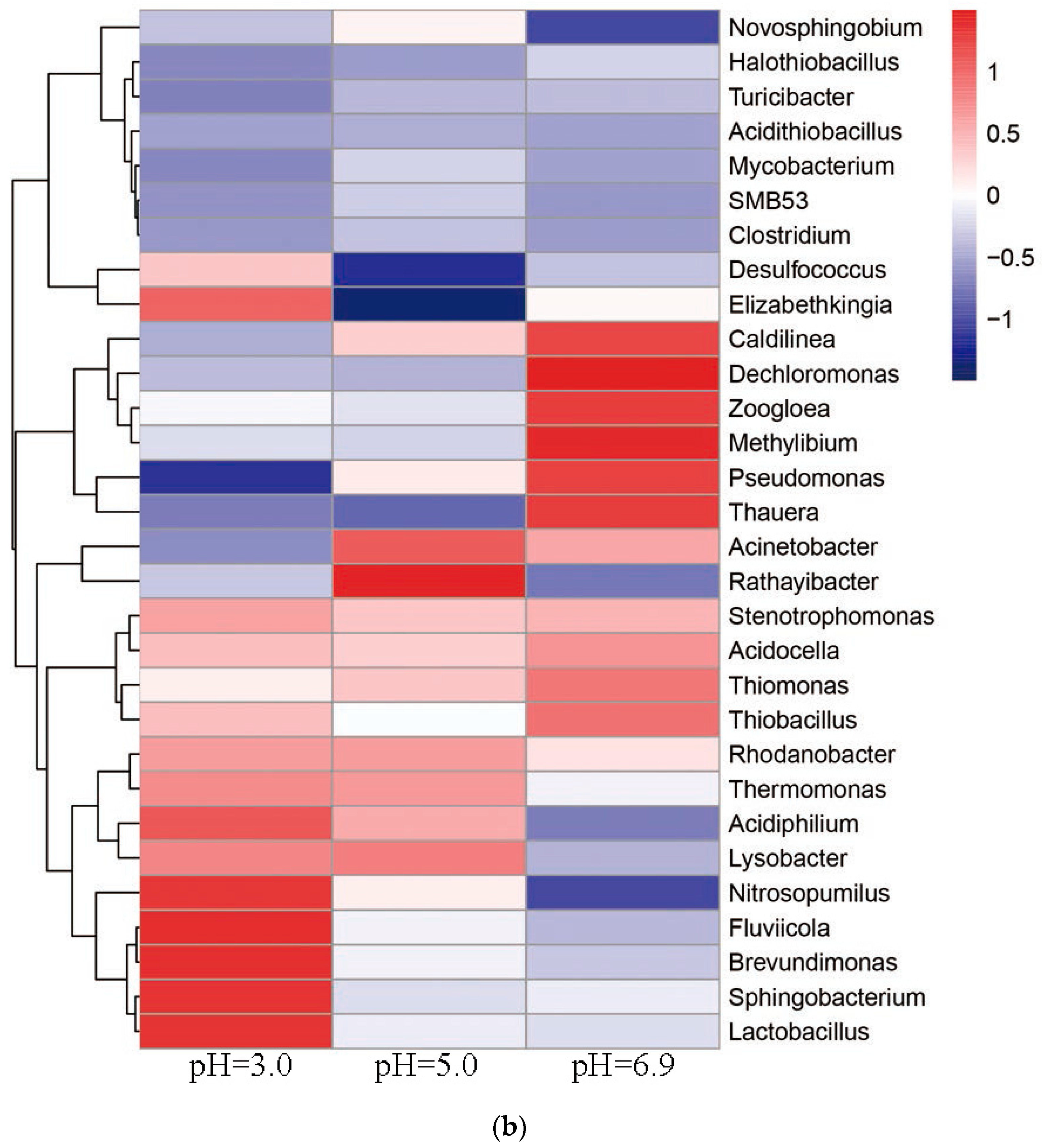
3.6. Relationship between Microbial Community Structure Succession and Sludge Initial pH
3.6.1. Changes of the Microbial Community
3.6.2. Microbial Similarity Analysis
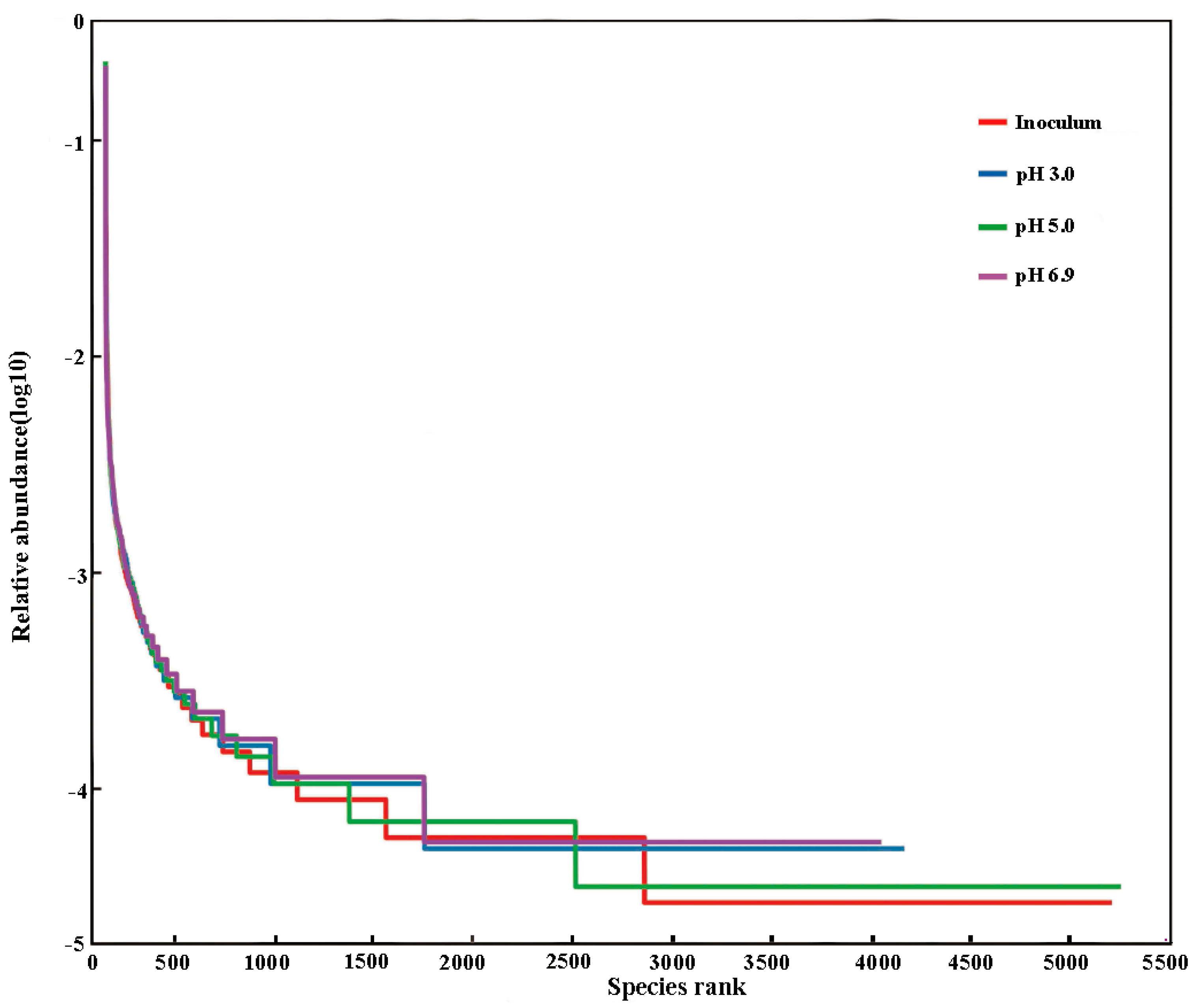
3.6.3. Analysis of Microbial Community Diversity
4. Conclusions
- (1)
- After bioleaching, the pH of the sludge decreased from (6.62~7.04) to (2.02~2.90), and the decrease rate was 56.06~70.07%, with the energy source reductive sulfur oxidation rate increasing by 34.64~63.28% and the Fe3+ concentration increasing by 20.91~65.89%.
