The Combination of Low-Frequency Ultrasound and Antibiotics Improves the Killing of In Vitro Staphylococcus aureus and Pseudomonas aeruginosa Biofilms
Abstract
:1. Introduction
2. Materials and Methods
2.1. Strains and Growth Conditions
2.2. Minimum Inhibitory Concentration (MIC)
2.3. Filter-Biofilms
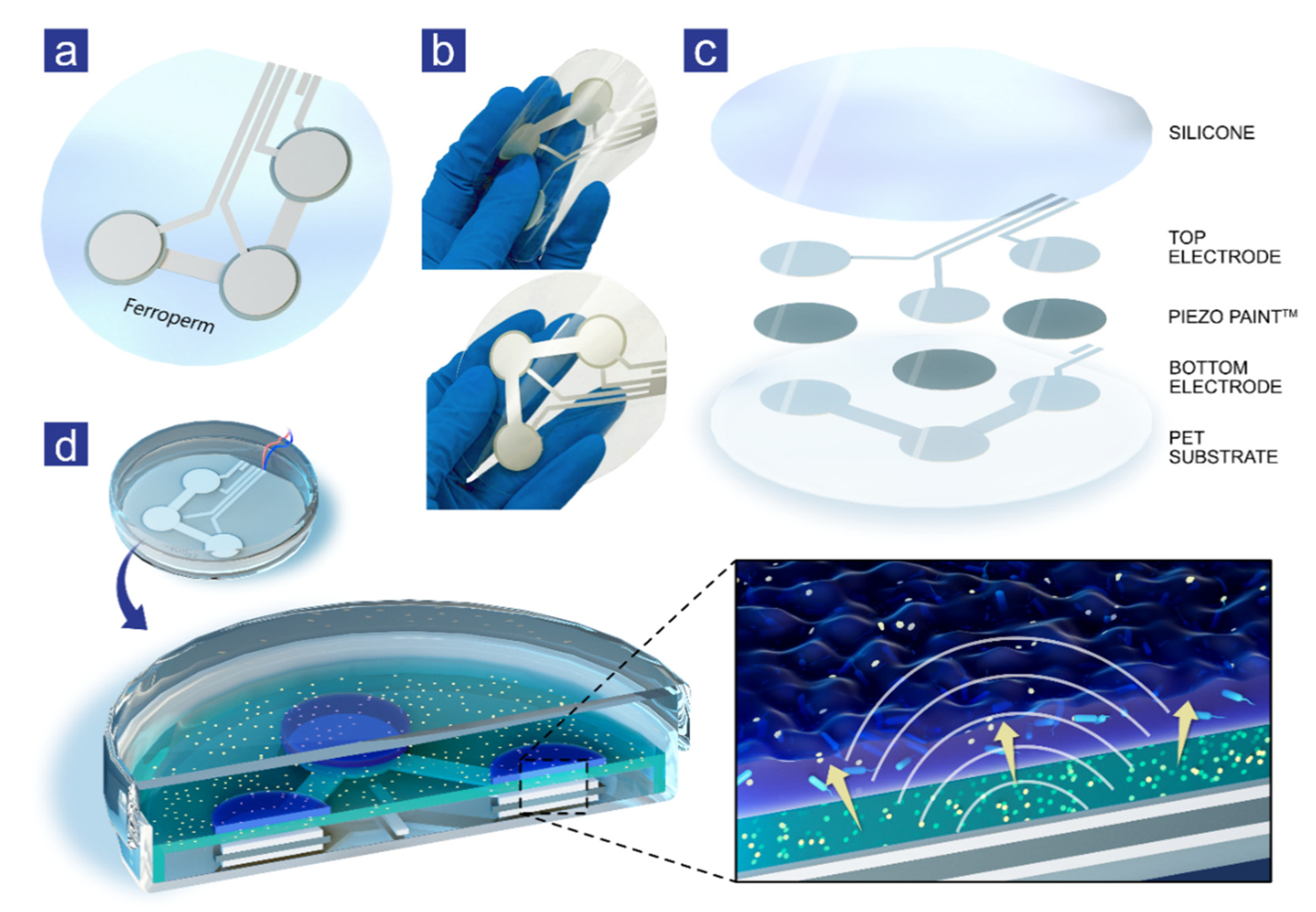
2.4. Ultrasound Patches
2.5. Experimental Setup for Ultrasound Stimulation
2.6. Experimental Setup with Ultrasound Patches
2.7. Colony-Forming Units Per Milliliter (CFU/mL)
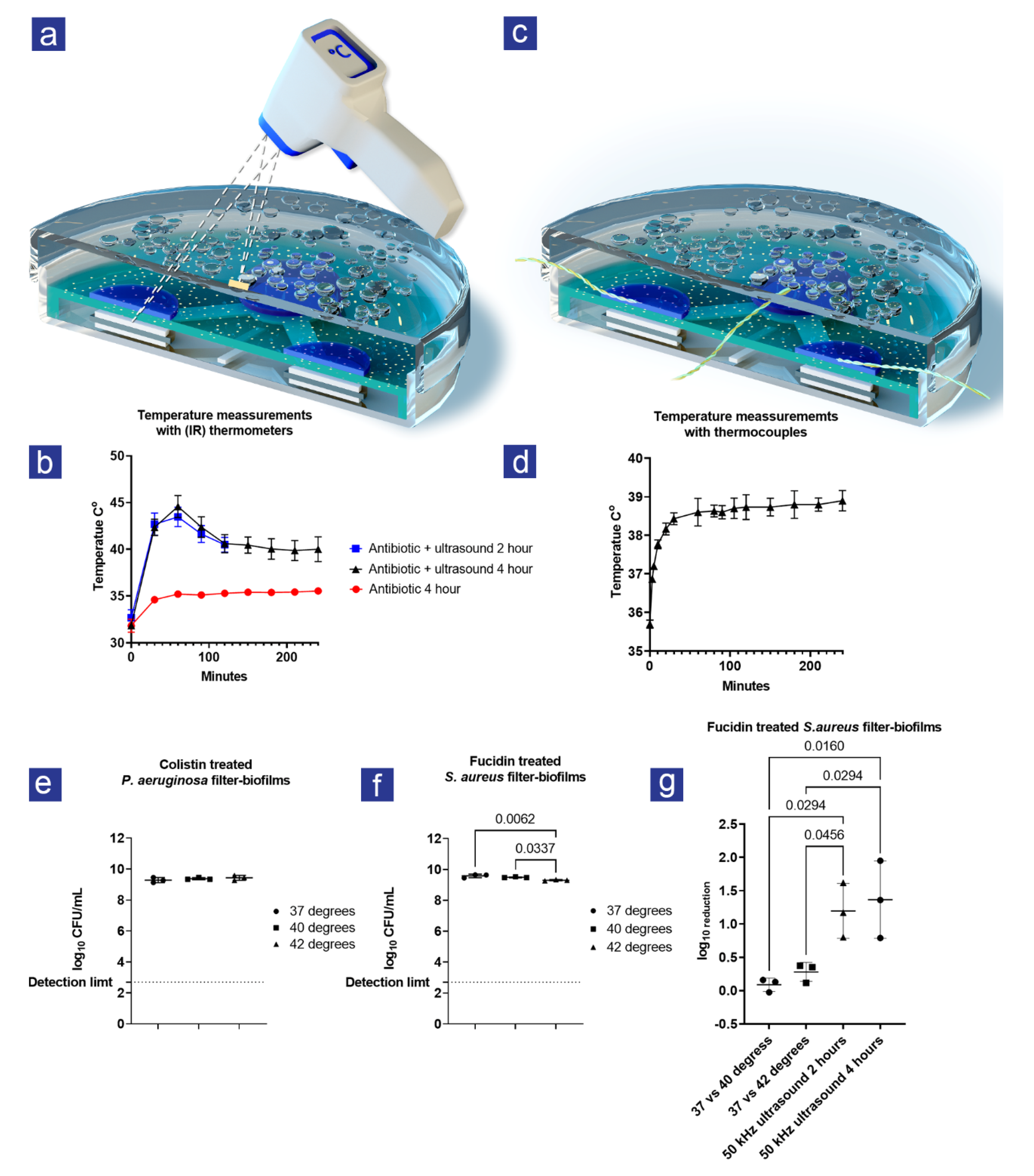
2.8. Measurement of Temperature
2.9. The combined Effect of Temperature and Antibiotics on the Viability
2.10. Statistics
3. Results
3.1. A synergistic Effect Was Observed between Ultrasound and Antibiotic Treatment
3.2. Heat Was Created during Ultrasound Treatment
3.3. The Increase in Heat Could Not Explain the Combined Effect of Ultrasound and Antibiotics
4. Discussion
4.1. Main Findings
4.2. Limitations and Strengths
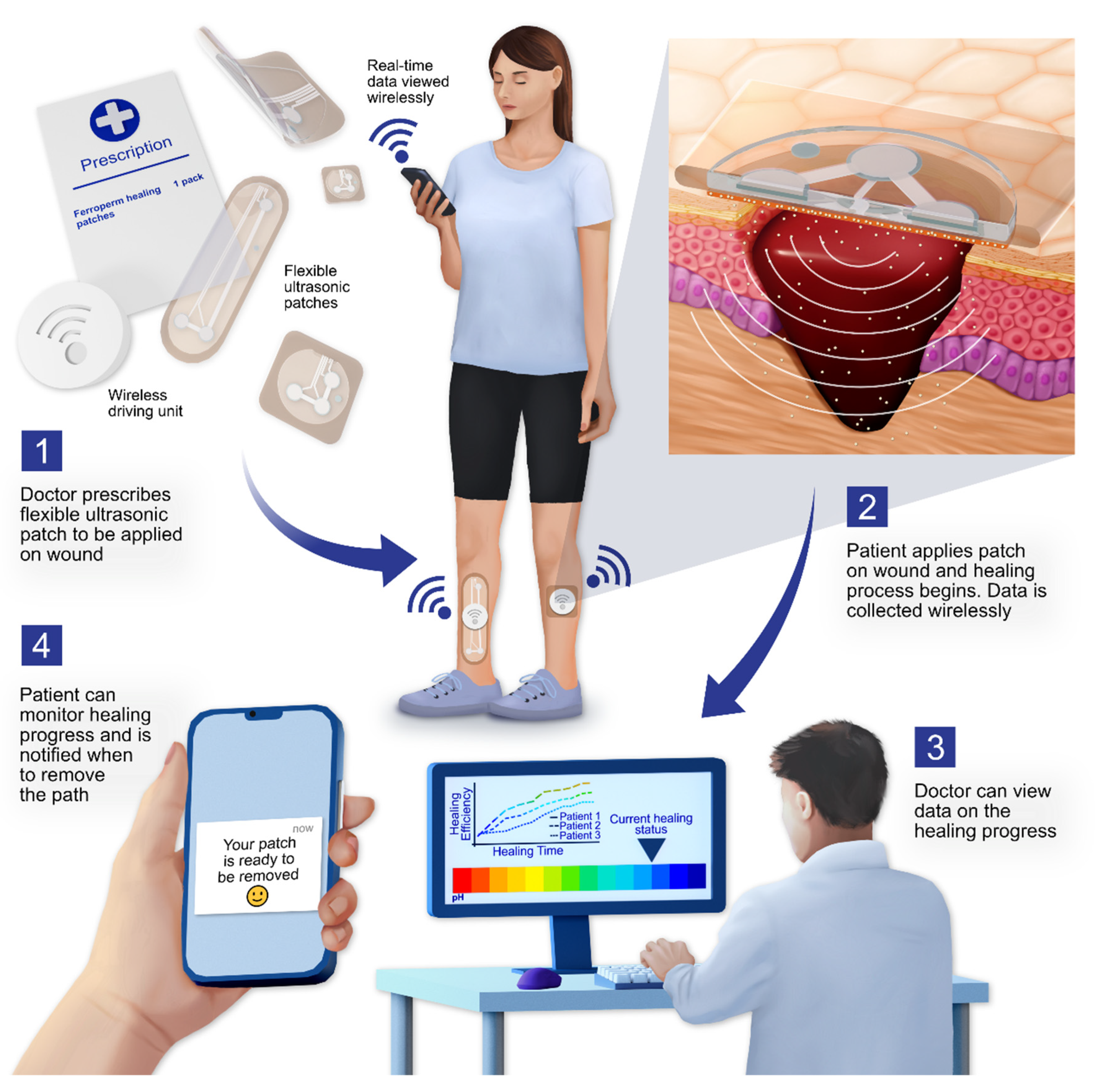
4.3. Future Perspectives
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Bjarnsholt, T.; Alhede, M.; Alhede, M.; Eickhardt-Sørensen, S.R.; Moser, C.; Kühl, M.; Jensen, P.Ø.; Høiby, N. The in vivo biofilm. Trends Microbiol. 2013, 21, 466–474. [Google Scholar] [CrossRef] [PubMed]
- Kolpen, M.; Kragh, K.N.; Enciso, J.B.; Faurholt-Jepsen, D.; Lindegaard, B.; Egelund, G.B.; Jensen, A.V.; Ravn, P.; Mathiesen, I.H.M.; Gheorge, A.G.; et al. Bacterial biofilms predominate in both acute and chronic human lung infections. Thorax 2022, 77, 1015–1022. [Google Scholar] [CrossRef] [PubMed]
