Green Micro- and Nanoemulsions for Managing Parasites, Vectors and Pests
Abstract
:1. Introduction
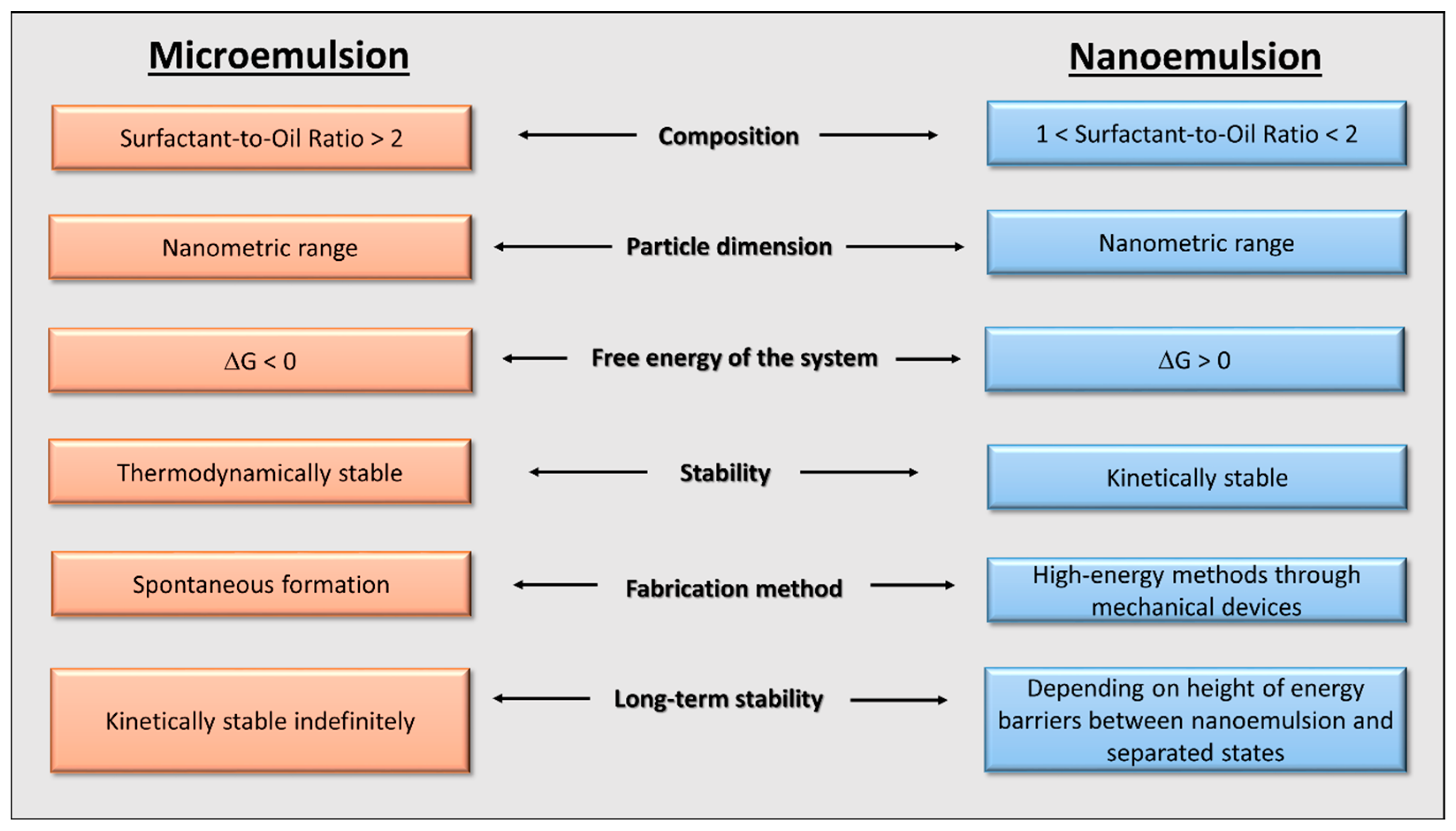
1.1. Micro- and Nanoemulsions
- (i)
- The SOR and relative concentrations; they influence the interfacial tension. It is not possible to stabilize a fixed relationship between these parameters because they are strictly related to the nature of the compounds that confer unique properties to the systems, which, in turn, differ from each other.
- (ii)
- The ionic strength of the dispersion medium; it affects the repulsive forces between the droplets of the dispersed phase. As the ionic strength increases, the repulsive forces decrease and the systems will be prone to instability.
- (iii)
- The solubility of the dispersed phase; it allows droplets to move towards the continuous phase with the appearance of Ostwald ripening.
- (iv)
- The temperature; it affects the solubility with the above-mentioned consequences. Moreover, it influences the energy balance of the system as well.
1.2. Applications
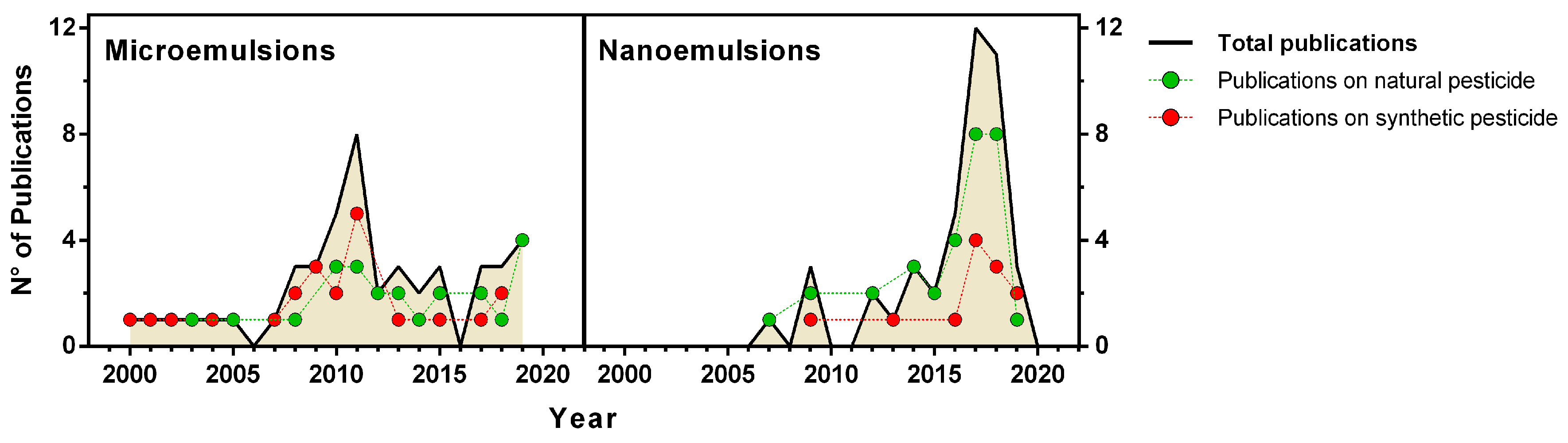
2. Green Micro- and Nanoemulsions
3. Green Micro- and Nanoemulsions as Insecticides
3.1. Hemiptera
3.2. Mosquitoes
3.3. Stored Product Beetles
4. Green Micro- and Nanoemulsions as Insect and Tick Repellents
5. Green Micro- and Nanoemulsions as Acaricides
6. Green Micro- and Nanoemulsions for Developing Antiparasitic Drugs
6.1. Parasitic Protozoa
6.1.1. Toxoplasma gondii
6.1.2. Leishmania spp.
6.1.3. Plasmodium spp.
6.2. Helminths
Echinococcus granulosus
7. Green Formulations against Nematodes Attacking Plants
Meloidogyne spp.
