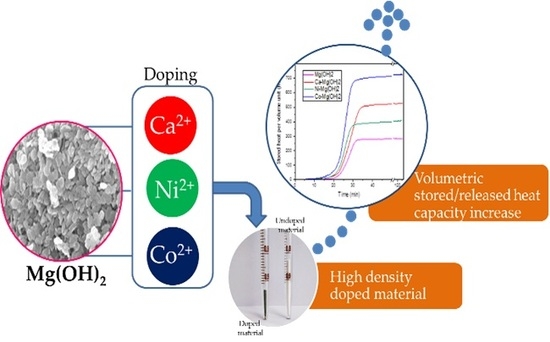
Synthesis of Me Doped Mg(OH)2 Materials for Thermochemical Heat Storage
Abstract
:1. Introduction
2. Materials and Methods
2.1. Samples Preparation
2.2. Samples Characterization
2.3. Thermochemical Performance
3. Results and Discussion
3.1. Me Doped Mg(OH)2 Preparation
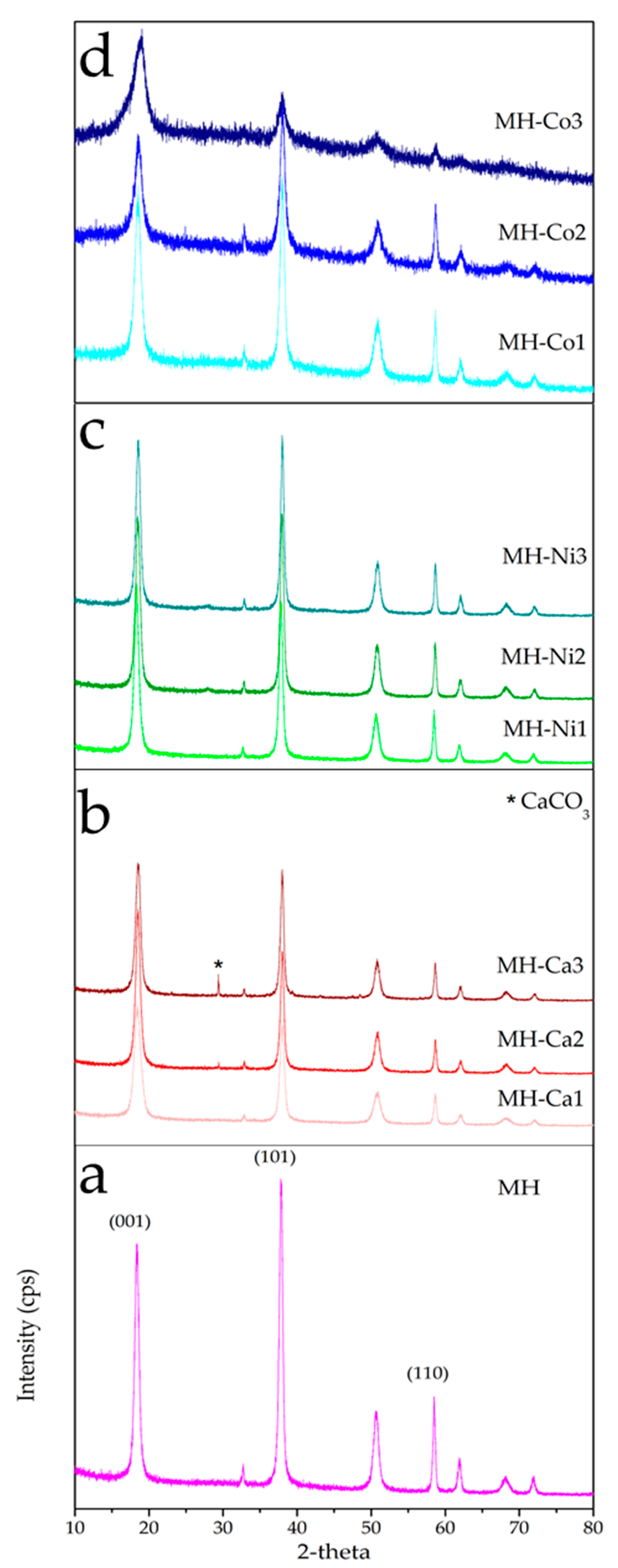
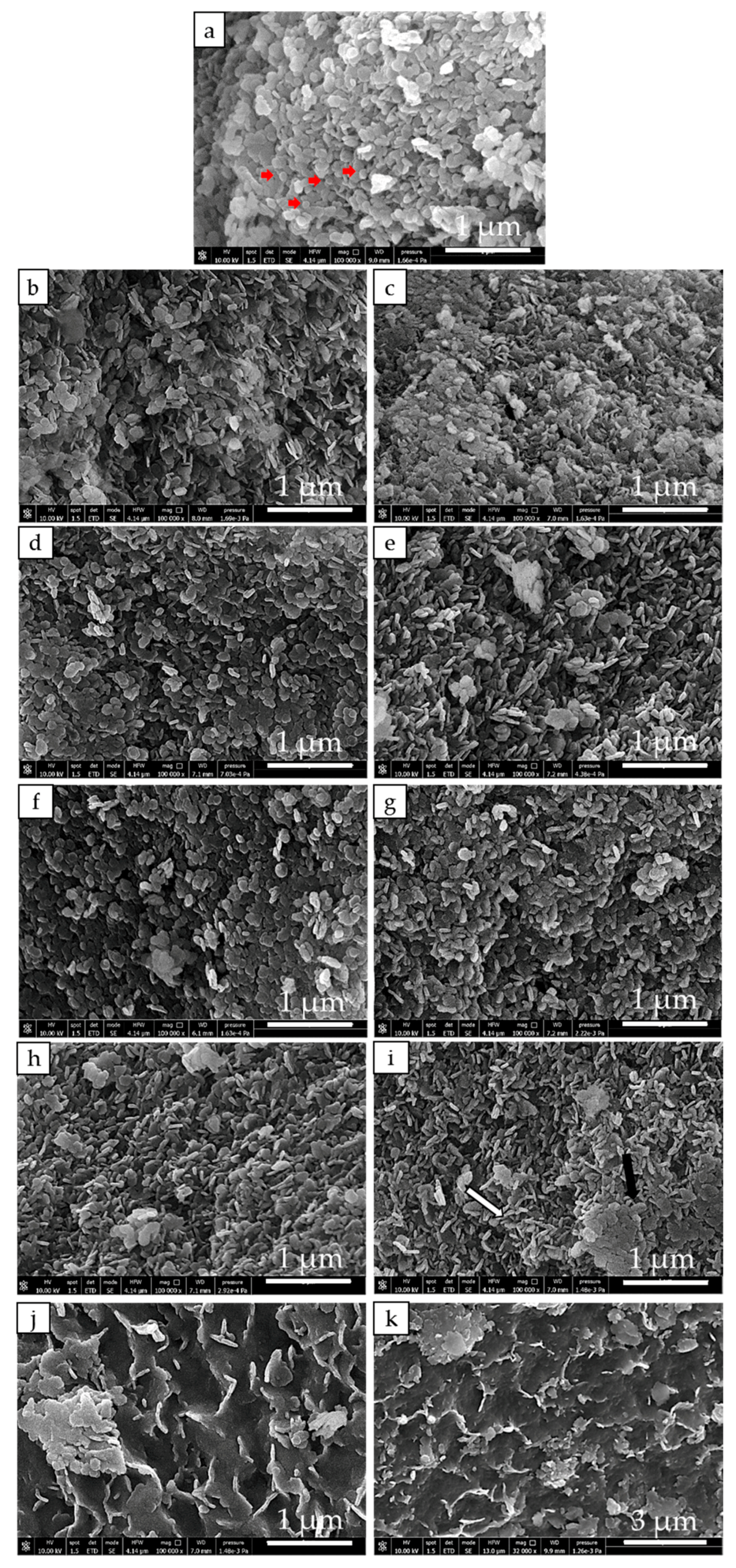
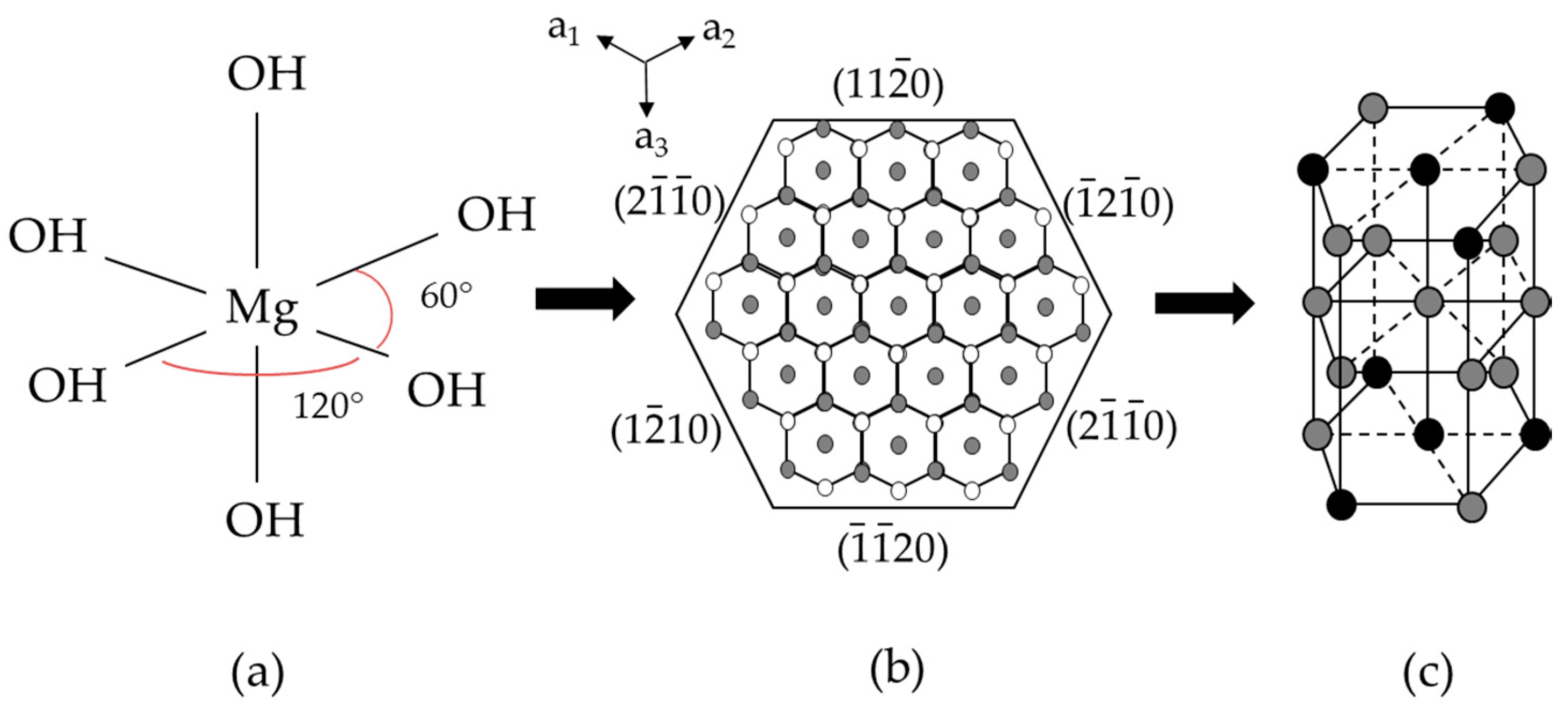
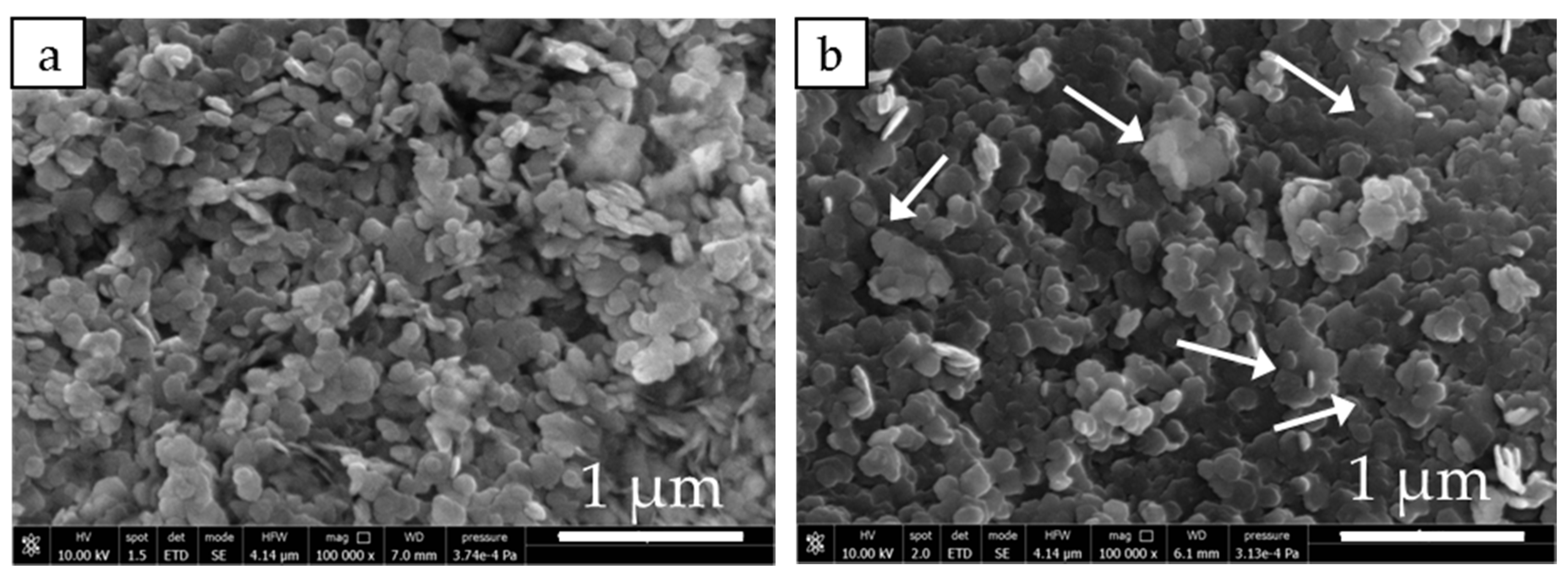
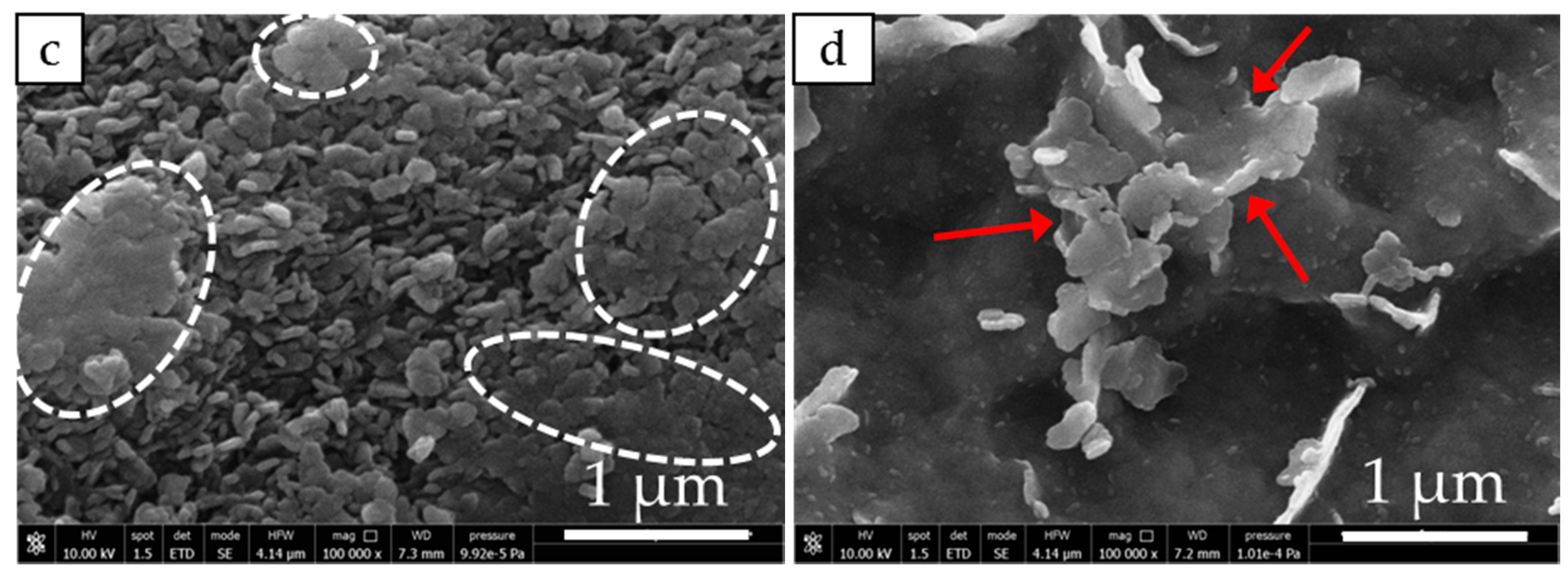
3.2. Structure and Morphology of Samples
3.3. Thermochemical Behavior
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Tescari, S.; Singh, A.; Agrafiotis, C.; de Oliveira, L.; Breuer, S.; Schlögl-Knothe, B.; Roeb, M.; Sattler, C. Experimental evaluation of a pilot-scale thermochemical storage system for a concentrated solar power plant. Appl. Energy 2017, 189, 66–75. [Google Scholar] [CrossRef]
- Li, G. Sensible heat thermal storage energy and exergy performance evaluations. Renew. Sustain. Energy Rev. 2016, 53, 897–923. [Google Scholar] [CrossRef]
- De Gracia, A.; Cabeza, L.F. Phase change materials and thermal energy storage for buildings. Energy Build. 2015, 103, 414–419. [Google Scholar] [CrossRef]
- Aydin, D.; Casey, S.P.; Riffat, S. The latest advancements on thermochemical heat storage systems. Renew. Sustain. Energy Rev. 2015, 41, 356–367. [Google Scholar] [CrossRef]
