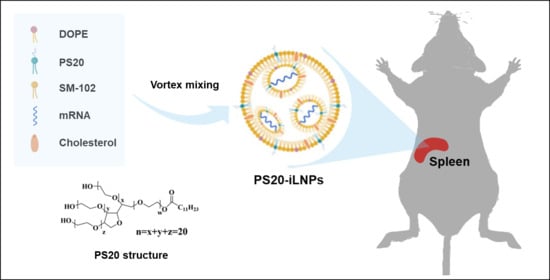
Construction of Spleen-Accumulated Polysorbate 20-Containing Ionizable Lipid Nanoparticles for mRNA Delivery
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
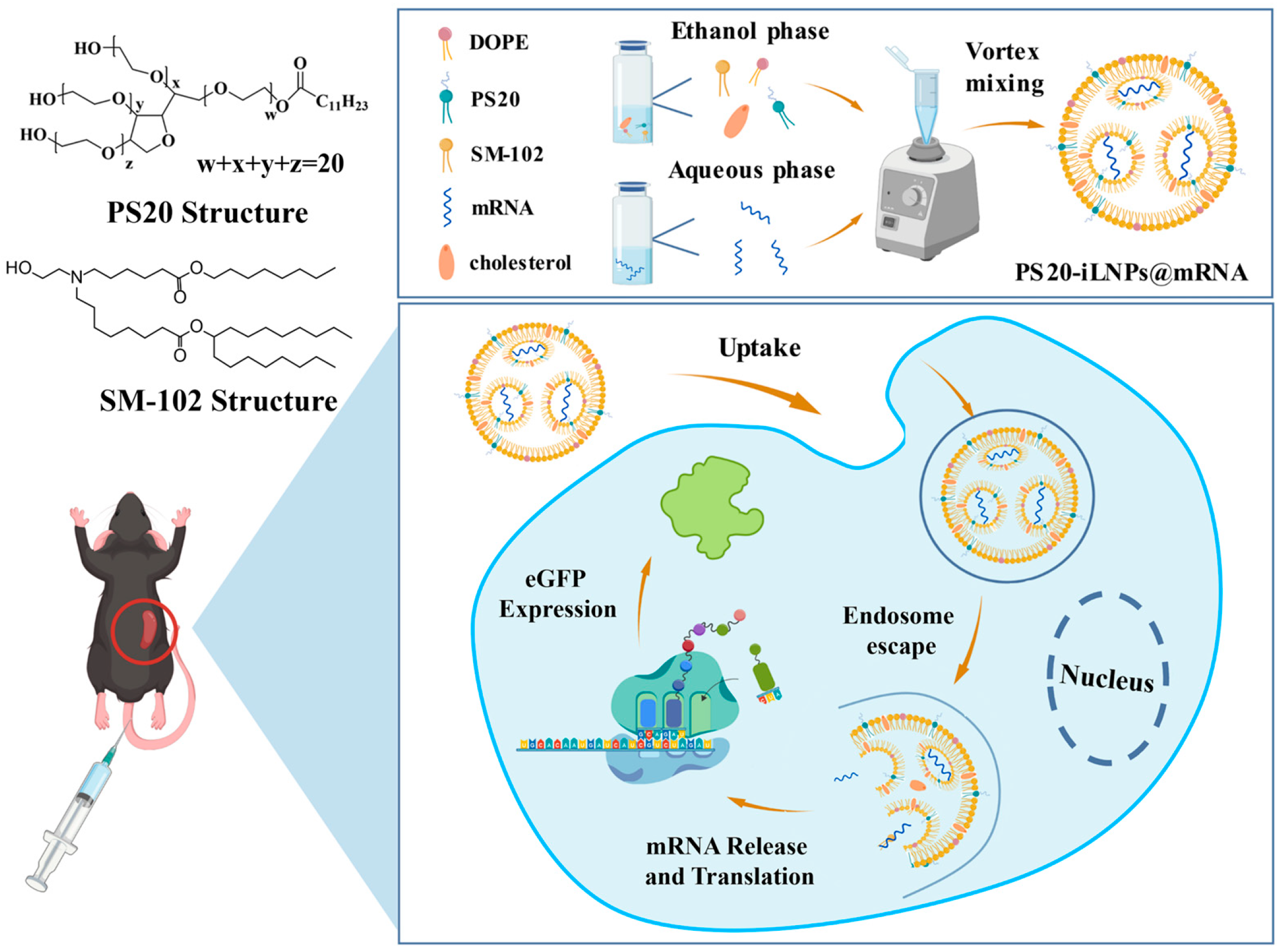
2.2. Preparation of iLNPs by Vortex Mixing Method
2.3. iLNPs Characterization
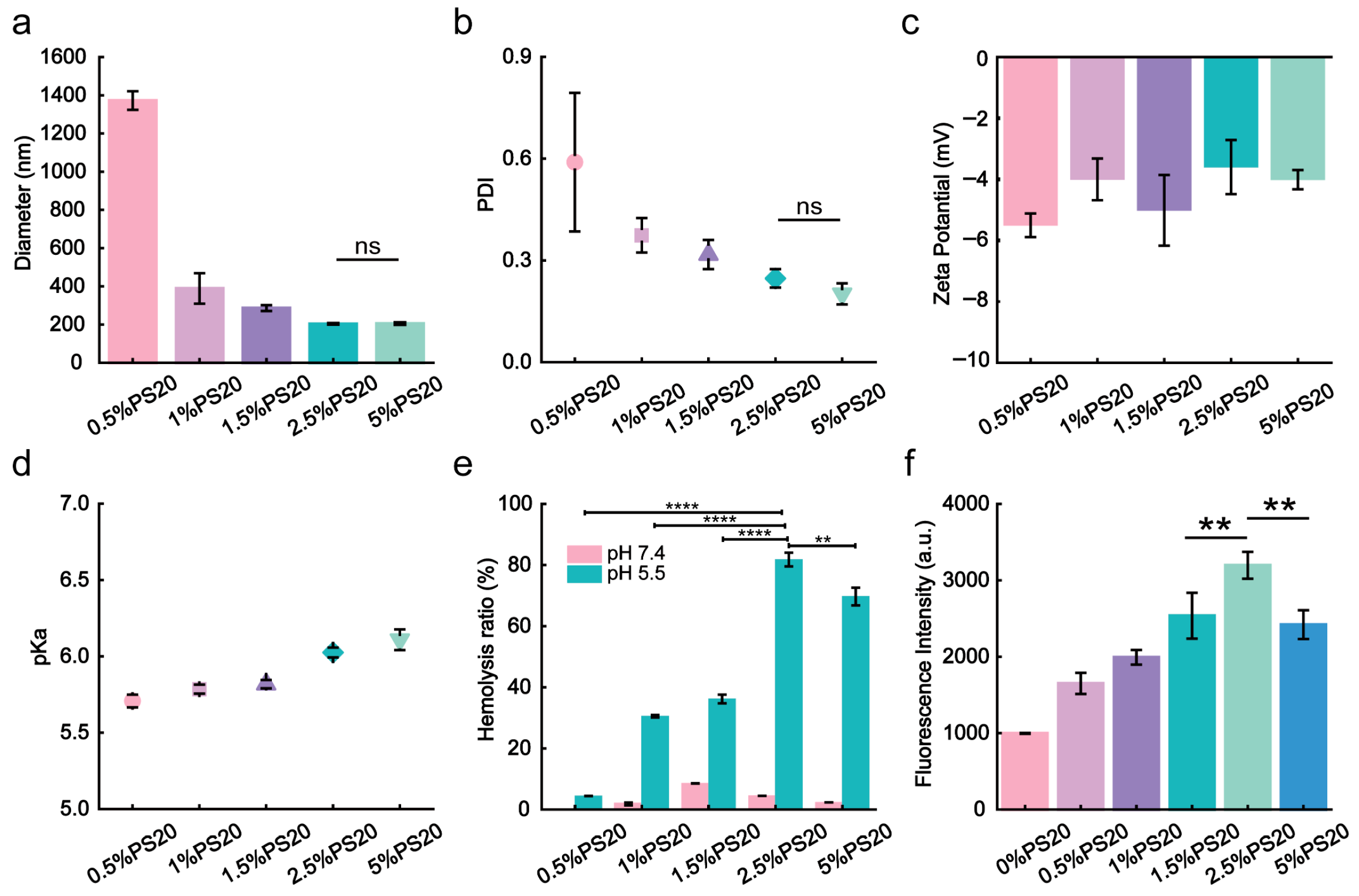
2.4. Apparent pKa Analysis
2.5. Membrane Disruption Capacity Experiment
2.6. Cellular Uptake of iLNPs with Different Ratio of PS20
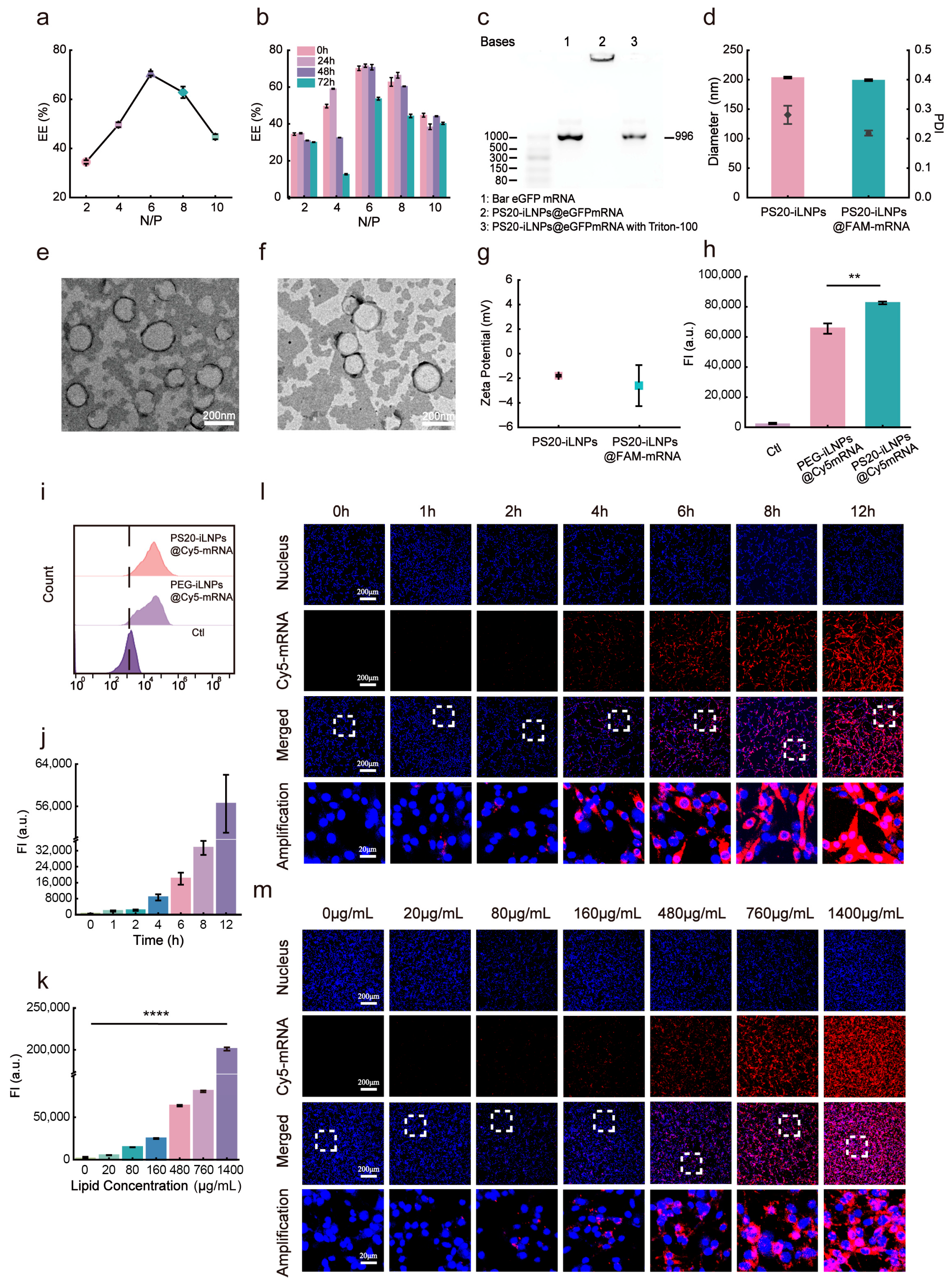
2.7. Exploration of Optimal Ratio of mRNA to Lipid (N/P)
2.8. Agarose Gel Electrophoresis
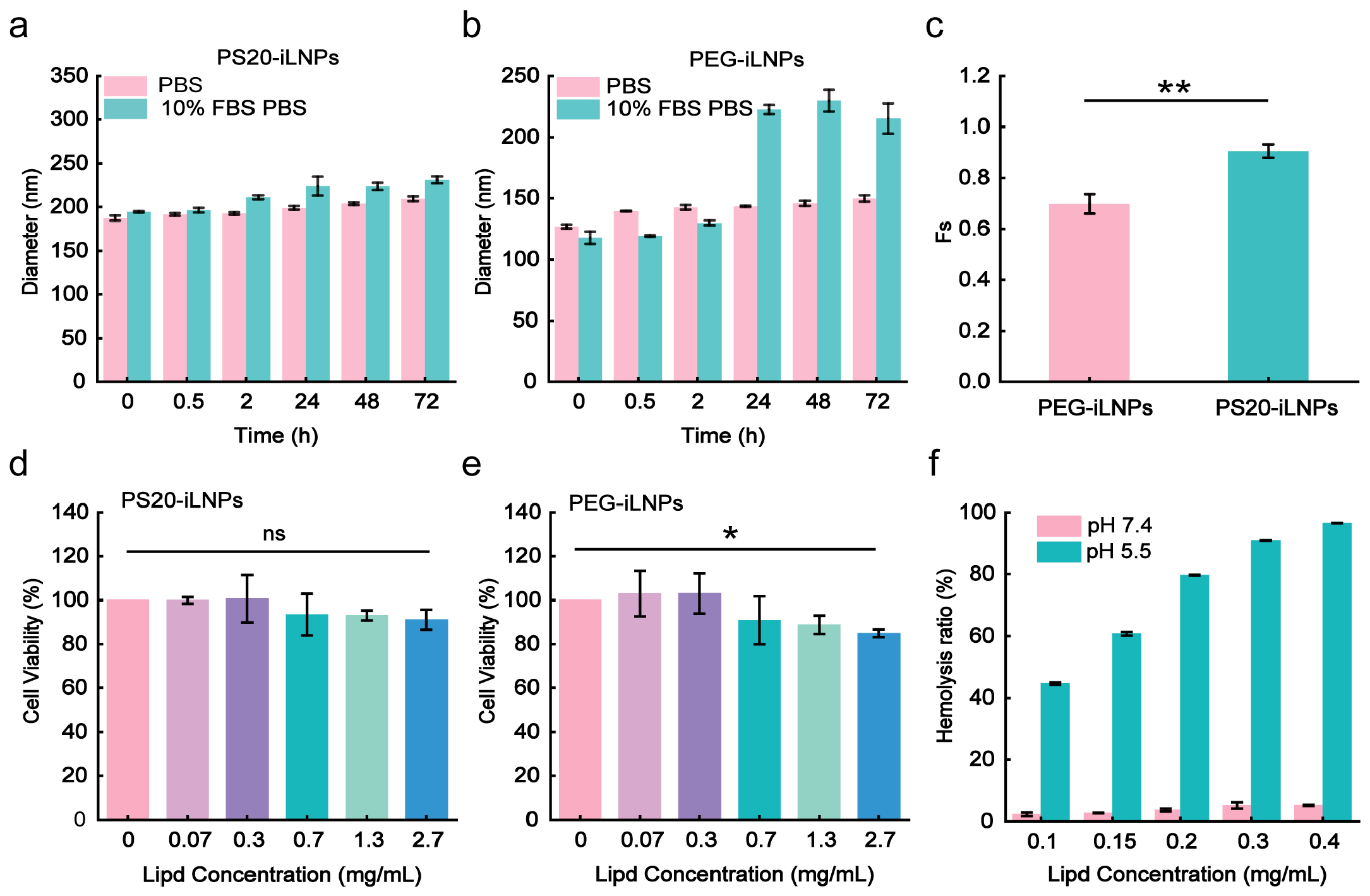
2.9. Stability of iLNPs
2.10. Protein Adsorption Test
2.11. Safety of iLNPs
2.12. Intracellular iLNPs@mRNA Delivery
2.13. PS20-iLNPs@Cy5-mRNA Cell Internalization Pathway
2.14. In Vitro Transfection Efficiency
2.15. In Vivo Biodistribution
2.16. Statistical Analysis
3. Results
3.1. Synthesis and Characterization of PS20-iLNPs
3.2. Stability and Stealthiness of PS20-iLNPs
3.3. In Vitro Safety
3.4. Delivery Capacity of PS20-iLNPs@Cy5mRNA In Vitro
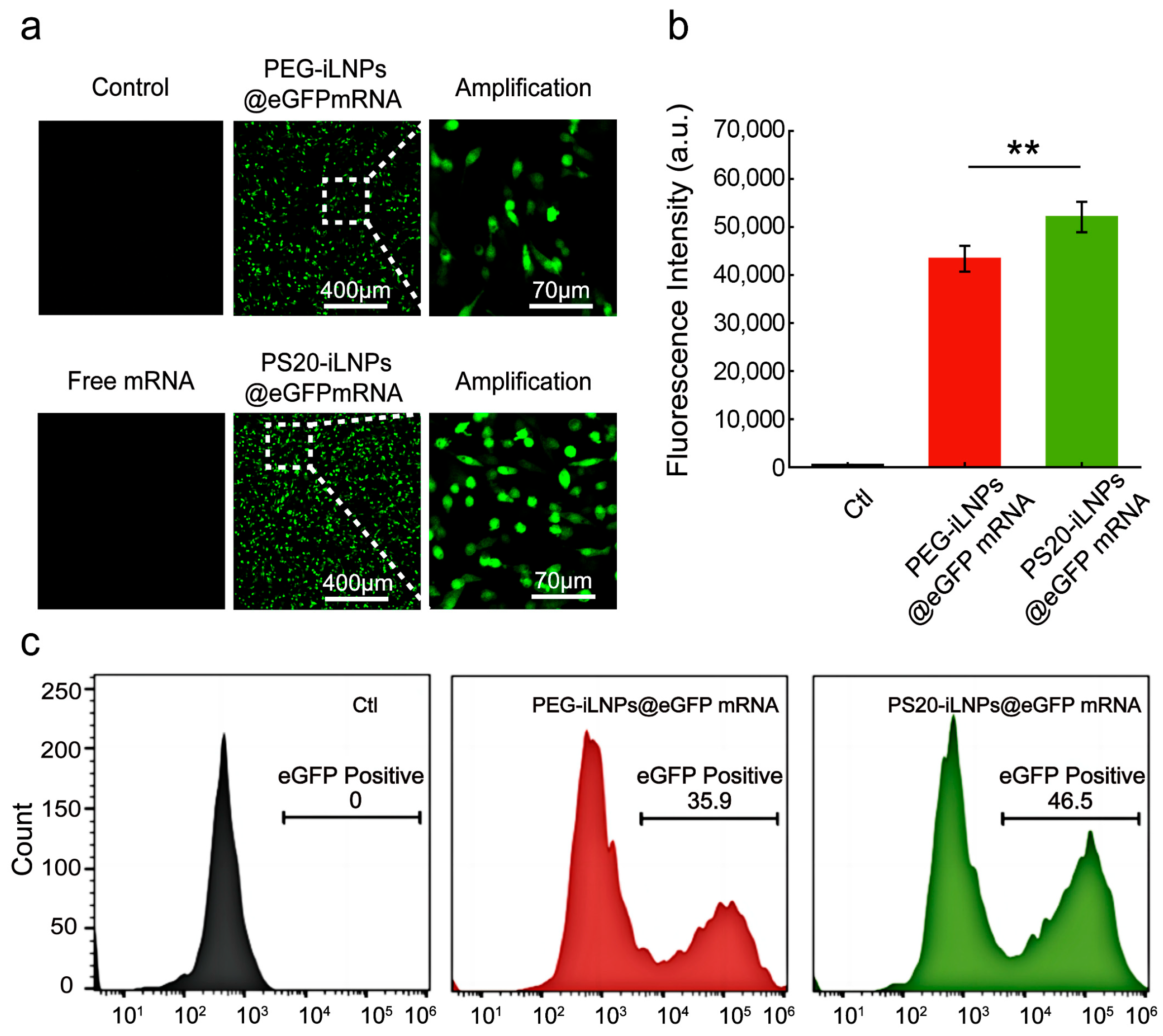
3.5. In Vitro eGFPmRNA Transfection
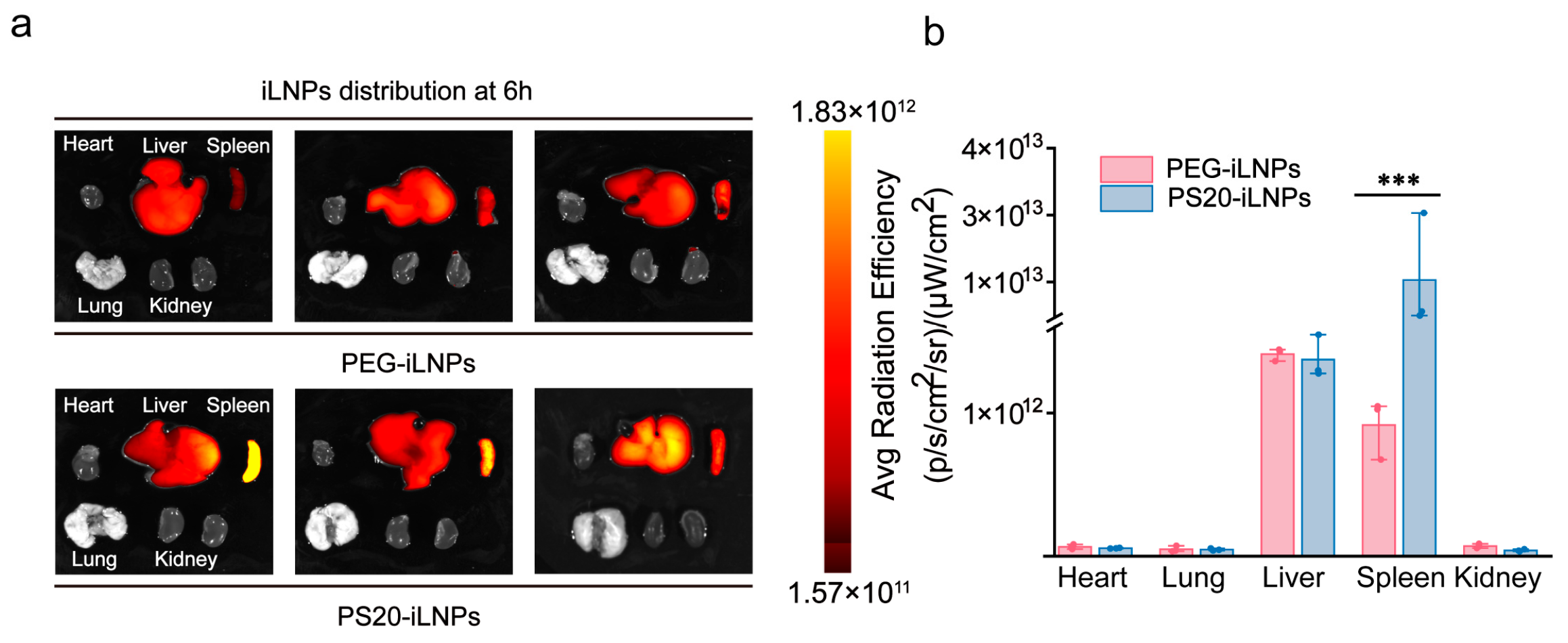
3.6. In Vivo Biodistribution of PS20-iLNPs
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Sharma, P.; Hoorn, D.; Aitha, A.; Breier, D.; Peer, D. The immunostimulatory nature of mRNA lipid nanoparticles. Adv. Drug Deliv. Rev. 2024, 205, 115175. [Google Scholar] [CrossRef]
- Senti, M.E.; del Valle, L.G.; Schiffelers, R.M. mRNA delivery systems for cancer immunotherapy: Lipid nanoparticles and beyond. Adv. Drug Deliv. Rev. 2024, 206, 115190. [Google Scholar] [CrossRef]
- Zhang, Y.B.; Hou, X.C.; Du, S.; Xue, Y.E.; Yan, J.Y.; Kang, D.D.; Zhong, Y.C.; Wang, C.; Deng, B.B.; McComb, D.W.; et al. Close the cancer-immunity cycle by integrating lipid nanoparticle-mRNA formulations and dendritic cell therapy. Nat. Nanotechnol. 2023, 18, 1364–1374. [Google Scholar] [CrossRef] [PubMed]