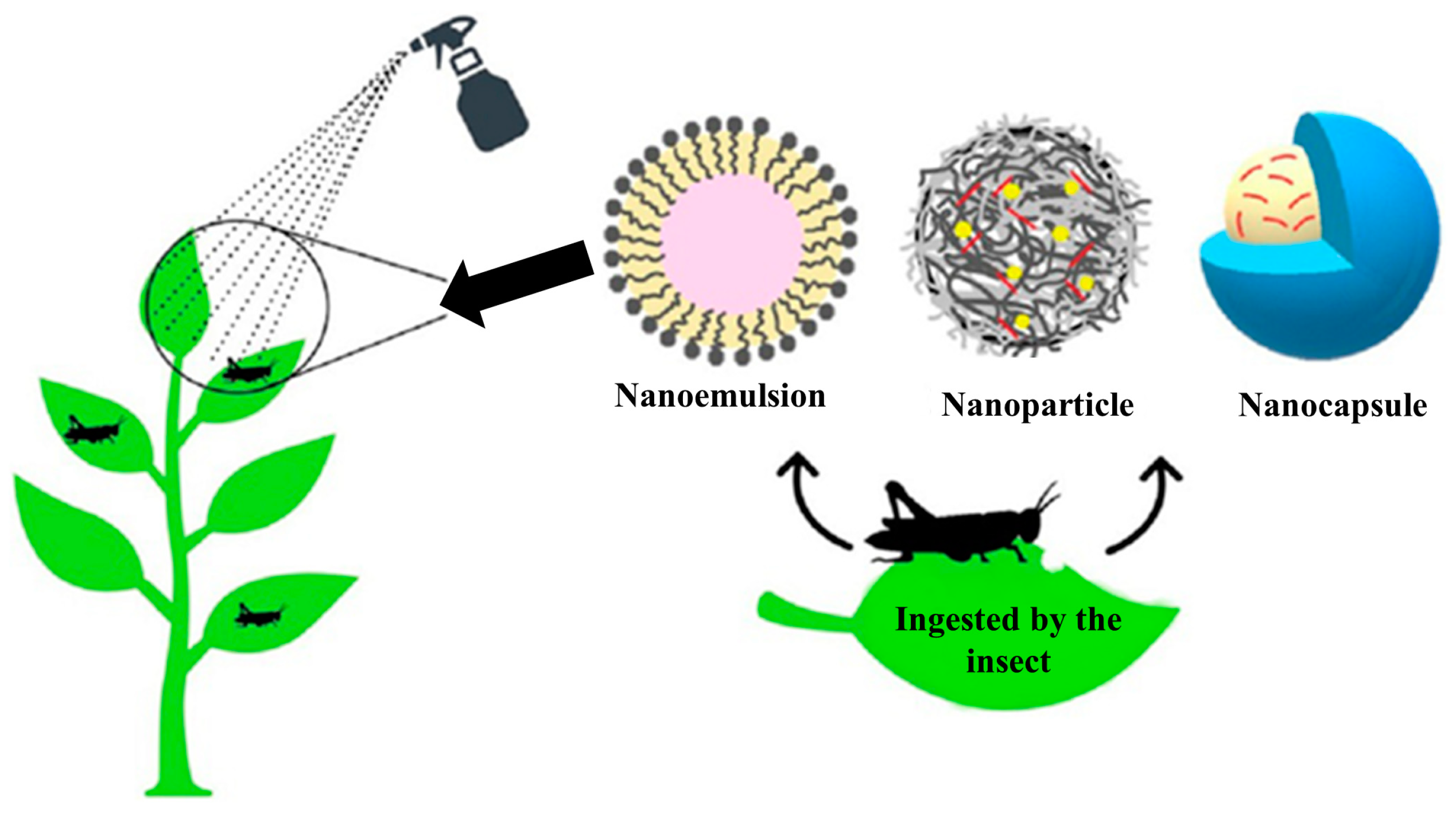
8. Green Micro- and Nanoemulsions in the Real World
9. Regulatory Remarks
10. Conclusions and Key Challenges for Future Research
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Anton, N.; Vandamme, T.F. Nano-emulsions and micro-emulsions: Clarifications of the critical differences. Pharm. Res. 2011, 28, 978–985. [Google Scholar] [CrossRef] [PubMed]
- Tadros, T.; Izquierdo, P.; Esquena, J.; Solans, C. Formation and stability of nano-emulsions. Adv. Colloid Interface Sci. 2004, 108, 303–318. [Google Scholar] [CrossRef] [PubMed]
- Talegaonkar, S.; Azeem, A.; Ahmad, F.; Khar, R.; Pathan, S.; Khan, Z. Microemulsions: A Novel Approach to Enhanced Drug Delivery. Recent Pat. Drug Deliv. 2008, 2, 238–257. [Google Scholar] [CrossRef]
- Gasco, M.R. Microemulsions in the pharmaceutical field: Perspectives and applications. Surfactant Sci. Ser. 1997, 66, 97–122. [Google Scholar]
- McClements, D.J. Nanoemulsions versus microemulsions: Terminology, differences, and similarities. Soft Matter 2012, 8, 1719–1729. [Google Scholar] [CrossRef]
- Rao, J.; McClements, D.J. Formation of flavor oil microemulsions, nanoemulsions and emulsions: Influence of composition and preparation method. J. Agric. Food Chem. 2011, 59, 5026–5035. [Google Scholar] [CrossRef]
- Danielsson, I.; Lindman, B. The definition of microemulsion. Colloids Surf. 1981, 3, 391–392. [Google Scholar] [CrossRef]
- Hoar, T.P.; Schulman, J.H. Transparent Water-in-Oil Dispersions: The Oleopathic Hydro-Micelle. Nature 1943, 152, 102–103. [Google Scholar] [CrossRef]
- Bera, A.; Mandal, A. Microemulsions: A novel approach to enhanced oil recovery: A review. J. Pet. Explor. Prod. Technol. 2015, 5, 255–268. [Google Scholar] [CrossRef]
- Pavoni, L.; Benelli, G.; Maggi, F.; Bonacucina, G. Green nanoemulsion interventions for biopesticide formulations. In Nano-Biopesticides Today and Future Perspectives; Academic Press: Cambridge, MA, USA, 2019; pp. 133–160. ISBN 978-0-12-815829-6. [Google Scholar]
- Venhuis, S.H.; Mehrvar, M. Health effects, environmental impacts, and photochemical degradation of selected surfactants in water. Int. J. Photoenergy 2004, 6, 115–125. [Google Scholar] [CrossRef]
- Wilhelm, K.P.; Cua, A.B.; Wolff, H.H.; Maibach, H.I. Surfactant-induced stratum corneum hydration in vivo: Prediction of the irritation potential of anionic surfactants. J. Investig. Dermatol. 1993, 101, 310–315. [Google Scholar] [CrossRef] [PubMed]
- Lawrence, M.J.; Rees, G.D. Microemulsion-based media as novel drug delivery systems. Adv. Drug Deliv. Rev. 2012, 64, 175–193. [Google Scholar] [CrossRef]
- Schulman, J.H.; Stoeckenius, W.; Prince, L.M. Mechanism of Formation and Structure of Micro Emulsions by Electron Microscopy. J. Phys. Chem 1959, 63, 1677–1680. [Google Scholar] [CrossRef]
- Alany, R.G.; Rades, T.; Agatonovic-Kustrin, S.; Davies, N.M.; Tucker, I.G. Effects of alcohols and diols on the phase behaviour of quaternary systems. Int. J. Pharm. 2000, 196, 141–145. [Google Scholar] [CrossRef]
- Lam, A.C.; Schechter, R.S. The theory of diffusion in microemulsion. J. Colloid Interface Sci. 1987, 120, 56–63. [Google Scholar] [CrossRef]
- Salvia-Trujillo, L.; Rojas-Graü, M.A.; Soliva-Fortuny, R.; Martín-Belloso, O. Effect of processing parameters on physicochemical characteristics of microfluidized lemongrass essential oil-alginate nanoemulsions. Food Hydrocoll. 2013, 30, 401–407. [Google Scholar] [CrossRef]
- Chang, Y.; McClements, D.J. Optimization of orange oil nanoemulsion formation by isothermal low-energy methods: Influence of the oil phase, surfactant, and temperature. J. Agric. Food Chem. 2014, 62, 2306–2312. [Google Scholar] [CrossRef]
- Tadros, T. Ostwald Ripening BT Encyclopedia of Colloid and Interface Science; Tadros, T., Ed.; Springer: Berlin/Heidelberg, Germany, 2013; p. 820. ISBN 978-3-642-20665-8. [Google Scholar]
- Chang, Y.; McLandsborough, L.; McClements, D.J. Physical properties and antimicrobial efficacy of thyme oil nanoemulsions: Influence of ripening inhibitors. J. Agric. Food Chem. 2012, 60, 12056–12063. [Google Scholar] [CrossRef]
- Donsì, F.; Annunziata, M.; Vincensi, M.; Ferrari, G. Design of nanoemulsion-based delivery systems of natural antimicrobials: Effect of the emulsifier. J. Biotechnol. 2012, 159, 342–350. [Google Scholar] [CrossRef]
- Terjung, N.; Löffler, M.; Gibis, M.; Hinrichs, J.; Weiss, J. Influence of droplet size on the efficacy of oil-in-water emulsions loaded with phenolic antimicrobials. Food Funct. 2012, 3, 290–301. [Google Scholar] [CrossRef]
- Shah, D.; Micelles, D.O. Microemulsions and Monolayers: Science and Technology; CRC Press: New York, NY, USA, 1998. [Google Scholar]
- Holmberg, K. Organic and bioorganic reactions in microemulsions. Adv. Colloid Interface Sci. 1994, 51, 137–174. [Google Scholar] [CrossRef]
- Lopez-Quintela, M.A. Synthesis of nanomaterials in microemulsions: Formation mechanisms and growth control. Curr. Opin. Colloid Interface Sci. 2003, 8, 137–144. [Google Scholar] [CrossRef]
- Yu, H.; Huang, Q. Improving the oral bioavailability of curcumin using novel organogel-based nanoemulsions. J. Agric. Food Chem. 2012, 60, 5373–5379. [Google Scholar] [CrossRef] [PubMed]
- Saifullah, M.; Ahsan, A.; Shishir, M.R.I. Production, Stability and Application of Micro and Nanoemulsion in Food Production and the food Processing Industry. Emulsions 2016, 3, 405–442. [Google Scholar] [CrossRef]
- Chee, C.P.; Gallaher, J.J.; Djordjevic, D.; Faraji, H.; McClements, D.J.; Decker, E.A.; Hollender, R.; Peterson, D.G.; Roberts, R.F.; Coupland, J.N. Chemical and sensory analysis of strawberry flavoured yogurt supplemented with an algae oil emulsion. J. Dairy Res. 2005, 72, 311–316. [Google Scholar] [CrossRef]
- Silva, H.D.; Cerqueira, M.Â.; Vicente, A.A. Nanoemulsions for Food Applications: Development and Characterization. Food Bioprocess Technol. 2012, 5, 854–867. [Google Scholar] [CrossRef]
- Donsi, F.; Ferrari, G. Essential oil nanoemulsions as antimicrobial agents in food. J. Biotechnol. 2016, 233, 106–120. [Google Scholar] [CrossRef]
- Alexandre, E.M.C.; Lourenço, R.V.; Bittante, A.M.Q.B.; Moraes, I.C.F.; do Amaral Sobral, P.J. Gelatin-based films reinforced with montmorillonite and activated with nanoemulsion of ginger essential oil for food packaging applications. Food Packag. Shelf Life 2016, 10, 87–96. [Google Scholar] [CrossRef]
- Flanagan, J.; Singh, H. Microemulsions: A potential delivery system for bioactives in food. Crit. Rev. Food Sci. Nutr. 2006, 46, 221–237. [Google Scholar] [CrossRef]
- Kralova, I.; Sjöblom, J. Surfactants used in food industry: A review. J. Dispers. Sci. Technol. 2009, 30, 1363–1383. [Google Scholar] [CrossRef]
- Araya, H.; Tomita, M.; Hayashi, M. The novel formulation design of O/W microemulsion for improving the gastrointestinal absorption of poorly water soluble compounds. Int. J. Pharm. 2005, 305, 61–74. [Google Scholar] [CrossRef] [PubMed]