- (2)
- After bioleaching, the SRF of sludge decreased to the lowest value (0.12~1.43) × 1012 m/kg from (4.05~4.83) × 1012 m/kg. When the sludge initial pH was 5.0 and the leaching duration was 48 h, the SRF decreased from 14.50 × 1011 m/kg to 0.12 × 1012 m/kg, showing a 91.66% reduction, and the dewatering performance of the sludge reached an optimum state.
- (3)
- The dominant microorganisms did not show significant variation in relative abundance at the phylum level with variation in the sludge initial pH; however, the community did differ significantly at the genus level. When the initial pH value was 5.0, after 48 h of bioleaching, autotrophic bacteria that can oxidize S0 and Fe2+, namely Thiomonas, Halothiobacillus and Acidithiobacillus, dominated the community. The relative abundances of these genera were 4.91%, 2.79% and 0.80% respectively. At this time, the ORP and pH reached the maximum and minimum values, respectively, compared with the blank test results. This shows that the acidification and oxidation that occurs during the bioleaching of sludge may be due to the synergistic effects of such microbial community members, which was also the fundamental reason for the improved sludge dewatering performance.
Author Contributions
Funding
Conflicts of Interest
References
- GEP Research. Global and China Sludge Treatment and Disposal Industry Development Research Report; GER Research, Dezhong Environmental Consulting: Beijing, China, 2018. [Google Scholar]
- Liu, C.; Zhang, P.; Jiang, J.; Zeng, C.; Huang, Y.; Xu, G. sewage sludge conditioning by bioleaching combined with fenton-like oxidation. Environ. Sci. 2015, 36, 333. [Google Scholar]
- Tuan, P.A.; Sillanpää, M. Effect of freeze/thaw conditions, polyelectrolyte addition, and sludge loading on sludge electro-dewatering process. Chem. Eng. J. 2010, 164, 85–91. [Google Scholar] [CrossRef]
- Mowla, D.; Tran, H.N.; Allen, D.G. A review of the properties of biosludge and its relevance to enhanced dewatering processes. Biomass Bioenergy 2013, 58, 365–378. [Google Scholar] [CrossRef]
- Feng, G.; Tan, W.; Zhong, N.; Liu, L. Effects of thermal treatment on physical and expression dewatering characteristics of municipal sludge. Chem. Eng. J. 2014, 247, 223–230. [Google Scholar] [CrossRef]
- Wang, H.F.; Hu, H.; Wang, H.J.; Zeng, R.J. Impact of dosing order of the coagulant and flocculant on sludge dewatering performance during the conditioning process. Sci. Total Environ. 2018, 643, 1065–1073. [Google Scholar] [CrossRef]
- Diak, J.; Proux, C.; Örmeci, B. Freeze-thaw treatment of RBC sludge: A sustainable option for sludge dewatering in cold regions. Proc. Water Environ. Fed. 2010, 59, 204–212. [Google Scholar] [CrossRef]
- Wang, L.; Zhang, L.; Li, A. Hydrothermal treatment coupled with mechanical expression at increased temperature for excess sludge dewatering: Influence of operating conditions and the process energetics. Water Res. 2014, 65, 85–97. [Google Scholar] [CrossRef]
- Zhu, J.; Zheng, H.; Jiang, Z.; Zhang, Z.; Liu, L.; Sun, Y.; Tshukudu, T. Synthesis and characterization of a dewatering reagent: Cationic polyacrylamide (P(AM–DMC–DAC)) for activated sludge dewatering treatment. Desalin. Water Treat. 2013, 51, 2791–2801. [Google Scholar] [CrossRef]
