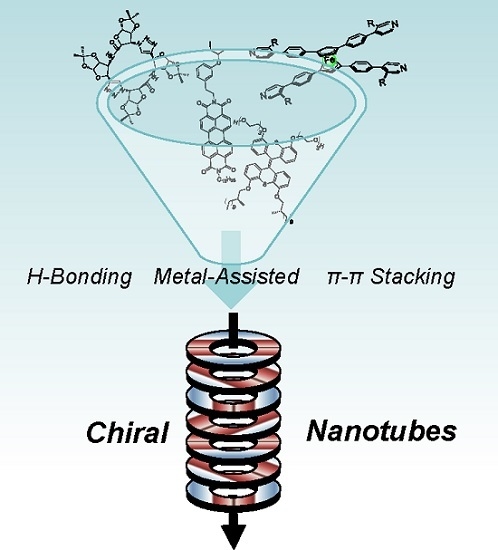
Chiral Nanotubes
Abstract
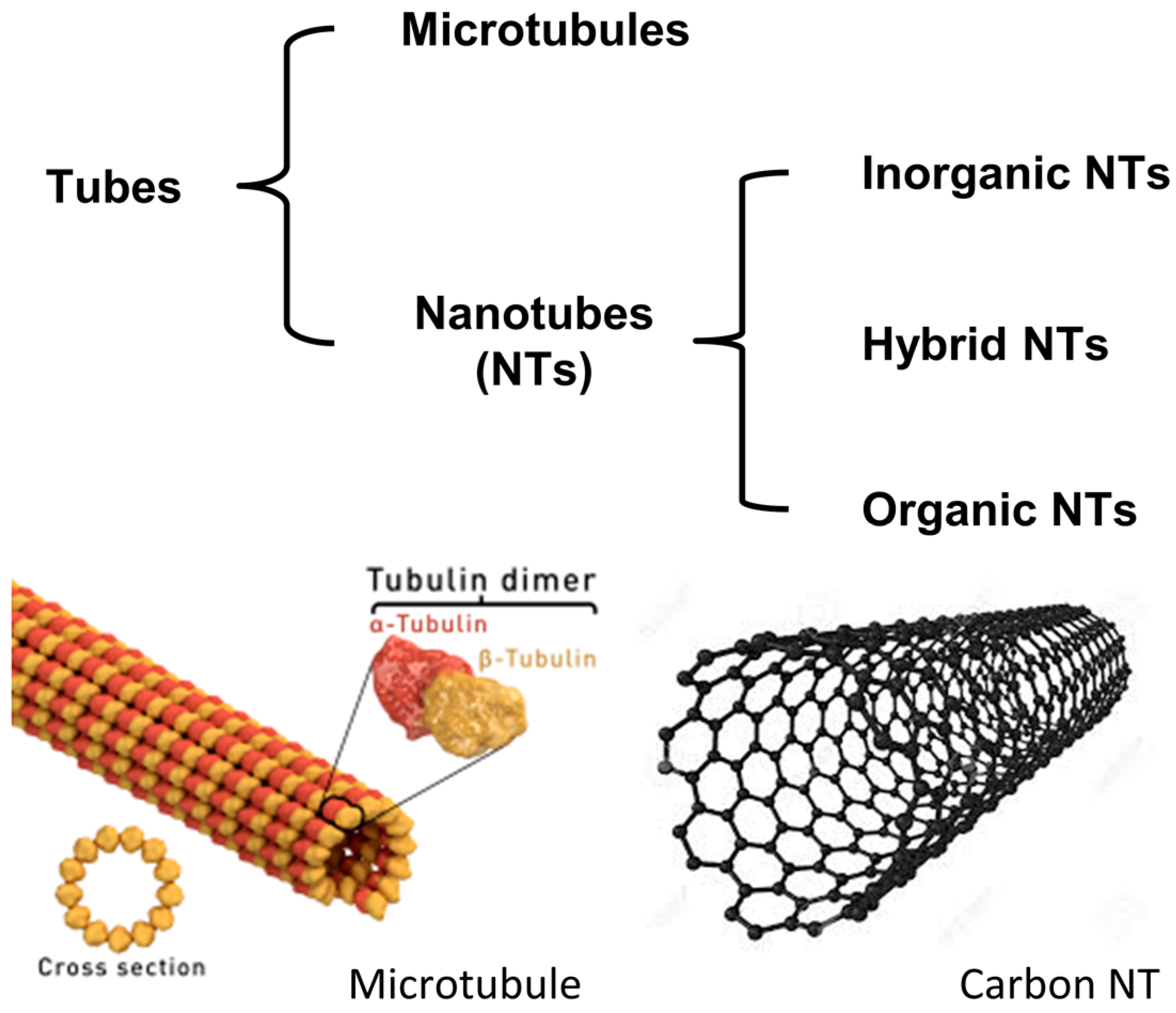
:1. Introduction
2. Discussion
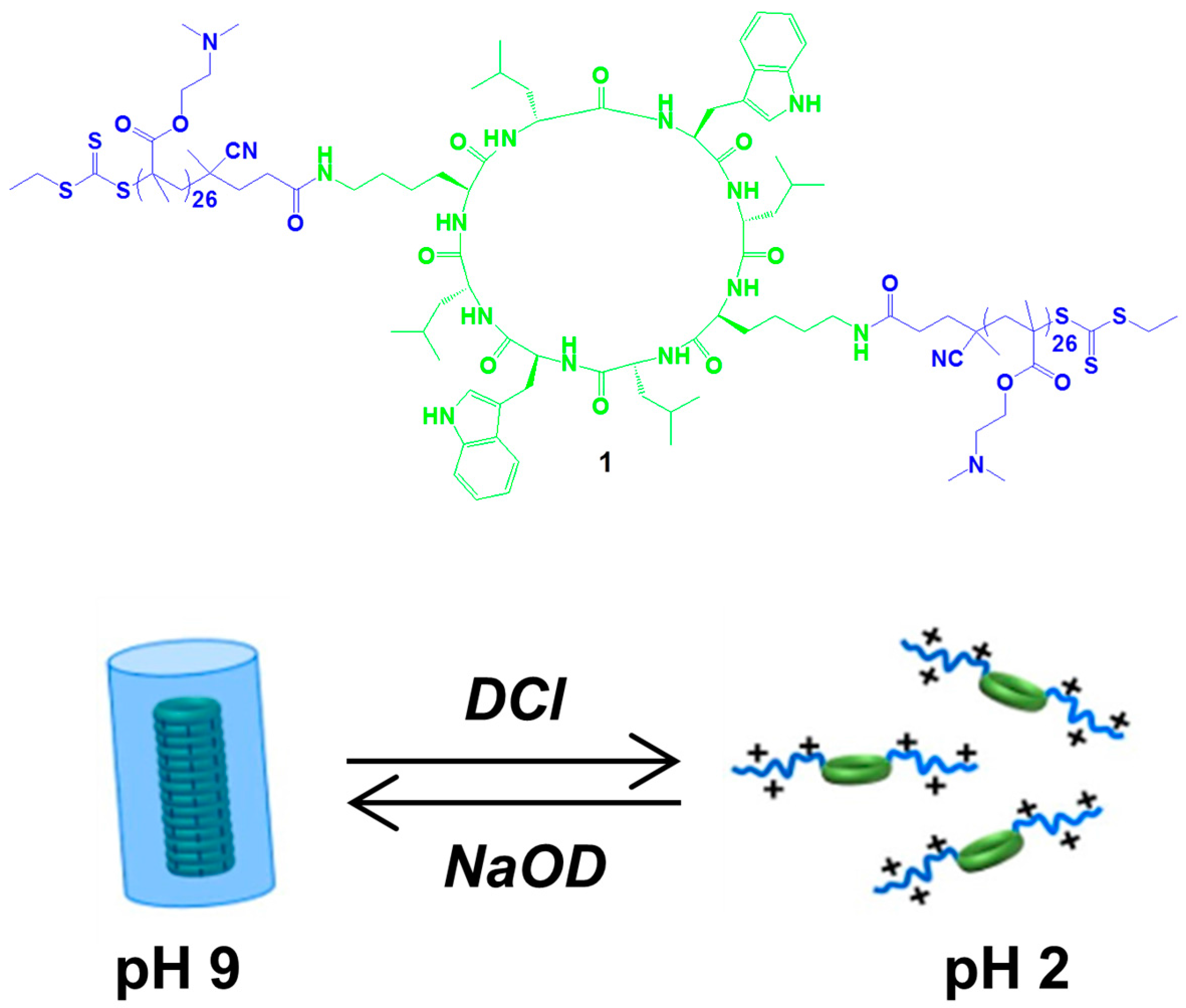
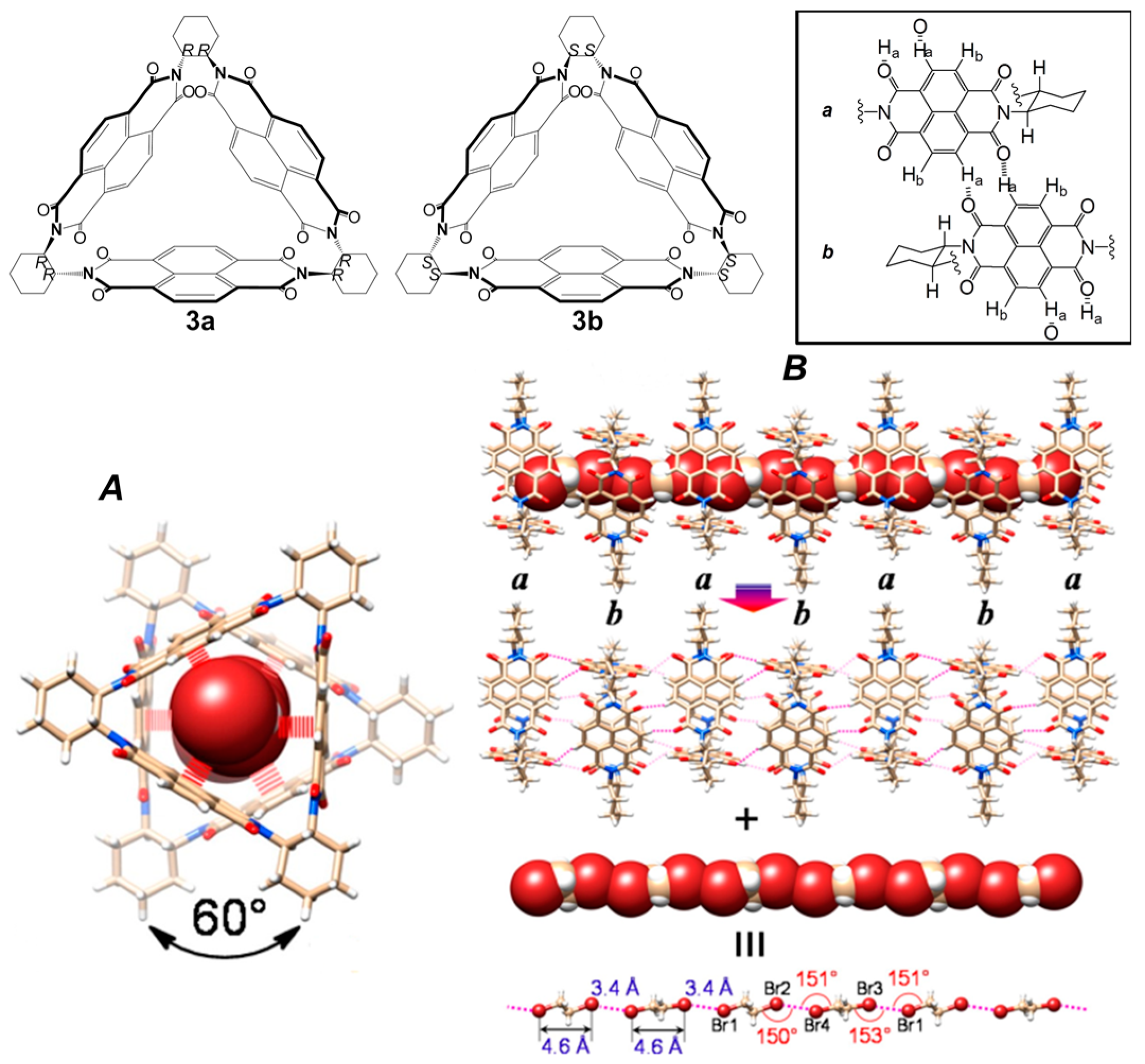
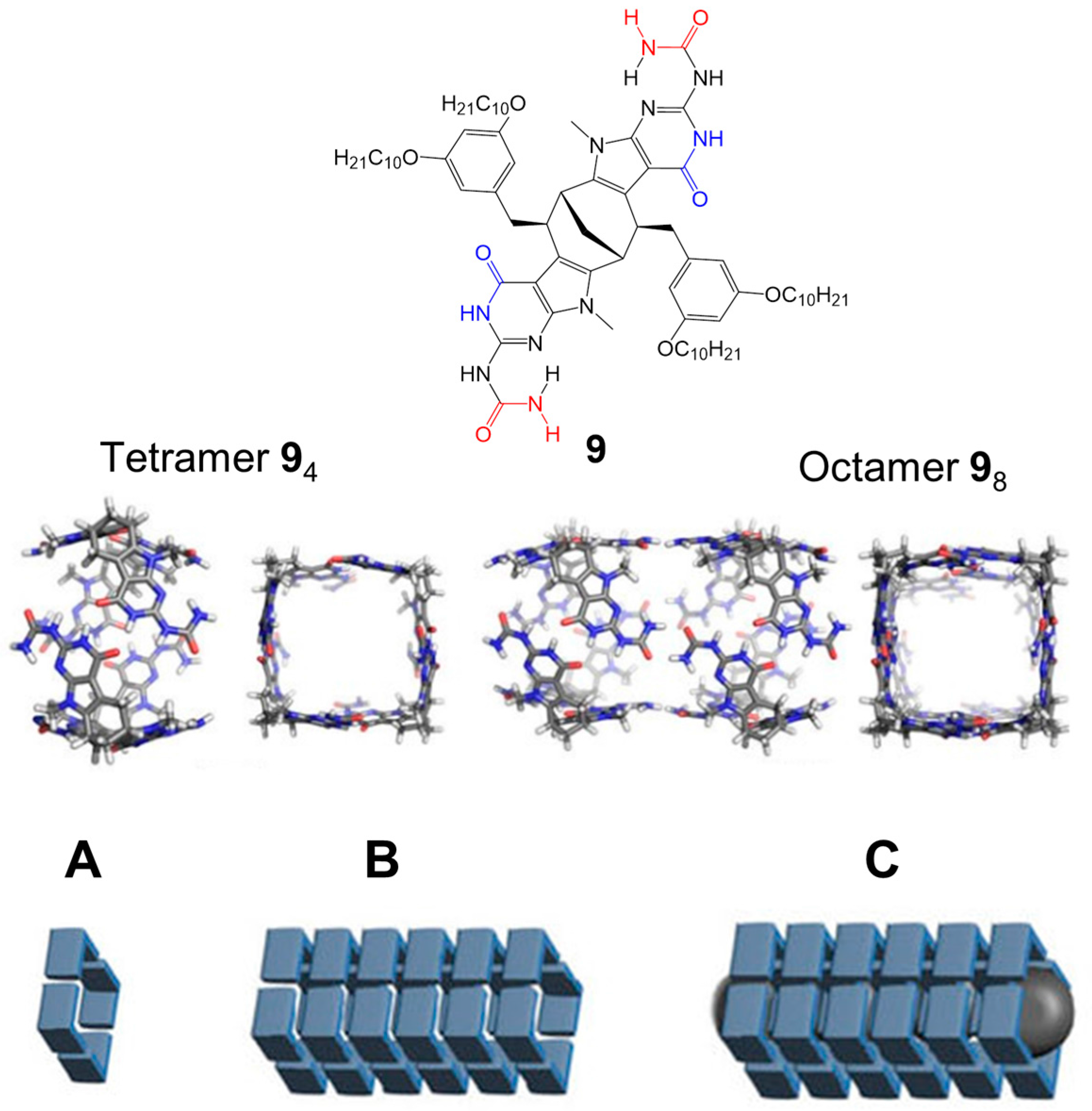
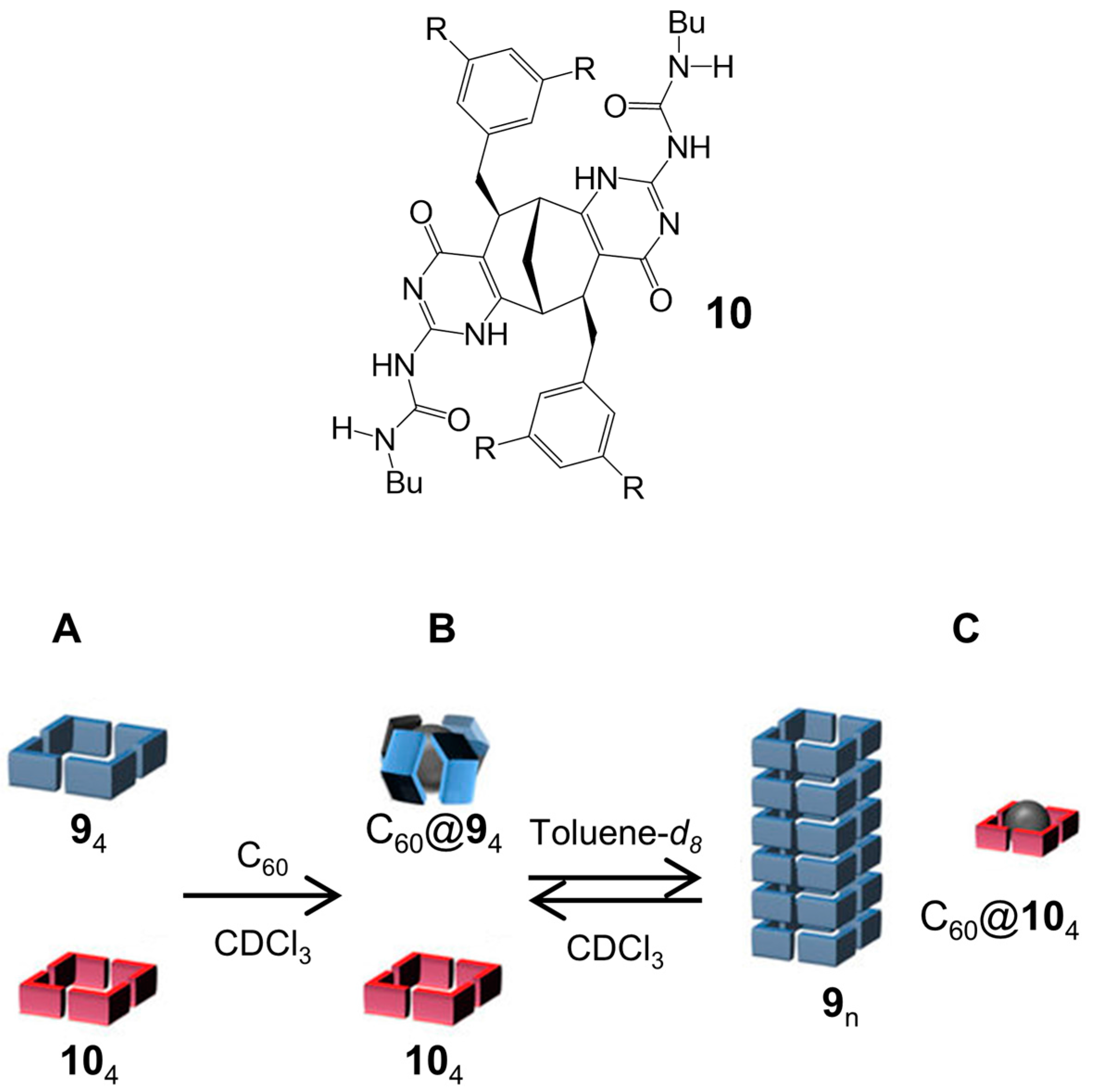
2.1. Hydrogen Bonds
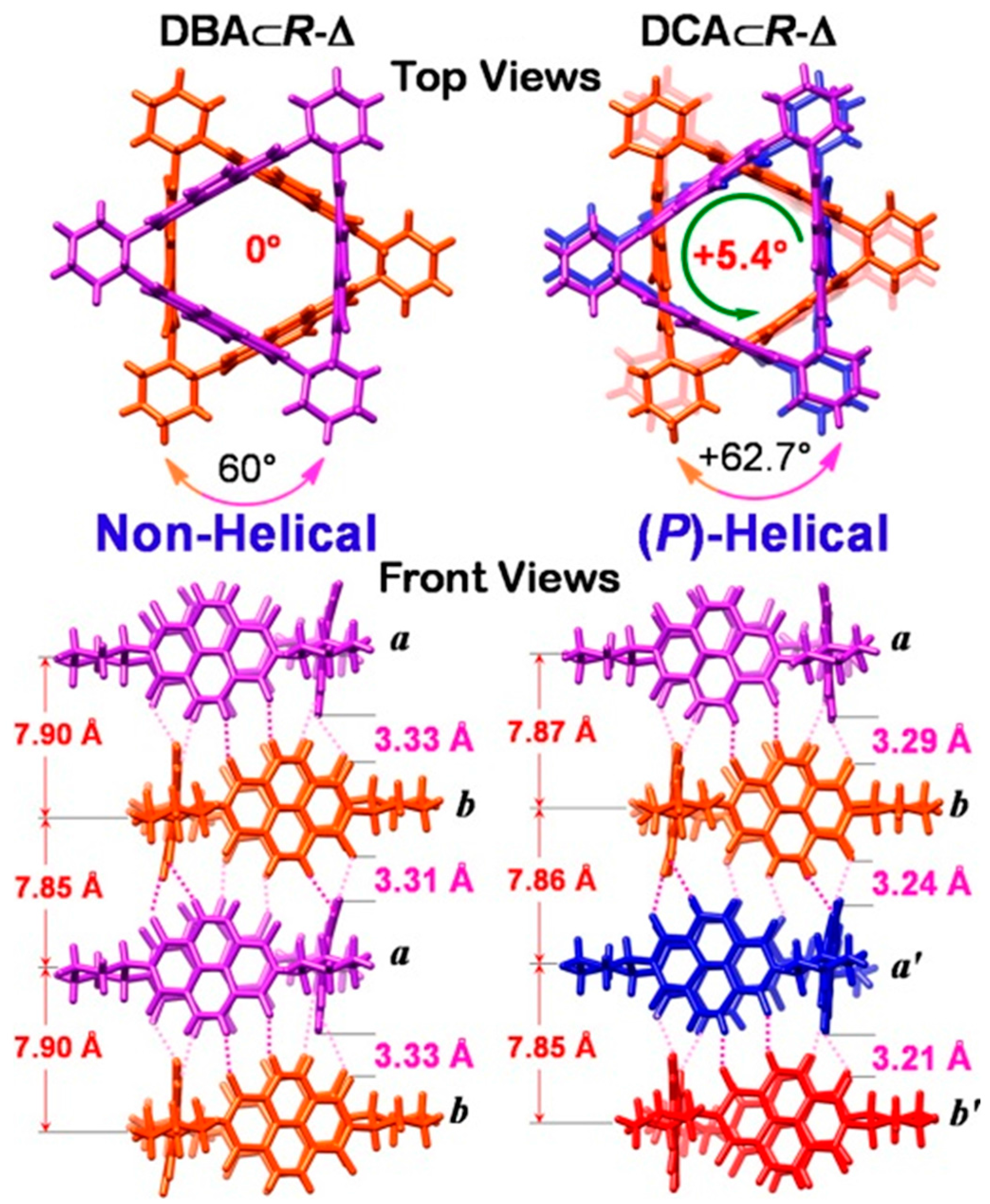
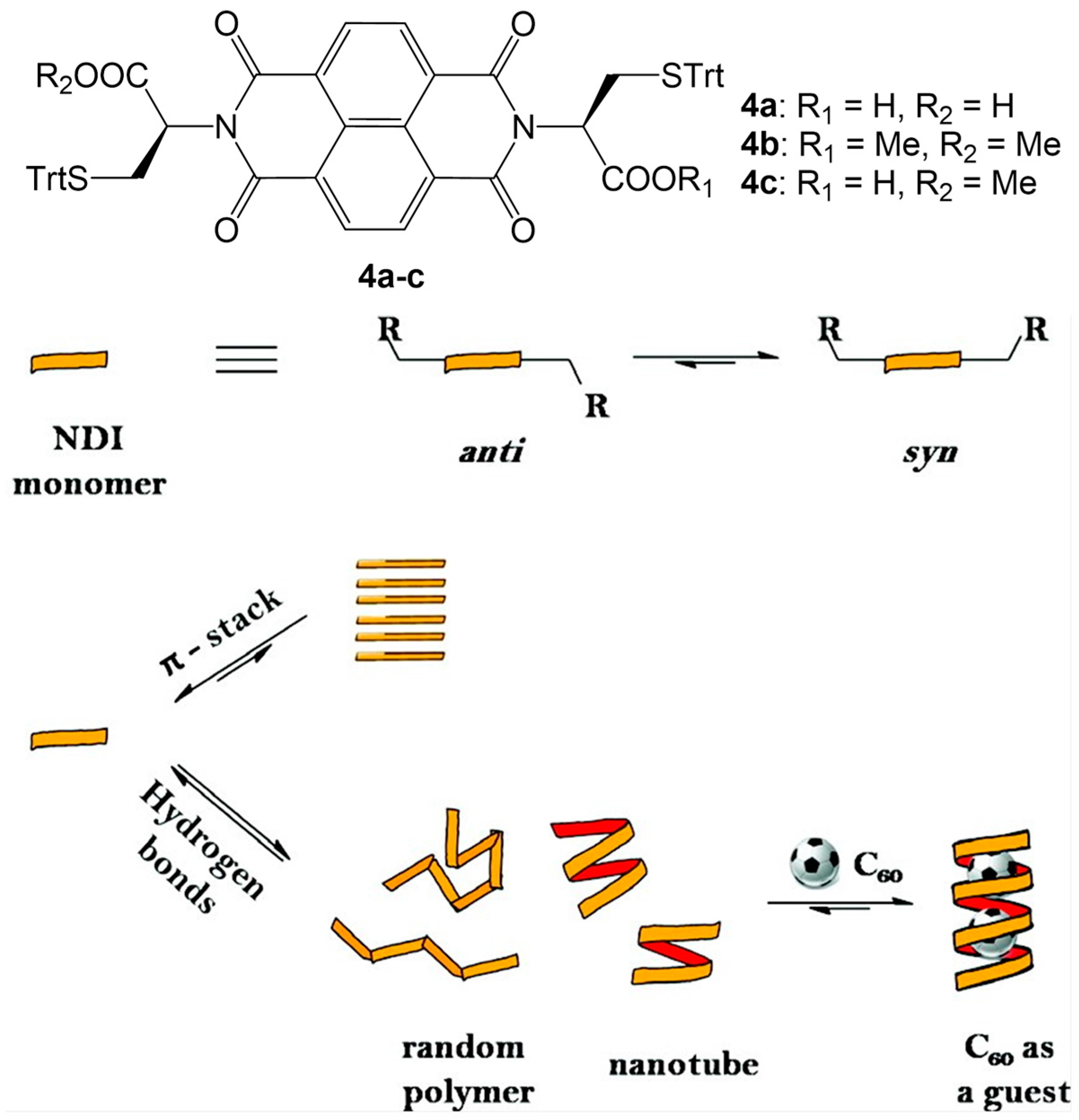
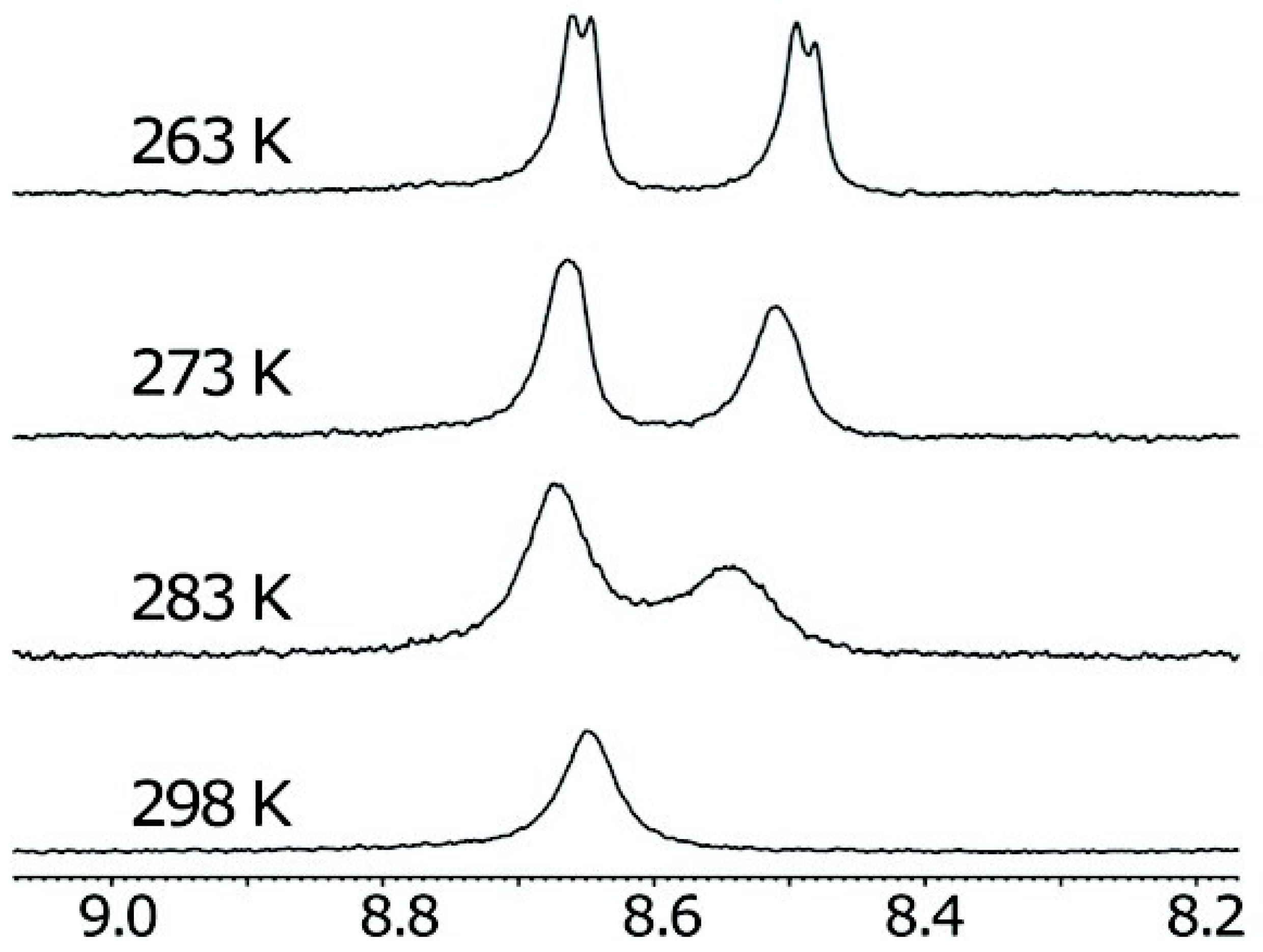
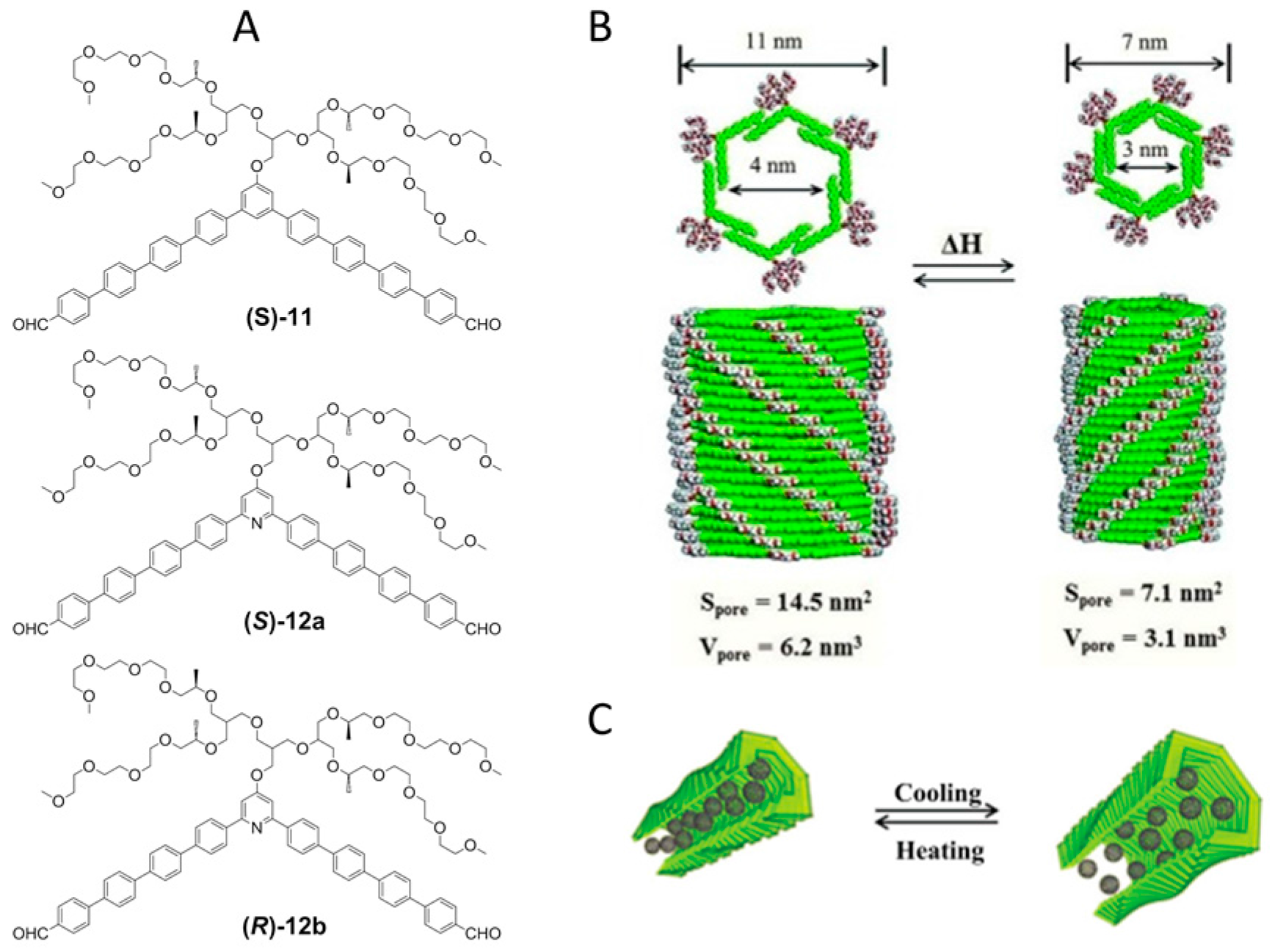
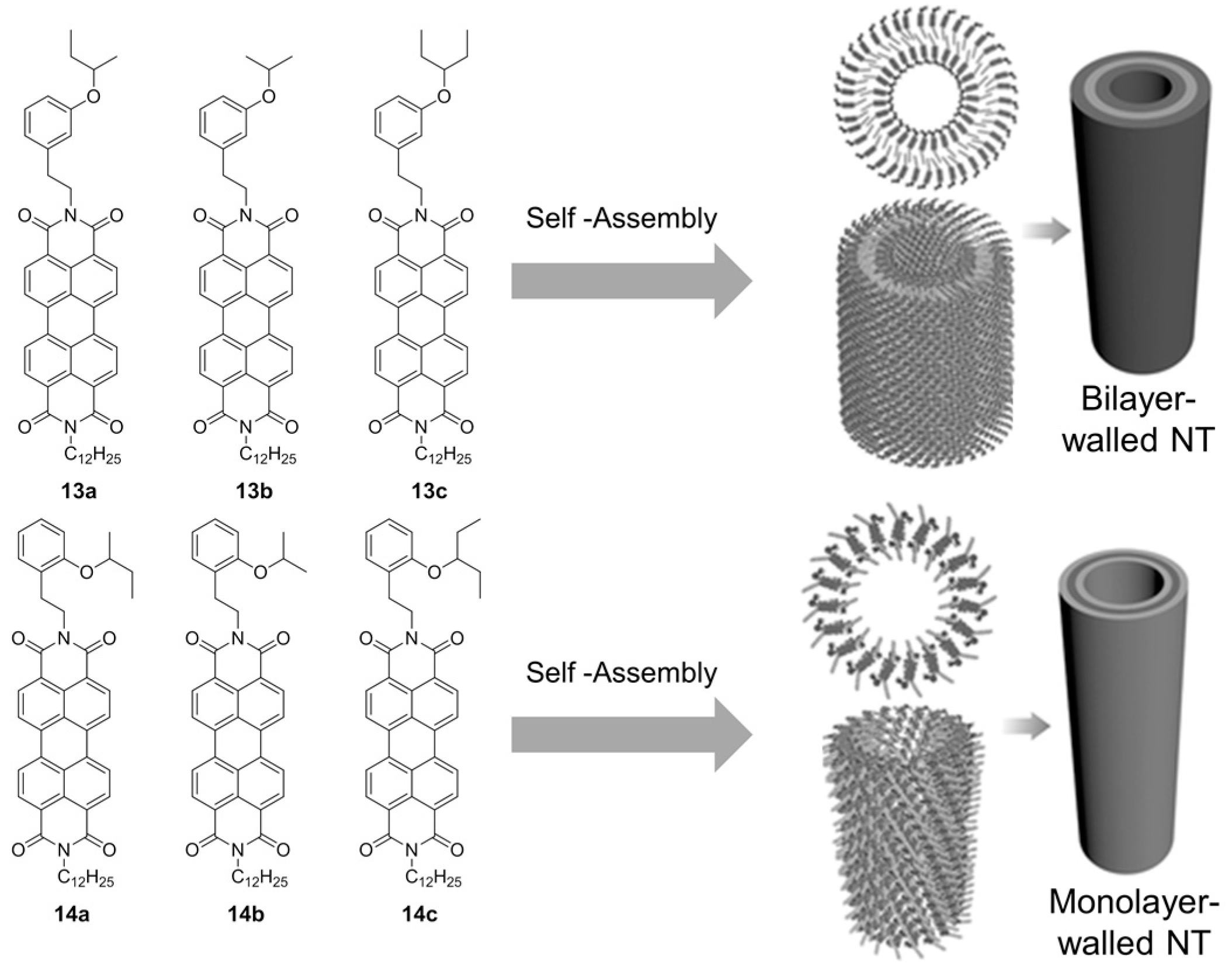
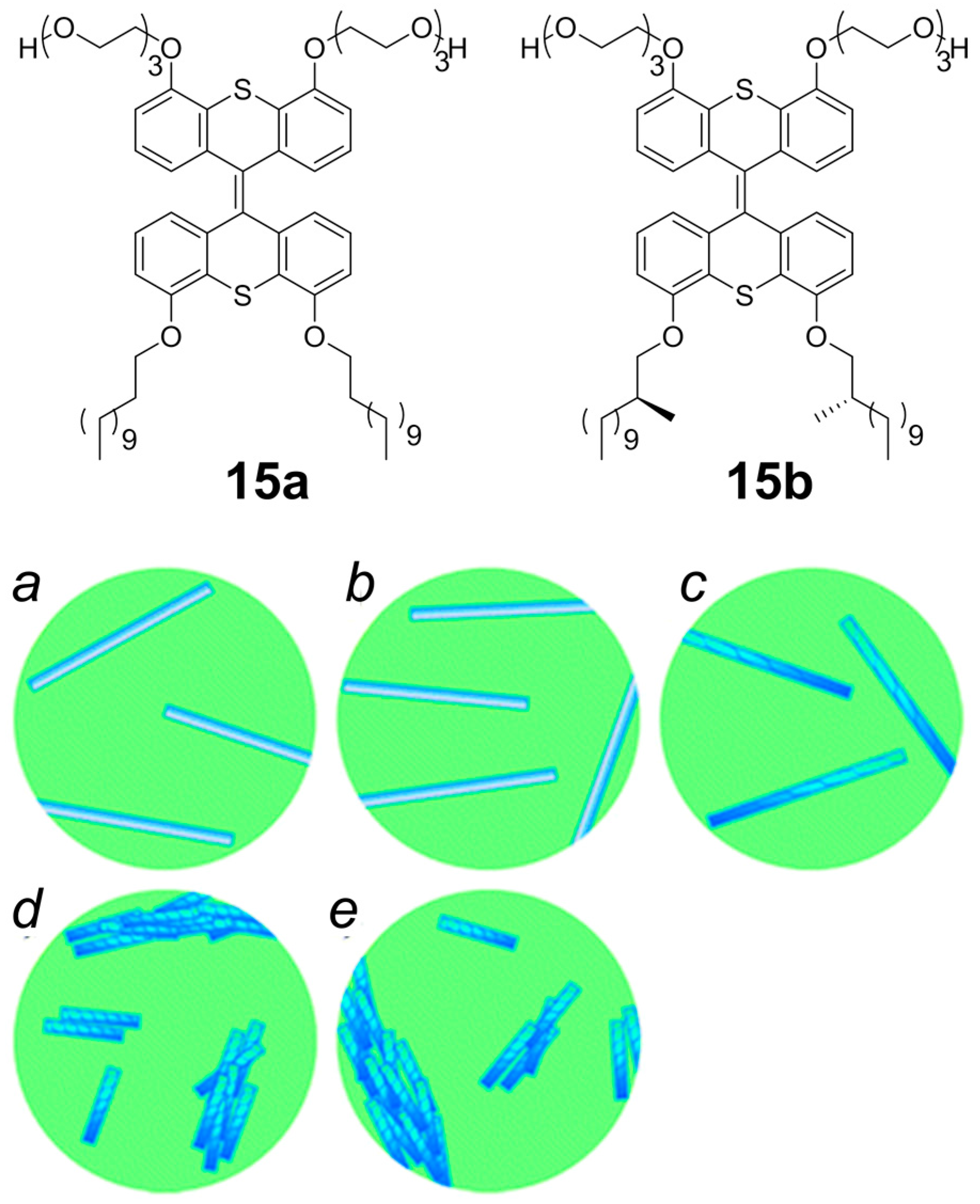
2.2. π–π Stacking Interactions
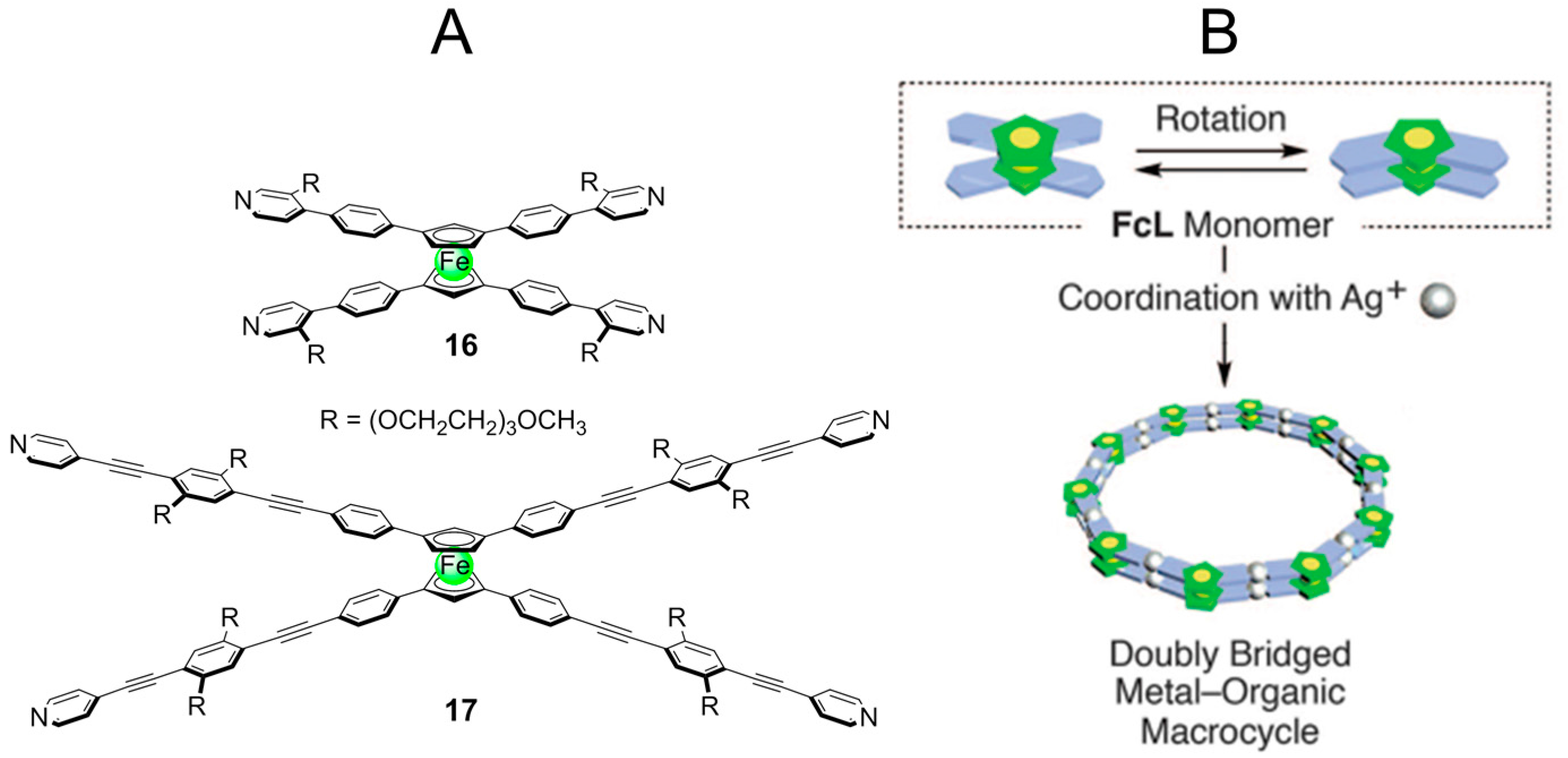
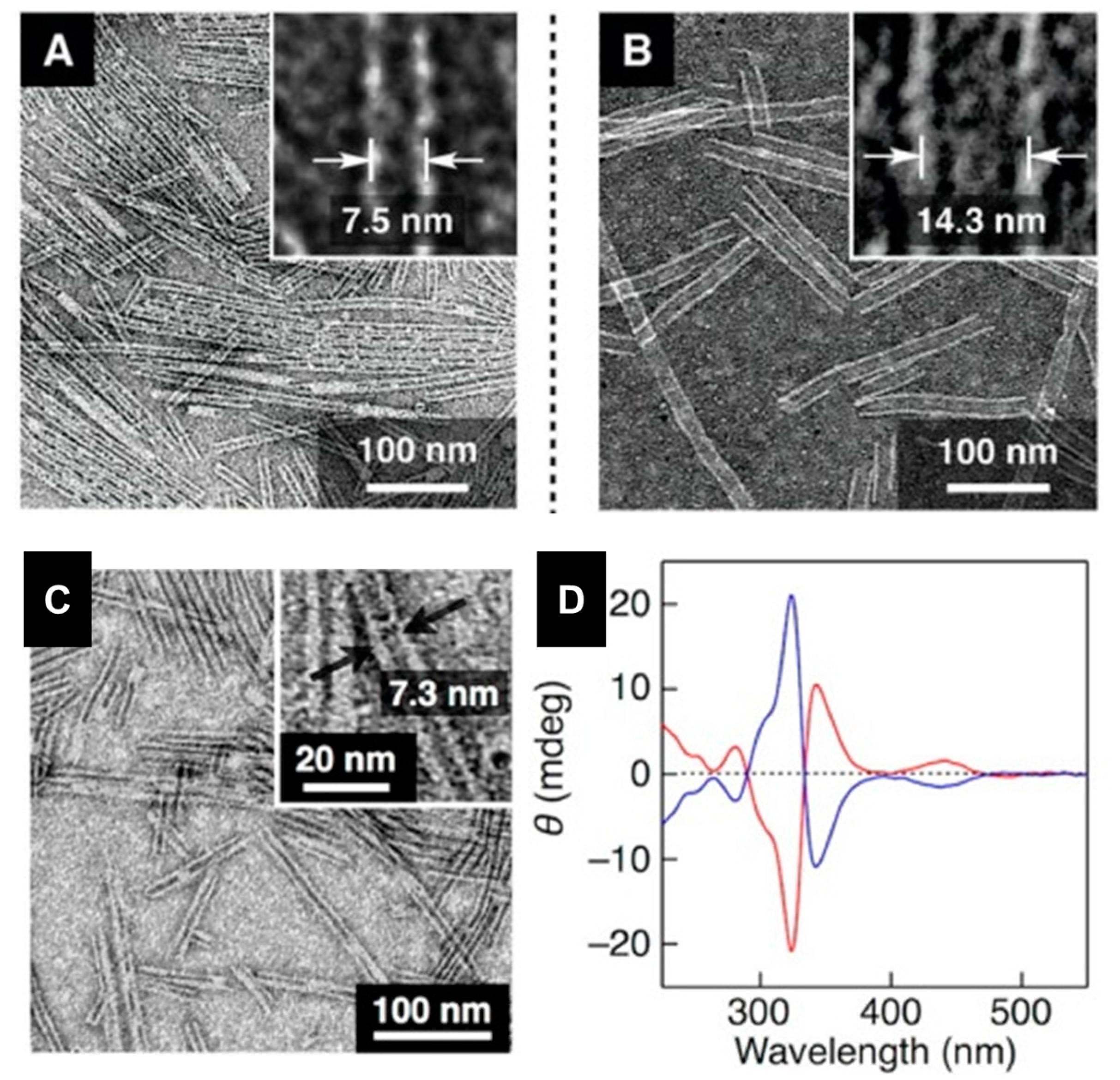
2.3. Metal-Organic Interactions
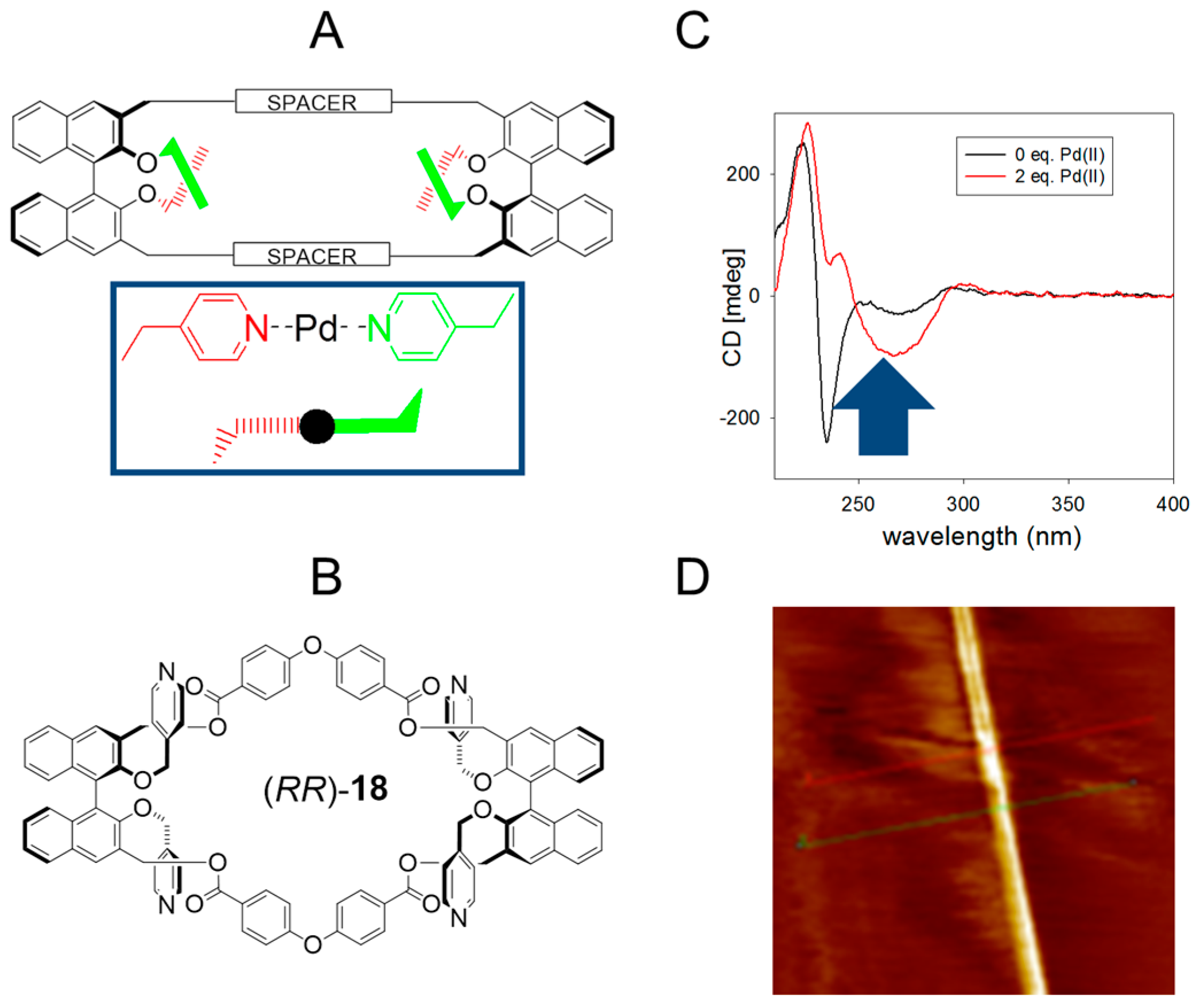
2.4. Multiple Interactions
3. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Pilhofer, M.; Ladinsky, M.S.; McDowall, A.W.; Petroni, G.; Jensen, G.J. Microtubules in bacteria: Ancient tubulins build a five-protofilament homolog of the eukaryotic cytoskeleton. PLoS Biol. 2011, 9, e1001213. [Google Scholar] [CrossRef] [PubMed]
- Tenne, R.; Redlich, M. Recent progress in the research of inorganic fullerene-like nanoparticles and inorganic nanotubes. Chem. Soc. Rev. 2010, 39, 1423–1434. [Google Scholar] [CrossRef] [PubMed]
- Soldano, C. Hybrid metal-based carbon nanotubes: Novel platform for multifunctional applications. Prog. Mater. Sci. 2015, 69, 183–212. [Google Scholar] [CrossRef]
- Ebbesen, T.W.; Ajayan, P.M. Large-scale synthesis of carbon nanotubes. Nature 1992, 358, 220–222. [Google Scholar] [CrossRef]
- Guo, T.; Nikolaev, P.; Thess, A.; Colbert, D.T.; Smalley, R.E. Catalytic growth of single-walled nanotubes by laser vaporization. Chem. Phys. Lett. 1995, 243, 49–54. [Google Scholar] [CrossRef]
- Endo, M.; Takeuchi, K.; Igarashi, S.; Kobori, K.; Shiraishi, M.; Kroto, H.W. The production and structure of pyrolytic carbon nanotubes. J. Phys. Chem. Solids 1993, 54, 1841–1848. [Google Scholar] [CrossRef]
- Hersam, M.C. Progress Towards Monodisperse Single-Walled Carbon Nanotubes. Nat. Nanotechnol. 2008, 3, 387–394. [Google Scholar] [CrossRef] [PubMed]
- Martel, R. Sorting Carbon Nanotubes for Electronics. ACS Nano 2008, 2, 2195–2199. [Google Scholar] [CrossRef] [PubMed]