- Zhang, H.; Baeyens, J.; Cáceres, G.; Degrève, J.; Lv, Y. Thermal energy storage: Recent developments and practical aspects. Prog. Energy Combust. Sci. 2016, 53, 1–40. [Google Scholar] [CrossRef]
- Wu, J.; Long, X.F. Research progress of solar thermochemical energy storage. Int. J. Energy Res. 2015, 39, 869–888. [Google Scholar] [CrossRef]
- Sarbu, I.; Sebarchievici, C. A Comprehensive review of thermal energy storage. Sustainability 2018, 10. [Google Scholar] [CrossRef]
- Bowery, R.G.; Justen, J. Energy storage using the reversible oxidation of barium oxide. Sol. Energy 1978, 21, 523–525. [Google Scholar] [CrossRef]
- Carrillo, A.J.; Sastre, D.; Serrano, D.P.; Pizarro, P.; Coronado, J.M. Revisiting the BaO2/BaO redox cycle for solar thermochemical energy storage. Phys. Chem. Chem. Phys. 2016, 18, 8039–8048. [Google Scholar] [CrossRef] [PubMed]
- Carrillo, A.J.; Serrano, D.P.; Pizarro, P.; Coronado, J.M. Manganese oxide-based thermochemical energy storage: Modulating temperatures of redox cycles by Fe–Cu co-doping. J. Energy Storage 2016, 5, 169–176. [Google Scholar] [CrossRef]
- Carrillo, A.J.; Moya, J.; Bayón, A.; Jana, P.; de la Peña O’Shea, V.A.; Romero, M.; Gonzalez-Aguilar, J.; Serrano, D.P.; Pizarro, P.; Coronado, J.M. Thermochemical energy storage at high temperature via redox cycles of Mn and Co oxides: Pure oxides versus mixed ones. Sol. Energy Mater. Sol. Cells 2014, 123, 47–57. [Google Scholar] [CrossRef]
- Block, T.; Schmücker, M. Metal oxides for thermochemical energy storage: A comparison of several metal oxide systems. Sol. Energy 2016, 126, 195–207. [Google Scholar] [CrossRef]