- Bonacucina, G.; Cespi, M.; Misici-falzi, M.; Palmieri, G.F. Colloidal Soft Matter as Drug Delivery System. J. Pharm. Sci. 2009, 98, 1–42. [Google Scholar] [CrossRef] [PubMed]
- Paul, B.K.; Moulik, S.P. Uses and applications of microemulsions. Curr. Sci. Assoc. 2001, 80, 990–1001. [Google Scholar]
- Kim, C.K.; Cho, Y.J.; Gao, Z.G. Preparation and evaluation of biphenyl dimethyl dicarboxylate microemulsions for oral delivery. J. Control. Release 2001, 70, 149–155. [Google Scholar] [CrossRef]
- Yin, Y.M.; Cui, F.D.; Mu, C.F.; Choi, M.K.; Kim, J.S.; Chung, S.J.; Shim, C.K.; Kim, D.D. Docetaxel microemulsion for enhanced oral bioavailability: Preparation and in vitro and in vivo evaluation. J. Control. Release 2009, 140, 86–94. [Google Scholar] [CrossRef] [PubMed]
- Von Corswant, C.; Thorén, P.; Engström, S. Triglyceride-based microemulsion for intravenous administration of sparingly soluble substances. J. Pharm. Sci. 1998, 87, 200–208. [Google Scholar] [CrossRef] [PubMed]
- Đorđević, S.M.; Santrač, A.; Cekić, N.D.; Marković, B.D.; Divović, B.; Ilić, T.M.; Savić, M.M.; Savić, S.D. Parenteral nanoemulsions of risperidone for enhanced brain delivery in acute psychosis: Physicochemical and in vivo performances. Int. J. Pharm. 2017, 533, 421–430. [Google Scholar] [CrossRef] [PubMed]
- Gupta, S.; Moulik, S.P. Biocompatible microemulsions and their prospective uses in drug delivery. J. Pharm. Sci. 2008, 97, 22–45. [Google Scholar] [CrossRef] [PubMed]
- Majeed, A.; Bashir, R.; Farooq, S.; Maqbool, M. Preparation, Characterization and Applications of Nanoemulsions: An Insight. J. Drug Deliv. 2019, 9, 520–527. [Google Scholar] [CrossRef] [Green Version]
- Vyas, T.K.; Babbar, A.K.; Sharma, R.K.; Singh, S.; Misra, A. Intranasal Mucoadhesive Microemulsions of Clonazepam: Preliminary Studies on Brain Targeting. J. Pharm. Sci. 2006, 95, 570–580. [Google Scholar] [CrossRef]
- Shiokawa, T.; Hattori, Y.; Kawano, K.; Ohguchi, Y.; Kawakami, H.; Toma, K.; Maitani, Y. Effect of polyethylene glycol linker chain length of folate-linked microemulsions loading aclacinomycln A on targeting ability and antitumor effect in vitro and in vivo. Clin. Cancer Res. 2005, 11, 2018–2025. [Google Scholar] [CrossRef] [PubMed]
- Medina-Pérez, G.; Fernández-Luqueño, F.; Campos-Montiel, R.G.; Sánchez-López, K.B.; Afanador-Barajas, L.N.; Prince, L. Nanotechnology in crop protection: Status and future trends. In Nano-Biopesticides Today and Future Perspectives; Academic Press: Cambridge, MA, USA, 2019; pp. 17–45. ISBN 978-0-12-815829-6. [Google Scholar]
- Khater, H.; Govindarajan, M.; Benelli, G. Natural Remedies in the Fight Against Parasites; InTech, BoD–Books on Demand: London, UK, 2017; ISBN 953513289X. [Google Scholar]
- Du, Z.; Wang, C.; Tai, X.; Wang, G.; Liu, X. Optimization and Characterization of Biocompatible Oil-in-Water Nanoemulsion for Pesticide Delivery. ACS Sustain. Chem. Eng. 2016, 4, 983–991. [Google Scholar] [CrossRef]
- Perlatti, B.; de Souza Bergo, P.L.; Fernandes, J.B.; Forim, M.R. Polymeric nanoparticle-based insecticides: A controlled release purpose for agrochemicals. In Insecticides-Development of Safer and More Effective Technologies; IntechOpen: London, UK, 2013. [Google Scholar]
- Song, S.; Liu, X.; Jiang, J.; Qian, Y.; Zhang, N.; Wu, Q. Stability of triazophos in self-nanoemulsifying pesticide delivery system. Colloids Surf. A Physicochem. Eng. Asp. 2009, 350, 57–62. [Google Scholar] [CrossRef]
- Lubbe, A.; Verpoorte, R. Cultivation of medicinal and aromatic plants for specialty industrial materials. Ind. Crops Prod. 2011, 34, 785–801. [Google Scholar] [CrossRef]
- Fahn, A. Structure and function of secretory cells. Adv. Bot. Res. 2000, 31, 37–75. [Google Scholar]
- Isman, M.B. Botanical insecticides, deterrents, and repellents in modern agriculture and an increasingly regulated world. Annu. Rev. Entomol. 2006, 51, 45–66. [Google Scholar] [CrossRef] [PubMed]
- Chen, M.; Chang, C.H.; Tao, L.; Lu, C. Residential exposure to pesticide during childhood and childhood cancers: A meta-analysis. Pediatrics 2015, 136, 719–729. [Google Scholar] [CrossRef]
- Goulson, D. An overview of the environmental risks posed by neonicotinoid insecticides. J. Appl. Ecol. 2013, 50, 977–987. [Google Scholar] [CrossRef]
- McCaffery, A.; Nauen, R. The insecticide resistance action committee (IRAC): Public responsibility and enlightened industrial self-interest. Outlooks Pest Manag. 2006, 17, 11–14. [Google Scholar]
- Thakore, Y. The biopesticide market for global agricultural use. Ind. Biotechnol. 2006, 2, 194–208. [Google Scholar] [CrossRef]
- Isman, M.B. A renaissance for botanical insecticides? Pest Manag. Sci. 2015, 71, 1587–1590. [Google Scholar] [CrossRef] [PubMed]
- Pavela, R. History, presence and perspective of using plant extracts as commercial botanical insecticides and farm products for protection against insects—A review. Plant Prot. Sci. 2016, 52, 229–241. [Google Scholar]
- Isman, M.B. Problems and opportunities for the commercialization of botanical insecticides. In Biopesticides of Plant Origin; Regnault-Roger, C., Philogene, B.J.R., Vincent, C., Eds.; Lavoisier: Paris, France, 2005; pp. 283–291. [Google Scholar]
- Collins, D.A. A review of alternatives to organophosphorus compounds for the control of storage mites. J. Stored Prod. Res. 2006, 42, 395–426. [Google Scholar] [CrossRef]
- Singh, A.; Srivastava, V.K. Toxic effect of synthetic pyrethroid permethrin on the enzyme system of the freshwater fish Channa striatus. Chemosphere 1999, 39, 1951–1956. [Google Scholar] [CrossRef]
- Guleria, S.; Jammu, T. Integrated Pest Management: Innovation-Development Process; Springer: Dordrecht, The Netherlands; Heidelberg, Germany, 2009. [Google Scholar]
- Benelli, G.; Canale, A.; Toniolo, C.; Higuchi, A.; Murugan, K.; Pavela, R.; Nicoletti, M. Neem (Azadirachta indica): Towards the ideal insecticide? Nat. Prod. Res. 2017, 31, 369–386. [Google Scholar] [CrossRef] [PubMed]
- Raizada, R.B.; Srivastava, M.K.; Kaushal, R.A.; Singh, R.P. Azadirachtin, a neem biopesticide: Subchronic toxicity assessment in rats. Food Chem. Toxicol. 2001, 39, 477–483. [Google Scholar] [CrossRef]
- Mehlhorn, H.; Al-Rasheid, K.A.S.; Abdel-Ghaffar, F. The Neem tree story: Extracts that really work. In Nature Helps; Springer: Heidelberg, Germany, 2011; pp. 77–108. [Google Scholar]
- Pavela, R.; Benelli, G. Essential Oils as Ecofriendly Biopesticides? Challenges and Constraints. Trends Plant Sci. 2016, 21, 1000–1007. [Google Scholar] [CrossRef] [PubMed]
- Burt, S. Essential oils: Their antibacterial properties and potential applications in foods—A review. Int. J. Food Microbiol. 2004, 94, 223–253. [Google Scholar] [CrossRef] [PubMed]
- Stroh, J.; Wan, M.T.; Isman, M.B.; Moul, D.J. Evaluation of the acute toxicity to juvenile Pacific coho salmon and rainbow trout of some plant essential oils, a formulated product, and the carrier. Bull. Environ. Contam. Toxicol. 1998, 60, 923–930. [Google Scholar] [CrossRef]