- Ishihara, S.; Azzarelli, J.M.; Krikorian, M.; Swager, T.M. Ultratrace Detection of Toxic Chemicals: Triggered Disassembly of Supramolecular Nanotube Wrappers. J. Am. Chem. Soc. 2016, 138, 8221–8227. [Google Scholar] [CrossRef] [PubMed]
- Rikken, G.L.J.A.; Raupach, E. Observation of magneto-chiral dichroism. Nature 1997, 390, 493–494. [Google Scholar] [CrossRef]
- Nagaosa, N.; Tokura, Y. Topological properties and dynamics of magnetic skyrmions. Nat. Nanotechnol. 2013, 8, 899–911. [Google Scholar] [CrossRef] [PubMed]
- Naaman, R.; Waldeck, D.H. Spintronics and chirality: Spin selectivity in electron transport through chiral molecules. Annu. Rev. Phys. Chem. 2015, 66, 263–281. [Google Scholar] [CrossRef] [PubMed]
- Bong, D.T.; Clark, T.D.; Granja, J.R.; Ghadiri, M.R. Self-Assembling Organic Nanotubes. Angew. Chem. Int. Ed. 2001, 40, 988. [Google Scholar] [CrossRef]
- Shimizu, T.; Masuda, M.; Minamikawa, H. Supramolecular Nanotube Architectures Based on Amphiphilic Molecules. Chem. Rev. 2005, 105, 1401. [Google Scholar] [CrossRef] [PubMed]
- Pasini, D.; Ricci, M. Macrocycles as Precursors for Organic Nanotubes. Curr. Org. Synth. 2007, 4, 59–80. [Google Scholar] [CrossRef]
- Montenegro, J.; Ghadiri, M.R.; Granja, J.R. Ion Channel Models Based on Self-Assembling Cyclic Peptide Nanotubes. Acc. Chem. Res. 2013, 46, 2955–2965. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Zheng, L. Towards chirality-pure carbon nanotubes. Nanoscale 2010, 2, 1919–1929. [Google Scholar] [CrossRef] [PubMed]
- Peng, X.; Komatsu, N.; Bhattacharya, S.; Shimawaki, T.; Aonuma, S.; Kimura, T.; Osuka, A. Optically active single-walled carbon nanotubes. Nat. Nanotechnol. 2007, 2, 361–365. [Google Scholar] [CrossRef] [PubMed]
- Goodman, C.M.; Choi, S.; Shandler, S.; DeGrado, W.F. Foldamers as versatile frameworks for the design and evolution of function. Nat. Chem. Biol. 2007, 3, 252. [Google Scholar] [CrossRef] [PubMed]
- Seebach, D.; Gardiner, J. β-Peptidic Peptidomimetics. Acc. Chem. Res. 2008, 41, 1366. [Google Scholar] [CrossRef] [PubMed]
- Horne, W.S.; Gellman, S.H. Foldamers with Heterogeneous Backbones. Acc. Chem. Res. 2008, 41, 1399–1408. [Google Scholar] [CrossRef] [PubMed]
- Saraogi, I.; Hamilton, A.D. Recent Advances in the Development of Aryl-Based Foldamers. Chem. Soc. Rev. 2009, 38, 1726–1743. [Google Scholar] [CrossRef] [PubMed]