- Schmidt, M.; Szczukowski, C.; Roßkopf, C.; Linder, M.; Wörner, A. Experimental results of a 10 kW high temperature thermochemical storage reactor based on calcium hydroxide. Appl. Therm. Eng. 2014, 62, 553–559. [Google Scholar] [CrossRef] [Green Version]
- Criado, Y.A.; Alonso, M.; Abanades, J.C. Conceptual process design of a CaO/Ca(OH)2 thermochemical energy storage system using fluidized bed reactors. Appl. Therm. Eng. 2014, 73, 1089–1094. [Google Scholar] [CrossRef]
- Criado, Y.A.; Alonso, M.; Abanades, J.C. Kinetics of the CaO/Ca(OH)2 hydration/dehydration reaction for thermochemical energy storage applications. Ind. Eng. Chem. Res. 2014, 53, 12594–12601. [Google Scholar] [CrossRef]
- Sakellariou, K.G.; Karagiannakis, G.; Criado, Y.A.; Konstandopoulos, A.G. Calcium oxide based materials for thermochemical heat storage in concentrated solar power plants. Sol. Energy 2015, 122, 215–230. [Google Scholar] [CrossRef]
- Mastronardo, E.; Bonaccorsi, L.; Kato, Y.; Piperopoulos, E.; Lanza, M.; Milone, C. Thermochemical performance of carbon nanotubes based hybrid materials for MgO/H2O/Mg(OH)2 chemical heat pumps. Appl. Energy 2016, 181, 232–243. [Google Scholar] [CrossRef]
- Shkatulov, A.; Aristov, Y. Thermochemical Energy Storage by LiNO3-doped Mg(OH)2: Dehydration Study. Energy Technol. Available online: https://onlinelibrary.wiley.com/doi/abs/10.1002/ente.201800050 (accessed on 14 May 2018).
