Abstract
Due to enhanced properties at the nanoscale, nanomaterials (NMs) have been incorporated into foods, food additives, and food packaging materials. Knowledge gaps related to (but not limited to) fate, transport, bioaccumulation, and toxicity of nanomaterials have led to an expedient need to expand research efforts in the food research field. While classical techniques can provide information on dilute suspensions, these techniques sample a low throughput of nanoparticles (NPs) in the suspension and are limited in the range of the measurement metrics so orthogonal techniques must be used in tandem to fill in measurement gaps. New and innovative characterization techniques have been developed and optimized for employment in food nano-characterization. Single particle inductively coupled plasma mass spectrometry, a high-throughput nanoparticle characterization technique capable of providing vital measurands of NP-containing samples such as size distribution, number concentration, and NP evolution has been employed as a characterization technique in food research since its inception. Here, we offer a short, critical review highlighting existing studies that employ spICP-MS in food research with a particular focus on method validation and trends in sample preparation and spICP-MS methodology. Importantly, we identify and address areas in research as well as offer insights into yet to be addressed knowledge gaps in methodology.
1. Introduction
Food is one major source of inorganic nanoparticle (NPs) exposure to consumers via the oral route/ingestion [1]. NPs that are potentially present in foods are naturally occurring or from anthropogenic origins. The latter can be divided into engineered and incidental NPs. Engineered NPs might be intentionally added to food. Currently, no engineered NPs are approved for addition to food in Europe; however, the first novel food in nanoparticulate form, iron hydroxide adipate tartrate, was recently evaluated as safe by the European Food Safety Authority [2]. Engineered NPs have the potential of release from food contact materials or can enter the food chain via the environment when they are used in other applications, e.g., construction and buildings. Incidental NPs might be formed and released during the preparation or production of food. In addition, several approved food additives such as the color additive forms of silver [3] and titanium dioxide (TiO2) [4] can release or contain a fraction of particles at the nanoscale. Despite almost three decades of intense research into the toxicology of engineered nanomaterials (NMs), the understanding of their direct impact on human health is still limited [5]. It is expected that, following oral exposure, the largest fraction of ingested NPs quickly passes through the gastrointestinal tract and is lost via fecal matter with a typical translocation to distal organs of less than 1% [5]. Due to the lack of suitable studies, it is not yet possible to conclude or even rank the toxicity of different types of NPs following oral exposure [5]. Many knowledge gaps still exist including the direct effects of NPs in food on gastrointestinal tissues and microbiota within the gastrointestinal tract [6].
The United States Food and Drug Administration (US FDA), e.g., regulates a wide range of US products including food, cosmetic products, drugs and drug formulations, devices, veterinary products, and tobacco products that may utilize nanotechnology in production or contain NMs [7]. More specifically, the incorporation of NMs into food and cosmetics is regulated by the Center for Food Safety and Applied Nutrition (CFSAN). This center is focused on improving information regarding the safety assessments for NMs in order to inform regulatory decision-making. While information is still being gathered by the US FDA to make regulatory decisions regarding safety, research has been utilized to inform uses and restrictions, and the allowable quantities of a given substance (in weight percent) when incorporated as a food additive, as well as recommendations for labeling. All this information is kept in an up-to-date database designated as the Code of Federal Regulations under Title 21—Food and Drugs [8].
The European Food Safety Authority (EFSA) has, e.g., developed specific guidelines regarding the risk assessment of NMs and small particles to be applied in the food and feed chain [9,10]. In the ongoing re-evaluation and follow-up activities regarding the safety of permitted food additives by EFSA, the presence of small particles is considered and the conventional risk assessment, if necessary, is complemented with nano-specific considerations. For example, a detailed risk assessment was performed for the food additive titanium dioxide (E171). A concern for genotoxicity could not be ruled out, and given many uncertainties, the EFSA Panel concluded that E171 could no longer be considered as safe when used as a food additive [11]. The aforementioned discussions and the following ban in Europe initiated several activities toward the analysis of E171 in foods.
Countries worldwide have examined the suitability of their regulatory frameworks for dealing with nanotechnologies in the agricultural, feed, and food sectors. The European Union (EU), along with Switzerland, was identified by Amenta et al. as the only world region where nano-specific provisions have been incorporated in existing legislation in 2015 [12]. In other regions, nanomaterials were regulated more implicitly by mainly building on guidance for industry [12]. Labeling for the presence of NMs as ingredients in food is, e.g., mandatory in the EU since December 2014 in accordance with Regulation No. 1169/2011 [12]. Regulation 1169/2011 states that all ingredients present in the form of engineered NMs shall be clearly indicated in the list of ingredients and that the names of such ingredients shall be followed by the word “nano” in brackets. EngineeredNMs are considered a novel food (if they had not been used for human consumption to a significant degree within the EU before 15 May 1997) and are covered by the novel food regulation [13]. Specific provisions for their safety assessment and authorization as food apply from 2018 onwards [13].
In the contexts of risk assessment, food labeling, and the development of novel foods, reliable detection and characterization methods for NPs in foods are needed. Studies are required to determine the level of NPs in food to allow an assessment of consumer exposure. For food control purposes, it is necessary to know whether intentionally added engineered NPs and food additives containing small particles can be distinguished from the background level of natural and incidental NPs [14].
Several analytical techniques for the characterization of NPs in food exist. The ones most frequently applied are electron microscopy (EM), asymmetric flow field-flow fractionation (AF4) coupled to inductively coupled plasma-mass spectrometry (ICP-MS), and ICP-MS in single particle mode (spICP-MS) [15,16]. Electron microscopy is considered the gold standard for the determination of particle sizes and provides additional information on particle shape, aggregation state, crystal structure, and if combined with spectroscopy, chemical composition. However, it requires special instrumentation that is not typically used in food control laboratories. AF4 faces reproducibility issues (mainly because of particle losses on the membrane) and requires experienced operators [15,16]. Single particle ICP-MS is a promising technique for the screening of food samples for the presence of metal-containing NPs, as it provides information on particle size and particle number concentration with high sensitivity and elemental specificity. Further advantages of spICP-MS are fast analysis, relatively simple sample pre-treatment, and easy implementation in state-of-the-art ICP−MS instruments, which otherwise can be used for metal analysis and speciation. There have been several reviews focusing on the topic of spICP-MS [17,18,19,20,21] discussing its principles, potential, benefits, limitations, and selected applications.
Sample preparation is a very critical step when it comes to the spICP-MS analysis of NPs in food. When using conventional sample introduction systems, i.e., pneumatic nebulizers, combined with a spray chamber, aqueous suspensions of the NPs are required. This means that the matrix of semi-solid or solid foods needs to be degraded. This can be achieved by acidic, alkaline, or enzymatic digestion. However, changes of the NPs, in particular dissolution and agglomeration, need to be avoided. For this reason, acid digestion (as used classically for elemental analysis) is usually not applied. When the aim of the analysis is to study the size of the constituent particles, the highest possible degree of de-agglomeration is desired, as spICP-MS cannot distinguish between individual particles and particles in an agglomerated state. De-agglomeration might, e.g., be achieved by applying probe sonication. However, sonication probes can release particles especially after a certain time of operation due to erosion. This might be problematic if the probe material contains the same element as the NPs present in the samples. An example is Ti-containing NPs which can be released from probes made of titanium alloy. Contamination can be reduced by using new/visually undamaged probes and monitored using procedural blanks. Another alternative is the use of indirect sonication using cup horns, vial tweeters, and similar devices.
Further, clogging of the instrument’s sample introduction system, especially the nebulizer, needs to be prevented by removing any large matrix components/residues of the matrix degradation by filtration, settling (sedimentation), or centrifugation.
A systematic literature review highlighting the determination of metallic NPs in biological samples by spICP-MS was recently performed by Laycock et al. [22]. The review included the organs or tissues collected from animals, plant tissues, and body fluids. The authors identified 83 relevant papers, with the latest search conducted in January 2021. The aim of this short review is to give an overview of existing studies where spICP-MS is used to study NPs in food additives, food, and food-relevant matrices and to identify knowledge gaps for future research.
2. Literature Search
The literature search was performed in the Web of Science database, accessed on 30 May 2023. The search commands included the terms: “SP-ICP-MS” or “SP-ICPMS” or “sp-ICPMS” or “spICPMS” or “single particle ICPMS” or “single particle ICP-MS” or “single particle inductively coupled plasma mass spectrometry” or “single particle inductively coupled plasma mass-spectrometry” and “food”, “feed”, “food additive”, “food matrix” (the characters are not case sensitive). The search was then extended to the terms: “plants”, “animals”, “tissues”, “bread”, “biscuity”, “cereal”, “pastry”, “oil”, “nut”, “margarine”, “mayonnaise”, “vinaigrette”, “salad dressing”, “egg”, “milk”, “cream”, “butter”, “yogurt”, “cheese”, “whey”, “casein”, “fruit”, “vegetables”, “legumes”, “seafood”, “seaweed”, “fish”, “mussels”, “shellfish”, “crustaceans”, “meat”, “offal”, “soft drinks”, “beverages”, “soda”, “juice”, “coffee”, “tea”, “beer”, “wine”, “bottled water”, “candy”, “confectionery”, “chewing gum”, “E171”, “E 171”, “E172”, “E 172”, “E173”, “E 173”, “E174”, “E 174”, “E551”, “E 551”. A total of 49 papers were considered relevant and will be thoroughly discussed in this short review article.