- Huc, I. Aromatic Oligoamide Foldamers. Eur. J. Org. Chem. 2004, 2004, 17–29. [Google Scholar] [CrossRef]
- Li, Z.T.; Hou, J.L.; Li, C. Peptide Mimics by Linear Arylamides: A Structural and Functional Diversity Test. Acc. Chem. Res. 2008, 41, 1343–1353. [Google Scholar] [CrossRef] [PubMed]
- Hecht, S.; Huc, I. Foldamers: Structure, Properties and Applications; Wiley-VCH: Weinheim, Germany, 2007. [Google Scholar]
- Martinek, T.A.; Fülöp, F. Peptidic foldamers: Ramping up diversity. Chem. Soc. Rev. 2012, 41, 687–702. [Google Scholar] [CrossRef] [PubMed]
- Ulijn, R.V.; Smith, A.M. Designing peptide based nanomaterials. Chem. Soc. Rev. 2008, 37, 664–675. [Google Scholar] [CrossRef] [PubMed]
- Chapman, R.; Danial, M.; Koh, M.L.; Jolliffe, K.A.; Perrier, S. Design and properties of functional nanotubes from the self-assembly of cyclic peptide templates. Chem. Soc. Rev. 2012, 41, 6023–6041. [Google Scholar] [CrossRef] [PubMed]
- Baillargeon, P.; Bernard, S.; Gauthier, D.; Skouta, R.; Dory, Y.L. Efficient Synthesis and Astonishing Supramolecular Architectures of Several Symmetric Macrolactams. Chem. Eur. J. 2007, 13, 9223–9235. [Google Scholar] [CrossRef] [PubMed]
- Ghorai, A.; Achari, B.; Chattopadhyay, P. Self-Assembly of Cyclic Peptides and Peptidomimetic Macrocycles: Linking Structure with Function. Tetrahedron 2016, 72, 3379–3387. [Google Scholar] [CrossRef]
- Ghadiri, M.R.; Granja, J.R.; Milligan, R.A.; McRee, D.E.; Khazanovich, N. Self-assembling organic nanotubes based on a cyclic peptide architecture. Nature 1993, 366, 324–327. [Google Scholar] [CrossRef] [PubMed]
- Cuerva, M.; Garcia-Fandino, R.; Vazquez-Vazquez, C.; Lopez-Quintela, M.A.; Montenegro, J.; Granja, J.R. Self-Assembly of Silver Metal Clusters of Small Atomicity on Cyclic Peptide Nanotubes. ACS Nano 2015, 9, 10834–10843. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez-Vazquez, N.; Garcia-Fandino, R.; Amorin, M.; Granja, J.R. Self-assembling alpha, gamma-cyclic peptides that generate cavities with tunable properties. Chem. Sci. 2016, 7, 183–187. [Google Scholar] [CrossRef]
- Danial, M.; Tran, C.M.N.; Jolliffe, K.A.; Perrier, S. Thermal Gating in Lipid Membranes Using Thermoresponsive Cyclic Peptide-Polymer Conjugates. J. Am. Chem. Soc. 2014, 136, 8018–8026. [Google Scholar] [CrossRef] [PubMed]