- Ryu, J.; Hirao, N.; Takahashi, R.; Kato, Y. Dehydration behavior of metal-salt-added magnesium hydroxide as chemical heat storage media. Chem. Lett. 2008, 37, 1140–1141. [Google Scholar] [CrossRef]
- Müller, D.; Knoll, C.; Ruh, T.; Artner, W.; Welch, J.M.; Peterlik, H.; Eitenberger, E.; Friedbacher, J.; Harasek, M.; Blaha, P.; et al. Thermochemical Energy Storage: Calcium Doping Facilitates Water Dissociation in Magnesium Oxide. Adv. Sustain. Syst. 2018, 2, 1700096. [Google Scholar] [CrossRef]
- Zamengo, M.; Junichi, R.Y.U.; Kato, Y. Chemical Heat Storage of Thermal Energy from a Nuclear Reactor by Using a Magnesium Hydroxide/Expanded Graphite Composite Material. Energy Procedia 2015, 71, 293–305. [Google Scholar] [CrossRef]
- Piperopoulos, E.; Mastronardo, E.; Fazio, M.; Lanza, M.; Galvagno, S.; Milone, C. Enhancing the volumetric heat storage capacity of Mg(OH)2 by the addition of a cationic surfactant during its synthesis. Appl. Energy 2018, 215, 512–522. [Google Scholar] [CrossRef]
- Mastronardo, E.; Bonaccorsi, L.; Kato, Y.; Piperopoulos, E.; Lanza, M.; Milone, C. Strategies for the enhancement of heat storage materials performances for MgO/H2O/Mg(OH)2 thermochemical storage system. Appl. Therm. Eng. 2017, 120, 626–634. [Google Scholar] [CrossRef]
- Mastronardo, E.; Kato, Y.; Bonaccorsi, L.; Piperopoulos, E.; Milone, C. Thermochemical Storage of Middle Temperature Wasted Heat by Functionalized C/Mg(OH)2 Hybrid Materials. Energies 2017, 10, 70. [Google Scholar] [CrossRef]