- Thelen, J.L.; Leite, W.; Urban, V.S.; O’Neill, H.M.; Grishaev, A.V.; Curtis, J.E.; Krueger, S.; Castellanos, M.M. Morphological Characterization of Self-Amplifying mRNA Lipid Nanoparticles. Acs Nano 2024, 18, 1464–1476. [Google Scholar] [CrossRef]
- Kim, Y.K. RNA therapy: Rich history, various applications and unlimited future prospects. Exp. Mol. Med. 2022, 54, 455–465. [Google Scholar] [CrossRef]
- Tang, X.F.; Zhang, Y.; Han, X.J. Ionizable Lipid Nanoparticles for mRNA Delivery. Adv. NanoBiomed Res. 2023, 3, 2300006. [Google Scholar] [CrossRef]
- Zong, W.; Hu, Y.; Su, Y.C.; Luo, N.; Zhang, X.N.; Li, Q.C.; Han, X.J. Polydopamine-coated liposomes as pH-sensitive anticancer drug carriers. J. Microencapsul. 2016, 33, 257–262. [Google Scholar] [CrossRef]
- Li, M.H.; Tang, X.F.; Liu, X.Y.; Cui, X.Y.; Lian, M.M.; Zhao, M.; Peng, H.S.; Han, X.J. Targeted miR-21 loaded liposomes for acute myocardial infarction. J. Mater. Chem. B 2020, 8, 10384–10391. [Google Scholar] [CrossRef] [PubMed]
- Segel, M.; Lash, B.; Song, J.W.; Ladha, A.; Liu, C.C.; Jin, X.; Mekhedov, S.L.; Macrae, R.K.; Koonin, E.V.; Zhang, F. Mammalian retrovirus-like protein PEG10 packages its own mRNA and can be pseudotyped for mRNA delivery. Science 2021, 373, 882–889. [Google Scholar] [CrossRef] [PubMed]
- Yang, D.; Wu, X.Q.; Yao, Y.A.; Duan, M.S.; Wang, X.; Li, G.L.; Guo, A.G.; Wu, M.X.; Liu, Y.H.; Zheng, J.; et al. An RNA editing strategy rescues gene duplication in a mouse model of MEPC2 duplication syndrome and nonhuman primates. Nat. Neurosci. 2025, 28, 72–83. [Google Scholar] [CrossRef]
- Yang, C.Y.; Huang, W.C.; Gao, Y.; Liu, Z.; An, N.; Mu, W.; Pan, Q.M.; Yang, B.; Guo, C.S.; Han, X.J. Phototherapy ablation of rabbit orthotopic tumors by non-stoichiometric BiPO4-x nanoparticles. Chem. Eng. J. 2020, 386, 123961. [Google Scholar] [CrossRef]
- Cui, X.Y.; Cheng, W.L.; Han, X.J. Lipid bilayer modified gold nanorod@mesoporous silica nanoparticles for controlled drug delivery triggered by near-infrared light. J. Mater. Chem. B 2018, 6, 8078–8084. [Google Scholar] [CrossRef]
- Zhu, P.Y.; Li, Y.S.; Zhang, D.P. One-Component Ionizable Amphiphilic Janus Dendrimers for Targeted mRNA Delivery. Angew. Chem. Int. Ed. 2025, 64, e202505304. [Google Scholar] [CrossRef] [PubMed]