- Catrouillet, S.; Brendel, J.C.; Larnaudie, S.; Barlow, T.; Jolliffe, K.A.; Perrier, S. Tunable Length of Cyclic Peptide-Polymer Conjugate Self-Assemblies in Water. ACS Macro Lett. 2016, 5, 1119–1123. [Google Scholar] [CrossRef]
- Ghorai, A.; Gayen, A.; Kulsi, G.; Padmanaban, E.; Laskar, A.; Achari, B.; Mukhopadhyay, C.; Chattopadhyay, P. Simultaneous Parallel and Antiparallel Self-Assembly in a Triazole/Amide Macrocyle Conformationally Homologous to D-,L-α-Amino Acid Based Cyclic Peptides: NMR and Molecular Modeling Study. Org. Lett. 2011, 13, 5512–5515. [Google Scholar] [CrossRef] [PubMed]
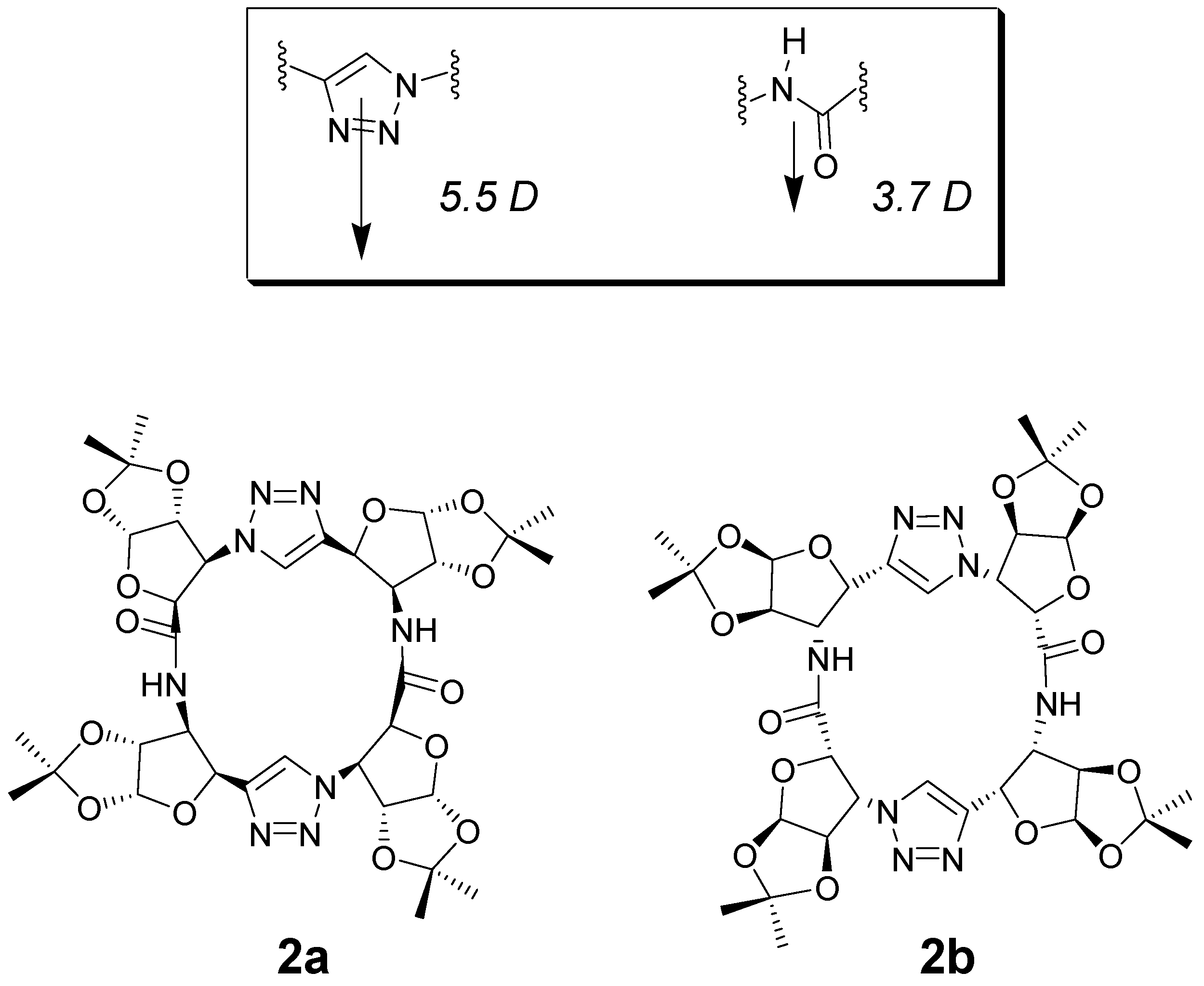
- Ghorai, A.; Padmanaban, E.; Mukhopadhyay, C.; Acharia, B.; Chattopadhyay, P. Design and synthesis of regioisomeric triazole based peptidomimetic macrocycles and their dipole moment controlled self-assembly. Chem. Commun. 2012, 48, 11975–11977. [Google Scholar] [CrossRef] [PubMed]
- Hennig, A.; Fischer, L.; Guichard, G.; Matile, S. Anion-Macrodipole Interactions: Self-Assembling Oligourea/Amide Macrocycles as Anion Transporters that Respond to Membrane Polarization. J. Am. Chem. Soc. 2009, 131, 16889–16895. [Google Scholar] [CrossRef] [PubMed]
- Reches, M.; Gazit, E. Casting metal nanowires within discrete self-assembled peptide nanotubes. Science 2003, 300, 625–627. [Google Scholar] [CrossRef] [PubMed]
- Adler-Abramovich, L.; Gazit, E. The physical properties of supramolecular peptide assemblies: from building block association to technological applications. Chem. Soc. Rev. 2014, 43, 6881–6893. [Google Scholar] [CrossRef] [PubMed]
- Baumeister, B.; Sakai, N.; Matile, S. Giant artificial ion channels formed by self-assembled, cationic rigid-rod beta-barrels. Angew. Chem. Int. Ed. 2000, 39, 1955–1958. [Google Scholar] [CrossRef]
- Percec, V.; Dulcey, A.E.; Balagurusamy, V.S.K.; Miura, Y.; Smidrkal, J.; Peterca, M.; Nummelin, S.; Edlund, U.; Hudson, S.D.; Heiney, P.A.; et al. Self-assembly of amphiphilic dendritic dipeptides into helical pores. Nature 2004, 430, 764–768. [Google Scholar] [CrossRef] [PubMed]
- Gattuso, G.; Menzer, S.; Nepogodiev, S.A.; Stoddart, J.F.; Williams, D.J. Carbothdrate Nanotubes. Angew. Chem. Int. Ed. 1997, 36, 1451–1454. [Google Scholar] [CrossRef]
- Ashton, P.R.; Cantrill, S.J.; Gattuso, G.; Menzer, S.; Nepogodiev, S.A.; Shipway, A.N.; Stoddart, J.F.; Williams, D.J. Achiral Cyclodextrin Analogues. Chem. Eur. J. 1997, 8, 1299–1314. [Google Scholar] [CrossRef]
