New Insights into Amino-Functionalization of Magnetic Nanoplatelets with Silanes and Phosphonates
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
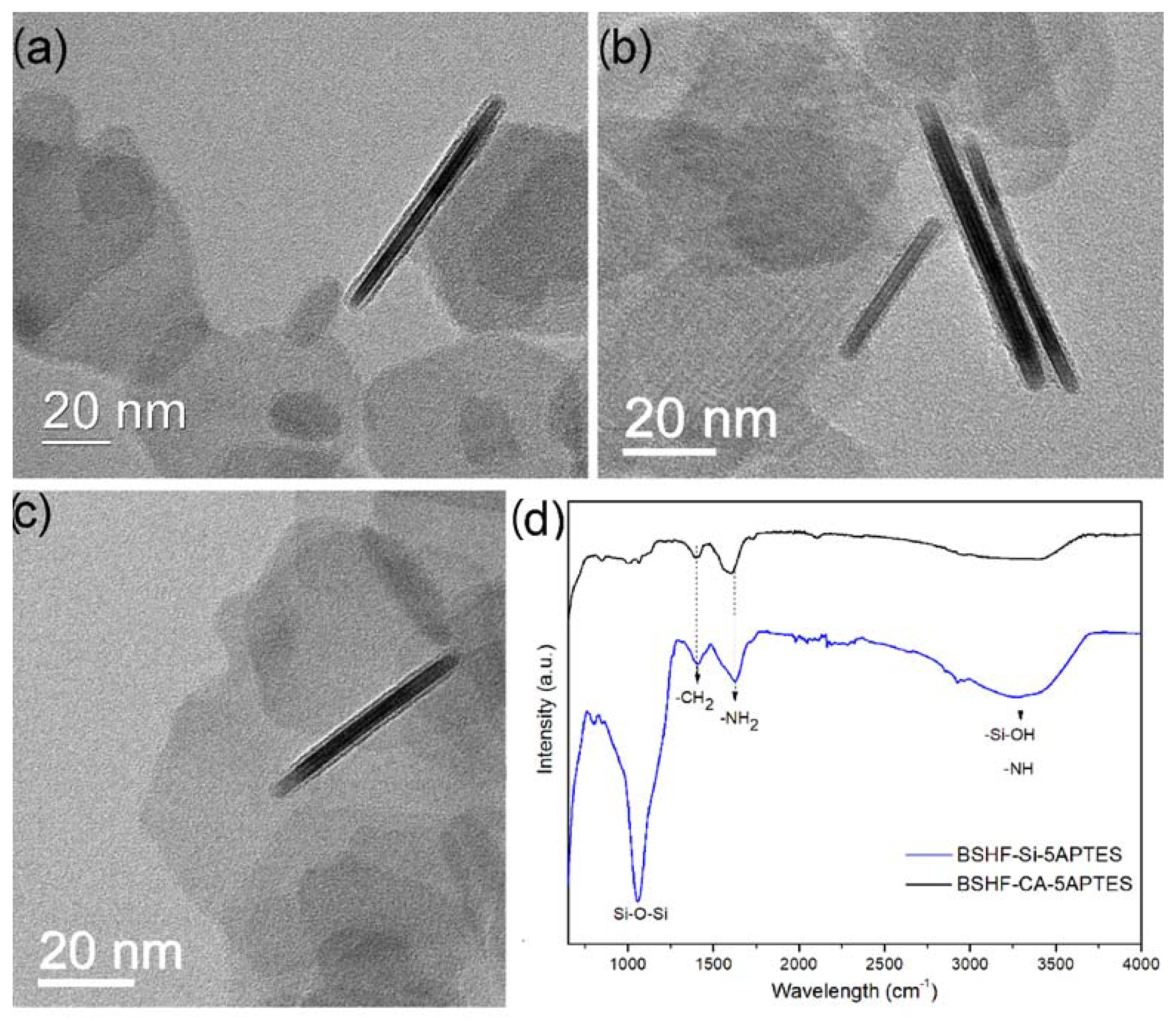
2.2. Coating BSHF NPLs with APTES
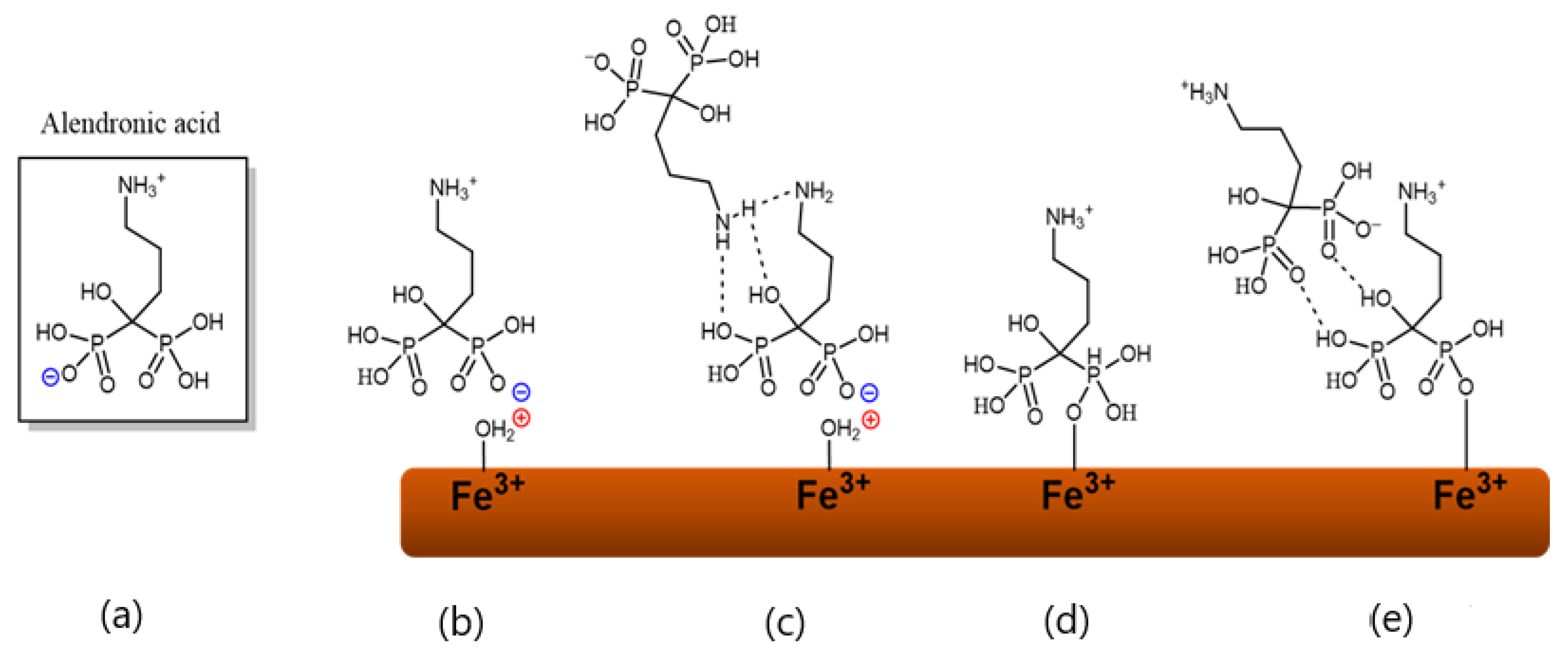
2.3. Coating BSHF NPLs with AL
2.4. Characterization
3. Results
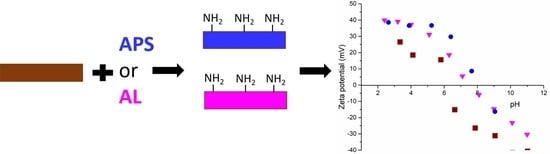
3.1. Coatings with APTES
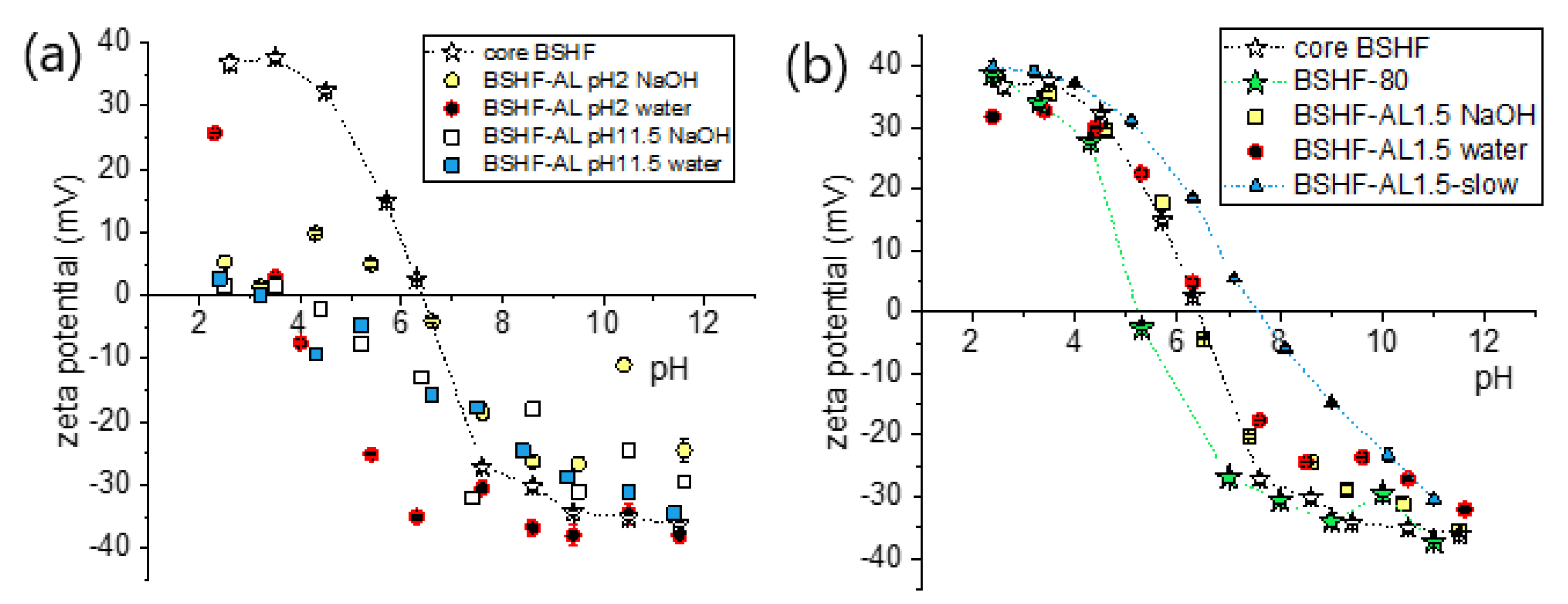
3.2. Coatings with AL
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Pullar, R.C. Hexagonal Ferrites: A Review of the Synthesis, Properties and Applications of Hexaferrite Ceramics. Prog. Mater. Sci. 2012, 57, 1191–1334. [Google Scholar] [CrossRef]
- Mertelj, A.; Lisjak, D.; Drofenik, M.; Čopič, M. Ferromagnetism in suspensions of magnetic platelets in liquid crystal. Nature 2013, 504, 237–241. [Google Scholar] [CrossRef] [PubMed]
- Shuai, M.; Klittnick, A.; Shen, Y.; Smith, G.P.; Tuchband, M.R.; Zhu, C.; Petschek, R.G.; Mertelj, A.; Lisjak, D.; Čopič, M.; et al. Spontaneous liquid crystal and ferromagnetic ordering of colloidal magnetic nanoplates. Nat. Commun. 2016, 7, 10394. [Google Scholar] [CrossRef] [PubMed]
- Mertelj, A.; Lisjak, D. Ferromagnetic nematic liquid crystals. Liq. Cryst. Rev. 2017, 5, 1–33. [Google Scholar] [CrossRef]
- Lisjak, D.; Boštjančič, P.H.; Mertelj, A.; Mavrič, A.; Valant, M.; Kovač, J.; Hudelja, H.; Kocjan, A.; Makovec, D. Formation of Fe(III)-phosphonate Coatings on Barium Hexaferrite Nanoplatelets for Porous Nanomagnets. ACS Omega 2020, 5, 14086–14095. [Google Scholar] [CrossRef]