- Sahoo, D.; Atochina-Vasserman, E.N.; Maurya, D.S.; Arshad, M.; Chenna, S.S.; Ona, N.; Vasserman, J.A.; Ni, H.P.; Weissman, D.; Percec, V. The Constitutional Isomerism of One-Component Ionizable Amphiphilic Janus Dendrimers Orchestrates the Total and Targeted Activities of mRNA Delivery. J. Am. Chem. Soc. 2024, 146, 3627–3634. [Google Scholar] [CrossRef] [PubMed]
- Tang, X.F.; Wang, Z.; Zhang, Y.; Mu, W.; Han, X.J. Non-viral nanocarriers for CRISPR-Cas9 gene editing system delivery. Chem. Eng. J. 2022, 435, 135116. [Google Scholar] [CrossRef]
- Wang, C.; Zhang, Y.B.; Dong, Y.Z. Lipid Nanoparticle-mRNA Formulations for Therapeutic Applications. Acc. Chem. Res. 2021, 54, 4283–4293. [Google Scholar] [CrossRef]
- Qiu, M.; Glass, Z.; Chen, J.J.; Haas, M.; Jin, X.; Zhao, X.W.; Rui, X.H.; Ye, Z.F.; Li, Y.M.; Zhang, F.; et al. Lipid nanoparticle-mediated codelivery of Cas9 mRNA and single-guide RNA achieves liver-specific in vivo genome editing of Angptl3. Proc. Natl. Acad. Sci. USA 2021, 118, e2020401118. [Google Scholar] [CrossRef] [PubMed]
- Arteta, M.Y.; Kjellman, T.; Bartesaghi, S.; Wallin, S.; Wu, X.Q.; Kvist, A.J.; Dabkowska, A.; Székely, N.; Radulescu, A.; Bergenholtz, J.; et al. Successful reprogramming of cellular protein production through mRNA delivered by functionalized lipid nanoparticles. Proc. Natl. Acad. Sci. USA 2018, 115, E3351–E3360. [Google Scholar] [CrossRef]
- Parhiz, H.; Shuvaev, V.V.; Pardi, N.; Khoshnejad, M.; Kiseleva, R.Y.; Brenner, J.S.; Uhler, T.; Tuyishime, S.; Mui, B.L.; Tam, Y.K.; et al. PECAM-1 directed re-targeting of exogenous mRNA providing two orders of magnitude enhancement of vascular delivery and expression in lungs independent of apolipoprotein E-mediated uptake. J. Control. Release 2018, 291, 106–115. [Google Scholar] [CrossRef]
- Salehirozveh, M.; Larsen, A.K.K.; Stojmenovic, M.; Thei, F.; Dong, M.D. In-situ PLL-g-PEG Functionalized Nanopore for Enhancing Protein Characterization. Chem. Asian J. 2023, 18, e202300515. [Google Scholar] [CrossRef]