- Cámara, M.; Green, W.; MacPhee, C.E.; Rakowska, P.D.; Raval, R.; Richardson, M.C.; Slater-Jefferies, J.; Steventon, K.; Webb, J.S. Economic significance of biofilms: A multidisciplinary and cross-sectoral challenge. NPJ Biofilms Microbiomes 2022, 8, 42. [Google Scholar] [CrossRef] [PubMed]
- Malone, M.; Bjarnsholt, T.; McBain, A.J.; James, G.A.; Stoodley, P.; Leaper, D.; Tachi, M.; Schultz, G.; Swanson, T.; Wolcott, R.W. The prevalence of biofilms in chronic wounds: A systematic review and meta-analysis of published data. J. Wound Care 2017, 26, 20–25. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Flemming, H.C.; Wingender, J.; Szewzyk, U.; Steinberg, P.; Rice, S.A.; Kjelleberg, S. Biofilms: An emergent form of bacterial life. Nat. Rev. Microbiol. 2016, 14, 563–575. [Google Scholar] [CrossRef]
- Høiby, N.; Bjarnsholt, T.; Givskov, M.; Molin, S.; Ciofu, O. Antibiotic resistance of bacterial biofilms. Int. J. Antimicrob. Agents. 2010, 35, 322–332. [Google Scholar] [CrossRef] [Green Version]
- Narayan, K.M.V.; Boyle, J.P.; Geiss, L.S.; Saaddine, J.B.; Thompson, T.J. Impact of Recent Increase in Incidence on Future Diabetes Burden: U.S., 2005–2050. Diabetes Care 2006, 29, 2114–2116. [Google Scholar] [CrossRef] [Green Version]
- Hjort, A.; Gottrup, F. Cost of wound treatment to increase significantly in Denmark over the next decade. J. Wound Care 2010, 19, 173–174. [Google Scholar] [CrossRef] [Green Version]