- Mastronardo, E.; Bonaccorsi, L.; Kato, Y.; Piperopoulos, E.; Milone, C. Efficiency improvement of heat storage materials for MgO/H2O/Mg(OH)2 chemical heat pumps. Appl Energy 2016, 162, 31–39. [Google Scholar] [CrossRef]
- Inoue, M.; Hirasawa, I. The relationship between crystal morphology and XRD peak intensity on CaSO4·2H2O. J. Cryst. Growth 2013, 380, 169–175. [Google Scholar] [CrossRef]
- Lv, Y.; Zhang, Z.; Lai, Y.; Li, J.; Liu, Y. Formation mechanism for planes (011) and (001) oriented Mg(OH)2 films electrodeposited on SnO2 coating glass. Cryst. Eng. Comm. 2011, 13, 3848–3851. [Google Scholar] [CrossRef]
- Chen, D.; Zhu, L.; Zhang, H.; Xu, K.; Chen, M. Magnesium hydroxide nanoparticles with controlled morphologies via wet coprecipitation. Mater. Chem. Phys. 2008, 109, 224–229. [Google Scholar] [CrossRef]
- Wu, Q.L.; Xiang, L.; Jin, Y. Influence of CaCl2 on the hydrothermal modification of Mg(OH)2. Powder Technol. 2006, 165, 100–104. [Google Scholar] [CrossRef]
- Cordero, Z.C.; Schuh, C.A. Phase strength effects on chemical mixing in extensively deformed alloys. Acta Mater. 2015, 82, 123–136. [Google Scholar] [CrossRef]
- Vanpoucke, D.E.P.; Cottenier, S.; Van Speybroeck, V.; Van Driessche, I.; Bultinck, P. Tetravalent Doping of CeO2: The Impact of Valence Electron Character on Group IV Dopant Influence. J. Am. Ceram. Soc. 2014, 97, 258–266. [Google Scholar] [CrossRef] [Green Version]