- Shimabukuro, T.T.; Cole, M.; Su, J.R. Reports of Anaphylaxis After Receipt of mRNA COVID-19 Vaccines in the US-December 14, 2020-January 18, 2021. Jama 2021, 325, 1101–1102. [Google Scholar] [CrossRef]
- Iguchi, T.; Umeda, H.; Kojima, M.; Kanno, Y.; Tanaka, Y.; Kinoshita, N.; Sato, D. Cumulative Adverse Event Reporting of Anaphylaxis After mRNA COVID-19 Vaccine (Pfizer-BioNTech) Injections in Japan: The First-Month Report. Drug Saf. 2021, 44, 1209–1214. [Google Scholar] [CrossRef]
- Castells, M.C.; Phillips, E.J. Maintaining Safety with SARS-CoV-2 Vaccines Reply. N. Engl. J. Med. 2021, 384, e37. [Google Scholar] [CrossRef]
- Mouri, M.; Imamura, M.; Suzuki, S.; Kawasaki, T.; Ishizaki, Y.; Sakurai, K.; Nagafuchi, H.; Matsumura, N.; Uchida, M.; Ando, T.; et al. Serum polyethylene glycol-specific IgE and IgG in patients with hypersensitivity to COVID-19 mRNA vaccines. Allergol. Int. 2022, 71, 512–519. [Google Scholar] [CrossRef]
- Kozma, G.T.; Shimizu, T.; Ishida, T.; Szebeni, J. Anti-PEG antibodies: Properties, formation, testing and role in adverse immune reactions to PEGylated nano-biopharmaceuticals. Adv. Drug Deliv. Rev. 2020, 154–155, 163–175. [Google Scholar] [CrossRef] [PubMed]
- Grenier, P.; Viana, I.M.O.; Lima, E.M.; Bertrand, N. Anti-polyethylene glycol antibodies alter the protein corona deposited on nanoparticles and the physiological pathways regulating their fate in vivo. J. Control. Release 2018, 287, 121–131. [Google Scholar] [CrossRef]
- Shen, L.; Li, Z.; Ma, A.; Cruz-Teran, C.; Talkington, A.; Shipley, S.T.; Lai, S.K. Free PEG Suppresses Anaphylaxis to PEGylated Nanomedicine in Swine. ACS Nano 2024, 18, 8733–8744. [Google Scholar] [CrossRef] [PubMed]
- Hershfield, M.S.; Ganson, N.J.; Kelly, S.J.; Scarlett, E.L.; Jaggers, D.A.; Sundy, J.S. Induced and pre-existing anti-polyethylene glycol antibody in a trial of every 3-week dosing of pegloticase for refractory gout, including in organ transplant recipients. Arthritis Res. Ther. 2014, 16, R63. [Google Scholar] [CrossRef] [PubMed]