This review excluded studies of biological samples that are not typically consumed by humans. These include tissues from humans, rats, mice, whales, earthworms, amphipods, soil nematodes, etc., as well as fish intestines, liver, gills, and brains. Regarding studies on plant tissues, this review focuses on plants that are a part of the typical diet and their eatable parts. Roots of wheat plants and leaves of tomato plants, e.g., were not considered. Garden cress (Lepidium sativum) which is used for culinary seasoning is included but thale cress/mouse-ear cress (Arabidopsis thaliana) which is a model organism in plant research but without any culinary use is excluded.
It was further decided to exclude papers dealing with the analysis of NPs in all types of waters and only include drinks and beverages.
3. Studied Food Matrices and Nanoparticles
Table 1 presents an overview of the sample characteristics, sampling, and sample preparation addressed in these papers. It was decided to divide the studies based on the characteristics of the food matrix as this largely impacts sample preparation.

Table 1.
Overview of existing studies where spICP-MS is used to study NPs in food additives, food, and food-relevant matrices with focus on sample characteristics (including food matrix category, food matrix, analyzed element, assumed nanoparticle (NP) composition, reported NP shape, NP origin, and origin of the food sample), sampling (including homogenization approach, number/amount of samples analyzed), and sample preparation (including matrix degradation approach and further sample pre-treatment). Used abbreviations: BSA = bovine serum albumin, SDS = sodium dodecyl sulfate, TMAH = tetramethylammonium hydroxide (TMAH).
Many of the papers focus on protein-rich foods (Figure 1A), where the main food type is seafood (shellfish and fish), followed by sugar-rich foods (chewing gum, candies, cake decorations, and similar) and starch-/dietary fiber-rich foods. The last category is mainly comprised of leaf vegetables but also radish and seaweed. There are almost no studies on foods rich in starch, with the exceptions of wheat flour and noodles. The number of studies on fat-rich foods and emulsions is rather limited and includes salad dressing and sour cream.
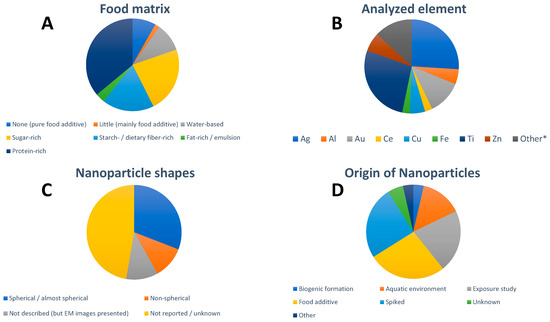
Figure 1.
Overview of (A) studied food matrix, (B) analyzed element in the NPs, (C) NP shapes, and (D) origin of the studied NPs in the 49 identified studies focusing on spICP-MS analysis of food. * The designation “Other” refers to elements that were only studied in single publications, respectively (Hg, La, Nb, Pb, Pr, Pt, Se, Si, Y). For visual aid, the entries in the pie charts appear in order in a clockwise fashion following the legend beginning at the 12 o’clock position.
The most frequently studied particle types were silver- and titanium-containing NPs (Figure 1B). The composition of NPs studied was rarely confirmed but certain assumptions were made, mainly based on the origin of the samples; whereas AuNPs and AgNPs typically contained no other elements, detected Ti was typically assumed to be TiO2, Ce to be CeO2, and Zn to be ZnO. Al, Cu, and Fe can be in NPs of various compositions. The influence of choosing a certain NP composition on NP diameter and NP mass concentration was, e.g., discussed by Vidmar et al. [14].
Screening studies looking for different elements in one food matrix are currently limited and include the investigation of Al-, Ag-, and Au-containing NPs in powders for decoration of confectionery and coated candy beads (all containing the respective food additives) [27], Ag-, Ti-, Cu-, and Zn- bearing NPs in diverse marine bivalve mollusks [52], 20 selected elements for NPs analysis in clams and oysters of which only six rare earth elements (Y, La, Ce, Pr, Nd, Gd) were detected [54] and eight elements (Ag, Al, Cr, Cu, Fe, Si, Ti, Zn) in 13 food products [14].
All presented studies assume spherical NPs when calculating particle size. Many publications (17 out of 49) report that the studied NPs were in fact spherical or near/almost spherical shaped (Figure 1C). Approximately half of the papers did not provide information highlighting NP shape. In some cases, electron microscopy images were presented but the shapes were neither described nor discussed. Some studies reported clear deviations from a spherical shape, including irregular, ellipsoidal, polygonal, rod-, and flake-like shapes as well as fractal aggregate structures. The relation between the obtained sizes by spICP-MS and the geometric size of the NPs was not always discussed.
The origin of the studied NPs was dominated by the NPs present in food additives, followed by spiking experiments and exposure studies with engineered NPs (Figure 1D). Studies on seafood/aquatic organisms most often referred to the presence of NPs in the aquatic environment, which can be either naturally occurring or anthropogenic NPs (including incidental and engineered NPs) [51]. Otherwise, studies focusing on naturally occurring NPs in food were missing. More “exotic cases” were biogenically formed NPs and included selenium NPs in bacterial strains used in the dairy industry [29] and mercury selenide NPs in fish and mollusks [67], lead NPs in game meat following the use of lead-containing bullets [64] and zinc oxide NPs migrating from a food contact material into chicken breast [63]. In some studies, the origin of the NPs was unknown but possible sources were discussed [14,39].
Thus far, the following food additives were studied: titanium dioxide (E171), iron oxides and hydroxides (E172), aluminum (E173), silver (E174), gold (E175), silicon dioxide (E551), and mica (potassium aluminum silicate (E555) with the focus being on titanium dioxide (13 articles). Titanium dioxide (E171) was studied as pure food additive but also incorporated in candies and chewing gums (coating), crab sticks, and salad dressing as a color. Food additive silver (E174) was investigated in five publications both as a pure additive and in candies/pastry decoration. Food additive gold (E175) was only studied in one paper where no NPs could be detected [27]. The absence of NPs in food additive gold was confirmed in a scientific report to the EFSA [69].
For iron oxides and hydroxides (E172), aluminum (E173), silicon dioxide (E551), and mica (E555), only one paper each exists [14] (E172 and E551), [27] (E173 and E555). None of the papers presented results related to method validation, as will be discussed in detail in Section 6.
4. Sample Collection and Sample Preparation
As seen in Figure 2A, a majority of the samples in the studies we highlight here were procured from local stores and markets, with a small size of samples originating from national control programs and online stores. In studies where sugar-rich foods such as candies were analyzed, sample sizes varied greatly; either single candy pieces from a package or batch (12 studies) were prepared or candies were pooled together to reach a critical mass of sample (eight studies). For vegetative samples, common practices included analysis of 1 g of dry leaves or ground plant tissues; however, there were also instances where individual leaves were cut into smaller squares. For meat products, 0.5 g samples were the preferred and most prevalently used sample weight for sample treatment and subsequent analysis. With regard to seafood samples, either dry or wet homogenized samples of 0.5 g or 1 g were prepared and analyzed. As far as the pure food additives themselves, sample sizes as small as 3.5 mg were used as these are typically rich in metal content.
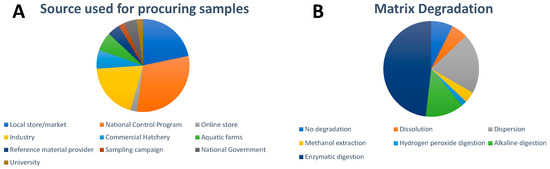
Figure 2.
Overview of (A) sources used for procuring samples and (B) matrix degradation approach used for the spICP-MS analysis of inorganic NPs in food. For visual aid, the entries appear in order in a clockwise fashion following the legend beginning at the 12 o’clock position.
Regarding homogenization approaches, there is no standard, agreed-upon protocol among the studies. Physical homogenization techniques were rarely employed for candy-type samples, but efforts of pooling samples were often used (i.e., pooling of chewing gum samples). Vegetative sample types underwent manual grinding and/or probe sonication procedures. In isolated studies, meat product samples underwent cryo-milling protocols to produce a slurry mix. Seafood samples underwent mechanical blending of large masses of sample (1 kg) and in some cases freeze-drying was employed. Overall, the homogenization procedures utilized in the studies herein were highly unreported among all of the research papers.
No matrix degradation (Figure 2B) was used in the case of pure food additives and liquid-based food such as drinks. In the latter case, simple dilution of the samples is usually applied. Dissolution of the matrix, typically in ultrapure water, was commonly applied for sugar-rich foods. Dispersion of the matrix with the help of sonication was only used in a few cases, e.g., for a salad dressing. NPs in protein-rich foods such as seafood and meat were either extracted by alkaline digestion with tetramethylammonium hydroxide (TMAH) or enzymatic digestion. Proteinase K, a broad-spectrum serine protease, was often used for protein-rich foods such as meat, milk, and seafood. A mixture of pancreatin and lipase has been applied to seafood in several works due to its fat content. An extensive comparison of existing enzymatic and alkaline digestion protocols for bivalve mollusks was performed by Sun et al. [51] which concluded that the optimal extraction was based on the employment of TMAH. Enzymatic digestion with Macerozyme R-10, a mixture of pectinase, cellulase, and hemicellulose, is often utilized for plant-based foods, e.g., lettuce and radish, as well as seaweed. The enzyme α-amylase has been applied for the detection of NPs in noodles and wheat flour. One group developed a methanol extraction procedure for the determination of Au, CuO, and ZnO NPs in plant leaf materials (lettuce, corn, and kale) after an enzyme-based approach with Macerozyme R-10 caused changes in the recovered size distribution of CuONPs [42]. One article described the use of hydrogen peroxide digestion [34]. Following matrix degradation, further sample preparation steps can include filtration, settling, centrifugation, and dilution with surfactant-containing solutions. The influence of these steps on the obtained size distributions and recoveries is rarely investigated or discussed.
5. Analytical Approaches
Table 2 presents an overview of the main experimental parameters, calibration, and data analysis approaches described in the 49 selected papers reviewed herein. The references appear in the same order as Table 1.

Table 2.
Overview of existing studies where spICP-MS is used to study NPs in food additives, food, and food-relevant matrices wit focus on experimental parameters (including instrument, sample introduction system, nebulizer, spray chamber, sample uptake, optimization of operating conditions, dwell time, analysis time, type of element/isotope detection, rinsing procedure), calibration (including type of used nanoparticle (NP) calibration standard, transport efficiency method), and data analysis. Used abbreviations: PEEK = polyether ether ketone, PEG = polyethylene glycol, PFA = perfluoroalkoxy, PVP = polyvinylpyrrolidone, SPCT = single particle calculation tool.
With regard to the ICP-MS instrumentation platforms (Figure 3A), single quadrupole ICP-MS are the mass analyzers most widely used for single particle analysis in this emerging field (33 out of 49 papers) because of their comparatively low cost, higher robustness, and capability for fast NP detection. However, since single quadrupole instruments are sequential analyzers, only one m/z can be monitored at a time, limiting their multielemental detection and resolution. The capability of triple quadrupole technology allows for overcoming matrix interference in NP analysis and has been utilized in 12 out of the 49 selected papers, with half of them reporting the determination of metal oxides NPs, mostly TiO2, and the other half highlighting the analysis of AgNPs. Although double-focusing or sector field ICP-MS, when operating at low-resolution mode, can achieve higher sensitivity than quadrupole ICP-MS because of its geometry, this kind of instrumentation has been rarely used in the analysis of inorganic NPs in food to this date (two out of 49 papers). Interestingly, Noireaux et al. compared the performances of both high-resolution ICP-MS and triple quadrupole ICP-MS to characterize TiO2 NPs in different food products, concluding that double-focusing ICP-MS was able to detect smaller NPs than triple quadrupole ICP-MS [31]. While significant improvements developed within the last decade have increased the interest to revisit the use of time-of-flight ICP-MS for spICP-MS analysis, this trend has not translated yet into the analysis of inorganic NPs in food additives and food. In fact, only one study reported the use of time-of-flight ICP-MS technology for the quasi-simultaneous multi-elemental detection of Au, CuO, and ZnO NPs extracted from three different plant leaf materials (lettuce, corn, and kale) [42].
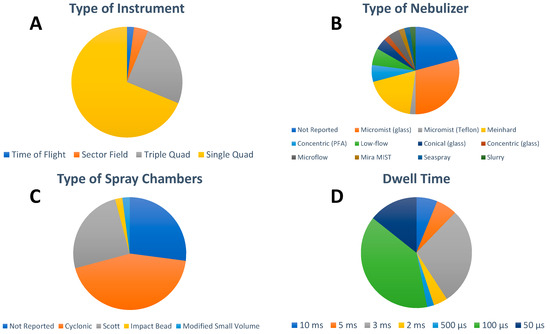
Figure 3.
Overview of (A) type of instrument, (B) type of nebulizer, (C) type of spray chamber, and (D) detector dwell time used for the spICP-MS analysis of inorganic NPs in food. For visual aid, the entries in the pie charts appear in order in a clockwise fashion following the legend beginning at the 12 o’clock position.
Typical sample introduction systems for the spICP-MS analysis of inorganic NP in food additives and food consists of a wide range of pneumatic nebulizers (Figure 3B) in combination with different spray chambers (Figure 3C) resulting in transport efficiencies in the range of 1% to 10% by applying sample flow rates of 0.1 mL min−1 to 1 mL min−1 (Table 2). Optimization of the operating conditions was largely unreported, with manual daily tuning for maximum sensitivity of the isotope(s) of interest carried out in a small fraction of the publications (12 out of 49) and autotune procedure reported in one case [55]. In spICP-MS, data acquisition was traditionally performed with millisecond dwell times in the past while a time resolution of hundreds of microseconds has been used more often in the last five to seven years. This trend is clearly manifested in the food analysis field where 27 papers reported the use of microsecond dwell times and the remaining 22 papers operated at millisecond dwell times (Figure 3D). The analysis time reported ranged from 60 s to 300 s, with 60 s being preferred by most of the laboratories (31 out of 49). As discussed above, the dominating use of sequential single quadrupole ICP-MS in this field clearly correlates with the fact that single element detection per analysis time was reported by 47 of the papers. Only two exceptions reported dual isotope detection [36] and simultaneous multi-elemental detection [42]. Rinsing procedures were highly unreported, with a sequence of diluted acid mixtures and surfactants or 2% nitric acid (v/v) being the most used approaches.
Overall, provided that there is suitable calibration of the instrument sensitivity, sample uptake rate, and transport efficiency (defined as the fraction of introduced sample that is transported to the plasma), spICP-MS allows the ability to obtain simultaneous information on both the number of NPs and the mass of the analyte per NP after a very short analysis time using off-the-shelf ICP-MS instruments. Calibration, sizing, and quantification strategies were summarized and described in detail in several reviews [17,18,19,20,21], so these factors will be only outlined below. Briefly speaking, when using pneumatic nebulizers, spICP-MS calibration is typically performed using NP standards of the same elemental composition and/or dissolved standard solutions of the element after taking into account the measure of the transport efficiency. This approach was reported in all 49 papers selected in this review.
With regard to the chemical composition of the NP used as calibration standards, AuNPs were reported in 43 papers and AgNPs in the remaining six papers. Monodispersed National Institute of Standards and Technology (NIST) Reference Material (RM) 8012 (nominal size 30 nm [70] and RM 8013 (nominal size 60 nm [71]) citrate-stabilized AuNPs have been widely adopted as calibration standards for spICP-MS in general, and for the spICP-MS analysis of inorganic NPs in food in particular. As illustrated in Figure 4A, NIST RM AuNPs were selected as calibration standards for 23 papers published between 2013 and 2021. Their prevalent use for spICP-MS calibration can be explained because of their well-defined and thoroughly characterized mean size, size distribution, and Au mass fraction, as well as their homogeneity and stability. Unfortunately, both NIST RM AuNPs have been out of stock since 2017. To date, the Quality Control Material LGCQC5050 Colloidal citrate-stabilized AuNPs (nominal diameter 30 nm), issued on February 2019 [72], has been used in one publication in this field [63]. In fact, the scarcity of appropriate NP RMs for spICP-MS calibration resulted in the wide use of commercially available NP suspensions of different sizes and coatings in 26 out of 49 papers (Figure 4A) for spICP-MS calibration. However, the accuracy of spICP-MS calibration based on commercial NPs can be compromised since value assignments provided by manufacturers are typically limited with respect to the number of NPs analyzed, just 100 NPs or so, and a more thorough in-house characterization is required [73]. Unfortunately, these required in-house characterization efforts for NP suspensions selected as calibration standards were rarely reported in the publications included in this review. Surprisingly, commercially available NPs, with very limited characterization information from the vendor, were often erroneously referred to as NP RMs, which are defined as materials, sufficiently homogeneous and stable with respect to one or more specified properties, which have been established to be fit for its intended use in a measurement process [74].
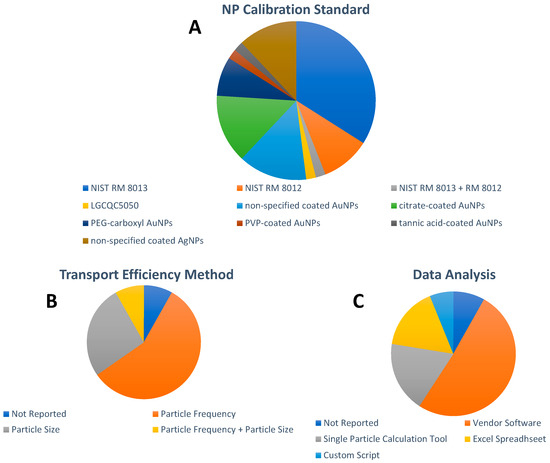
Figure 4.
Overview of (A) NP calibration standards, (B) transport efficiency method, and (C): data analysis approach. For visual aid, the entries in the pie charts appear in order in a clockwise fashion following the legend beginning at the 12 o’clock position.
Two of the most popular approaches used in the spICP-MS analysis of inorganic NPs in food are to calculate transport efficiency, specifically employing the particle frequency and the particle size methods outlined by Pace et al. [75]. Both approaches require measurement of the sample flow, analysis of dissolved standard solutions (size method), and analysis of a standard NP suspension (i.e., NP RM) of known size and mass concentration or particle number concentration. It can be seen in Figure 4B that while the method for the calculation of the transport efficiency was not reported in four papers, the particle frequency method was the method of choice in 28 papers, followed by the particle size method in 13 papers.
Calculation of the transport efficiency using both methods was reported in four papers. Surprisingly, the dynamic mass flow, an indirect NP RM free method that solely relies on continuous mass measurements of the waste and sample uptake over time in a well-equilibrated ICP-MS, proposed by Cuello-Nuñez et al. in 2020 [76] has yet to be implemented in this field.
Due to the high time resolution, large data sets are generated in spICP-MS analysis and data reduction can be cumbersome. Aiming at automated data reduction, different strategies for increasing the sophistication of data processing have developed over the past decade, as illustrated in Figure 4C. For millisecond dwell time resolution, as particle events are detected as discrete pulses, datasets can be more easily handled by using simple algorithms implemented in spreadsheets such as the single particle calculation tool (SPCT) [77], used in nine papers, or spreadsheets developed in-house as employed in eight papers. For microsecond dwell time analysis, NPs are recorded as peaks that require more complex algorithms and software. Thus, proprietary software has been developed by most instrument manufacturers, allowing relatively easy processing of the acquired data; this is the preferred choice for data processing of spICP-MS analysis of NPs in food with a total of 25 papers. Alternatively, two different groups have also reported the development of custom scripts as data analysis tools in this field [42,44,67].
6. Method Validation
In this section, validation of spICP-MS for the analysis of inorganic NP in food additives and food will be discussed through a selection of classical analytical figures of merit. Thus, Table 3 includes detailed information (where applicable) about the limit of detection and quantification, repeatability, reproducibility, linear range, and trueness of spICP-MS in this arena. On Table 3, references appear in the same order as Table 1 and Table 2.

Table 3.
Overview of existing studies where spICP-MS is used to study NPs in food additives, food, and food-relevant matrices with focus on analytical figures of merit for method validation (including limit of detection (LOD) and limit of quantification (LOQ) for size, LOD/LOQ for mass and number concentration, size linear range, repeatability, reproducibility, trueness of size and concentration). Use abbreviations: AF4-ICP-MS = asymmetric flow-field flow fractionation coupled to inductively coupled plasma-mass spectrometry, AAS = atomic absorption spectroscopy, CLS = centrifugal liquid sedimentation, DLS = dynamic light scattering, SEM = Scanning electron microscopy, TEM = Transmission electron microscopy.
The size detection limit (LOD) was the figure of merit most often reported, for a total of 36 papers. While the size LOD is typically defined as the smallest ion burst which can be distinguished as a particle event, details on how it is mathematically calculated are not often provided. In spICP-MS, size LOD varies between NPs of different chemical compositions (i.e., its sensitivity, potential spectral interferences, and stoichiometry of NP) and the concentration of the dissolved analyte in the sample. Smallest-size LODs reported in this field ranged from 9 nm for Ag, 15 nm for Au, 18 nm for Se, 21 nm for HgSe, 26 nm for ZnO, 27 nm for TiO2, 35 nm for Fe2O3, 37 nm for Al2O3, 42 nm for CuO, 43 nm for Pb, and 89 nm for SiO2; these size LODs are comparable to values reported in general spICP-MS literature. In fact, measured NP diameters for smaller NP materials, reported in Table 1, are often very close to the reported size LODs, whose measured signals are at the method LOD and subject to high uncertainty. Interestingly, a clear correlation between smaller size LOD and the popularity of spICP-MS in measuring the size distribution of inorganic NPs, presented in Section 3, could not be established. Surprisingly, the size quantification limit (LOQ) was only reported by Waegeneers et al. for the analysis of AgNPs in confectionary [23].
One of the most important strengths of spICP-MS is its superb detection capability in terms of NP mass or number concentration comparable to NP concentrations in real-world environmental samples (on the order of ng L−1). However, mass concentration and particle number concentration LODs were reported to a lower extent with a total of 12 and 13 papers, respectively, with values in the ng L−1, and 105 to 107 L−1, respectively. Table 3 also shows the very limited information regarding the size linear dynamic range of NPs detectable in different food samples by spICP−MS, about one order of magnitude in particle diameter, reported in only two studies.
The assessment of the precision for the determination of particle size, mass concentration, and number concentration of inorganic NPs in food by spICP-MS was expressed in terms of repeatability and/or reproducibility, as displayed in Table 3. Repeatability for the measurement of particle size was reported in 14 papers and for mass and/or number concentration in 12 papers, while reproducibility was provided only in eight studies for particle size and four for mass concentration and/or number concentration. In short, results for the determination of median/mean particle diameter (1% to 10% repeatability standard deviation (SD), and 5% to 25% reproducibility SD) were more repeatable and reproducible compared to the determination of particle mass concentration or number concentration (2% to 47% repeatability SD and 7.5% to 90% reproducibility SD). It is important to highlight that the term reproducibility was erroneously used in the selected single laboratory studies. In fact, the intermediate precision was the actual indicator of precision, which is considered the most practically realizable estimate that can be achieved without evaluating a material in an interlaboratory study [78].
A summary of the trueness associated with the determination of particle size, mass concentration, and number concentrations, is also presented in the last two columns of Table 3. Information on the trueness of particle size measurements was largely reported, with a total of 40 papers, ranging from 60% to 116%. In practice, the most adopted approach for the evaluation of the trueness of particle size was through confirmation by Transmission Electron Microscopy (TEM) or Scanning Electron Microscopy (SEM) data either provided by the manufacturer, previously published, or acquired in-house. Alternatively, a comparison of electron microscopy results with spICP-MS size results of pristine NP suspensions used for spiking was performed in nine cases. The trueness of particle mass and/or number concentration was also largely reported, with a total of 32 papers, ranging from 12% to 127%. This larger bias compared to particle size can be understood because of the more challenging particle mass and number concentration measurements using spICP-MS (already discussed).
The comparison with expected concentration values, provided by manufacturers or RM producers, was the most used approach (15 papers), followed by the comparison with total concentration with conventional ICP-MS after acid digestion (12 papers).
While several in-house validation research projects have been developed at a single laboratory level [26,28,32,37,42,61], at this point it is necessary to highlight the publication of two international interlaboratory studies [24,62] that have established how well spICP-MS performs for the analysis of Ag and TiO2 NPs in food matrices. Under the framework of the NanoLyse project, Weigel et al. [62] organized the first interlaboratory comparison in this field for the size determination and quantification of AgNPs in chicken meat. In this interlaboratory study, results indicated greater variability in the particle number quantification than in the size characterization, yielding non-quantitative particle number concentration recoveries. These results could stem from numerous factors including the lack of stability of the NPs in initial suspensions and different matrices depending on the handling and storage conditions. Moreover, Geiss et al. [24] performed an interlaboratory comparison for the spICP-MS analysis of pristine TiO2 food additive E171, and in two types of confectionary products involving seven experienced participants. While spICP-MS resulted in significantly larger mean and median particle diameter than TEM, due to higher particle size LOD for spICP-MS and the difficulty of overcoming agglomeration in the sample preparation, the authors concluded that the study provided a good evaluation of spICP-MS practical validation.
7. Overview and Future Perspectives
This review identified several knowledge gaps in the field of spICP-MS analysis of NPs in food. In general, more screening studies are required to determine the background level of natural and incidental NPs in other food groups than seafood, e.g., fruits and vegetables. Single particle ICP-MS appears to be the ideal technique for it. The applicability of spICP-MS for the characterization of additional food additives other than titanium dioxide and silver should be investigated, e.g., iron oxides and hydroxides (E172) and aluminum (E173). Sample preparation procedures for foods with high starch and fat contents should be developed to investigate, e.g., the presence of inorganic NPs in bread or vegetable oils. The presence of NPs in feed and feed additives could be investigated with spICP-MS as well. Existing good practices of sampling that consider sample homogeneity and representativeness of subsamples should be applied for NP analysis by spICP-MS in the same way as for other methods.
Food additive SiO2 (used as an anti-clumping agent in powdered food products and a stabilizer in the production of beer ([79] accessed on 12 June 2023, [80] accessed on 12 June 2023)) is considered to be less toxic, especially at the allowable limit of 2% by weight; however, nanosized SiO2 may be created in the incorporation process and the toxicological effects then become unclear [80,81]. Hence, the toxicological effects of SiO2 are still under investigation. Research has been recently conducted toward the multi-technique characterization of food additive/food grade SiO2, with spICP-MS being employed as a main characterization avenue [82]. Although detection of Si is hampered heavily by isobaric interferences at m/z 28, this can be overcome through optimization of ICP-MS operating conditions (cold plasma and microsecond dwell time acquisition; Khan et al. (in preparation)) or switching to sector field ICP-mass spectrometers. Similar to the metal NPs discussed in this review, as more optimal spICP-MS methodologies present themselves toward the measurement and characterization of SiO2 in foods and food additive materials, more results can be gathered to inform regulatory decisions. This is also the case for the emergence of nano- and microplastics in the food industry.
Ideally, spICP-MS studies should always be supplemented with additional techniques to determine particle shape and composition. If this is not possible, limitations of spICP-MS should be communicated more clearly in publications when it comes to the assumptions that are made when determining particle size. Particle sizes could be presented as mass-equivalent sizes to highlight that the NP shape is either nonspherical or unknown. Considerations about the influence of NP composition and density on the NP size and mass concentration should be presented.
It is necessary to highlight that a general trend for using conventional spICP-MS experimental set-ups for the analysis of inorganic NPs in food additives and food is clearly prevalent. This can be explained due to the predominant application focus of the selected publications in this emerging field. It is expected to experience a gradual incorporation of alternative ICP-MS platforms, other than the single quadrupole instruments currently barely used in this field, over the course of the next years. Whereas higher sensitivity of double-focusing or sector field ICP-MS instruments will result in the lowering of the size detection limits, the trending use of time-of-flight ICP-MS will open the door to the identification, quantification, and classification of NPs of unknown sources based on their multielement fingerprint (i.e., engineered, incidental, and natural NPs).
A substantial collective effort should be made to report the optimization of the operating conditions and rinsing procedures that are largely unreported currently, to minimize this knowledge gap and to enable the transferability of measurement procedures more consistently across laboratories.
While the shortage of suitable NP RMs is not only an exclusive issue for spICP-MS but for any analytical technique for the characterization of NPs, it can be considered a major issue and current limitation affecting the accuracy of spICP-MS calibration, and sizing and quantification results of spICP-MS in general and in the food analysis field in particular. To overcome this lack of appropriate NP RMs, commercially available NP suspensions of different sizes and coatings are typically used instead. However, value assignments provided by the manufacturers have been demonstrated to be very limited with respect to the number of NPs analyzed, and therefore, a more thorough in-house characterization of commercial NP suspensions is required prior to their reliable use as calibration standards as recently outlined in [73,83]. This challenging task is currently considered an unexplored territory towards significant progress from the spICP-MS community in the food analysis field.
Unlike main experimental parameters, calibration, and data analysis approaches, information on the key analytical figures of merit for spICP-MS validation was generally unreported among all the selected studies. From the two interlaboratory studies and from the several in-house validation publications, two main remarks can be extracted with regard to spICP-MS validation in food analysis. While spICP-MS tends to perform reasonably well for the characterization of the central tendency of NP size distributions, important challenges remain in obtaining accurate and consistent particle number concentration measurements. The less than reliable number concentration results are related, but not limited to several factors: inaccurate calibration of transport efficiency, instability of NPs after extraction from food matrices, poor performance with regard to elemental sensitivity (leading to incorrect element responses factors), and loss of particles to the surface of the sample introduction system or the sidewalls walls of sample containers.
Author Contributions
Conceptualization, K.L., M.E.J. and A.R.M.B.; writing—original draft preparation, K.L., M.E.J. and A.R.M.B.; writing—review and editing, K.L., M.E.J. and A.R.M.B. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Data Availability Statement
Not applicable.
Acknowledgments
The authors would like to thank Carolyn Q. Burdette (Chemical Sciences Division, NIST) and Diana L. Ortiz-Montalvo (Materials Measurement Science Division, NIST) for their thorough review of the manuscript. Certain commercial products or equipment are described in this paper in order to specify adequately the experimental procedure. In no case does such identification imply recommendation or endorsement by the National Institute of Standards and Technology, nor does it imply that it is necessarily the best available for the purpose.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Tschiche, H.R.; Bierkandt, F.S.; Creutzenberg, O.; Fessard, V.; Franz, R.; Greiner, R.; Gruber-Traub, C.; Haas, K.-H.; Haase, A.; Hartwig, A.; et al. Analytical and Toxicological Aspects of Nanomaterials in Different Product Groups: Challenges and Opportunities. NanoImpact 2022, 28, 100416. [Google Scholar] [CrossRef]
- Turck, D.; Bohn, T.; Castenmiller, J.; De Henauw, S.; Ildico Hirsch-Ernst, K.; Maciuk, A.; Mangelsdorf, I.; McArdle, H.J.; Naska, A.; Pelaez, C.; et al. Safety of Iron Hydroxide Adipate Tartrate as a Novel Food Pursuant to Regulation (EU) 2015/2283 and as a Source of Iron in the Context of Directive 2002/46/EC. EFSA J. 2021, 19, e06935. [Google Scholar] [CrossRef] [PubMed]
- De Vos, S.; Waegeneers, N.; Verleysen, E.; Smeets, K.; Mast, J. Physico-Chemical Characterisation of the Fraction of Silver (Nano)Particles in Pristine Food Additive E174 and in E174-Containing Confectionery. Food Addit. Contam. Part A 2020, 37, 1831–1846. [Google Scholar] [CrossRef] [PubMed]
- Geiss, O.; Ponti, J.; Senaldi, C.; Bianchi, I.; Mehn, D.; Barrero, J.; Gilliland, D.; Matissek, R.; Anklam, E. Characterisation of Food Grade Titania with Respect to Nanoparticle Content in Pristine Additives and in Their Related Food Products. Food Addit. Contam. Part A 2020, 37, 239–253. [Google Scholar] [CrossRef] [PubMed]
- Kermanizadeh, A.; Cassee, F.R.; de Jong, W. The Potential Adverse Effects of Engineered Nanomaterial Exposure. In Molecular and Integrative Toxicology; Springer Science and Business Media B.V.: Berlin/Heidelberg, Germany, 2021; pp. 41–58. [Google Scholar]
- Bouwmeester, H.; van der Zande, M.; Jepson, M.A. Effects of Food-borne Nanomaterials on Gastrointestinal Tissues and Microbiota. WIREs Nanomed. Nanobiotechnol. 2018, 10, e1481. [Google Scholar] [CrossRef] [PubMed]
- Nanotechnology Programs at FDA. Available online: https://www.fda.gov/science-research/science-and-research-special-topics/nanotechnology-programs-fda (accessed on 29 May 2023).
- CFR—Code of Federal Regulations Title 21. Available online: https://www.accessdata.fda.gov/scripts/cdrh/cfdocs/cfcfr/CFRSearch.cfm?CFRPart=73 (accessed on 31 May 2023).
- More, S.; Bampidis, V.; Benford, D.; Bragard, C.; Halldorsson, T.; Hernández-Jerez, A.; Hougaard Bennekou, S.; Koutsoumanis, K.; Lambré, C.; Machera, K.; et al. Guidance on Risk Assessment of Nanomaterials to Be Applied in the Food and Feed Chain: Human and Animal Health. EFSA J. 2021, 19, 6768. [Google Scholar] [CrossRef]
- More, S.; Bampidis, V.; Benford, D.; Bragard, C.; Halldorsson, T.; Hernández-Jerez, A.; Bennekou, S.H.; Koutsoumanis, K.; Lambré, C.; Machera, K.; et al. Guidance on Technical Requirements for Regulated Food and Feed Product Applications to Establish the Presence of Small Particles Including Nanoparticles. EFSA J. 2021, 19, 6769. [Google Scholar] [CrossRef]
- Younes, M.; Aquilina, G.; Castle, L.; Engel, K.; Fowler, P.; Frutos Fernandez, M.J.; Fürst, P.; Gundert-Remy, U.; Gürtler, R.; Husøy, T.; et al. Safety Assessment of Titanium Dioxide (E171) as a Food Additive. EFSA J. 2021, 19, 6585. [Google Scholar] [CrossRef]
- Amenta, V.; Aschberger, K.; Arena, M.; Bouwmeester, H.; Botelho Moniz, F.; Brandhoff, P.; Gottardo, S.; Marvin, H.J.P.; Mech, A.; Quiros Pesudo, L.; et al. Regulatory aspects of nanotechnology in the agri/feed/food sector in EU and non-EU countries. Regul. Toxicol. Pharmacol. 2015, 73, 463–476. [Google Scholar] [CrossRef]
- Rauscher, H.; Rasmussen, K.; Sokull-Klüttgen, B. Regulatory Aspects of Nanomaterials in the EU. Chem. Ing. Tech. 2017, 89, 224–231. [Google Scholar] [CrossRef]
- Vidmar, J.; Hässmann, L.; Loeschner, K. Single-Particle ICP–MS as a Screening Technique for the Presence of Potential Inorganic Nanoparticles in Food. J. Agric. Food Chem. 2021, 69, 9979–9990. [Google Scholar] [CrossRef] [PubMed]
- Mattarozzi, M.; Suman, M.; Cascio, C.; Calestani, D.; Weigel, S.; Undas, A.; Peters, R. Analytical Approaches for the Characterization and Quantification of Nanoparticles in Food and Beverages. Anal. Bioanal. Chem. 2017, 409, 63–80. [Google Scholar] [CrossRef] [PubMed]
- Correia, M.; Verleysen, E.; Loeschner, K. Analytical Challenges and Practical Solutions for Enforcing Labeling of Nanoingredients in Food Products in the European Union. In Nanomaterials for Food Applications; Elsevier: Amsterdam, The Netherlands, 2019; pp. 273–311. [Google Scholar] [CrossRef]
- Montaño, M.D.; Olesik, J.W.; Barber, A.G.; Challis, K.; Ranville, J.F. Single Particle ICP-MS: Advances toward Routine Analysis of Nanomaterials. Anal. Bioanal. Chem. 2016, 408, 5053–5074. [Google Scholar] [CrossRef] [PubMed]
- Meermann, B.; Nischwitz, V. ICP-MS for the Analysis at the Nanoscale—A Tutorial Review. J. Anal. At. Spectrom. 2018, 33, 1432–1468. [Google Scholar] [CrossRef]
- Mozhayeva, D.; Engelhard, C. A Critical Review of Single Particle Inductively Coupled Plasma Mass Spectrometry—A Step towards an Ideal Method for Nanomaterial Characterization. J. Anal. At. Spectrom. 2020, 35, 1740–1783. [Google Scholar] [CrossRef]
- Resano, M.; Aramendía, M.; García-Ruiz, E.; Bazo, A.; Bolea-Fernandez, E.; Vanhaecke, F. Living in a Transient World: ICP-MS Reinvented via Time-Resolved Analysis for Monitoring Single Events. Chem. Sci. 2022, 13, 4436–4473. [Google Scholar] [CrossRef]
- Laborda, F.; Abad-Álvaro, I.; Jiménez, M.S.; Bolea, E. Catching Particles by Atomic Spectrometry: Benefits and Limitations of Single Particle—Inductively Coupled Plasma Mass Spectrometry. Spectrochim. Acta Part B At. Spectrosc. 2023, 199, 106570. [Google Scholar] [CrossRef]
- Laycock, A.; Clark, N.J.; Clough, R.; Smith, R.; Handy, R.D. Determination of Metallic Nanoparticles in Biological Samples by Single Particle ICP-MS: A Systematic Review from Sample Collection to Analysis. Environ. Sci. Nano 2022, 9, 420–453. [Google Scholar] [CrossRef]
- Waegeneers, N.; De Vos, S.; Verleysen, E.; Ruttens, A.; Mast, J. Estimation of the Uncertainties Related to the Measurement of the Size and Quantities of Individual Silver Nanoparticles in Confectionery. Materials 2019, 12, 2677. [Google Scholar] [CrossRef]
- Geiss, O.; Bianchi, I.; Senaldi, C.; Bucher, G.; Verleysen, E.; Waegeneers, N.; Brassinne, F.; Mast, J.; Loeschner, K.; Vidmar, J.; et al. Particle Size Analysis of Pristine Food-Grade Titanium Dioxide and E 171 in Confectionery Products: Interlaboratory Testing of a Single-Particle Inductively Coupled Plasma Mass Spectrometry Screening Method and Confirmation with Transmission Electron Micr. Food Control 2021, 120, 107550. [Google Scholar] [CrossRef]
- Helsper, J.P.F.G.; Peters, R.J.B.; van Bemmel, M.E.M.; Rivera, Z.E.H.; Wagner, S.; von der Kammer, F.; Tromp, P.C.; Hofmann, T.; Weigel, S. Physicochemical Characterization of Titanium Dioxide Pigments Using Various Techniques for Size Determination and Asymmetric Flow Field Flow Fractionation Hyphenated with Inductively Coupled Plasma Mass Spectrometry. Anal. Bioanal. Chem. 2016, 408, 6679–6691. [Google Scholar] [CrossRef]
- Verleysen, E.; Waegeneers, N.; Brassinne, F.; De Vos, S.; Jimenez, I.O.; Mathioudaki, S.; Mast, J. Physicochemical Characterization of the Pristine E171 Food Additive by Standardized and Validated Methods. Nanomaterials 2020, 10, 592. [Google Scholar] [CrossRef] [PubMed]
- Laborda, F.; Gimenez-Ingalaturre, A.C.; Bolea, E.; Castillo, J.R. Single Particle Inductively Coupled Plasma Mass Spectrometry as Screening Tool for Detection of Particles. Spectrochim. Acta Part B At. Spectrosc. 2019, 159, 105654. [Google Scholar] [CrossRef]
- Witzler, M.; Küllmer, F.; Hirtz, A.; Günther, K. Validation of Gold and Silver Nanoparticle Analysis in Fruit Juices by Single-Particle ICP-MS without Sample Pretreatment. J. Agric. Food Chem. 2016, 64, 4165–4170. [Google Scholar] [CrossRef] [PubMed]
- Kantorová, V.; Krausová, G.; Hyršlová, I.; Loula, M.; Mestek, O.; Kaňa, A. Determination of Selenium Nanoparticles in Fermented Dairy Products. Spectrochim. Acta Part B At. Spectrosc. 2023, 199, 106592. [Google Scholar] [CrossRef]
- Li, B.; Chua, S.L.; Yu, D.; Chan, S.H.; Li, A. Detection, Identification and Size Distribution of Silver Nanoparticles (AgNPs) in Milk and Migration Study for Breast Milk Storage Bags. Molecules 2022, 27, 2539. [Google Scholar] [CrossRef]
- Noireaux, J.; López-Sanz, S.; Vidmar, J.; Correia, M.; Devoille, L.; Fisicaro, P.; Loeschner, K. Titanium Dioxide Nanoparticles in Food: Comparison of Detection by Triple-Quadrupole and High-Resolution ICP-MS in Single-Particle Mode. J. Nanoparticle Res. 2021, 23, 102. [Google Scholar] [CrossRef]
- de la Calle, I.; Menta, M.; Klein, M.; Séby, F. Study of the Presence of Micro- and Nanoparticles in Drinks and Foods by Multiple Analytical Techniques. Food Chem. 2018, 266, 133–145. [Google Scholar] [CrossRef]
- Candás-Zapico, S.; Kutscher, D.J.; Montes-Bayón, M.; Bettmer, J. Single Particle Analysis of TiO2 in Candy Products Using Triple Quadrupole ICP-MS. Talanta 2018, 180, 309–315. [Google Scholar] [CrossRef]
- Peters, R.J.B.; van Bemmel, G.; Herrera-Rivera, Z.; Helsper, H.P.F.G.; Marvin, H.J.P.; Weigel, S.; Tromp, P.C.; Oomen, A.G.; Rietveld, A.G.; Bouwmeester, H. Characterization of Titanium Dioxide Nanoparticles in Food Products: Analytical Methods To Define Nanoparticles. J. Agric. Food Chem. 2014, 62, 6285–6293. [Google Scholar] [CrossRef]
- Givelet, L.; Truffier-Boutry, D.; Noël, L.; Damlencourt, J.-F.; Jitaru, P.; Guérin, T. Optimisation and Application of an Analytical Approach for the Characterisation of TiO2 Nanoparticles in Food Additives and Pharmaceuticals by Single Particle Inductively Coupled Plasma-Mass Spectrometry. Talanta 2021, 224, 121873. [Google Scholar] [CrossRef]
- Bucher, G.; Auger, F. Combination of 47 Ti and 48 Ti for the Determination of Highly Polydisperse TiO2 Particle Size Distributions by SpICP-MS. J. Anal. At. Spectrom. 2019, 34, 1380–1386. [Google Scholar] [CrossRef]
- de la Calle, I.; Menta, M.; Klein, M.; Maxit, B.; Séby, F. Towards Routine Analysis of TiO2 (Nano-)Particle Size in Consumer Products: Evaluation of Potential Techniques. Spectrochim. Acta Part B At. Spectrosc. 2018, 147, 28–42. [Google Scholar] [CrossRef]
- Verleysen, E.; Van Doren, E.; Waegeneers, N.; De Temmerman, P.-J.; Abi Daoud Francisco, M.; Mast, J. TEM and SP-ICP-MS Analysis of the Release of Silver Nanoparticles from Decoration of Pastry. J. Agric. Food Chem. 2015, 63, 3570–3578. [Google Scholar] [CrossRef] [PubMed]
- Loeschner, K.; Correia, M.; López Chaves, C.; Rokkjær, I.; Sloth, J.J. Detection and Characterisation of Aluminium-Containing Nanoparticles in Chinese Noodles by Single Particle ICP-MS. Food Addit. Contam. Part A 2018, 35, 86–93. [Google Scholar] [CrossRef] [PubMed]
- López-Mayán, J.J.; Álvarez-Fernández, B.; Peña-Vázquez, E.; Barciela-Alonso, M.C.; Moreda-Piñeiro, A.; Bermejo-Barrera, P. Ultrasonication Followed by Enzymatic Hydrolysis as a Sample Pre-Treatment for the Determination of Ag Nanoparticles in Edible Seaweed by SP-ICP-MS. Talanta 2022, 247, 123556. [Google Scholar] [CrossRef]
- Jiménez-Lamana, J.; Wojcieszek, J.; Jakubiak, M.; Asztemborska, M.; Szpunar, J. Single Particle ICP-MS Characterization of Platinum Nanoparticles Uptake and Bioaccumulation by Lepidium Sativum and Sinapis Alba Plants. J. Anal. At. Spectrom. 2016, 31, 2321–2329. [Google Scholar] [CrossRef]
- Laughton, S.; Laycock, A.; Bland, G.; von der Kammer, F.; Hofmann, T.; Casman, E.A.; Lowry, G.V. Methanol-Based Extraction Protocol for Insoluble and Moderately Water-Soluble Nanoparticles in Plants to Enable Characterization by Single Particle ICP-MS. Anal. Bioanal. Chem. 2021, 413, 299–314. [Google Scholar] [CrossRef]
- Keller, A.A.; Huang, Y.; Nelson, J. Detection of Nanoparticles in Edible Plant Tissues Exposed to Nano-Copper Using Single-Particle ICP-MS. J. Nanoparticle Res. 2018, 20, 101. [Google Scholar] [CrossRef]
- Laughton, S.; Laycock, A.; von der Kammer, F.; Hofmann, T.; Casman, E.A.; Rodrigues, S.M.; Lowry, G.V. Persistence of Copper-Based Nanoparticle-Containing Foliar Sprays in Lactuca Sativa (Lettuce) Characterized by SpICP-MS. J. Nanoparticle Res. 2019, 21, 174. [Google Scholar] [CrossRef]
- Wojcieszek, J.; Jiménez-Lamana, J.; Bierla, K.; Asztemborska, M.; Ruzik, L.; Jarosz, M.; Szpunar, J. Elucidation of the Fate of Zinc in Model Plants Using Single Particle ICP-MS and ESI Tandem MS. J. Anal. At. Spectrom. 2019, 34, 683–693. [Google Scholar] [CrossRef]
- Wei, W.-J.; Li, L.; Gao, Y.-P.; Wang, Q.; Zhou, Y.-Y.; Liu, X.; Yang, Y. Enzyme Digestion Combined with SP-ICP-MS Analysis to Characterize the Bioaccumulation of Gold Nanoparticles by Mustard and Lettuce Plants. Sci. Total Environ. 2021, 777, 146038. [Google Scholar] [CrossRef] [PubMed]
- Wojcieszek, J.; Jiménez-Lamana, J.; Bierła, K.; Ruzik, L.; Asztemborska, M.; Jarosz, M.; Szpunar, J. Uptake, Translocation, Size Characterization and Localization of Cerium Oxide Nanoparticles in Radish (Raphanus sativus L.). Sci. Total Environ. 2019, 683, 284–292. [Google Scholar] [CrossRef] [PubMed]
- Wojcieszek, J.; Jiménez-Lamana, J.; Ruzik, L.; Asztemborska, M.; Jarosz, M.; Szpunar, J. Characterization of TiO2 NPs in Radish (Raphanus sativus L.) by Single-Particle ICP-QQQ-MS. Front. Environ. Sci. 2020, 8, 100. [Google Scholar] [CrossRef]
- Xiao, B.; Zhang, Y.; Wang, X.; Chen, M.; Sun, B.; Zhang, T.; Zhu, L. Occurrence and Trophic Transfer of Nanoparticulate Ag and Ti in the Natural Aquatic Food Web of Taihu Lake, China. Environ. Sci. Nano 2019, 6, 3431–3441. [Google Scholar] [CrossRef]
- Taboada-López, M.V.; Alonso-Seijo, N.; Herbello-Hermelo, P.; Bermejo-Barrera, P.; Moreda-Piñeiro, A. Determination and Characterization of Silver Nanoparticles in Bivalve Molluscs by Ultrasound Assisted Enzymatic Hydrolysis and Sp-ICP-MS. Microchem. J. 2019, 148, 652–660. [Google Scholar] [CrossRef]
- Sun, Y.; Yang, Y.; Tou, F.-Y.; Niu, Z.-S.; Guo, X.-P.; Liu, C.; Yan, J.; Wu, J.-Y.; Xu, M.; Hou, L.-J.; et al. Extraction and Quantification of Metal-Containing Nanoparticles in Marine Shellfish Based on Single Particle Inductively Coupled Plasma-Mass Spectrometry Technique. J. Hazard. Mater. 2022, 424, 127383. [Google Scholar] [CrossRef]
- Xu, L.; Wang, Z.; Zhao, J.; Lin, M.; Xing, B. Accumulation of Metal-Based Nanoparticles in Marine Bivalve Mollusks from Offshore Aquaculture as Detected by Single Particle ICP-MS. Environ. Pollut. 2020, 260, 114043. [Google Scholar] [CrossRef]
- Kuehr, S.; Meisterjahn, B.; Schröder, N.; Knopf, B.; Völker, D.; Schwirn, K.; Schlechtriem, C. Testing the Bioaccumulation of Manufactured Nanomaterials in the Freshwater Bivalve Corbicula Fluminea Using a New Test Method. Environ. Sci. Nano 2020, 7, 535–553. [Google Scholar] [CrossRef]
- Zhou, Q.; Liu, L.; Liu, N.; He, B.; Hu, L.; Wang, L. Determination and Characterization of Metal Nanoparticles in Clams and Oysters. Ecotoxicol. Environ. Saf. 2020, 198, 110670. [Google Scholar] [CrossRef]
- Gallocchio, F.; Biancotto, G.; Moressa, A.; Pascoli, F.; Pretto, T.; Toffan, A.; Arcangeli, G.; Montesi, F.; Peters, R.; Ricci, A. Bioaccumulation and In Vivo Formation of Titanium Dioxide Nanoparticles in Edible Mussels. Food Chem. 2020, 323, 126841. [Google Scholar] [CrossRef] [PubMed]
- Taboada-López, M.V.; Iglesias-López, S.; Herbello-Hermelo, P.; Bermejo-Barrera, P.; Moreda-Piñeiro, A. Ultrasound Assisted Enzymatic Hydrolysis for Isolating Titanium Dioxide Nanoparticles from Bivalve Mollusk before Sp-ICP-MS. Anal. Chim. Acta 2018, 1018, 16–25. [Google Scholar] [CrossRef] [PubMed]
- Grasso, A.; Ferrante, M.; Arena, G.; Salemi, R.; Zuccarello, P.; Fiore, M.; Copat, C. Chemical Characterization and Quantification of Silver Nanoparticles (Ag-NPs) and Dissolved Ag in Seafood by Single Particle ICP-MS: Assessment of Dietary Exposure. Int. J. Environ. Res. Public Health 2021, 18, 4076. [Google Scholar] [CrossRef] [PubMed]
- Grasso, A.; Ferrante, M.; Zuccarello, P.; Filippini, T.; Arena, G.; Fiore, M.; Cristaldi, A.; Conti, G.O.; Copat, C. Chemical Characterization and Quantification of Titanium Dioxide Nanoparticles (TiO2-NPs) in Seafood by Single-Particle ICP-MS: Assessment of Dietary Exposure. Int. J. Environ. Res. Public Health 2020, 17, 9547. [Google Scholar] [CrossRef]
- Grasso, A.; Ferrante, M.; Moreda-Piñeiro, A.; Arena, G.; Magarini, R.; Oliveri Conti, G.; Cristaldi, A.; Copat, C. Dietary Exposure of Zinc Oxide Nanoparticles (ZnO-NPs) from Canned Seafood by Single Particle ICP-MS: Balancing of Risks and Benefits for Human Health. Ecotoxicol. Environ. Saf. 2022, 231, 113217. [Google Scholar] [CrossRef]
- Loeschner, K.; Navratilova, J.; Købler, C.; Mølhave, K.; Wagner, S.; von der Kammer, F.; Larsen, E.H. Detection and Characterization of Silver Nanoparticles in Chicken Meat by Asymmetric Flow Field Flow Fractionation with Detection by Conventional or Single Particle ICP-MS. Anal. Bioanal. Chem. 2013, 405, 8185–8195. [Google Scholar] [CrossRef] [PubMed]
- Peters, R.J.B.; Rivera, Z.H.; van Bemmel, G.; Marvin, H.J.P.; Weigel, S.; Bouwmeester, H. Development and Validation of Single Particle ICP-MS for Sizing and Quantitative Determination of Nano-Silver in Chicken Meat. Anal. Bioanal. Chem. 2014, 406, 3875–3885. [Google Scholar] [CrossRef]
- Weigel, S.; Peters, R.; Loeschner, K.; Grombe, R.; Linsinger, T.P.J. Results of an Interlaboratory Method Performance Study for the Size Determination and Quantification of Silver Nanoparticles in Chicken Meat by Single-Particle Inductively Coupled Plasma Mass Spectrometry (Sp-ICP-MS). Anal. Bioanal. Chem. 2017, 409, 4839–4848. [Google Scholar] [CrossRef]
- Gomez-Gomez, B.; Perez-Corona, M.T.; Madrid, Y. Using Single-Particle ICP-MS for Unravelling the Effect of Type of Food on the Physicochemical Properties and Gastrointestinal Stability of ZnONPs Released from Packaging Materials. Anal. Chim. Acta 2020, 1100, 12–21. [Google Scholar] [CrossRef]
- Kollander, B.; Widemo, F.; Ågren, E.; Larsen, E.H.; Loeschner, K. Detection of Lead Nanoparticles in Game Meat by Single Particle ICP-MS Following Use of Lead-Containing Bullets. Anal. Bioanal. Chem. 2017, 409, 1877–1885. [Google Scholar] [CrossRef]
- Gray, E.P.; Coleman, J.G.; Bednar, A.J.; Kennedy, A.J.; Ranville, J.F.; Higgins, C.P. Extraction and Analysis of Silver and Gold Nanoparticles from Biological Tissues Using Single Particle Inductively Coupled Plasma Mass Spectrometry. Environ. Sci. Technol. 2013, 47, 14315–14323. [Google Scholar] [CrossRef] [PubMed]
- Gallocchio, F.; Biancotto, G.; Cibin, V.; Losasso, C.; Belluco, S.; Peters, R.; van Bemmel, G.; Cascio, C.; Weigel, S.; Tromp, P.; et al. Transfer Study of Silver Nanoparticles in Poultry Production. J. Agric. Food Chem. 2017, 65, 3767–3774. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, Y.; Kondo, M.; Akiyama, H.; Ogra, Y. Presence of Nano-Sized Mercury-Containing Particles in Seafoods, and an Estimate of Dietary Exposure. Environ. Pollut. 2022, 307, 119555. [Google Scholar] [CrossRef] [PubMed]
- Taboada-López, M.V.; Herbello-Hermelo, P.; Domínguez-González, R.; Bermejo-Barrera, P.; Moreda-Piñeiro, A. Enzymatic Hydrolysis as a Sample Pre-Treatment for Titanium Dioxide Nanoparticles Assessment in Surimi (Crab Sticks) by Single Particle ICP-MS. Talanta 2019, 195, 23–32. [Google Scholar] [CrossRef] [PubMed]
- Verleysen, E.; Waegeneers, N.; De Vos, S.; Brassinne, F.; Ledecq, M.; Van Steen, F.; Andjelkovic, M.; Janssens, R.; Mathioudaki, S.; Delfosse, L.; et al. Physicochemical Characterization of Nanoparticles in Food Additives in the Context of Risk Identification. EFSA Support. Publ. 2021, 18, 6678E. [Google Scholar] [CrossRef]
- NIST. Reference Material® 8012 Gold Nanoparticles, Nominal 30 Nm Diameter; National Institute of Standards and Technology: Gaithersburg, MD, USA, 2015.
- NIST. Reference Material® 8013 Gold Nanoparticles, Nominal 60 Nm Diameter; National Institute of Standards and Technology: Gaithersburg, MD, USA, 2015.
- Colloidal Gold NPs—Nominal Diameter 30 Nm Quality Control Material LGCQC5050, LGC. 2019. Available online: https://www.Lgcstandards.Com/Medias/Sys_master/Root/H84/H04/10435846012958/LGCQC5050.Pdf (accessed on 31 May 2023).
- Montoro Bustos, A.R.; Purushotham, K.P.; Possolo, A.; Farkas, N.; Vladár, A.E.; Murphy, K.E.; Winchester, M.R. Validation of Single Particle ICP-MS for Routine Measurements of Nanoparticle Size and Number Size Distribution. Anal. Chem. 2018, 90, 14376–14386. [Google Scholar] [CrossRef] [PubMed]
- International Organization for Standardization (ISO). ISO Guide 30; International Organization for Standardization (ISO): Geneva, Switzerland, 2015. [Google Scholar]
- Pace, H.E.; Rogers, N.J.; Jarolimek, C.; Coleman, V.A.; Higgins, C.P.; Ranville, J.F. Determining Transport Efficiency for the Purpose of Counting and Sizing Nanoparticles via Single Particle Inductively Coupled Plasma Mass Spectrometry. Anal. Chem. 2011, 83, 9361–9369. [Google Scholar] [CrossRef]
- Cuello-Nuñez, S.; Abad-Álvaro, I.; Bartczak, D.; Del Castillo Busto, M.E.; Ramsay, D.A.; Pellegrino, F.; Goenaga-Infante, H. The Accurate Determination of Number Concentration of Inorganic Nanoparticles Using SpICP-MS with the Dynamic Mass Flow Approach. J. Anal. At. Spectrom. 2020, 35, 1832–1839. [Google Scholar] [CrossRef]
- Peters, R.; Herrera-Rivera, Z.; Undas, A.; Van Der Lee, M.; Marvin, H.; Bouwmeester, H.; Weigel, S. Single Particle ICP-MS Combined with a Data Evaluation Tool as a Routine Technique for the Analysis of Nanoparticles in Complex Matrices. J. Anal. At. Spectrom. 2015, 30, 1274–1285. [Google Scholar] [CrossRef]
- Beauchamp, C.R.; Camara, J.E.; Carney, J.; Choquette, S.J.; Cole, K.D.; DeRose, P.C.; Duewer, D.L.; Epstein, M.S.; Kline, M.C.; Lippa, K.A.; et al. Metrological Tools for the Reference Materials and Reference Instruments of the NIST Material Measurement Laboratory; NIST: Gaithersburg, MD, USA, 2021.
- CFR—Code of Federal Regulations Title 21. 172.480 Silicon Dioxide. Available online: https://www.ecfr.gov/current/title-21/chapter-I/subchapter-B/part-172/subpart-E/section-172.480 (accessed on 5 December 2022).
- Younes, M.; Aggett, P.; Aguilar, F.; Crebelli, R.; Dusemund, B.; Filipič, M.; Frutos, M.J.; Galtier, P.; Gott, D.; Gundert-Remy, U.; et al. Re-Evaluation of Silicon Dioxide (E 551) as a Food Additive. EFSA J. 2018, 16, e05088. [Google Scholar] [CrossRef]
- Lozano, O.; Silva-Platas, C.; Chapoy-Villanueva, H.; Pérez, B.E.; Lees, J.G.; Ramachandra, C.J.A.; Contreras-Torres, F.F.; Lázaro-Alfaro, A.; Luna-Figueroa, E.; Bernal-Ramírez, J.; et al. Amorphous SiO2 Nanoparticles Promote Cardiac Dysfunction via the Opening of the Mitochondrial Permeability Transition Pore in Rat Heart and Human Cardiomyocytes. Part Fibre Toxicol. 2020, 17, 15. [Google Scholar] [CrossRef] [PubMed]
- Khan, S.A.; Johnson, M.E.; Kalan, M.S.; Bustos, A.R.M.; Rabb, S.A.; Strenge, I.H.; Murphy, K.E.; Timothy, R. Croley Characterization of Nanoparticles in Silicon Dioxide Food Additives. 2023; in preparation. [Google Scholar]
- Geiss, O.; Bianchi, I.; Bucher, G.; Verleysen, E.; Brassinne, F.; Mast, J.; Loeschner, K.; Givelet, L.; Cubadda, F.; Ferraris, F.; et al. Determination of the Transport Efficiency in SpICP-MS Analysis Using Conventional Sample Introduction Systems: An Interlaboratory Comparison Study. Nanomaterials 2022, 12, 725. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).