- Wu, J.S.; Du, J.; Gao, Y.M. Crystal growth morphology of magnesium hydroxide. Turk. J. Chem. 2014, 38, 402–412. [Google Scholar] [CrossRef]
- Pardo, P.; Deydier, A.; Anxionnaz-Minvielle, Z.; Rougé, S.; Cabassud, M.; Cognet, P. A review on high temperature thermochemical heat energy storage. Renew. Sustain. Energ. Rev. 2014, 32, 591–610. [Google Scholar] [CrossRef] [Green Version]
- Tang, X.; Guo, L.; Chen, C.; Liu, Q.; Li, T.; Zhu, Y. The analysis of magnesium oxide hydration in three-phase reaction system. J. Solid State. Chem. 2014, 2013, 32–37. [Google Scholar] [CrossRef]
- Rocha, S.D.F.; Mansur, M.B.; Ciminelli, V.S.T. Kinetics and mechanistic analysis of caustic magnesia hydration. J. Chem. Technol. Biotechnol. 2004, 79, 816–821. [Google Scholar] [CrossRef]
- Fruhwirth, O.; Herzog, G.W.; Hollerer, I.; Rachetti, A. Dissolution and hydration kinetics of MgO. Surf. Technol. 1985, 24, 301–317. [Google Scholar] [CrossRef]
- Smithson, G.L.; Bakhshi, N.N. The kinetics and mechanism of the hydration of magnesium oxide in a batch reactor. Can. J. Chem. Eng. 1969, 47, 508–513. [Google Scholar] [CrossRef]
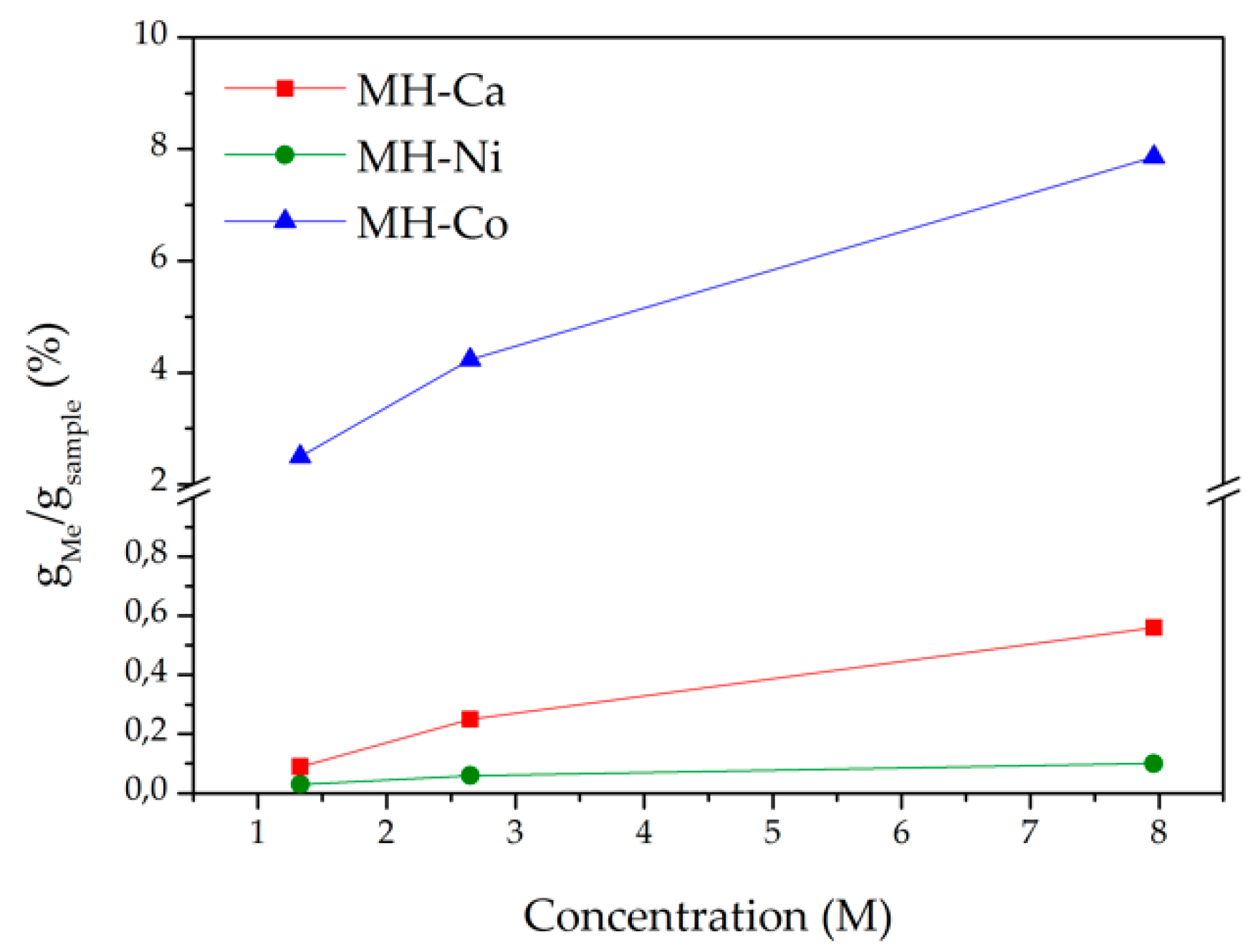


| Sample Code | Type of Me2+ | [Me] | Me/Mg2+ Nominal Molar Ratio |
|---|---|---|---|
| MH | - | - | - |
| MH-Ca1 | Ca2+ | 0.0003 | 0.033 |
| MH-Ca2 | Ca2+ | 0.0007 | 0.067 |
| MH-Ca3 | Ca2+ | 0.0020 | 0.200 |
| MH-Ni1 | Ni2+ | 0.0003 | 0.033 |
| MH-Ni2 | Ni2+ | 0.0007 | 0.067 |
| MH-Ni3 | Ni2+ | 0.0020 | 0.200 |