- Yang, Q.; Jacobs, T.M.; McCallen, J.D.; Moore, D.T.; Huckaby, J.T.; Edelstein, J.N.; Lai, S.K. Analysis of Pre-existing IgG and IgM Antibodies against Polyethylene Glycol (PEG) in the General Population. Anal. Chem. 2016, 88, 11804–11812. [Google Scholar] [CrossRef]
- Garay, R.P.; El-Gewely, R.; Armstrong, J.K.; Garratty, G.; Richette, P. Antibodies against polyethylene glycol in healthy subjects and in patients treated with PEG-conjugated agents. Expert Opin. Drug Deliv. 2012, 9, 1319–1323. [Google Scholar] [CrossRef]
- Bauernfeind, S.; Salzberger, B.; Hitzenbichler, F.; Scigala, K.; Einhauser, S.; Wagner, R.; Gessner, A.; Koestler, J.; Peterhoff, D. Association between Reactogenicity and Immunogenicity after Vaccination with BNT162b2. Vaccines 2021, 9, 1089. [Google Scholar] [CrossRef]
- Szebeni, J.; Simberg, D.; Gonzalez-Fernandez, A.; Barenholz, Y.; Dobrovolskaia, M.A. Roadmap and strategy for overcoming infusion reactions to nanomedicines. Nat. Nanotechnol. 2018, 13, 1100–1108. [Google Scholar] [CrossRef]
- Fulop, T.; Kozma, G.T.; Vashegyi, I.; Meszaros, T.; Rosivall, L.; Urbanics, R.; Storm, G.; Metselaar, J.M.; Szebeni, J. Liposome-induced hypersensitivity reactions: Risk reduction by design of safe infusion protocols in pigs. J. Control. Release 2019, 309, 333–338. [Google Scholar] [CrossRef]
- Mohamed, M.; Abu Lila, A.S.; Shimizu, T.; Alaaeldin, E.; Hussein, A.; Sarhan, H.A.; Szebeni, J.; Ishida, T. PEGylated liposomes: Immunological responses. Sci. Technol. Adv. Mater. 2019, 20, 710–724. [Google Scholar] [CrossRef]
- Holick, C.T.; Klein, T.; Mehnert, C.; Adermann, F.; Anufriev, I.; Streiber, M.; Harder, L.; Traeger, A.; Hoeppener, S.; Franke, C.; et al. Poly(2-ethyl-2-oxazoline) (POx) as Poly(ethylene glycol) (PEG)-Lipid Substitute for Lipid Nanoparticle Formulations. Small 2025, 21, 2411354. [Google Scholar] [CrossRef]
- Golba, B.; Zhong, Z.F.; Romio, M.; Almey, R.; Deforce, D.; Dhaenens, M.; Sanders, N.N.; Benetti, E.M.; De Geest, B.G. Cyclic Poly(2-methyl-2-oxazoline)-Lipid Conjugates Are Good Alternatives to Poly(ethylene glycol)-Lipids for Lipid Nanopartcile mRNA Formulation. Biomacromolecules 2025, 26, 1816–1825. [Google Scholar] [CrossRef]