- Liu, Z.; Liu, G.; Wu, Y.; Cao, D.; Sun, J.; Schneebeli, S.T.; Nassar, M.S.; Mirkin, C.A.; Stoddart, J.F. Assembly of Supramolecular Nanotubes from Molecular Triangles and 1,2-Dihalohydrocarbons. J. Am. Chem. Soc. 2014, 136, 16651–16660. [Google Scholar] [CrossRef] [PubMed]
- Ponnuswamy, N.; Dan Pantoş, G.; Smulders, M.M.J.; Sanders, J.K.M. Thermodynamics of Supramolecular Naphthalenediimide Nanotube Formation: The Influence of Solvents, Side Chains, and Guest Templates. J. Am. Chem. Soc. 2012, 134, 566–573. [Google Scholar] [CrossRef] [PubMed]
- Cook, J.L.; Hunter, C.A.; Low, C.M.R.; Perez-Velasco, A.; Vinter, J.G. Solvent Effects on Hydrogen Bonding. Angew. Chem. Int. Ed. 2007, 46, 3706–3709. [Google Scholar] [CrossRef] [PubMed]
- Shi, Q.; Javorskis, T.; Bergquist, K.; Ulčinas, A.; Niaura, G.; Matulaitiene, I.; Orentas, E.; Wӓrnmark, K. Stimuli-Controlled Self-Assembly of Diverse Tubular Aggregates From One Single Small Monomer. Nat. Commun. 2017, 8, 14943. [Google Scholar] [CrossRef] [PubMed]
- Hunter, C.A.; Sanders, J.K.M. The Nature of π-π Interactions. J. Am. Chem. Soc. 1990, 112, 5525–5534. [Google Scholar] [CrossRef]
- Huang, Z.; Kang, S.-K.; Banno, M.; Yamaguchi, T.; Lee, D.; Seok, C.; Yashima, E.; Lee, M. Pulsating Tubules from Noncovolaent Macrocycles. Science 2012, 337, 1521–1526. [Google Scholar] [CrossRef] [PubMed]
- Ma, X.; Zhang, Y.; Zheng, Y.; Zhang, Y.; Tao, X.; Che, Y.; Zhao, J. Highly Fluorescent One-Handed Nanotubes Assembled from a Chiral Asymmetric Perylene Diimide. Chem. Commun. 2015, 51, 4231–4233. [Google Scholar] [CrossRef] [PubMed]
- Peng, C.; Zhang, Y.; Zhang, Y.; Hu, Y.; Che, Y.; Zhao, J. Highly fluorescent nanotubes with tunable diameter and wall thickness self-assembled from asymmetric perylene diimide. Small 2016, 12, 4363–4369. [Google Scholar] [CrossRef] [PubMed]
- Van Dijken, D.J.; Stacko, P.; Stuart, M.C.A.; Browne, W.R.; Feringa, B.L. Chirality Controlled Responsive Self-Assembled Nanotubes in Water. Chem. Sci. 2017, 8, 1783–1789. [Google Scholar] [CrossRef] [PubMed]
- Wei, Y.; Sun, D.; Yuan, D.; Liu, Y.; Zhao, Y.; Li, X.; Wang, S.; Dou, J.; Wang, X.; Haoa, A.; Sun, D. Pb(II) Metal–Organic Nanotubes Based on Cyclodextrins: Biphasic Synthesis, Structures and Properties. Chem. Sci. 2012, 3, 2282–2287. [Google Scholar] [CrossRef]