| MH-Co1 | Co2+ | 0.0003 | 0.033 |
| MH-Co2 | Co2+ | 0.0007 | 0.067 |
| MH-Co3 | Co2+ | 0.0020 | 0.200 |
| Hydroxide | [Me2+] [OH−]n | Ksp (at 25 °C) | Supersaturation Condition |
|---|---|---|---|
| Mg(OH)2 | 3.98 × 10−7 | 1.80 × 10−11 | Y |
| Ca(OH)2 | 1.33 × 10−8 | 7.90 × 10−06 | N |
| Ca(OH)2 | 2.65 × 10−8 | 7.90 × 10−06 | N |
| Ca(OH)2 | 7.96 × 10−8 | 7.90 × 10−06 | N |
| Ni(OH)2 | 1.33 × 10−8 | 2.80 × 10−16 | Y |
| Ni(OH)2 | 2.65 × 10−8 | 2.80 × 10−16 | Y |
| Ni(OH)2 | 7.96 × 10−8 | 2.80 × 10−16 | Y |
| Co(OH)2 | 1.33 × 10−8 | 2.50 × 10−16 | Y |
| Co(OH)2 | 2.65 × 10−8 | 2.50 × 10−16 | Y |
| Co(OH)2 | 7.96 × 10−8 | 2.50 × 10−16 | Y |
| Entry | Sample Code | Intensity Ratios | Rietveld Refinement | Morphological Properties | |||
|---|---|---|---|---|---|---|---|
| I001/101 | I001/110 | V(Å3) | Mean Particle Size (nm) * | ρ (kg/m3) | Vpore (cm3/g) | ||
| 1 | MH | 0.78 | 2.63 | 40.9 | 180.5 ± 24.0 | 350 | 0.618 |
| 2 | MH-Ca1 | 1.16 | 3.64 | 40.7 | 78.9 ± 44.5 | 685 | 0.614 |
| 3 | MH-Ca2 | 1.34 | 4.77 | 40.8 | 131.3 ± 33.1 | 644 | 0.497 |
| 4 | MH-Ca3 | 1.04 | 3.57 | 41.3 | 117.1 ± 45.6 | 740 | 0.525 |
| 5 | MH-Ni1 | 1.09 | 3.26 | 40.8 | 97.8 ± 50.1 | 752 | 0.885 |
| 6 | MH-Ni2 | 0.97 | 3.19 | 41.3 | 105.8 ± 38.1 | 712 | 0.478 |
| 7 | MH-Ni3 | 0.95 | 3.26 | 41.0 | 118.4 ± 20.6 | 663 | 0.515 |
| 8 | MH-Co1 | 0.89 | 2.36 | 40.4 | 85.8 ± 34.0 | 616 | 0.778 |
| 9 | MH-Co2 | 0.72 | 1.69 | 40.9 | 67.1 ± 40.0 | 587 | 0.914 |