- Khunsuk, P.O.; Pongma, C.; Palaga, T.; Hoven, V.P. Zwitterionic Polymer-Decorated Lipid Nanoparticles for mRNA Delivery in Mammalian Cells. Biomacromolecules 2023, 24, 5654–5665. [Google Scholar] [CrossRef] [PubMed]
- Adler, A.; Inoue, Y.; Sato, Y.; Ishihara, K.; Ekdahl, K.N.; Nilsson, B.; Teramura, Y. Synthesis of poly(2-methacryloyloxyethyl phosphorylcholine)-conjugated lipids and their characterization and surface properties of modified liposomes for protein interactions. Biomater. Sci. 2021, 9, 5854–5867. [Google Scholar] [CrossRef] [PubMed]
- Xiao, Y.F.; Lian, X.Z.; Sun, Y.H.; Sung, Y.C.; Vaidya, A.; Chen, Z.X.; Gupta, A.; Chatterjee, S.; Zheng, L.N.; Guerrero, E.; et al. High-density brush-shaped polymer lipids reduce anti-PEG antibody binding for repeated administration of mRNA therapeutics. Nat. Mater. 2025, 24, 1840–1851. [Google Scholar] [CrossRef] [PubMed]
- Nogueira, S.S.; Schlegel, A.; Maxeiner, K.; Weber, B.; Barz, M.; Schroer, M.A.; Blanchet, C.E.; Svergun, D.I.; Ramishetti, S.; Peer, D.; et al. Polysarcosine-Functionalized Lipid Nanoparticles for Therapeutic mRNA Delivery. ACS Appl. Nano Mater. 2020, 3, 10634–10645. [Google Scholar] [CrossRef]
- Hu, Y.L.; Hou, Y.Q.; Wang, H.; Lu, H. Polysarcosine as an Alternative to PEG for Therapeutic Protein Conjugation. Bioconjugate Chem. 2018, 29, 2232–2238. [Google Scholar] [CrossRef] [PubMed]
- Permana, Y.S.; Jang, M.; Yeom, K.; Fagan, E.; Kim, Y.J.; Choi, J.H.; Park, J.H. Ganglioside-incorporating lipid nanoparticles as a polyethylene glycol-free mRNA delivery platform. Biomater. Sci. 2025, 13, 1222–1232. [Google Scholar] [CrossRef]
- Titapiccolo, G.I.; Alexander, M.; Corredig, M. Rennet-induced aggregation of homogenized milk: Impact of the presence of fat globules on the structure of casein gels. Dairy Sci. Technol. 2010, 90, 623–639. [Google Scholar] [CrossRef]
- Hu, X.; Zhang, X.; Chen, D.; Li, N.; Hemar, Y.; Yu, B.; Tang, S.; Sun, Y. How much can we trust polysorbates as food protein stabilizers—The case of bovine casein. Food Hydrocoll. 2019, 96, 81–92. [Google Scholar] [CrossRef]