- Li, Y.; Yuan, X.; Wu, Z.; Hou, W.; Xiao, Z.; Yan, W.; Chen, X.; Zeng, G. Enhancing the sludge dewaterability by electrolysis/electrocoagulation combined with zero-valent iron activated persulfate process. Chem. Eng. J. 2016, 303, 636–645. [Google Scholar] [CrossRef]
- Zhang, W.; Peng, Y.; Yang, X.; Zhan, C.; Wang, D. Insights into the respective role of acidification and oxidation for enhancing anaerobic digested sludge dewatering performance with Fenton process. Bioresour. Technol. 2015, 181, 247–253. [Google Scholar] [CrossRef]
- Liu, F.; Zhou, L.; Zhou, J.; Feng, J.; Wang, D. Improvement of municipal sewage sludge dewaterability by bioleaching:A pilot-scale study with sequence batch reaction model. Environ. Sci. 2011, 32, 2023–2029. [Google Scholar]
- Yu, R.; Shi, L.; Gu, G.; Zhou, D.; You, L.; Chen, M.; Qiu, G.; Zeng, W. The shift of microbial community under the adjustment of initial and processing pH during bioleaching of chalcopyrite concentrate by moderate thermophiles. Bioresour. Technol. 2014, 162, 300–307. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Li, K.; Chen, X.; Zhou, H. Responses of microbial community to pH stress in bioleaching of low grade copper sulfide. Bioresour. Technol. 2017, 249, 146–153. [Google Scholar] [CrossRef] [PubMed]
- Lei, F.; Feng, X.; Xiong, C. Study on the production of landfill leachate blow-off pretreatment combined with municipal wastewater treatment plant. Technol. Water Treat. 2018, 44, 88–90. (In Chinese) [Google Scholar]
- Wang, S. The Effect of Landfill Leachate on Operation of Municipal Wastewater Treatment. Ph.D. Thesis, Tianjin University, Tianjin, China, 2014. (In Chinese). [Google Scholar]
- Mannarino, C.F.; Ferreira, J.O.A.; Moreira, J.C.; Bila, D.M.; Es, D.P.M. Assessment of combined treatment of landfill urban solid waste leachate and sewage using danio rerio and daphnia similis. Bull. Environ. Contam. Toxicol. 2010, 85, 274. [Google Scholar] [CrossRef] [PubMed]
- Yu, J.H.; Zhao, S.X.; Xia, G.D.; Deng, W.C.; Zhou, S.Q.; Wang, W.F. Impact and countermeasures of treating landfill leachate in municipal sewage treatment plant. China Water Wastewater 2010, 26, 95–97. [Google Scholar]
- Zeng, X.; Ding, W.; Zhang, Z.; Wan, P.; Deng, Y.; Wang, S. Effect of the mixing ratio during co-treatment of landfill leachate and sewage with a combined stripping and reversed A2/O process. Environ. Technol. 2015, 36, 2668. [Google Scholar] [CrossRef]
- Collivignarelli, M.C.; Carnevale Miino, M.; Caccamo, F.M.; Baldi, M. Evaluation of foaming potential for water treatment: Limits and developments. Environ. Sci. Pollut. Res. 2020, 27, 27952–27960. [Google Scholar] [CrossRef]
- Collivignarelli, M.C.; Baldi, M.; Abbà, A.; Caccamo, F.M.; Carnevale Miino, M.; Rada, E.C.; Torretta, V. Foams in wastewater treatment plants: From causes to control methods. Appl. Sci. 2020, 10, 2716. [Google Scholar] [CrossRef] [Green Version]
- Yun, C.; Xie, J.; Qin, Y.; Wan, Y.S.; Zhu, W.; Yan, Z. Variations in physical, chemical and biological properties in relation to sludge dewaterability under Fe (II)—Oxone conditioning. Water Res. 2017, 109, 13–23. [Google Scholar]