- Xin, X.; Wang, J.; Gong, C.; Xu, H.; Wang, R.; Ji, S.; Dong, H.; Meng, Q.; Zhang, L.; Dai, F.; et al. Cyclodextrin-Based Metal-Organic Nanotube as Fluorescent Probe for Selective Turn-On Detection of Hydrogen Sulfide in Living Cells Based on H2S-Involved Coordination Mechanism. Sci. Rep. 2016, 6, 21951. [Google Scholar] [CrossRef] [PubMed]
- Rebilly, J.-N.; Bacsa, J.; Rosseinsky, M.J. 1D Tubular and 2D Metal–Organic Frameworks Based on a Flexible Amino Acid Derived Organic Spacer. Chem. Asian J. 2009, 4, 892–903. [Google Scholar] [CrossRef] [PubMed]
- Lykourinou, V.; Chen, Y.; Wang, X.S.; Meng, L.; Hoang, T.; Ming, L.J.; Musselman, R.L.; Ma, S.Q. Immobilization of MP-11 into a Mesoporous Metal–Organic Framework, MP-11@mesoMOF: A New Platform for Enzymatic Catalysis. J. Am. Chem. Soc. 2011, 133, 10382–10385. [Google Scholar] [CrossRef] [PubMed]
- Valente, C.; Choi, E.; Belowich, M.E.; Doonan, C.J.; Li, Q.; Gasa, T.B.; Botros, Y.Y.; Yaghi, O.M.; Stoddart, J.F. Metal–organic frameworks with designed chiral recognition sites. Chem. Commun. 2010, 46, 4911–4913. [Google Scholar] [CrossRef] [PubMed]
- Sawano, T.; Ji, P.; McIsaac, A.R.; Lin, Z.; Abney, C.W.; Lin, W. The first chiral diene-based metal–organic frameworks for highly enantioselective carbon–carbon bond formation reactions. Chem. Sci. 2015, 6, 7163–7168. [Google Scholar] [CrossRef]
- Fukino, T.; Joo, H.; Hisada, Y.; Obana, M.; Yamagishi, H.; Hikima, T.; Takata, M.; Fujita, N.; Aida, T. Manipulation of Discrete Nanostructures by Selective Modulation of Noncovalent Forces. Science 2014, 344, 499–504. [Google Scholar] [CrossRef] [PubMed]
- Yamagishi, H.; Fukino, T.; Hashizume, D.; Mori, T.; Inoue, Y.; Hikima, T.; Takata, M.; Aida, T. Metal–Organic Nanotube with Helical and Propeller-Chiral Motifs Composed of a C10-Symmetric Double-Decker Nanoring. J. Am. Chem. Soc. 2015, 137, 7628–7631. [Google Scholar] [CrossRef] [PubMed]
- Caricato, M.; Deforge, A.; Bonifazi, D.; Dondi, D.; Mazzanti, A.; Pasini, D. Chiral Nanostructuring of Multivalent Macrocycles in Solution and on Surfaces. Org. Biomol. Chem. 2015, 13, 3593–3601. [Google Scholar] [CrossRef] [PubMed]