- Liu, S.; Cheng, Q.; Wei, T.; Yu, X.; Johnson, L.T.; Farbiak, L.; Siegwart, D.J. Membrane-destabilizing ionizable phospholipids for organ-selective mRNA delivery and CRISPR-Cas gene editing. Nat. Mater. 2021, 20, 701–710. [Google Scholar] [CrossRef]
- Li, Y.; Yang, T.; Yu, Y.; Shi, N.; Yang, L.; Glass, Z.; Bolinger, J.; Finkel, I.J.; Li, W.; Xu, Q. Combinatorial library of chalcogen-containing lipidoids for intracellular delivery of genome-editing proteins. Biomaterials 2018, 178, 652–662. [Google Scholar] [CrossRef]
- Zhao, X.; Glass, Z.; Chen, J.; Yang, L.; Kaplan, D.L.; Xu, Q. mRNA Delivery Using Bioreducible Lipidoid Nanoparticles Facilitates Neural Differentiation of Human Mesenchymal Stem Cells. Adv. Healthc. Mater. 2021, 10, 2000938. [Google Scholar] [CrossRef]
- Chen, L.T.; Weiss, L. The role of the sinus wall in the passage of erythrocytes through the spleen. Blood 1973, 41, 529–537. [Google Scholar] [CrossRef]
- Cheng, Q.; Wei, T.; Farbiak, L.; Johnson, L.T.; Dilliard, S.A.; Siegwart, D.J. Selective organ targeting (SORT) nanoparticles for tissue-specific mRNA delivery and CRISPR-Cas gene editing. Nat. Nanotechnol. 2020, 15, 313–320. [Google Scholar] [CrossRef] [PubMed]
- Moghimi, S.M.; Porter, C.J.H.; Muir, I.S.; Illum, L.; Davis, S.S. Non-Phagocytic Uptake of Intravenously Injected Microspheres in Rat Spleen—Influence of Particle-Size and Hydrophilic Coating. Biochem. Biophys. Res. Commun. 1991, 177, 861–866. [Google Scholar] [CrossRef] [PubMed]
- Moghimi, S.M.; Hunter, A.C.; Andresen, T.L. Factors Controlling Nanoparticle Pharmacokinetics: An Integrated Analysis and Perspective. Annu. Rev. Pharmacol. Toxicol. 2012, 52, 481–503. [Google Scholar] [CrossRef]
- Xu, M.Z.; Qi, Y.M.; Liu, G.S.; Song, Y.Q.; Jiang, X.Y.; Du, B.J. Size-Dependent Transport of Nanoparticles: Implications for Delivery, Targeting, and Clearance. Acs Nano 2023, 17, 20825–20849. [Google Scholar] [CrossRef] [PubMed]