| 10 | MH-Co3 | 1.85 | 4.84 | 41.5 | 188.6 ± 23.4 | 1.050 | 0.245 |
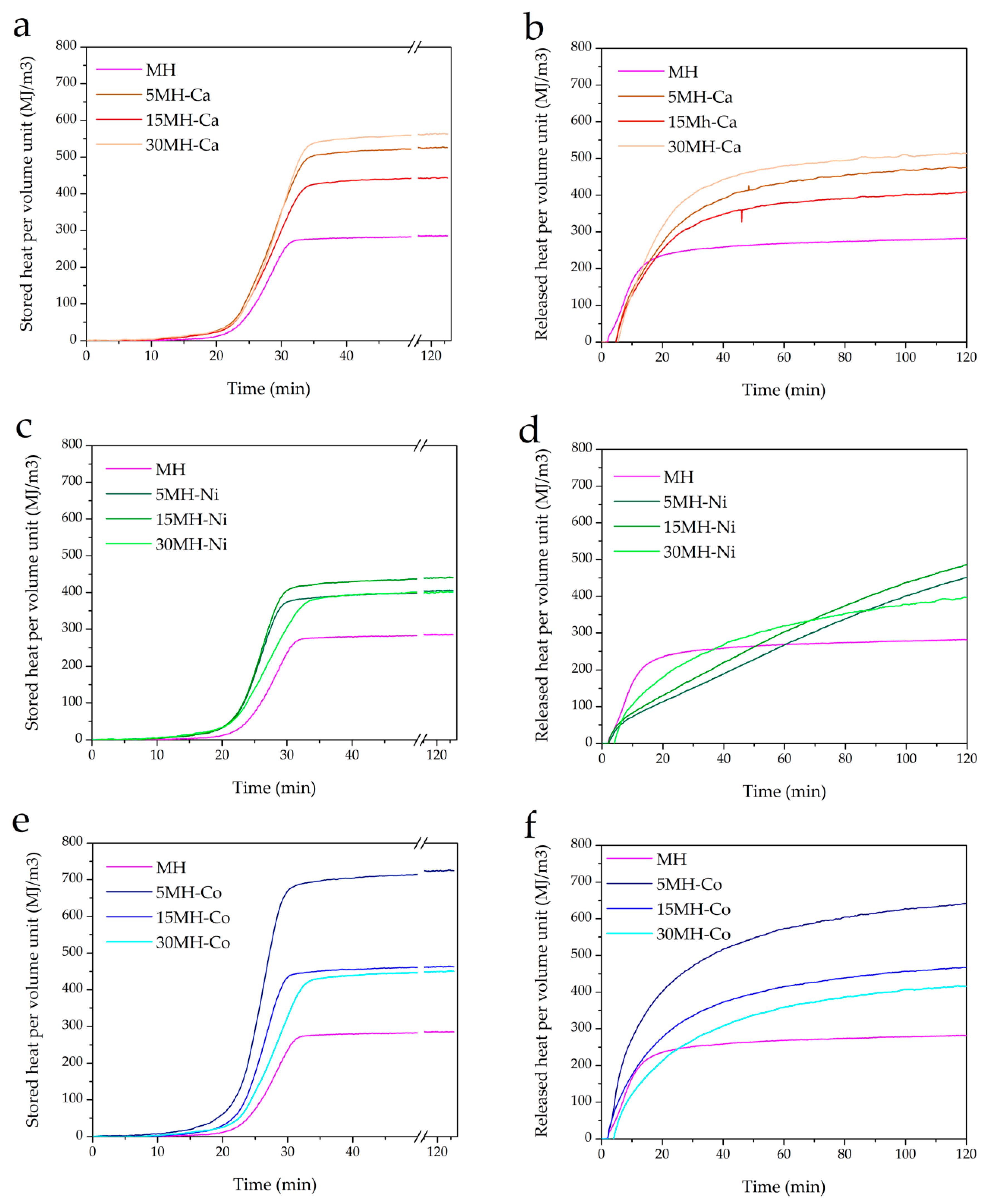
| Entry | Sample Code | 1st Cycle | 3rd Cyle | QsV (MJ/m3) | QsV (MJ/m3) | ||
|---|---|---|---|---|---|---|---|
| βd (%) | βh (%) | βd (%) | βh (%) | ||||
| 1 | MH | 89.0 | 61.6 | 58.8 | 58.0 | 285.4 | 281.95 |
| 2 | MH-Ca1 | 90.0 | 62.7 | 58.8 | 54.0 | 536.4 | 513.98 |
| 3 | MH-Ca2 | 90.0 | 52.7 | 49.4 | 45.6 | 444.9 | 408.44 |
| 4 | MH-Ca3 | 88.3 | 55.8 | 51.2 | 45.6 | 525.7 | 475.5 |
| 5 | MH-Ni1 | 93.7 | 36.6 | 38.2 | 38.0 | 401.7 | 397.01 |
| 6 | MH-Ni2 | 97.7 | 42.0 | 44.4 | 49.0 | 441.0 | 485.01 |
| 7 | MH-Ni3 | 94.8 | 41.1 | 44.4 | 49.0 | 407.0 | 449.36 |
| 8 | MH-Co1 | 89.0 | 55.5 | 52.5 | 48.6 | 450.6 | 416.22 |
| 9 | MH-Co2 | 89.0 | 56.6 | 56.8 | 57.2 | 463.3 | 466.98 |
| 10 | MH-Co3 | 87.2 | 55.5 | 49.7 | 43.8 | 724.8 | 640.75 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Piperopoulos, E.; Fazio, M.; Mastronardo, E. Synthesis of Me Doped Mg(OH)2 Materials for Thermochemical Heat Storage. Nanomaterials 2018, 8, 573. https://doi.org/10.3390/nano8080573
Piperopoulos E, Fazio M, Mastronardo E. Synthesis of Me Doped Mg(OH)2 Materials for Thermochemical Heat Storage. Nanomaterials. 2018; 8(8):573. https://doi.org/10.3390/nano8080573
Chicago/Turabian StylePiperopoulos, Elpida, Marianna Fazio, and Emanuela Mastronardo. 2018. "Synthesis of Me Doped Mg(OH)2 Materials for Thermochemical Heat Storage" Nanomaterials 8, no. 8: 573. https://doi.org/10.3390/nano8080573