- Xing, D.; Sun, S.; Zhang, L.; Ai, Y. Determination of sulfate in water by barium chromate spectrophotometry. J. Environ. Health 2007, 12, 990–991. [Google Scholar]
- Kim, J.H.; Nam, S.Y. Conditioning and dewatering properties of digested and thickened sludge with inorganic conditioner. Korean J. Environ. Health Sci. 2011, 37, 467–473. [Google Scholar] [CrossRef] [Green Version]
- Quilliam, R.S.; Rangecroft, S.; Emmett, B.A.; Deluca, T.H.; Jones, D.L. Is biochar a source or sink for polycyclic aromatic hydrocarbon (PAH) compounds in agricultural soils? GCB Bioenergy 2013, 5, 96–103. [Google Scholar] [CrossRef]
- Guillem, L.B.; Marina, B.F.; Daniel, L.; Sara, R.M.; Damià, B.; Taina, P.; Gloria, C.; Paqui, B. Degradation of pharmaceuticals from membrane biological reactor sludge with Trametes versicolor. Environ. Sci. Proc.Imp. 2015, 17, 429. [Google Scholar]
- Zheng, Y.; Lin, H.; Lin, Z.; Zeng, Y.; Yu, Y.; Liu, C. The optimization of thermal pre-treatment time for extracellular polymeric substances extraction from sewage sludge. Appl. Mech. Mater. 2015, 768, 496–505. [Google Scholar] [CrossRef]
- Liu, F.; Zhou, L.; Zhou, J.; Song, X.; Wang, D. Improvement of sludge dewaterability and removal of sludge-borne metals by bioleaching at optimum pH. J. Hazard. Mater. 2012, 221–222, 170–177. [Google Scholar] [CrossRef]
- Misra, M.; Bukka, K.; Chen, S. The effect of growth medium of Thiobacillus ferrooxidans on pyrite flotation. Miner. Eng. 2015, 9, 157–168. [Google Scholar] [CrossRef]
- Chen, M.; Lu, G.; Guo, C.; Yang, C.; Wu, J.; Huang, W.; Yee, N.; Dang, Z. Sulfate migration in a river affected by acid mine drainage from the Dabaoshan mining area, South China. Chemosphere 2015, 119, 734–743. [Google Scholar] [CrossRef]
- Zeng, J.; Gou, M.; Tang, Y.Q.; Li, G.Y.; Sun, Z.Y.; Kida, K. Effective bioleaching of chromium in tannery sludge with an enriched sulfur-oxidizing bacterial community. Bioresour. Technol. 2016, 218, 859–866. [Google Scholar] [CrossRef]
- Fortin, D.; Davis, B.; Beveridge, T.J. Role of Thiobacillus and sulfate-reducing bacteria in iron biocycling in oxic and acidic mine tailings. FEMS Microbiol. Ecol. 2010, 21, 11–24. [Google Scholar] [CrossRef]
- Winarko, R.; Mubarok, M.Z.; Rizki, I.N.; Chaerun, S.K. Biooxidation of carbonaceous refractory gold ores by an iron-sulfur-oxidizing mixotrophic bacterium at neutral pH. Adv. Mater. Res. 2015, 1130, 440–444. [Google Scholar] [CrossRef]
- Yu, J.; Yang, H.Y.; Tong, L.L.; Zhu, J. Intensified bioleaching of low-grade molybdenite concentrate by ferrous sulfate and pyrite. Rare Metals 2015, 34, 207–214. [Google Scholar] [CrossRef]
- Wang, W.; Liu, W.; Wang, L.; Yang, T.; Li, R. Characteristics and distribution research on extracellular polymer substance extracted from sewage sludge. J. Environ. Biol. 2016, 37, 305–312. [Google Scholar]
- Ni, L.; Li, D.; Rong, S.; Su, L.; Zhou, W.; Wang, P.; Wang, C.; Li, S.; Acharya, K. Characterization of extracellular polymeric substance (EPS) fractions produced by Microcystis aeruginosa under the stress of linoleic acid sustained-release microspheres. Environ. Sci. Pollut. Res. 2017, 24, 1–12. [Google Scholar] [CrossRef]