- Frykberg, R.G.; Banks, J. Challenges in the Treatment of Chronic Wounds. Adv. Wound Care 2015, 4, 560–582. [Google Scholar] [CrossRef] [Green Version]
- Liu, B.; Wang, D.-J.; Liu, B.-M.; Wang, X.; He, L.-L.; Wang, J.; Xu, S.-K. The influence of ultrasound on the fluoroquinolones antibacterial activity. Ultrason. Sonochem. 2011, 18, 1052–1056. [Google Scholar] [CrossRef]
- Ensing, G.T.; Neut, D.; van Horn, J.R.; van der Mei, H.C.; Busscher, H.J. The combination of ultrasound with antibiotics released from bone cement decreases the viability of planktonic and biofilm bacteria: An in vitro study with clinical strains. J. Antimicrob. Chemother. 2006, 58, 1287–1290. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Crone, S.; Garde, C.; Bjarnsholt, T.; Alhede, M. A novel in vitro wound biofilm model used to evaluate low-frequency ultrasonic-assisted wound debridement. J. Wound Care 2015, 24, 64. [Google Scholar] [CrossRef] [PubMed]
- Pitt, W.G.; McBride, M.O.; Lunceford, J.K.; Roper, R.J.; Sagers, R.D. Ultrasonic enhancement of antibiotic action on gram-negative bacteria. Antimicrob. Agents. Chemother. 1994, 38, 2577–2582. [Google Scholar] [CrossRef] [Green Version]
- Pitt, W.G.; Ross, S.A. Ultrasound increases the rate of bacterial cell growth. Biotechnol. Prog. 2003, 19, 1038–1044. [Google Scholar] [CrossRef] [PubMed]