- Caricato, M.; Olmo, A.; Gargiulli, C.; Gattuso, G.; Pasini, D. A “clicked” macrocyclic probe incorporating Binol as the signalling unit for the chiroptical sensing of anions. Tetrahedron 2012, 68, 7861–7866. [Google Scholar] [CrossRef]
- Bencini, A.; Coluccini, C.; Garau, A.; Giorgi, C.; Lippolis, V.; Messori, L.; Pasini, D.; Puccioni, S. A BINOL-based chiral polyammonium receptor for highly enantioselective recognition and fluorescence sensing of (S,S)-tartaric acid in aqueous solution. Chem. Commun. 2012, 48, 10428–10430. [Google Scholar] [CrossRef] [PubMed]
- Coluccini, C.; Mazzanti, A.; Pasini, D. Locked Chromophores as CD and NMR Probes for the Helical Conformation of Tetraamidic Macrocycles. Org. Biomol. Chem. 2010, 8, 1807–1815. [Google Scholar] [CrossRef] [PubMed]
- Colombo, S.; Coluccini, C.; Caricato, M.; Gargiulli, C.; Gattuso, G.; Pasini, D. Shape Selectivity in the Synthesis of Chiral Macrocyclic Amides. Tetrahedron 2010, 66, 4206–4211. [Google Scholar] [CrossRef]
- Coluccini, C.; Dondi, D.; Caricato, M.; Taglietti, A.; Boiocchi, M.; Pasini, D. Structurally-Variable, Rigid and Optically-Active D2 and D3 Macrocycles Possessing Recognition Properties towards C60. Org. Biomol. Chem. 2010, 8, 1640–1649. [Google Scholar] [CrossRef] [PubMed]
- Caricato, M.; Coluccini, C.; Dondi, D.; Vander Griend, D.A.; Pasini, D. Nesting Complexation of C60 with Large, Rigid D2 Symmetrical Macrocycles. Org. Biomol. Chem. 2010, 8, 3272–3280. [Google Scholar] [CrossRef] [PubMed]
- Caricato, M.; Leza, N.J.; Roy, K.; Dondi, D.; Gattuso, G.; Shimizu, L.S.; Vander Griend, D.A.; Pasini, D. A Chiroptical Probe for Sensing Metal Ions in Water. Eur. J. Org. Chem. 2013, 27, 6078–6083. [Google Scholar] [CrossRef]
- Agnes, M.; Nitti, A.; Vander Griend, D.A.; Dondi, D.; Merli, D.; Pasini, D. A chiroptical molecular sensor for ferrocene. Chem. Commun. 2016, 52, 11492–11495. [Google Scholar] [CrossRef] [PubMed]
- Pasini, D.; Nitti, A. Recent Advances in Chirality Sensing using Atropoisomeric Molecular Receptors. Chirality 2016, 28, 116–123. [Google Scholar] [CrossRef] [PubMed]
- Caricato, M.; Sharma, A.K.; Coluccini, C.; Pasini, D. Nanostructuring with Chirality: Binaphthyl-Based Synthons for the Production of Functional Oriented Nanomaterials. Nanoscale 2014, 6, 7165–7174. [Google Scholar] [CrossRef] [PubMed]
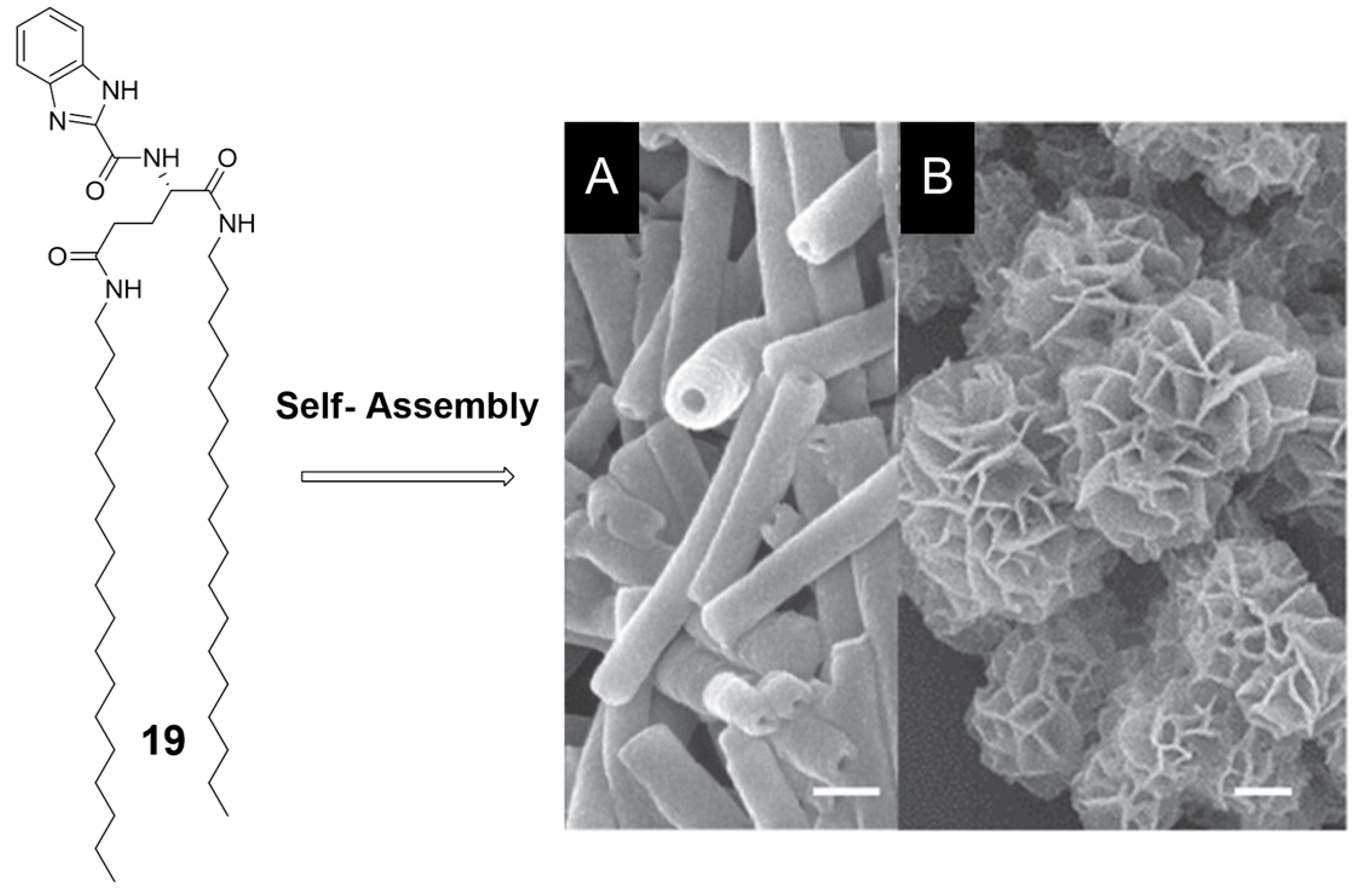