- Koplovitz, G.; Primc, D.; Dor, O.B.; Yochelis, S.; Rotem, D.; Porath, D.; Paltiel, Y. Magnetic Nanoplatelet-Based Spin Memory Device Operating at Ambient Temperatures. Adv. Mater. 2017, 29, 1606748. [Google Scholar] [CrossRef]
- Hu, J.; Gorsak, T.; Rodríguez, E.M.; Calle, D.; Muñoz-Ortiz, T.; Jaque, D.; Fernández, N.; Cussó, L.; Rivero, F.; Torres, R.A.; et al. Magnetic Nanoplatelets for High Contrast Cardiovascular Imaging by Magnetically Modulated Optical Coherence Tomography. ChemPhotoChem 2019, 3, 529–539. [Google Scholar] [CrossRef]
- Goršak, T.; Drab, M.; Križaj, D.; Jeran, M.; Genova, J.; Kralj, S.; Lisjak, D.; Kralj-Iglič, V.; Iglič, A.; Makovec, D. Magneto-mechanical actuation of barium-hexaferrite nanoplatelets for the disruption of phospholipid membranes. J. Colloid Interface Sci. 2020, 579, 508–519. [Google Scholar] [CrossRef]
- Lisjak, D.; Drofenik, M. Chemical substitution-an alternative strategy for controlling the particle size of barium ferrite. Cryst. Growth Des. 2012, 12, 5174–5179. [Google Scholar] [CrossRef]
- Makovec, D.; Komelj, M.; Dražić, G.; Belec, B.; Goršak, T.; Gyergyek, S.; Lisjak, D. Incorporation of Sc into the structure of barium- hexaferrite nanoplatelets and its extraordinary finite-size effect on the magnetic properties. Acta Mater. 2019, 172, 84–91. [Google Scholar] [CrossRef]
- Boštjančič, P.H.; Gregorin, Ž.; Sebastián, N.; Osterman, N.; Lisjak, D.; Mertelj, A. Isotropic to nematic transition in alcohol ferrofluids of barium hexaferrite nanoplatelets. J. Mol. Liq. 2022, 348, 118038. [Google Scholar] [CrossRef]
- Boštjančič, P.H.; Tomšič, M.; Jamnik, A.; Lisjak, D.; Mertelj, A. Electrostatic Interactions between Barium Hexaferrite Nanoplatelets in Alcohol Suspensions. J. Phys. Chem. C 2019, 123, 23272–23279. [Google Scholar] [CrossRef] [Green Version]