- Runyan, C.M.; Carmen, J.C.; Beckstead, B.L.; Nelson, J.L.; Robison, R.A.; Pitt, W.G. Low-frequency ultrasound increases outer membrane permeability of Pseudomonas aeruginosa. J. Gen. Appl. Microbiol. 2006, 52, 295–301. [Google Scholar] [CrossRef] [Green Version]
- Kim, D.-H.; Lu, N.; Ma, R.; Kim, Y.-S.; Kim, R.-H.; Wang, S.; Wu, J.; Won, S.M.; Tao, H.; Islam, A.; et al. Epidermal Electronics. Science 2011, 333, 838–843. [Google Scholar] [CrossRef] [Green Version]
- Rogers, J.A.; Chen, X.; Feng, X. Flexible Hybrid Electronics. Adv. Mater. 2020, 32, e1905590. [Google Scholar] [CrossRef] [Green Version]
- Nahid, A.; Griffin, J.M.; Lustik, M.B.; Hayes, J.J.; Fong, K.S.; Horseman, T.S.; Menguito, M.; Snesrud, E.C.; Barnhill, J.C.; Washington, M.A. A Longitudinal Evaluation of the Bacterial Pathogens Colonizing Chronic Non-Healing Wound Sites at a United States Military Treatment Facility in the Pacific Region. Infect. Drug Resist. 2021, 14, 1–10. [Google Scholar] [CrossRef]
- Gjødsbøl, K.; Christensen, J.J.; Karlsmark, T.; Jørgensen, B.; Klein, B.M.; Krogfelt, K.A. Multiple bacterial species reside in chronic wounds: A longitudinal study. Int. Wound J. 2006, 3, 225–231. [Google Scholar] [CrossRef]
- Frees, D.; Chastanet, A.; Qazi, S.; Sørensen, K.; Hill, P.; Msadek, T.; Ingmer, H. Clp ATPases are required for stress tolerance, intracellular replication and biofilm formation in Staphylococcus aureus. Mol. Microbiol. 2004, 54, 1445–1462. [Google Scholar] [CrossRef]