- Braet, F.; Wisse, E.; Bomans, P.; Frederik, P.; Geerts, W.; Koster, A.; Soon, L.; Ringer, S. Contribution of high-resolution correlative imaging techniques in the study of the liver sieve in three-dimensions. Microsc. Res. Tech. 2007, 70, 230–242. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.X.; Jia, Y.B.; Huang, Y.R.; Liu, H.N.; Sun, X.M.; Cai, T.; Liu, R.T.; Xu, Z.P. Efficient delivery of clay-based nanovaccines to the mouse spleen promotes potent anti-tumor immunity for both prevention and treatment of lymphoma. Nano Res. 2021, 14, 1326–1334. [Google Scholar] [CrossRef]
- Okuda, K.; Sato, Y.; Iwakawa, K.; Sasaki, K.; Okabe, N.; Maeki, M.; Tokeshi, M.; Harashima, H. On the size-regulation of RNA-loaded lipid nanoparticles synthesized by microfluidic device. J. Control. Release 2022, 348, 648–659. [Google Scholar] [CrossRef]
- Diken, M.; Kreiter, S.; Selmi, A.; Britten, C.M.; Huber, C.; Tuereci, O.; Sahin, U. Selective uptake of naked vaccine RNA by dendritic cells is driven by macropinocytosis and abrogated upon DC maturation. Gene Ther. 2011, 18, 702–708. [Google Scholar] [CrossRef]
- Lin, X.P.; Mintern, J.D.; Gleeson, P.A. Macropinocytosis in Different Cell Types: Similarities and Differences. Membranes 2020, 10, 177. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, H.; Li, S.; Chen, K.; Yao, S.; Tang, X.; Han, X. Construction of Spleen-Accumulated Polysorbate 20-Containing Ionizable Lipid Nanoparticles for mRNA Delivery. Nanomaterials 2025, 15, 1844. https://doi.org/10.3390/nano15241844
Liu H, Li S, Chen K, Yao S, Tang X, Han X. Construction of Spleen-Accumulated Polysorbate 20-Containing Ionizable Lipid Nanoparticles for mRNA Delivery. Nanomaterials. 2025; 15(24):1844. https://doi.org/10.3390/nano15241844
Chicago/Turabian StyleLiu, Hanyu, Siqi Li, Kexin Chen, Shuyi Yao, Xuefeng Tang, and Xiaojun Han. 2025. "Construction of Spleen-Accumulated Polysorbate 20-Containing Ionizable Lipid Nanoparticles for mRNA Delivery" Nanomaterials 15, no. 24: 1844. https://doi.org/10.3390/nano15241844
APA StyleLiu, H., Li, S., Chen, K., Yao, S., Tang, X., & Han, X. (2025). Construction of Spleen-Accumulated Polysorbate 20-Containing Ionizable Lipid Nanoparticles for mRNA Delivery. Nanomaterials, 15(24), 1844. https://doi.org/10.3390/nano15241844