- Goršak, D.; Kralj, T.; Makovec, S.; Lisjak, D. The formation of silica coatings on barium hexaferrite nanoparticles and funct ionalization with 3-aminopropyl silane. In Proceedings of the 8th Jozef Stefan International Postgraduate Students’ Conference, Ljubljana, Slovenia, 31 May–1 June 2016; pp. 227–238. Available online: http://ipssc.mps.si/2016/proceedings/Proceedings8_IPSSC_2016_Part1.pdf (accessed on 18 June 2022).
- Goršak, T.; Makovec, D.; Javornik, U.; Belec, B.; Kralj, S.; Lisjak, D. A functionalization strategy for the dispersion of permanently magnetic barium-hexaferrite nanoplatelets in complex biological media. Colloids Surf. A Physicochem. Eng. Asp. 2019, 573, 119–127. [Google Scholar] [CrossRef]
- Schoth, A.; Keith, A.D.; Landfester, K.; Muñoz-Espí, R. Silanization as a versatile functionalization method for the synthesis of polymer/magnetite hybrid nanoparticles with controlled structure. RSC Adv. 2016, 6, 53903–53911. [Google Scholar] [CrossRef] [Green Version]
- Ahangaran, F.; Navarchian, A.H. Recent advances in chemical surface modification of metal oxide nanoparticles with silane coupling agents: A review. Adv. Colloid Interface Sci. 2020, 286, 102298. [Google Scholar] [CrossRef]
- Owen, M.J.; Dvornic, P.R. General Introduction to Silicone Surfaces. In Silicone Surface Science; Owen, M.J., Dvornic, P.R., Eds.; Springer: Dordrecht, The Netherlands, 2012; pp. 1–21. [Google Scholar] [CrossRef]
- Čampelj, S.; Makovec, D.; Drofenik, M. Functionalization of magnetic nanoparticles with 3-aminopropyl silane. J. Magn. Magn. Mater. 2009, 321, 1346–1350. [Google Scholar] [CrossRef]