- Pavela, R.; Benelli, G.; Pavoni, L.; Bonacucina, G.; Cespi, M.; Cianfaglione, K.; Bajalan, I.; Morshedloo, M.R.; Lupidi, G.; Romano, D.; et al. Microemulsions for delivery of Apiaceae essential oils—Towards highly effective and eco-friendly mosquito larvicides? Ind. Crops Prod. 2019, 129, 631–640. [Google Scholar] [CrossRef]
- Dubey, N.K. Natural Products in Plant Pest Management; CABI: Wallingford, UK, 2011; ISBN 184593671X. [Google Scholar]
- Isman, M.B. Botanical Insecticides, Deterrents, Repellents and Oils; CABI: Oxfordsh, UK, 2010; pp. 433–445. [Google Scholar]
- Koul, O.; Walia, S.; Dhaliwal, G.S. Essential oils as green pesticides: Potential and constraints. Biopestic. Int. 2008, 4, 63–84. [Google Scholar]
- Nerio, L.S.; Olivero-Verbel, J.; Stashenko, E. Repellent activity of essential oils: A review. Bioresour. Technol. 2010, 101, 372–378. [Google Scholar] [CrossRef] [PubMed]
- Pavela, R. Essential oils for the development of eco-friendly mosquito larvicides: A review. Ind. Crops Prod. 2015, 76, 174–187. [Google Scholar] [CrossRef]
- Rattan, R.S. Mechanism of action of insecticidal secondary metabolites of plant origin. Crop Prot. 2010, 29, 913–920. [Google Scholar] [CrossRef]
- Lambert, R.J.W.; Skandamis, P.N.; Coote, P.J.; Nychas, G. A study of the minimum inhibitory concentration and mode of action of oregano essential oil, thymol and carvacrol. J. Appl. Microbiol. 2001, 91, 453–462. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tian, J.; Ban, X.; Zeng, H.; He, J.; Chen, Y.; Wang, Y. The mechanism of antifungal action of essential oil from dill (Anethum graveolens L.) on Aspergillus flavus. PLoS ONE 2012, 7, e30147. [Google Scholar] [CrossRef] [PubMed]
- Ceylan, E.; Fung, D.Y.C. Antimicrobial activity of spices 1. J. Rapid Methods Autom. Microbiol. 2004, 12, 1–55. [Google Scholar] [CrossRef]
- Di Pasqua, R.; Hoskins, N.; Betts, G.; Mauriello, G. Changes in membrane fatty acids composition of microbial cells induced by addiction of thymol, carvacrol, limonene, cinnamaldehyde, and eugenol in the growing media. J. Agric. Food Chem. 2006, 54, 2745–2749. [Google Scholar] [CrossRef]
- Enan, E. Insecticidal activity of essential oils: Octopaminergic sites of action. Comp. Biochem. Physiol. Part C Toxicol. Pharm. 2001, 130, 325–337. [Google Scholar] [CrossRef]
- Jankowska, M.; Rogalska, J.; Wyszkowska, J.; Stankiewicz, M. Molecular targets for components of essential oils in the insect nervous system—A review. Molecules 2018, 23, 34. [Google Scholar] [CrossRef]
- Mills, C.; Cleary, B.V.; Walsh, J.J.; Gilmer, J.F. Inhibition of acetylcholinesterase by tea tree oil. J. Pharm. Pharm. 2004, 56, 375–379. [Google Scholar] [CrossRef]
- Priestley, C.M.; Williamson, E.M.; Wafford, K.A.; Sattelle, D.B. Thymol, a constituent of thyme essential oil, is a positive allosteric modulator of human GABAA receptors and a homo–oligomeric GABA receptor from Drosophila melanogaster. Br. J. Pharm. 2003, 140, 1363–1372. [Google Scholar] [CrossRef]
- Enan, E.E. Molecular response of Drosophila Melanogaster Tyramine Receptor Cascade to Plant Essential Oils. Insect Biochem. Mol. Biol. 2005, 35, 309–321. [Google Scholar] [CrossRef] [PubMed]
- Isman, M.B. Plant essential oils for pest and disease management. Crop Prot. 2000, 19, 603–608. [Google Scholar] [CrossRef]
- Benelli, G.; Pavela, R.; Canale, A.; Cianfaglione, K.; Ciaschetti, G.; Conti, F.; Nicoletti, M.; Senthil-Nathan, S.; Mehlhorn, H.; Maggi, F. Acute larvicidal toxicity of five essential oils (Pinus nigra, Hyssopus officinalis, Satureja montana, Aloysia citrodora and Pelargonium graveolens) against the filariasis vector Culex quinquefasciatus: Synergistic and antagonistic effects. Parasitol. Int. 2017, 66, 166–171. [Google Scholar] [CrossRef] [PubMed]
- Pavela, R. Acute toxicity and synergistic and antagonistic effects of the aromatic compounds of some essential oils against Culex quinquefasciatus Say larvae. Parasitol. Res. 2015, 114, 3835–3853. [Google Scholar] [CrossRef] [PubMed]
- Turek, C.; Stintzing, F.C. Stability of essential oils: A review. Compr. Rev. Food Sci. Food Saf. 2013, 12, 40–53. [Google Scholar] [CrossRef]
- Isman, M.B.; Miresmailli, S.; Machial, C. Commercial opportunities for pesticides based on plant essential oils in agriculture, industry and consumer products. Phytochem. Rev. 2011, 10, 197–204. [Google Scholar] [CrossRef]
- Benelli, G. Plant-mediated biosynthesis of nanoparticles as an emerging tool against mosquitoes of medical and veterinary importance: A review. Parasitol. Res. 2016, 115, 23–34. [Google Scholar] [CrossRef]
- Haldar, K.M.; Haldar, B.; Chandra, G. Fabrication, characterization and mosquito larvicidal bioassay of silver nanoparticles synthesized from aqueous fruit extract of putranjiva, Drypetes roxburghii (Wall.). Parasitol. Res. 2013, 112, 1451–1459. [Google Scholar] [CrossRef]
- Benelli, G. Gold nanoparticles–against parasites and insect vectors. Acta Trop. 2018, 178, 73–80. [Google Scholar] [CrossRef] [PubMed]
- Arjunan, N.K.; Murugan, K.; Rejeeth, C.; Madhiyazhagan, P.; Barnard, D.R. Green synthesis of silver nanoparticles for the control of mosquito vectors of malaria, filariasis, and dengue. Vector-Borne Zoonotic Dis. 2012, 12, 262–268. [Google Scholar] [CrossRef] [PubMed]
- Ghormade, V.; Deshpande, M.V.; Paknikar, K.M. Perspectives for nano-biotechnology enabled protection and nutrition of plants. Biotechnol. Adv. 2011, 29, 792–803. [Google Scholar] [CrossRef] [PubMed]
- Pavela, R.; Murugan, K.; Canale, A.; Benelli, G. Saponaria officinalis-synthesized silver nanocrystals as effective biopesticides and oviposition inhibitors against Tetranychus urticae Koch. Ind. Crops Prod. 2017, 97, 338–344. [Google Scholar] [CrossRef]
- Cespi, M.; Quassinti, L.; Perinelli, D.R.; Bramucci, M.; Iannarelli, R.; Papa, F.; Ricciutelli, M.; Bonacucina, G.; Palmieri, G.F.; Maggi, F. Microemulsions enhance the shelf-life and processability of Smyrnium olusatrum L. essential oil. Flavour Fragr. J. 2017, 32, 159–164. [Google Scholar] [CrossRef]
- Pavela, R.; Pavoni, L.; Bonacucina, G.; Cespi, M.; Kavallieratos, N.G.; Cappellacci, L.; Petrelli, R.; Maggi, F.; Benelli, G. Rationale for developing novel mosquito larvicides based on isofuranodiene microemulsions. J. Pest Sci. 2019, 92, 909–921. [Google Scholar] [CrossRef]
- Osman Mohamed Ali, E.; Shakil, N.A.; Rana, V.S.; Sarkar, D.J.; Majumder, S.; Kaushik, P.; Singh, B.B.; Kumar, J. Antifungal activity of nano emulsions of neem and citronella oils against phytopathogenic fungi, Rhizoctonia solani and Sclerotium rolfsii. Ind. Crops Prod. 2017, 108, 379–387. [Google Scholar] [CrossRef]
- Pavoni, L.; Maggi, F.; Mancianti, F.; Nardoni, S.; Ebani, V.V.; Cespi, M.; Bonacucina, G.; Palmieri, G.F. Microemulsions: An effective encapsulation tool to enhance the antimicrobial activity of selected EOs. J. Drug Deliv. Sci. Technol. 2019. [Google Scholar] [CrossRef]
- Liang, R.; Xu, S.; Shoemaker, C.F.; Li, Y.; Zhong, F.; Huang, Q. Physical and antimicrobial properties of peppermint oil nanoemulsions. J. Agric. Food Chem. 2012, 60, 7548–7555. [Google Scholar] [CrossRef]