- Liu, Y.; Liu, Z.; Zhang, A.; Chen, Y.; Wang, X. The role of EPS concentration on membrane fouling control: Comparison analysis of hybrid membrane bioreactor and conventional membrane bioreactor. Desalination 2012, 305, 38–43. [Google Scholar] [CrossRef]
- Liu, H.; Yang, S.; Shi, J.; Xu, X.; Liu, H.; Fu, B. Towards understanding the dewatering mechanism of sewage sludge improved by bioleaching processing. Sep. Purif. Technol. 2016, 165, 53–59. [Google Scholar] [CrossRef]
- Huo, M.; Zheng, G.; Zhou, L. Enhancement of the dewaterability of sludge during bioleaching mainly controlled by microbial quantity change and the decrease of slime extracellular polymeric substances content. Bioresour. Technol. 2014, 168, 190–197. [Google Scholar] [CrossRef]
- Xing, M.; Li, C.; Jiang, J.; Wang, Y.; Yang, J. Influence analysis for the behavior of dewaterability of excess sludge in a two-stage vermifilter. Appl. Microbiol. Biotechnol. 2016, 101, 1643–1652. [Google Scholar] [CrossRef]
- Yuan, H.; Wang, T.; Qi, L.; Niu, S. Effects of different pretreatment methods on sludge dewatering performance. Chin. J. Environ. Eng. 2015, 9, 4015–4020. [Google Scholar]
- Yu, W.Z.; Qu, J.H.; Gregory, J. Pre-coagulation on the submerged membrane fouling in nano-scale: Effect of sedimentation process. Chem. Eng. J. 2015, 262, 676–682. [Google Scholar] [CrossRef]
- Li, H.; Yue, W.; Cao, A.S.; Huang, J.; Qi, Z.; Somasundaran, P. The influence of additives (Ca2+, Al3+, and Fe3+) on the interaction energy and loosely bound extracellular polymeric substances (EPS) of activated sludge and their flocculation mechanisms. Bioresour. Technol. 2012, 114, 188–194. [Google Scholar] [CrossRef]
- Li, Z. Study of Particle Size Distribution in Activated Sludge Processes: Impacts of Solids Retention Time and Process Configurations. Ph.D. Thesis, University of Californ, Los Angeles, CA, USA, 2016. [Google Scholar]
- Liu, J.; Qi, J.; Han, S.; Zhou, Q. Changes of EPS in bioleaching sludge and the effect on Zeta potential and floc size. Jiangsu J. Agric. Sci. 2017, 33, 107–112. (In Chinese) [Google Scholar]
- Tang, Y.F.; Wang, R.C.; Ma, L.M.; Zhao, J.F. Optimization of enhanced flocculation for treating sediment dredging wastewater: Pollutant removal performance and zeta potential analysis. Adv. Mater. Res. 2011, 356–360, 1668–1674. [Google Scholar] [CrossRef]
- Mishra, D.; Rhee, Y.H. Microbial leaching of metals from solid industrial wastes. J. Micribiol. 2014, 52, 1–7. [Google Scholar] [CrossRef]
- Huber, B.; Herzog, B.; Drewes, J.E.; Koch, K.; Müller, E. Characterization of sulfur oxidizing bacteria related to biogenic sulfuric acid corrosion in sludge digesters. BMC Microbiol. 2016, 16, 153. [Google Scholar] [CrossRef] [Green Version]
- Kucera, J.; Pakostova, E.; Lochman, J.; Janiczek, O.; Mandl, M. Are there multiple mechanisms of anaerobic sulfur oxidation with ferric iron in Acidithiobacillus ferrooxidans? Res. Microbiol. 2016, 167, 357–366. [Google Scholar] [CrossRef]
- Wan, Y.; Zhao, G. Research on sulfur microorganisms in prokaryotes. Microbiol. China 2017, 44, 1471–1480. (In Chinese) [Google Scholar]
- Mehrotra, A.; Sreekrishnan, T.R. Heavy metal bioleaching and sludge stabilization in a single-stage reactor using indigenous acidophilic heterotrophs. Environ. Technol. 2017, 38, 1–16. [Google Scholar] [CrossRef]
- Dong, X. Study on Biogas Production of Two Phase Anaerobic Process of Vegetable Waste. Ph.D. Thesis, Shengyang Agricultural University, Liaoning, China, 2016. [Google Scholar]
- Chen, L.; Li, J.; Chen, Y.; Huang, L.; Hua, Z.; Hu, M.; Shu, W. Shifts in microbial community composition and function in the acidification of a lead/zinc mine tailings. Environ. Microbiol. 2013, 15, 2431–2444. [Google Scholar] [CrossRef]
- Slyemi, D.; Moinier, D.; Brochier-Armanet, C.; Bonnefoy, V.; Johnson, D.B. Characteristics of a phylogenetically ambiguous, arsenic-oxidizing Thiomonas sp., Thiomonas arsenitoxydans strain 3AsT sp. nov. Arch. Microbiol. 2011, 193, 439–449. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Guo, S.; Yu, R.; Zou, K.; Qiu, G. A new cytoplasmic monoheme cytochrome c from Acidithiobacillus ferrooxidans involved in sulfur oxidation. Curr. Microbiol. 2014, 68, 285–292. [Google Scholar] [CrossRef]


| Parameters | Value |
|---|---|
| pH | 6.00 ± 1.00 |
| Chemical Oxygen Demand(COD)/(mg L−1) | 15,800 ± 7200 |
| Ammonia-Nitrogen(NH3-N)/(mg L−1) | 4050 ± 1650 |
| Total Nitrogen (TN)/(mg L−1) | 4350 ± 1750 |
| Parameters | Value |
|---|---|
| pH | 6.83 ± 0.21 |
| Solid content % | 1.32 ± 0.32 |
| Sludge sedimentation rate % | 61 ± 4 |
| Specific Resistance to Filtration (SRF)/(mg L−1) | (4.44 ± 0.39) × 1012 |
| Parameter | Method |
|---|---|
| pH, ORP | MultiHQ40d portable pH/DO tester |
| Fe2+, Fe3+ | Phenanthroline spectrophotometry [22] |
| SO42− | Barium chromate spectrophotometry [23] |
| Specific Resistance to Filtration (SRF) | Buchner funnel test [24] |
| Sludge particle size | Mastersizer 2000 type laser particle size analyzer |
| Moisture content | Drying at 105 °C for 24 h and then weighing [25] |
| Volatile Suspended Solids (VSS) | Burning in a muffle furnace at 600 °C for 0.5 h and then weighing [26] |
| Total Organic Carbon (TOC) | TOC-VCPH type total organic carbon analyzer |
| EPS (replace by TOC and VSS ratio) | Heat extraction [27] |
| Sludge sedimentation rate | Gravity separation method |
| Simple | Chao1 | Shannon | Simpson |
|---|---|---|---|
| Inoculum | 6880.97 | 8.40 | 0.95 |
| pH = 3.0 | 7822.00 | 8.56 | 0.95 |
| pH = 5.0 | 7972.65 | 8.76 | 0.94 |
| pH = 6.9 | 7585.80 | 8.59 | 0.95 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lin, S.; Shi, M.; Wang, J.; Zhu, H.; Wen, G. Influence of Sludge Initial pH on Bioleaching of Excess Sludge to Improve Dewatering Performance. Coatings 2020, 10, 989. https://doi.org/10.3390/coatings10100989
Lin S, Shi M, Wang J, Zhu H, Wen G. Influence of Sludge Initial pH on Bioleaching of Excess Sludge to Improve Dewatering Performance. Coatings. 2020; 10(10):989. https://doi.org/10.3390/coatings10100989
Chicago/Turabian StyleLin, Shaonan, Mingyan Shi, Jiade Wang, Huijie Zhu, and Guicheng Wen. 2020. "Influence of Sludge Initial pH on Bioleaching of Excess Sludge to Improve Dewatering Performance" Coatings 10, no. 10: 989. https://doi.org/10.3390/coatings10100989