- Zhou, X.; Jin, Q.; Zhang, L.; Shen, Z.; Jiang, L.; Liu, M. Self-Assembly of Hierarchical Chiral Nanostructures Based on Metal–Benzimidazole Interactions: Chiral Nanofibers, Nanotubes, and Microtubular Flowers. Small 2016, 12, 4743–4752. [Google Scholar] [CrossRef] [PubMed]
- Grave, C.; Schlüter, A.D. Shape-Persistent, Nano-Sized Macrocycles. Eur. J. Org. Chem. 2002, 2002, 3075–3098. [Google Scholar] [CrossRef]
- Yamaguchi, Y.; Yoshida, Z.-I. Shape-persistency and Molecular Function in Heteromacrocycles: Creation of Heteroarenecyclynes and Arene–Azaarenecyclynes. Chem. Eur. J. 2003, 9, 5430–5440. [Google Scholar] [CrossRef] [PubMed]
- Höger, S. Shape-Persistent Phenylene-Acetylene Macrocycles: Large Rings—Low Yield? Angew. Chem. Int. Ed. 2005, 44, 3806–3808. [Google Scholar] [CrossRef] [PubMed]
- Zhao, D.; Moore, J.S. Shape-Persistent Arylene Ethynylene Macrocycles: Syntheses and Supramolecular Chemistry. Chem. Commun. 2003, 807–818. [Google Scholar] [CrossRef]
- Bunz, U.H.F.; Rubin, Y.; Tobe, Y. Polyethynylated cyclic π-systems: scaffoldings for novel two and three-dimensional carbon networks. Chem. Soc. Rev. 1999, 28, 107–119. [Google Scholar] [CrossRef]
- Haley, M.M.; Pak, J.J.; Brand, S.C. Macrocyclic Oligo (phenylacetylenes) and Oligo(phenyldiacetylenes). Curr. Chem. 1999, 201, 81–130. [Google Scholar] [CrossRef]
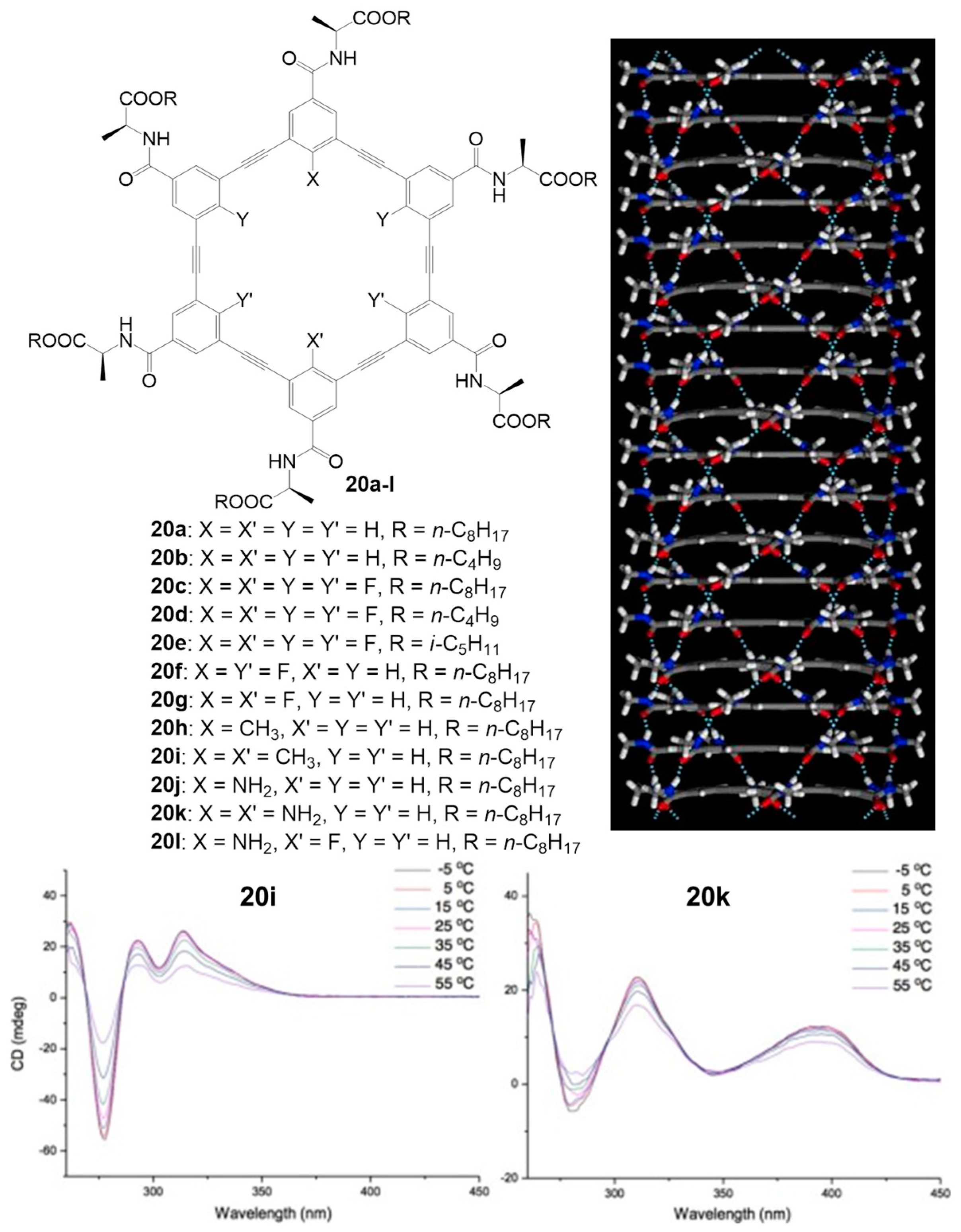
- Zhou, X.B.; Liu, G.D.; Yamato, K.; Shen, Y.; Cheng, R.X.; Wei, X.X.; Bai, W.L.; Gao, Y.; Li, H.; Liu, Y.; et al. Self-assembling Subnanometer Pores with Unusual Mass-Transport Properties. Nat. Commun. 2012, 3, 949. [Google Scholar] [CrossRef] [PubMed]
- Zhong, Y.; Wang, Q.; Yang, Y.; Lu, Z.; He, L.; Gong, B. Hexakis(m-phenylene ethynylene) Macrocycles with Multiple H-Bonding Side Chains and Modified Cavities: Altered Stacking Strength and Persistent Tubular Assembly. Org. Lett. 2016, 18, 2094–2097. [Google Scholar] [CrossRef] [PubMed]
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nitti, A.; Pacini, A.; Pasini, D. Chiral Nanotubes. Nanomaterials 2017, 7, 167. https://doi.org/10.3390/nano7070167
Nitti A, Pacini A, Pasini D. Chiral Nanotubes. Nanomaterials. 2017; 7(7):167. https://doi.org/10.3390/nano7070167
Chicago/Turabian StyleNitti, Andrea, Aurora Pacini, and Dario Pasini. 2017. "Chiral Nanotubes" Nanomaterials 7, no. 7: 167. https://doi.org/10.3390/nano7070167