- Sadeghi, S.; Nourmohammadi, J.; Ghaee, A.; Soleimani, N. Carboxymethyl cellulose-human hair keratin hydrogel with controlled clindamycin release as antibacterial wound dressing. Int. J. Biol. Macromol. 2020, 147, 1239–1247. [Google Scholar] [CrossRef] [PubMed]
- Jain, R.; Murthy, S.I.; Motukupally, S.R.; Jain, M. Use of topical colistin in multiple drug-resistant Pseudomonas aeruginosa bacterial keratitis. Cornea 2014, 33, 923–927. [Google Scholar] [CrossRef] [PubMed]
- Wu, P.; He, H.; Hong, W.D.; Wu, T.-R.; Huang, G.-Y.; Zhong, Y.-Y.; Tu, B.-R.; Gao, M.; Zhou, J.; Zhao, S.-Q.; et al. The biological evaluation of fusidic acid and its hydrogenation derivative as antimicrobial and anti-inflammatory agents. Infect. Drug Resist. 2018, 11, 1945–1957. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Miró, N. Controlled multicenter study on chronic suppurative otitis media treated with topical applications of ciprofloxacin 0.2% solution in single-dose containers or combination of polymyxin B, neomycin, and hydrocortisone suspension. Otolaryngol. Head Neck Surg. 2000, 123, 617–623. [Google Scholar] [CrossRef] [PubMed]
- Bjarnsholt, T.; Alhede, M.; Jensen, P.Ø.; Nielsen, A.K.; Johansen, H.K.; Homøe, P.; Høiby, N.; Givskov, M.; Kirketerp-Møller, K. Antibiofilm Properties of Acetic Acid. Adv. Wound Care 2015, 4, 363–372. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Flexible Piezoelectric Material, Production and Use Thereof—US10079336B2. Available online: https://patents.google.com/patent/US10079336B2/en (accessed on 17 October 2022).
- Flexible Piezoelectric Material, Production and Use Thereof—EP2608287B1. Available online: https://patents.google.com/patent/EP2608287B1/en?oq=EP2608287B1 (accessed on 17 October 2022).
- Fazli, M.; Bjarnsholt, T.; Kirketerp-Møller, K.; Jørgensen, B.; Andersen, A.S.; Krogfelt, K.A.; Givskov, M.; Tolker-Nielsen, T. Nonrandom distribution of Pseudomonas aeruginosa and Staphylococcus aureus in chronic wounds. J. Clin. Microbiol. 2009, 47, 4084–4089. [Google Scholar] [CrossRef] [Green Version]
- Murray, C.J.; Ikuta, K.S.; Sharara, F.; Swetschinski, L.; Aguilar, G.R.; Gray, A.; Han, C.; Bisignano, C.; Rao, P.; Wool, E.; et al. Global burden of bacterial antimicrobial resistance in 2019: A systematic analysis. Lancet 2022, 399, 629–655. [Google Scholar] [CrossRef]
- Rediske, A.M.; Rapoport, N.; Pitt, W.G. Reducing bacterial resistance to antibiotics with ultrasound. Lett. Appl. Microbiol. 1999, 28, 81–84. [Google Scholar] [CrossRef]
- Liu, X.; Wang, J.; Weng, C.X.; Wang, R.; Cai, Y. Low-Frequency Ultrasound Enhances Bactericidal Activity of Antimicrobial Agents against Klebsiella pneumoniae Biofilm. Biomed. Res. Int. 2020, 2020, 5916260. [Google Scholar] [CrossRef] [Green Version]
- Heman-Ackah, S.M. Microbial Kinetics of Drug Action against Gram-Positive and Gram-Negative Organisms 11: Effect of Clindamycin on Staphylococcus aureus and Escherichia coli. J. Pharm. Sci. 1975, 64, 1612–1620. [Google Scholar] [CrossRef]
- Wilkinson, J.D. Fusidic acid in dermatology. Br. J. Dermatol. 1998, 139 (Suppl. S53), 37–40. [Google Scholar] [CrossRef] [PubMed]
- Stokes, J.M.; Lopatkin, A.J.; Lobritz, M.A.; Collins, J.J. Bacterial Metabolism and Antibiotic Efficacy. Cell. Metab. 2019, 30, 251–259. [Google Scholar] [CrossRef] [PubMed]
- Ciofu, O.; Moser, C.; Jensen, P.; Høiby, N. Tolerance and resistance of microbial biofilms. Nat. Rev. Microbiol. 2022, 20, 621–635. [Google Scholar] [CrossRef] [PubMed]
- Carmen, J.C.; Runyan, C.; Robison, R.A.; Nelson, J.L.; Beckstead, B.L.; Pitt, W.G.; Schaalje, G.B. Ultrasonic-enhanced gentamicin transport through colony biofilms of Pseudomonas aeruginosa and Escherichia coli. J. Infect. Chemother. 2004, 10, 193–199. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- LuTheryn, G.; Glynne-Jones, P.; Webb, J.S.; Carugo, D. Ultrasound-mediated therapies for the treatment of biofilms in chronic wounds: A review of present knowledge. Microb. Biotechnol. 2020, 13, 613–628. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vyas, H.K.N.; Xia, B.; Mai-Prochnow, A. Clinically relevant in vitro biofilm models: A need to mimic and recapitulate the host environment. Biofilm 2022, 4, 100069. [Google Scholar] [CrossRef]
- Lindholm, C.; Searle, R. Wound management for the 21st century: Combining effectiveness and efficiency. Int. Wound J. 2016, 13 (Suppl. S2), 5–15. [Google Scholar] [CrossRef] [Green Version]
- Posnett, J.; Gottrup, F.; Lundgren, H.; Saal, G. The resource impact of wounds on health-care providers in Europe. J. Wound Care 2009, 18, 154–161. [Google Scholar] [CrossRef] [Green Version]
- Järbrink, K.; Ni, G.; Sönnergren, H.; Schmidtchen, A.; Pang, C.; Bajpai, R.; Car, J. The humanistic and economic burden of chronic wounds: A protocol for a systematic review. Syst. Rev. 2017, 6, 15. [Google Scholar] [CrossRef] [Green Version]
- Rosenblum, J.I.; Gazes, M.I.; Greenberg, N. Surface acoustic wave patch therapy affects tissue oxygenation in ischemic feet. Wounds 2014, 26, 301–305. [Google Scholar]
- Bajpai, A.; Nadkarni, S.; Neidrauer, M.; Weingarten, M.S.; Lewin, P.A.; Spiller, K.L. Effects of Non-thermal, Non-cavitational Ultrasound Exposure on Human Diabetic Ulcer Healing and Inflammatory Gene Expression in a Pilot Study. Ultrasound Med. Biol. 2018, 44, 2043–2049. [Google Scholar] [CrossRef] [PubMed]
- Ngo, O.; Niemann, E.; Gunasekaran, V.; Sankar, P.; Putterman, M.; Lafontant, A.; Nadkarni, S.; DiMaria-Ghalili, R.A.; Neidrauer, M.; Zubkov, L.; et al. Development of Low Frequency (20–100 kHz) Clinically Viable Ultrasound Applicator for Chronic Wound Treatment. IEEE Trans. Ultrason. Ferroelectr. Freq. Control. 2019, 66, 572–580. [Google Scholar] [CrossRef] [PubMed]
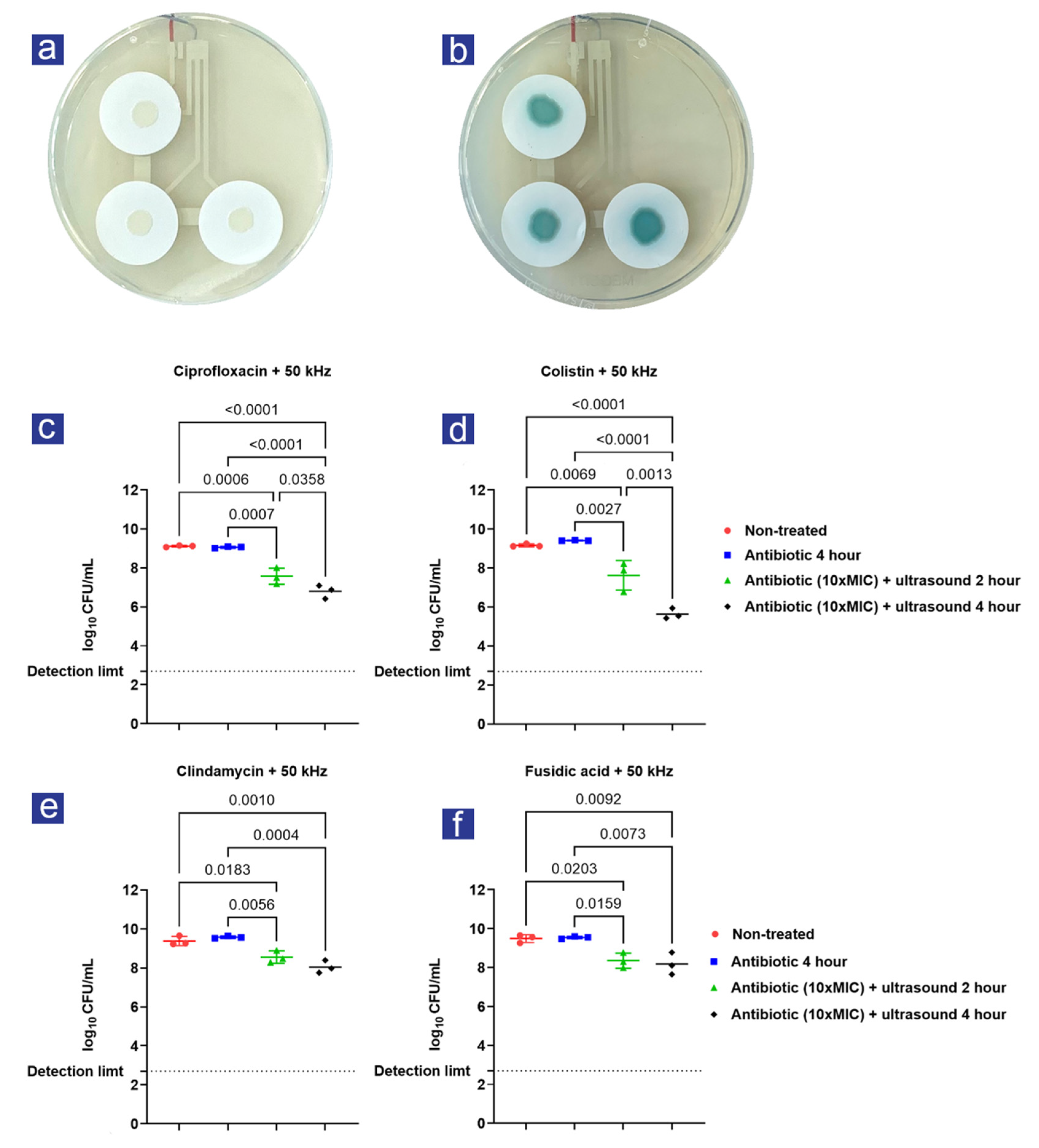
| Log-Reduction | % Reduction | p-Value | |
|---|---|---|---|
| Ultrasound + colistin 2-hour | 1.78 ± 0.63 | 98.34% | 0.003 |
| Ultrasound + colistin 4-hour | 3.77 ± 0.22 | 99.98% | <0.001 |
| Ultrasound + ciprofloxacin 2-hour | 1.49 ± 0.37 | 96.76% | 0.001 |
| Ultrasound + ciprofloxacin 4-hour | 2.26 ± 0.25 | 99.45% | <0.001 |
| Ultrasound + fusidic acid 2-hour | 1.19 ± 0.33 | 93.54% | 0.016 |
| Ultrasound + fusidic acid 4-hour | 1.37 ± 0.47 | 95.73% | 0.007 |
| Ultrasound + clindamycin 2-hour | 1.02 ± 0.23 | 90.45% | 0.006 |
| Ultrasound + clindamycin 4-hour | 1.54 ± 0.22 | 97.12% | <0.001 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kvich, L.; Christensen, M.H.; Pierchala, M.K.; Astafiev, K.; Lou-Moeller, R.; Bjarnsholt, T. The Combination of Low-Frequency Ultrasound and Antibiotics Improves the Killing of In Vitro Staphylococcus aureus and Pseudomonas aeruginosa Biofilms. Antibiotics 2022, 11, 1494. https://doi.org/10.3390/antibiotics11111494
Kvich L, Christensen MH, Pierchala MK, Astafiev K, Lou-Moeller R, Bjarnsholt T. The Combination of Low-Frequency Ultrasound and Antibiotics Improves the Killing of In Vitro Staphylococcus aureus and Pseudomonas aeruginosa Biofilms. Antibiotics. 2022; 11(11):1494. https://doi.org/10.3390/antibiotics11111494
Chicago/Turabian StyleKvich, Lasse, Mads H. Christensen, Malgorzata K. Pierchala, Konstantin Astafiev, Rasmus Lou-Moeller, and Thomas Bjarnsholt. 2022. "The Combination of Low-Frequency Ultrasound and Antibiotics Improves the Killing of In Vitro Staphylococcus aureus and Pseudomonas aeruginosa Biofilms" Antibiotics 11, no. 11: 1494. https://doi.org/10.3390/antibiotics11111494