- Kralj, S.; Drofenik, M.; Makovec, D. Controlled surface functionalization of silica-coated magnetic nanoparticles with terminal amino and carboxyl groups. J. Nanoparticle Res. 2011, 13, 2829–2841. [Google Scholar] [CrossRef]
- Dutta, B.; Nema, A.; Shetake, N.G.; Gupta, J.; Barick, K.C.; Lawande, M.A.; Pandey, B.N.; Priyadarsini, I.K.; Hassan, P.A. Glutamic acid-coated Fe3O4 nanoparticles for tumor-targeted imaging and therapeutics. Mater. Sci. Eng. C 2020, 112, 110915. [Google Scholar] [CrossRef]
- Zhu, M.; Lerum, M.Z.; Chen, W. How to Prepare Reproducible, Homogeneous, and Hydrolytically Stable Aminosilane-Derived Layers on Silica. Langmuir 2011, 28, 416–423. [Google Scholar] [CrossRef] [Green Version]
- Okhrimenko, D.V.; Budi, A.; Ceccato, M.; Cárdenas, M.; Johansson, D.B.; Lybye, D.; Bechgaard, K.; Andersson, M.P.; Stipp, S.L.S. Hydrolytic Stability of 3-Aminopropylsilane Coupling Agent on Silica and Silicate Surfaces at Elevated Temperatures. ACS Appl. Mater. Interfaces 2017, 9, 8344–8353. [Google Scholar] [CrossRef]
- Smith, E.A.; Chen, W. How to Prevent the Loss of Surface Functionality Derived from Aminosilanes. Langmuir 2008, 24, 12405–12409. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fadeev, A.Y.; McCarthy, T.J. Self-Assembly Is Not the Only Reaction Possible between Alkyltrichlorosilanes and Surfaces: Monomolecular and Oligomeric Covalently Attached Layers of Dichloro- and Trichloroalkylsilanes on Silicon. Langmuir 2000, 16, 7268–7274. [Google Scholar] [CrossRef]
- Queffélec, C.; Petit, M.; Janvier, P.; Knight, D.A.; Bujoli, B. Surface modification using phosphonic acids and esters. Chem. Rev. 2012, 112, 3777–3807. [Google Scholar] [CrossRef] [PubMed]
- Pujari, S.P.; Scheres, L.; Marcelis, A.T.M.; Zuilhof, H. Covalent surface modification of oxide surfaces. Angew. Chemie Int. Ed. 2014, 53, 6322–6356. [Google Scholar] [CrossRef]
- Milošev, I.; Zimerl, D.; Carriére, C.; Zanna, S.; Seyeux, A.; Iskra, J.; Stavber, S.; Chiter, F.; Poberžnik, M.; Costa, D.; et al. Editors’ Choice—The Effect of Anchor Group and Alkyl Backbone Chain on Performance of Organic Compounds as Corrosion Inhibitors for Aluminum Investigated Using an Integrative Experimental-Modeling Approach. J. Electrochem. Soc. 2020, 167, 061509. [Google Scholar] [CrossRef]
- Nowack, B.; Stone, A.T. Adsorption of phosphonates onto the Goethite-Water interface. J. Colloid Interface Sci. 1999, 214, 20–30. [Google Scholar] [CrossRef] [Green Version]
- Mo, X.; Qian, J.; Chen, Y.; Zhang, W.; Xian, P.; Tang, S.; Zhou, C.; Huang, N.; Ji, H.; Luo, E.; et al. Corrosion and degradation decelerating alendronate embedded zinc phosphate hybrid coating on biodegradable Zn biomaterials. Corros. Sci. 2021, 184, 109398. [Google Scholar] [CrossRef]
- Kostiv, U.; Lobaz, V.; Kučka, J.; Švec, P.; Sedláček, O.; Hrubý, M.; Janoušková, O.; Francová, P.; Kolářová, V.; Šefc, L.; et al. A simple neridronate-based surface coating strategy for upconversion nanoparticles: Highly colloidally stable125I-radiolabeled NaYF4:Yb3+/Er3+@PEG nanoparticles for multimodal in vivo tissue imaging. Nanoscale 2017, 9, 16680–16688. [Google Scholar] [CrossRef] [Green Version]
- Lisjak, D.; Vozlič, M.; Kostiv, U.; Horák, D.; Majaron, B.; