A Mechanochemical Route for ZnS Nanocrystals, and Batch Sorting along Size Distribution
Abstract
:1. Introduction
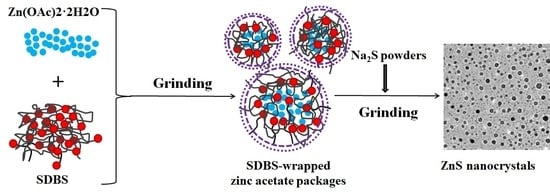
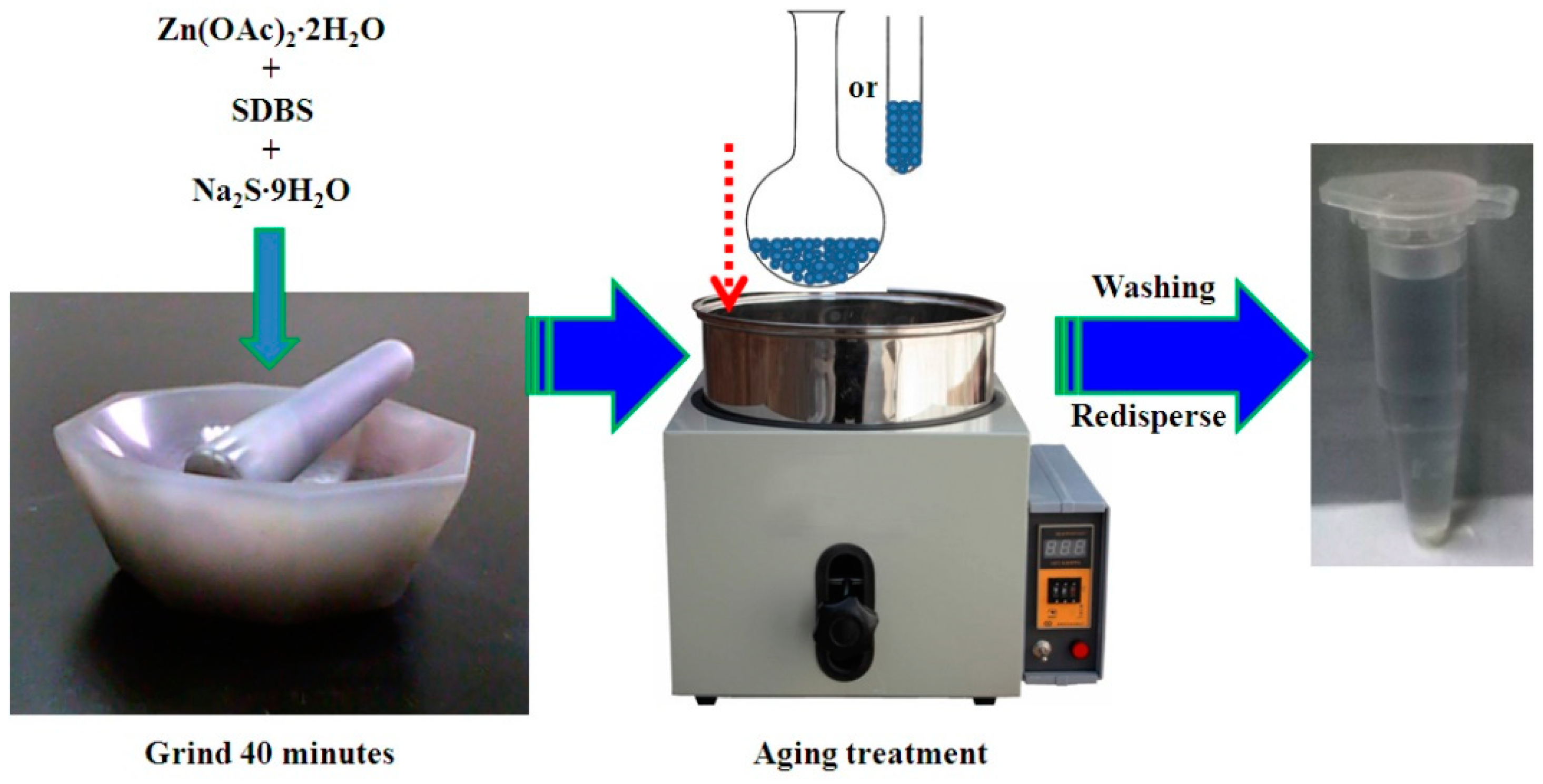
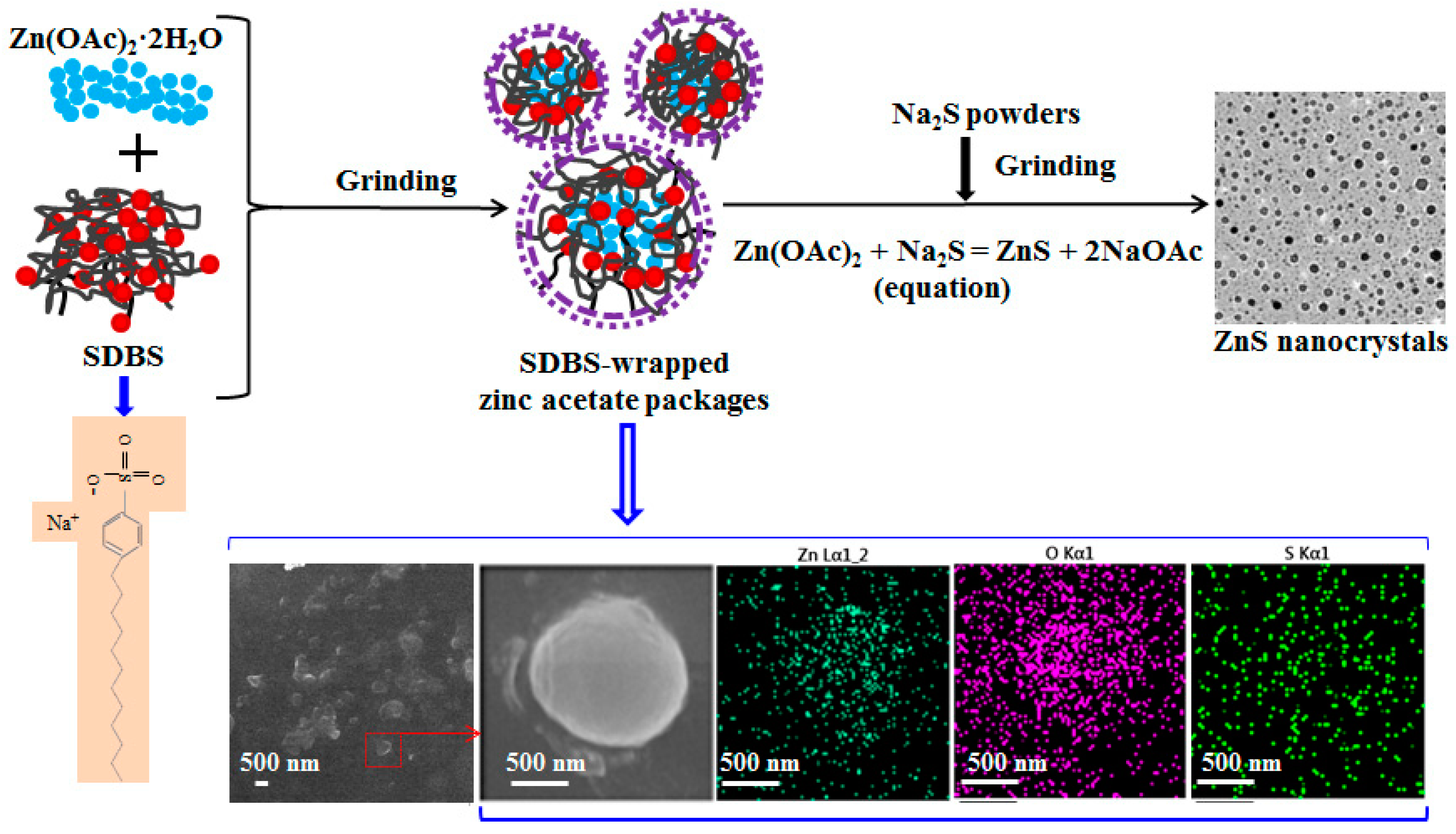
2. Experimental
3. Results and Discussion
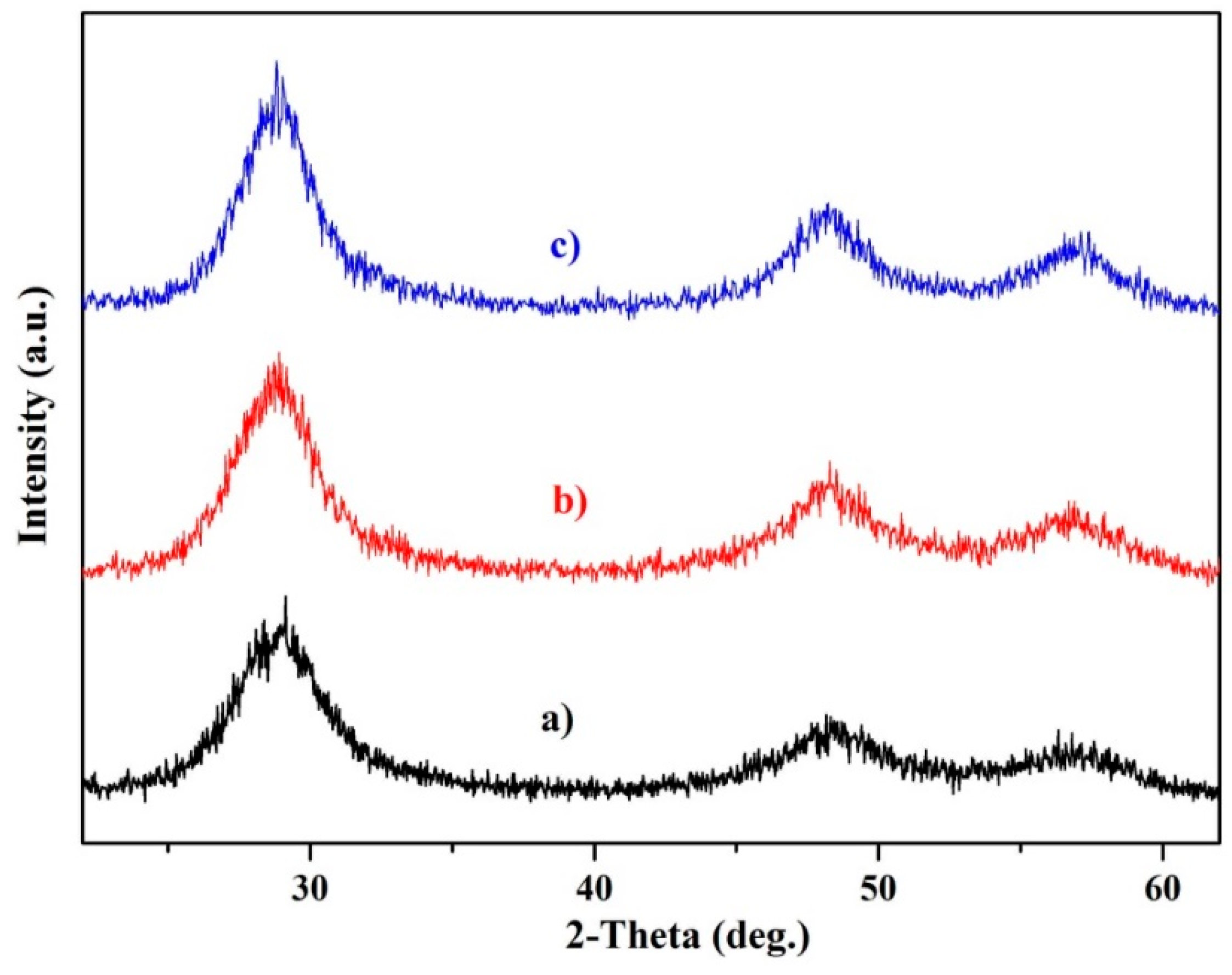
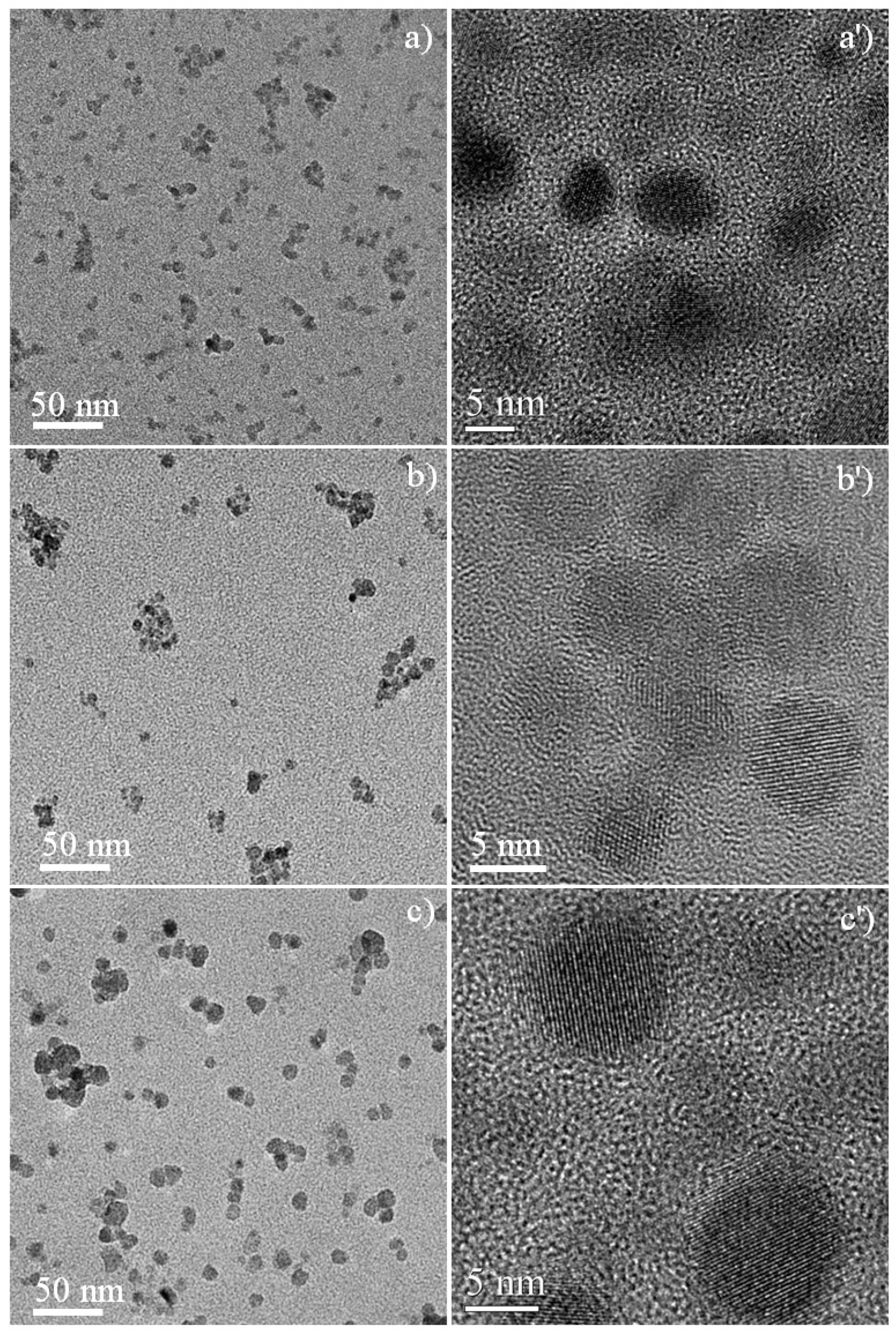
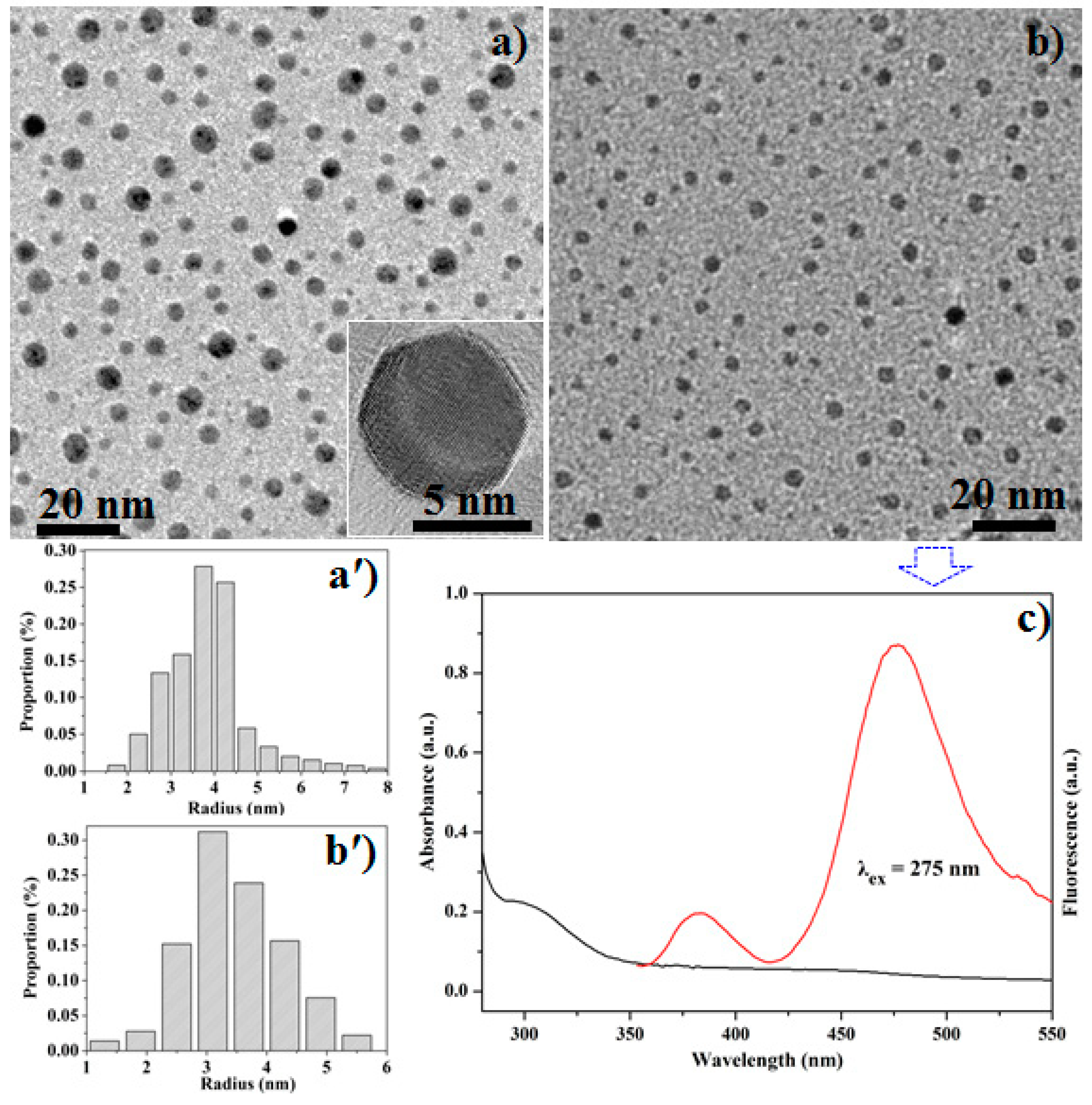
3.1. Crystal Structures of Zinc Sulfide (ZnS) Nanocrystals
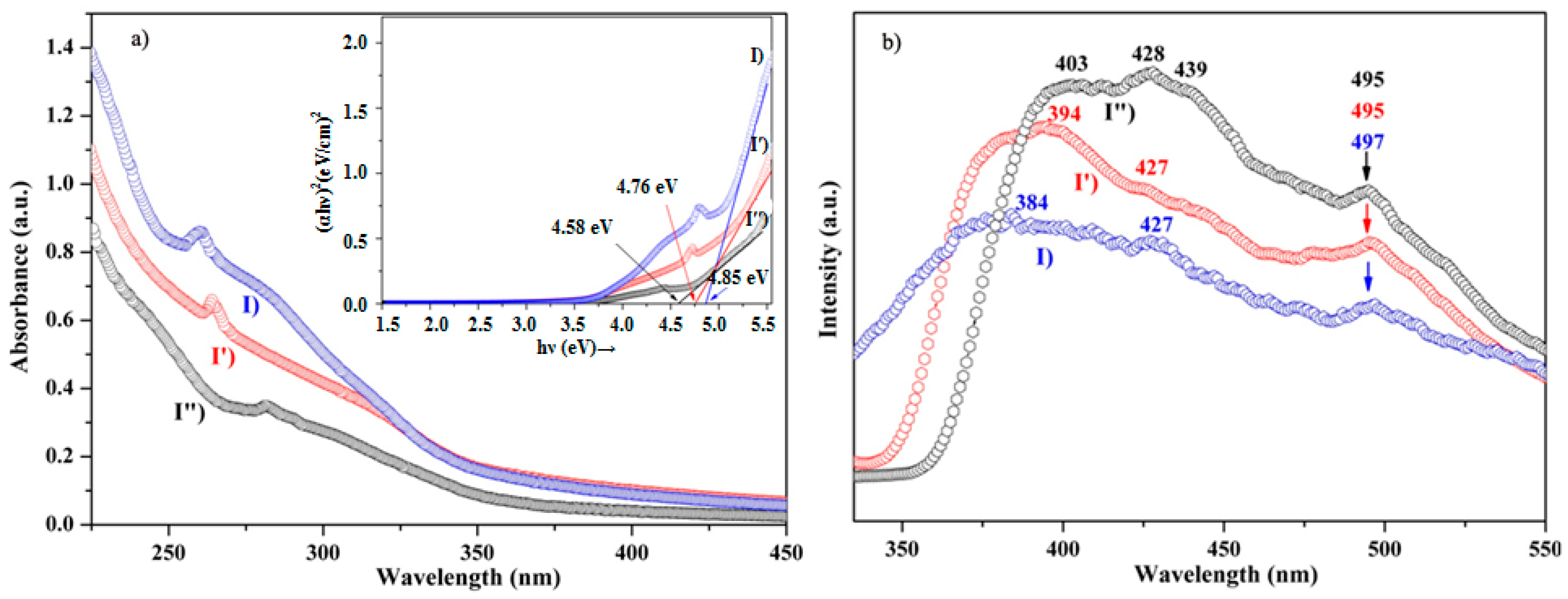
3.2. Optical Properties of ZnS Nanocrystals
3.2.1. Ultraviolet-Visible (UV-Vis) Spectral Characteristics
3.2.2. Photoluminescence Studies
3.3. Batch-Sorting of ZnS Nanocrystals
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Narayanaswamy, A.; Feiner, L.F.; Meijerink, A.; van der Zaag, P.J. The effect of temperature and dot size on the spectral properties of colloidal inp/ZnS core-shell quantum dots. ACS Nano 2009, 3, 2539–2546. [Google Scholar] [CrossRef] [PubMed]
- Sapra, S.; Sarma, D.D. Evolution of the electronic structure with size in ii-vi semiconductor nanocrystals. Phys. Rev. B 2004, 69, 125304. [Google Scholar] [CrossRef]
- Nirmal, M.; Brus, L. Luminescence photophysics in semiconductor nanocrystals. Acc. Chem. Res. 1999, 32, 407–414. [Google Scholar] [CrossRef]
- Kim, T.H.; Cho, K.S.; Lee, E.K.; Lee, S.J.; Chae, J.; Kim, J.W.; Kim, D.H.; Kwon, J.Y.; Amaratunga, G.; Lee, S.Y.; et al. Full-colour quantum dot displays fabricated by transfer printing. Nat. Photonics 2011, 5, 176–182. [Google Scholar] [CrossRef]
- Cowles, C.L.; Zhu, X. Sensitive detection of cardiac biomarker using ZnS nanoparticles as novel signal transducers. Biosens. Bioelectron. 2011, 30, 342–346. [Google Scholar] [CrossRef] [PubMed]
- Michalet, X.; Pinaud, F.; Bentolila, L.; Tsay, J.; Doose, S.; Li, J.; Sundaresan, G.; Wu, A.; Gambhir, S.; Weiss, S. Quantum dots for live cells, in vivo imaging, and diagnostics. Science 2005, 307, 538–544. [Google Scholar] [CrossRef]
- Zhang, R.; Xu, J.; Jia, M.; Pan, E.; Zhou, C.; Jia, M. Ultrafine ZnS quantum dots decorated reduced graphene oxide composites derived from zif-8/graphene oxide hybrids as anode for sodium-ion batteries. J. Alloys Compd. 2019, 781, 450–459. [Google Scholar] [CrossRef]
- Shen, H.; Cao, W.; Shewmon, N.T.; Yang, C.; Li, L.S.; Xue, J. High-efficiency, low turn-on voltage blue-violet quantum-dot-based light-emitting diodes. Nano Lett. 2015, 15, 1211–1216. [Google Scholar] [CrossRef]
- Koneswaran, M.; Narayanaswamy, R. L-cysteine-capped ZnS quantum dots based fluorescence sensor for Cu2+ ion. Sens. Actuators B 2009, 139, 104–109. [Google Scholar] [CrossRef]
- Joo, J.; Na, H.B.; Yu, T.; Yu, J.H.; Kim, Y.W.; Wu, F.X.; Zhang, J.Z.; Hyeon, T. Generalized and facile synthesis of semiconducting metal sulfide nanocrystals. J. Am. Chem. Soc. 2003, 125, 11100–11105. [Google Scholar] [CrossRef]
- Ma, D.; Zhong, J.; Li, J.; Li, W.; Peng, R. Enhanced photocatalytic activity of biocl by C70 modification and mechanism insight. Appl. Surf. Sci. 2018, 443, 497–505. [Google Scholar] [CrossRef]
- Si, Y.; Liu, H.; Li, N.; Zhong, J.; Li, J.; Ma, D. Sdbs-assisted hydrothermal treatment of tio2 with improved photocatalytic activity. Mater. Lett. 2018, 212, 147–150. [Google Scholar] [CrossRef]
- Zhong, J.B.; Li, J.Z.; Feng, F.M.; Lu, Y.; Zeng, J.; Hu, W.; Tang, Z. Improved photocatalytic performance of sio2-tio2 prepared with the assistance of sdbs. J. Mol. Catal. A Chem. 2012, 357, 101–105. [Google Scholar] [CrossRef]
- Cao, Y.; Hu, P.; Jia, D. Solvothermal synthesis, growth mechanism, and photoluminescence property of sub-micrometer pbs anisotropic structures. Nanoscale Res. Lett. 2012, 7, 668. [Google Scholar] [CrossRef] [PubMed]
- Fan, L.; Guo, R. Growth of dendritic silver crystals in ctab/sdbs mixed-surfactant solutions. Cryst. Growth Des. 2008, 8, 2150–2156. [Google Scholar] [CrossRef]
- Lu, B.; Zhan, F.; Gong, G.; Cao, Y.; Zhen, Q.; Hu, P. Room-temperature mechanochemical synthesis of silver nanoparticle homojunction assemblies for the surface-enhanced raman scattering substrate. RSC Adv. 2016, 6, 74662–74669. [Google Scholar] [CrossRef]
- Hu, P.; Cao, Y.; Jia, D.; Li, Q.; Liu, R. Engineering the metathesis and oxidation-reduction reaction in solid state at room temperature for nanosynthesis. Sci. Rep. 2014, 4, 4153. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hu, P.; Cao, Y. A new chemical route to a hybrid nanostructure: Room-temperature solid-state reaction synthesis of ag@agcl with efficient photocatalysis. Dalton Trans. 2012, 41, 8908–8912. [Google Scholar] [CrossRef]
- Russo, L.; Colangelo, F.; Cioffi, R.; Rea, I.; De Stefano, L. A mechanochemical approach to porous silicon nanoparticles fabrication. Materials 2011, 4, 1023–1033. [Google Scholar] [CrossRef]
- Service, R.F. Don’t sweat the small stuff. Science 2008, 320, 1584–1585. [Google Scholar]
- Hu, P.F.; Gong, G.D.; Zhan, F.Y.; Zhang, Y.; Li, R.; Cao, Y.L. The hydrothermal evolution of the phase and shape of ZnS nanostructures and their gas-sensing properties. Dalton Trans. 2016, 45, 2409–2416. [Google Scholar] [CrossRef] [PubMed]
- Nosaka, Y. Finite depth spherical well model for excited states of ultrasmall semiconductor particles. An application. J. Phys. Chem. 1991, 95, 3591–3597. [Google Scholar] [CrossRef]
- Slater, J.C.; Koster, G.F. Simplified lcao method for the periodic potential problem. Phys. Rev. 1954, 94, 1498. [Google Scholar] [CrossRef]
- Kulmala, S.; Suomi, J. Current status of modem analytical luminescence methods. Anal. Chim. Acta 2003, 500, 21–69. [Google Scholar] [CrossRef]
- Becker, W.G.; Bard, A.J. Photoluminescence and photoinduced oxygen adsorption of colloidal zinc sulfide dispersions. J. Phys. Chem. 1983, 87, 4888–4893. [Google Scholar] [CrossRef]
- Chestnoy, N.; Harris, T.D.; Hull, R.; Brus, L.E. Luminescence and photophysics of cadmium sulfide semiconductor clusters: The nature of the emitting electronic state. J. Phys. Chem. 1986, 90, 3393–3399. [Google Scholar] [CrossRef]
- Wageh, S.; Ling, Z.S.; Xu, X.R. Growth and optical properties of colloidal ZnS nanoparticles. J. Cryst. Growth 2003, 255, 332–337. [Google Scholar] [CrossRef]
- Selvaraj, J.; Mahesh, A.; Baskaralingam, V.; Dhayalan, A.; Paramasivam, T. Organic-to-water dispersible mn: ZnS-ZnS doped core-shell quantum dots: Synthesis, characterization and their application towards optical bioimaging and a turn-off fluorosensor. New J. Chem. 2019, 43, 11912–11925. [Google Scholar] [CrossRef]
- Li, H.; Shih, W.Y.; Shih, W.H. Stable aqueous ZnS quantum dots obtained using (3-mercaptopropyl) trimethoxysilane as a capping molecule. Nanotechnology 2007, 18, 495605. [Google Scholar] [CrossRef]

© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hu, P.; Xie, C.; Mao, Z.; Liang, X. A Mechanochemical Route for ZnS Nanocrystals, and Batch Sorting along Size Distribution. Nanomaterials 2019, 9, 1325. https://doi.org/10.3390/nano9091325
Hu P, Xie C, Mao Z, Liang X. A Mechanochemical Route for ZnS Nanocrystals, and Batch Sorting along Size Distribution. Nanomaterials. 2019; 9(9):1325. https://doi.org/10.3390/nano9091325
Chicago/Turabian StyleHu, Pengfei, Chen Xie, Zhihui Mao, and Xue Liang. 2019. "A Mechanochemical Route for ZnS Nanocrystals, and Batch Sorting along Size Distribution" Nanomaterials 9, no. 9: 1325. https://doi.org/10.3390/nano9091325