- Sasson, Y.; Levy-Ruso, G.; Toledano, O.; Ishaaya, I. Nanosuspensions: Emerging novel agrochemical formulations. In Insecticides Design Using Advanced Technologies; Springer: Berlin, Germany, 2007; pp. 1–39. [Google Scholar]
- Salvia-Trujillo, L.; Rojas-Graü, A.; Soliva-Fortuny, R.; Martín-Belloso, O. Physicochemical characterization and antimicrobial activity of food-grade emulsions and nanoemulsions incorporating essential oils. Food Hydrocoll. 2015, 43, 547–556. [Google Scholar] [CrossRef]
- Zhao, N.N.; Zhang, H.; Zhang, X.C.; Luan, X.B.; Zhou, C.; Liu, Q.Z.; Shi, W.P.; Liu, Z.L. Evaluation of acute toxicity of essential oil of garlic (Allium sativum) and its selected major constituent compounds against overwintering Cacopsylla chinensis (Hemiptera: Psyllidae). J. Econ. Entomol. 2013, 106, 1349–1354. [Google Scholar] [CrossRef] [PubMed]
- Mann, R.S.; Tiwari, S.; Smoot, J.M.; Rouseff, R.L.; Stelinski, L.L. Repellency and toxicity of plant-based essential oils and their constituents against Diaphorina citri Kuwayama (Hemiptera: Psyllidae). J. Appl. Entomol. 2012, 136, 87–96. [Google Scholar] [CrossRef]
- González, W.J.O.; Gutiérrez, M.M.; Murray, A.P.; Ferrero, A.A. Composition and biological activity of essential oils from Labiatae against Nezara viridula (Hemiptera: Pentatomidae) soybean pest. Pest Manag. Sci. 2011, 67, 948–955. [Google Scholar] [CrossRef] [PubMed]
- Tian, B.L.; Liu, Q.Z.; Liu, Z.L.; Li, P.; Wang, J.W. Insecticidal Potential of Clove Essential Oil and Its Constituents on Cacopsylla chinensis (Hemiptera: Psyllidae) in Laboratory and Field. J. Econ. Entomol. 2015, 108, 957–961. [Google Scholar] [CrossRef] [PubMed]
- Fernandes, C.P.; de Almeida, F.B.; Silveira, A.N.; Gonzalez, M.S.; Mello, C.B.; Feder, D.; Apolinário, R.; Santos, M.G.; Carvalho, J.C.T.; Tietbohl, L.A.C.; et al. Development of an insecticidal nanoemulsion with Manilkara subsericea (Sapotaceae) extract. J. Nanobiotechnol. 2014, 12, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Fernandes, C.P.; Xavier, A.; Pacheco, J.P.F.; Santos, M.G.; Mexas, R.; Ratcliffe, N.A.; Gonzalez, M.S.; Mello, C.B.; Rocha, L.; Feder, D. Laboratory evaluation of the effects of Manilkara subsericea (Mart.) Dubard extracts and triterpenes on the development of Dysdercus peruvianus and Oncopeltus fasciatus. Pest Manag. Sci. 2013, 69, 292–301. [Google Scholar] [CrossRef] [PubMed]
- Stanisçuaski, F.; Ferreira-DaSilva, C.T.; Mulinari, F.; Pires-Alves, M.; Carlini, C.R. Insecticidal effects of canatoxin on the cotton stainer bug Dysdercus peruvianus (Hemiptera: Pyrrhocoridae). Toxicon 2005, 45, 753–760. [Google Scholar] [CrossRef] [PubMed]
- Gutiérrez, C.; Fereres, A.; Reina, M.; Cabrera, R.; González-Coloma, A. Behavioral and Sublethal Effects of Structurally Related Lower Terpenes on Myzus persicae. J. Chem. Ecol. 1997, 23, 1641–1650. [Google Scholar] [CrossRef]
- Santana, O.; Cabrera, R.; Gimenez, C.; González-Coloma, A.; Sánchez-Vioque, R.; De los Mozos-Pascual, M.; Rodríguez-Conde, M.F.; Laserna-Ruiz, I.; Usano-Alemany, J.; Herraiz, D. Perfil químico y biológico de aceites esenciales de plantas aromáticas de interés agro-industrial en Castilla-La Mancha (España). Grasas Y Aceites 2012, 63. [Google Scholar]
- Blackman, R.L.; Eastop, V.F. Aphids on the World’s Crops: An Identification and Information Guide; John Wiley & Sons Ltd.: Hoboken, NJ, USA, 2000; ISBN 0471851914. [Google Scholar]
- Kalaitzaki, A.; Papanikolaou, N.E.; Karamaouna, F.; Dourtoglou, V.; Xenakis, A.; Papadimitriou, V. Biocompatible colloidal dispersions as potential formulations of natural pyrethrins: A structural and efficacy study. Langmuir 2015, 31, 5722–5730. [Google Scholar] [CrossRef] [PubMed]
- Pascual-Villalobos, M.J.; Cantó-Tejero, M.; Vallejo, R.; Guirao, P.; Rodríguez-Rojo, S.; Cocero, M.J. Use of nanoemulsions of plant essential oils as aphid repellents. Ind. Crops Prod. 2017, 110, 45–57. [Google Scholar] [CrossRef]
- Blackman, R.L.; Eastop, V.F. Taxonomic issues. Aphids Crop Pests 2007, 1–29. [Google Scholar]
- James, A.A. Mosquito molecular genetics: The hands that feed bite back. Science 1992, 257, 37–39. [Google Scholar] [CrossRef] [PubMed]
- Jambulingam, P.; Subramanian, S.; de Vlas, S.J.; Vinubala, C.; Stolk, W.A. Mathematical modelling of lymphatic filariasis elimination programmes in India: Required duration of mass drug administration and post-treatment level of infection indicators. Parasit. Vectors 2016, 9, 501. [Google Scholar] [CrossRef] [PubMed]
- Benelli, G.; Romano, D. Mosquito vectors of Zika virus. Entomol. Gen. 2017, 36, 309–318. [Google Scholar] [CrossRef]
- Oliveira, A.E.M.F.M.; Duarte, J.L.; Cruz, R.A.S.; Souto, R.N.P.; Ferreira, R.M.A.; Peniche, T.; Conceição, E.C.; Oliveira, L.A.R.; Faustino, S.M.M.; Florentino, A.C.; et al. Pterodon emarginatus oleoresin-based nanoemulsion as a promising tool for Culex quinquefasciatus (Diptera: Culicidae) control. J. Nanobiotechnol. 2017, 15, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Duarte, J.L.; Amado, J.R.R.; Oliveira, A.E.M.F.M.; Cruz, R.A.S.; Ferreira, A.M.; Souto, R.N.P.; Falcão, D.Q.; Carvalho, J.C.T.; Fernandesa, C.P. Evaluation of larvicidal activity of a nanoemulsion of Rosmarinus officinalis essential oil. Braz. J. Pharm. 2015, 25, 189–192. [Google Scholar] [CrossRef]
- Ghosh, V.; Mukherjee, A.; Chandrasekaran, N. Formulation and characterization of plant essential oil based nanoemulsion: Evaluation of its larvicidal activity against Aedes aegypti. Asian J. Chem. 2013, 25, S321. [Google Scholar]
- Balasubramani, S.; Rajendhiran, T.; Moola, A.K.; Kumari, R.; Diana, B. Development of nanoemulsion from Vitex negundo Lessential oil and their efficacy of antioxidant antimicrobial and larvicidal activities (Aedes aegypti L.). ) Environ. Sci. Pollut. Res. 2017, 24, 15125–15133. [Google Scholar] [CrossRef]
- Gaysinsky, S.; Taylor, T.M.; Davidson, P.M.; Bruce, B.D. Antimicrobial Efficacy of Eugenol Microemulsions in Milk against Listeria monocytogenes and Escherichia coli O157:H7. J. Food Prot. 2007, 70, 2631–2637. [Google Scholar] [CrossRef]
- Anjali, C.; Sharma, Y.; Mukherjee, A.; Chandrasekaran, N. Neem oil (Azadirachta indica) nanoemulsion-a potent larvicidal agent against Culex quinquefasciatus. Pest Manag. Sci. 2012, 68, 158–163. [Google Scholar] [CrossRef] [PubMed]
- Sugumar, S.; Clarke, S.K.; Nirmala, M.J.; Tyagi, B.K.; Mukherjee, A.; Chandrasekaran, N. Nanoemulsion of eucalyptus oil and its larvicidal activity against Culex quinquefasciatus. Bull. Entomol. Res. 2014, 104, 393–402. [Google Scholar] [CrossRef]
- Dwivedy, A.K.; Singh, V.K.; Prakash, B.; Dubey, N.K. Nanoencapsulated Illicium verum Hook. f. essential oil as an effective novel plant-based preservative against aflatoxin B1 production and free radical generation. Food Chem. Toxicol. 2018, 111, 102–113. [Google Scholar] [CrossRef] [PubMed]
- Alonso-Amelot, M.E.; Avila-Núñez, J.L. Comparison of seven methods for stored cereal losses to insects for their application in rural conditions. J. Stored Prod. Res. 2011, 47, 82–87. [Google Scholar] [CrossRef]
- Magan, N.; Hope, R.; Cairns, V.; Aldred, D. Post-harvest fungal ecology: Impact of fungal growth and mycotoxin accumulation in stored grain. In Epidemiology of Mycotoxin Producing Fungi; Springer: Berlin, Germany, 2003; pp. 723–730. [Google Scholar]
- Hodges, R.J.; Robinson, R.; Hall, D.R. Quinone contamination of dehusked rice by Tribolium castaneum (Herbst) (Coleoptera: Tenebrionidae). J. Stored Prod. Res. 1996, 32, 31–37. [Google Scholar] [CrossRef]
- Nenaah, G.E. Chemical composition, toxicity and growth inhibitory activities of essential oils of three Achillea species and their nano-emulsions against Tribolium castaneum (Herbst). Ind. Crops Prod. 2014, 53, 252–260. [Google Scholar] [CrossRef]
- Hashem, A.S.; Awadalla, S.S.; Zayed, G.M.; Maggi, F.; Benelli, G. Pimpinella anisum essential oil nanoemulsions against Tribolium castaneum—Insecticidal activity and mode of action. Environ. Sci. Pollut. Res. 2018, 25, 18802–18812. [Google Scholar] [CrossRef] [PubMed]
- Pant, M.; Dubey, S.; Patanjali, P.K.; Naik, S.N.; Sharma, S. Insecticidal activity of eucalyptus oil nanoemulsion with karanja and jatropha aqueous filtrates. Int. Biodeterior. Biodegrad. 2014, 91, 119–127. [Google Scholar] [CrossRef]
- Kesari, V.; Das, A.; Rangan, L. Physico-chemical characterization and antimicrobial activity from seed oil of Pongamia pinnata, a potential biofuel crop. Biomass Bioenergy 2010, 34, 108–115. [Google Scholar] [CrossRef]
- Sharma, S.; Verma, M.; Prasad, R.; Yadav, D. Efficacy of non-edible oil seedcakes against termite (Odontotermes obesus). J. Sci. Ind. Res. 2011, 70, 1037–1041. [Google Scholar]
- Mossa, A.T.H.; Abdelfattah, N.A.H.; Mohafrash, S.M.M. Nanoemulsion of camphor (Eucalyptus globulus) essential oil, formulation, characterization and insecticidal activity against wheat weevil, Sitophilus granarius. Asian J. Crop Sci. 2017, 9, 50–62. [Google Scholar] [CrossRef]
- Choupanian, M.; Omar, D.; Basri, M.; Asib, N. Preparation and characterization of neem oil nanoemulsion formulations against Sitophilus oryzae and Tribolium castaneum adults. J. Pestic. Sci. 2017, 42, 158–165. [Google Scholar] [CrossRef] [PubMed]
- van der Goes van Naters, W.; Carlson, J.R. Insects as chemosensors of humans and crops. Nature 2006, 444, 302. [Google Scholar] [CrossRef] [PubMed]
- Drapeau, J.; Verdier, M.; Touraud, D.; Kröckel, U.; Geier, M.; Rose, A.; Kunz, W. Effective insect repellent formulation in both surfactantless and classical microemulsions with a long-lasting protection for human beings. Chem. Biodivers. 2009, 6, 934–947. [Google Scholar] [CrossRef] [PubMed]
- Tavares, M.; da Silva, M.R.M.; de Oliveira de Siqueira, L.B.; Rodrigues, R.A.S.; Bodjolle-d’Almeira, L.; dos Santos, E.P.; Ricci-Júnior, E. Trends in insect repellent formulations: A review. Int. J. Pharm. 2018, 539, 190–209. [Google Scholar] [CrossRef] [PubMed]
- Pinto, I.C.; Cerqueira-Coutinho, C.S.; Santos, E.P.; Carmo, F.A.; Ricci-Junior, E. Development and characterization of repellent formulations based on nanostructured hydrogels. Drug Dev. Ind. Pharm. 2017, 43, 67–73. [Google Scholar] [CrossRef]
- Rowland, M.; Freeman, T.; Downey, G.; Hadi, A.; Saeed, M. DEET mosquito repellent sold through social marketing provides personal protection against malaria in an area of all–night mosquito biting and partial coverage of insecticide–treated nets: A case–control study of effectiveness. Trop. Med. Int. Heal. 2004, 9, 343–350. [Google Scholar] [CrossRef]
- Abou-Donia, M.B. Neurotoxicity resulting from coexposure to pyridostigmine bromide, DEET, and permethrin: Implications of Gulf War chemical exposures. J. Toxicol. Environ. Heal. Part A 1996, 48, 35–56. [Google Scholar] [CrossRef]
- Qiu, H.; McCall, J.W.; Jun, H.W. Formulation of topical insect repellent N, N-diethyl-m-toluamide (DEET): Vehicle effects on DEET in vitro skin permeation. Int. J. Pharm. 1998, 163, 167–176. [Google Scholar] [CrossRef]
- Moore, S.J.; Lenglet, A.; Hill, N. Plant-based insect repellents. In Insect Repellents: Principles Methods, and Use; CRC Press: Boca Raton, FL, USA, 2006. [Google Scholar]
- Seyoum, A.; Pålsson, K.; Kung’a, S.; Kabiru, E.W.; Lwande, W.; Killeen, G.F.; Hassanali, A.; Knots, B.G.J. Traditional use of mosquito-repellent plants in western Kenya and their evaluation in semi-field experimental huts against Anopheles gambiae: Ethnobotanical studies and application by thermal expulsion and direct burning. Trans. R. Soc. Trop. Med. Hyg. 2002, 96, 225–231. [Google Scholar] [CrossRef]
- Rehman, J.U.; Ali, A.; Khan, I.A. Plant based products: Use and development as repellents against mosquitoes: A review. Fitoterapia 2014, 95, 65–74. [Google Scholar] [CrossRef] [PubMed]
- Jaenson, T.G.T.; Pålsson, K.; Borg-Karlson, A.K. Evaluation of extracts and oils of mosquito (Diptera: Culicidae) repellent plants from Sweden and Guinea-Bissau. J. Med. Entomol. 2006, 43, 113–119. [Google Scholar] [CrossRef] [PubMed]
- Sukumar, K.; Perich, M.J.; Boobar, L.R. Botanical derivatives in mosquito control: A review. J. Am. Mosq. Control Assoc. 1991, 7, 210–237. [Google Scholar] [PubMed]
- Jantan, I.; Zaki, Z.M. Development of environment-friendly insect repellents from the leaf oils of selected Malaysian plants. Asean Rev. Biodivers. Environ. Conserv. 1998, 6, 1–7. [Google Scholar]
- Yang, Y.C.; Lee, E.H.; Lee, H.S.; Lee, D.K.; Ahn, Y.J. Repellency of aromatic medicinal plant extracts and a steam distillate to Aedes aegypti. J. Am. Mosq. Control Assoc. 2004, 20, 146–149. [Google Scholar] [PubMed]
- Nuchuchua, O.; Sakulku, U.; Uawongyart, N.; Puttipipatkhachorn, S.; Soottitantawat, A.; Ruktanonchai, U. In Vitro Characterization and Mosquito (Aedes aegypti) Repellent Activity of Essential-Oils-Loaded Nanoemulsions. AAPS PharmSciTech 2009, 10, 1234–1242. [Google Scholar] [CrossRef] [PubMed]
- Sakulku, U.; Nuchuchua, O.; Uawongyart, N.; Puttipipatkhachorn, S.; Soottitantawat, A.; Ruktanonchai, U. Characterization and mosquito repellent activity of citronella oil nanoemulsion. Int. J. Pharm. 2009, 372, 105–111. [Google Scholar] [CrossRef] [PubMed]
- Kogan, A.; Garti, N. Microemulsions as transdermal drug delivery vehicles. Adv. Colloid Interface Sci. 2006, 123–126, 369–385. [Google Scholar] [CrossRef] [PubMed]
- Steib, B.M. The Effect of Lactic Acid on Odour-Related Host Preference of Yellow Fever Mosquitoes. Chem. Senses 2001, 26, 523–528. [Google Scholar] [CrossRef]
- Bernier, U.R.; Kline, D.L.; Posey, K.H.; Booth, M.M.; Yost, R.A.; Barnard, D.R. Synergistic Attraction of Aedes aegypti (L.) to Binary Blends of L-Lactic Acid and Acetone, Dichloromethane, or Dimethyl Disulfide. J. Med. Entomol. 2009, 40, 653–656. [Google Scholar] [CrossRef] [PubMed]
- Navayan, A.; Moghimipour, E.; Khodayar, M.J.; Vazirianzadeh, B.; Siahpoosh, A.; Valizadeh, M.; Mansourzadeh, Z. Evaluation of the Mosquito Repellent Activity of Nano-sized Microemulsion of Eucalyptus globulus Essential Oil Against Culicinae. Jundishapur J. Nat. Pharm. Prod. 2017, 12. [Google Scholar] [CrossRef]
- Miresmailli, S.; Isman, M.B. Efficacy and persistence of rosemary oil as an acaricide against twospotted spider mite (Acari: Tetranychidae) on greenhouse tomato. J. Econ. Entomol. 2006, 99, 2015–2023. [Google Scholar] [CrossRef] [PubMed]
- Çalmaşur, Ö.; Aslan, İ.; Şahin, F. Insecticidal and acaricidal effect of three Lamiaceae plant essential oils against Tetranychus urticae Koch and Bemisia tabaci Genn. Ind. Crops Prod. 2006, 23, 140–146. [Google Scholar] [CrossRef]
- Laborda, R.; Manzano, I.; Gamón, M.; Gavidia, I.; Pérez-Bermúdez, P.; Boluda, R. Effects of Rosmarinus officinalis and Salvia officinalis essential oils on Tetranychus urticae Koch (Acari: Tetranychidae). Ind. Crops Prod. 2013, 48, 106–110. [Google Scholar] [CrossRef]
- Han, J.; Kim, S.; Choi, B.; Lee, S.; Ahn, Y. Fumigant toxicity of lemon eucalyptus oil constituents to acaricide–Susceptible and acaricide–Resistant Tetranychus urticae. Pest Manag. Sci. 2011, 67, 1583–1588. [Google Scholar] [CrossRef]
- Choi, W.I.; Lee, S.G.; Park, H.M.; Ahn, Y.J. Toxicity of plant essential oils to Tetranychus urticae (Acari: Tetranychidae) and Phytoseiulus persimilis (Acari: Phytoseiidae). J. Econ. Entomol. 2004, 97, 553–558. [Google Scholar] [CrossRef]
- Xu, J.; Fan, Q.J.; Yin, Z.Q.; Li, X.T.; Du, Y.H.; Jia, R.Y.; Wang, K.Y.; Lv, C.; Ye, G.; Geng, Y.; et al. The preparation of neem oil microemulsion (Azadirachta indica) and the comparison of acaricidal time between neem oil microemulsion and other formulations in vitro. Vet. Parasitol. 2010, 169, 399–403. [Google Scholar] [CrossRef]
- Chaisri, W.; Chaiyana, W.; Pikulkaew, S.; Okonogi, S.; Suriyasathaporn, W. Enhancement of acaricide activity of citronella oil after microemulsion preparation. Jpn. J. Vet. Res. 2019, 67, 15–23. [Google Scholar] [CrossRef]
- Pedrini, N.; Ortiz-Urquiza, A.; Zhang, S.; Keyhani, N. Targeting of insect epicuticular lipids by the entomopathogenic fungus Beauveria Bassiana: Hydrocarbon oxidation within the context of a host-pathogen interaction. Front. Microbiol. 2013, 4, 24. [Google Scholar] [CrossRef]
- dos Santos, D.S.; Boito, J.P.; Santos, R.C.V.; Quatrin, P.M.; Ourique, A.F.; dos Reis, J.H.; Gebert, R.R.; Glombowsky, P.; Klauck, V.; Boligon, A.A.; et al. Nanostructured cinnamon oil has the potential to control Rhipicephalus microplus ticks on cattle. Exp. Appl. Acarol. 2017, 73, 129–138. [Google Scholar] [CrossRef]
- Federal, U.; Maria, D.S.; Maria, S.; Maria, S.; Federal, U.; Maria, D.S.; Catarina, S. Archivos de Zootecnia. Agric. Biol. Sci. Anim. Sci. Zool. 2018, 67, 494–498. [Google Scholar]
- Volpato, A.; Grosskopf, R.K.; Santos, R.C.; Vaucher, R.A.; Raffin, R.P.; Boligon, A.A.; Athayde, M.L.; Stefani, L.M.; Da Silva, A.S. Influence of rosemary, andiroba and copaiba essential oils on different stages of the biological cycle of the tick Rhipicephalus microplus in vitro. J. Essent. Oil Res. 2015, 27, 244–250. [Google Scholar] [CrossRef]
- Mossa, A.T.H.; Afia, S.I.; Mohafrash, S.M.M.; Abou-Awad, B.A. Formulation and characterization of garlic (Allium sativum L.) essential oil nanoemulsion and its acaricidal activity on eriophyid olive mites (Acari: Eriophyidae). Environ. Sci. Pollut. Res. 2018, 25, 10526–10537. [Google Scholar] [CrossRef] [PubMed]
- Badawy, M.E.I.; Abdelgaleil, S.A.M.; Mahmoud, N.F.; Marei, A.E.S.M. Preparation and characterizations of essential oil and monoterpene nanoemulsions and acaricidal activity against two-spotted spider mite (Tetranychus urticae Koch). Int. J. Acarol. 2018, 44, 330–340. [Google Scholar] [CrossRef]
- Echeverría, J.; de Albuquerque, D.G.; Diego, R. Nanoemulsions of essential oils: New tool for control of vector–borne diseases and in vitro effects on some parasitic agents. Medicines 2019, 6, 42. [Google Scholar] [CrossRef] [PubMed]
- Montoya, J.G.; Liesenfeld, O. Toxoplasmosis. Lancet 2004, 363, 1965–1976. [Google Scholar] [CrossRef]
- Pinto, B.; Mattei, R.; Moscato, G.A.; Cristofano, M.; Giraldi, M.; Scarpato, R.; Buffolano, W.; Bruschi, F. Toxoplasma infection in individuals in central Italy: Does a gender-linked risk exist? Eur. J. Clin. Microbiol. Infect. Dis. 2017, 36, 739–746. [Google Scholar] [CrossRef] [PubMed]
- Dunay, I.R.; Gajurel, K.; Dhakal, R.; Liesenfeld, O.; Montoya, J.G. Treatment of toxoplasmosis: Historical perspective, animal models, and current clinical practice. Clin. Microbiol. Rev. 2018, 31, e00057-17. [Google Scholar] [CrossRef] [PubMed]
- Azami, S.J.; Amani, A.; Keshavarz, H.; Najafi-Taher, R.; Mohebali, M.; Faramarzi, M.A.; Mahmoudi, M.; Shojaee, S. Nanoemulsion of atovaquone as a promising approach for treatment of acute and chronic toxoplasmosis. Eur. J. Pharm. Sci. 2018, 117, 138–146. [Google Scholar] [CrossRef] [PubMed]
- Bruschi, F.; Gradoni, L. The Leishmaniases: Old Neglected Tropical Diseases; Springer: Berlin, Germany, 2018; ISBN 3319723863. [Google Scholar]
- da Silva Cardoso, V.; Vermelho, A.B.; Ricci Junior, E.; Almeida Rodrigues, I.; Mazotto, A.M.; Supuran, C.T. Antileishmanial activity of sulphonamide nanoemulsions targeting the β-carbonic anhydrase from Leishmania species. J. Enzym. Inhib. Med. Chem. 2018, 33, 850–857. [Google Scholar] [CrossRef] [PubMed]
- Dhorm Pimentel de Moraes, A.R.; Tavares, G.D.; Soares Rocha, F.J.; de Paula, E.; Giorgio, S. Effects of nanoemulsions prepared with essential oils of copaiba and andiroba against Leishmania infantum and Leishmania amazonensis infections. Exp. Parasitol. 2018, 187, 12–21. [Google Scholar] [CrossRef] [PubMed]
- de Oliveira de Siqueira, L.B.; da Silva Cardoso, V.; Rodrigues, I.A.; Vazquez-Villa, A.L.; dos Santos, E.P.; da Costa Leal Ribeiro Guimarães, B.; Dos Santos Cerqueira Coutinho, C.; Vermelho, A.B.; Junior, E.R. Development and evaluation of zinc phthalocyanine nanoemulsions for use in photodynamic therapy for Leishmania spp. Nanotechnology 2017, 28, 65101. [Google Scholar]
- Shokri, A.; Saeedi, M.; Fakhar, M.; Morteza-Semnani, K.; Keighobadi, M.; Teshnizi, S.H.; Kelidari, H.R.; Sadjadi, S. Antileishmanial activity of Lavandula angustifolia and Rosmarinus officinalis essential oils and nano-emulsions on Leishmania major (MRHO/IR/75/ER). Iran. J. Parasitol. 2017, 12, 622. [Google Scholar]
- Bouyahya, A.; Et-Touys, A.; Bakri, Y.; Talbaui, A.; Fellah, H.; Abrini, J.; Dakka, N. Chemical composition of Mentha pulegium and Rosmarinus officinalis essential oils and their antileishmanial, antibacterial and antioxidant activities. Microb. Pathog. 2017, 111, 41–49. [Google Scholar] [CrossRef] [PubMed]
- Baldissera, M.D.; Da Silva, A.S.; Oliveira, C.B.; Zimmermann, C.E.P.; Vaucher, R.A.; Santos, R.C.V.; Rech, V.C.; Tonin, A.A.; Giongo, J.L.; Mattos, C.B. Trypanocidal activity of the essential oils in their conventional and nanoemulsion forms: In vitro tests. Exp. Parasitol. 2013, 134, 356–361. [Google Scholar] [CrossRef] [PubMed]
- World Malaria Report 2018; World Health Organization: Geneva, Switzerland, 2018.
- Dwivedi, P.; Khatik, R.; Chaturvedi, P.; Khandelwal, K.; Taneja, I.; Raju, K.S.R.; Dwivedi, H.; kumar Singh, S.; Gupta, P.K.; Shukla, P. Arteether nanoemulsion for enhanced efficacy against Plasmodium yoelii nigeriensis malaria: An approach by enhanced bioavailability. Colloids Surf. B Biointerfaces 2015, 126, 467–475. [Google Scholar] [CrossRef] [PubMed]
- Torgerson, P.R. Economic effects of echinococcosis. Acta Trop. 2003, 85, 113–118. [Google Scholar] [CrossRef]
- Budke, C.M.; Deplazes, P.; Torgerson, P.R. Global socioeconomic impact of cystic echinococcosis. Emerg. Infect. Dis. 2006, 12, 296. [Google Scholar] [CrossRef]
- Moazeni, M.; Borji, H.; Darbandi, M.S.; Saharkhiz, M.J. In vitro and in vivo antihydatid activity of a nano emulsion of Zataria multiflora essential oil. Res. Vet. Sci. 2017, 114, 308–312. [Google Scholar] [CrossRef]
- Mahmoudvand, H.; Mirbadie, S.R.; Sadooghian, S.; Harandi, M.F.; Jahanbakhsh, S.; Saedi Dezaki, E. Chemical composition and scolicidal activity of Zataria multiflora Boiss essential oil. J. Essent. Oil Res. 2017, 29, 42–47. [Google Scholar] [CrossRef]
- Lymbery, A.J. Phylogenetic pattern, evolutionary processes and species delimitation in the genus Echinococcus. In Advances in Parasitology; Elsevier: Amsterdam, The Netherlands, 2017; Volume 95, pp. 111–145. ISBN 0065-308X. [Google Scholar]
- Monteiro, D.U.; Azevedo, M.I.; Weiblen, C.; Botton, S.D.A.; Funk, N.L.; Da Silva, C.D.B.; Zanette, R.A.; Schwanz, T.G.; De La Rue, M.L. In vitro and ex vivo activity of Melaleuca alternifolia against protoscoleces of Echinococcus ortleppi. Parasitology 2017, 144, 214–219. [Google Scholar] [CrossRef] [PubMed]
- Ntalli, N.G.; Caboni, P. Botanical nematicides in the mediterranean basin. Phytochem. Rev. 2012, 11, 351–359. [Google Scholar] [CrossRef]
- Ntalli, N.; Caboni, P. A review of isothiocyanates biofumigation activity on plant parasitic nematodes. Phytochem. Rev. 2017, 16, 827–834. [Google Scholar] [CrossRef]
- Ntalli, N.G.; Caboni, P. Botanical nematicides: A review. J. Agric. Food Chem. 2012, 60, 9929–9940. [Google Scholar] [CrossRef] [PubMed]
- Caboni, P.; Ntalli, N.G. Botanical nematicides, recent findings. In Biopesticides: State of the Art and Future Opportunities; ACS Publications: Washington, WA, USA, 2014; pp. 145–157. ISBN 1947-5918. [Google Scholar]
- Ntalli, N.G.; Ferrari, F.; Giannakou, I.; Menkissoglu-Spiroudi, U. Synergistic and antagonistic interactions of terpenes against Meloidogyne incognita and the nematicidal activity of essential oils from seven plants indigenous to Greece. Pest Manag. Sci. 2011, 67, 341–351. [Google Scholar] [CrossRef]
- Ntalli, N.G.; Ferrari, F.; Giannakou, I.; Menkissoglu-Spiroudi, U. Phytochemistry and nematicidal activity of the essential oils from 8 Greek Lamiaceae aromatic plants and 13 terpene components. J. Agric. Food Chem. 2010, 58, 7856–7863. [Google Scholar] [CrossRef]
- Kim, C.T.; Kim, C.J.; Cho, Y.J.; Choi, S.W.; Choi, A.J. Nanoemulsion and Nanoparticle Containing Plant Essential Oil and Method of Production Thereof. U.S. Patent US20100136207A1, 3 June 2010. [Google Scholar]
- Magdassi, S.; Dayan, B.; Levi-Ruso, G. Pesticide Nanoparticles Obtained from Microemulsions and Nanoemulsions. U.S. Patent US9095133B2, 4 August 2015. [Google Scholar]
- Enan, E.; Porpiglia, P.J.; Lindner, G.J. Methods for Pest Control Employing Microemulsion-Based Enhanced Pest Control Formulations. U.S. Patent US20120251641A1, 4 October 2012. [Google Scholar]
- ECHA REACH Guidance for Nanomaterials Published. Available online: https://echa.europa.eu/it/-/reach-guidance-for-nanomaterials-published (accessed on 2 August 2019).
- Villaverde, J.J.; Sevilla-morán, B.; López-goti, C.; Alonso-prados, J.L.; Sandín-españa, P. Considerations of nano-QSAR/QSPR models for nanopesticide risk assessment within the European legislative framework. Sci. Total Environ. 2018, 634, 1530–1539. [Google Scholar] [CrossRef]
- Puzyn, T.; Leszczynski, J.; Leszczynska, D.; Leszczynski, J. Toward the Development of Nano-QSARs: Advances and Challenges. Small 2009, 5, 2494–2509. [Google Scholar] [CrossRef]
- Gajewicz, A.; Rasulev, B.; Dinadayalane, T.C.; Urbaszek, P.; Puzyn, T.; Leszczynska, D.; Leszczynski, J. Advancing risk assessment of engineered nanomaterials: Application of computational approaches. Adv. Drug Deliv. Rev. 2012, 64, 1663–1693. [Google Scholar] [CrossRef]
- Puzyn, T.; Rasulev, B.; Gajewicz, A.; Hu, X.; Dasari, T.P.; Michalkova, A.; Hwang, H.M.; Toropov, A.; Leszczynska, D.; Leszczynski, J. Using nano-QSAR to predict the cytotoxicity of metal oxide nanoparticles. Nat. Nanotechnol. 2011, 6, 175. [Google Scholar] [CrossRef]
- Durdagi, S.; Mavromoustakos, T.; Papadopoulos, M.G. 3D QSAR CoMFA/CoMSIA, molecular docking and molecular dynamics studies of fullerene-based HIV-1 PR inhibitors. Bioorg. Med. Chem. Lett. 2008, 18, 6283–6289. [Google Scholar] [CrossRef] [PubMed]
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pavoni, L.; Pavela, R.; Cespi, M.; Bonacucina, G.; Maggi, F.; Zeni, V.; Canale, A.; Lucchi, A.; Bruschi, F.; Benelli, G. Green Micro- and Nanoemulsions for Managing Parasites, Vectors and Pests. Nanomaterials 2019, 9, 1285. https://doi.org/10.3390/nano9091285
Pavoni L, Pavela R, Cespi M, Bonacucina G, Maggi F, Zeni V, Canale A, Lucchi A, Bruschi F, Benelli G. Green Micro- and Nanoemulsions for Managing Parasites, Vectors and Pests. Nanomaterials. 2019; 9(9):1285. https://doi.org/10.3390/nano9091285
Chicago/Turabian StylePavoni, Lucia, Roman Pavela, Marco Cespi, Giulia Bonacucina, Filippo Maggi, Valeria Zeni, Angelo Canale, Andrea Lucchi, Fabrizio Bruschi, and Giovanni Benelli. 2019. "Green Micro- and Nanoemulsions for Managing Parasites, Vectors and Pests" Nanomaterials 9, no. 9: 1285. https://doi.org/10.3390/nano9091285





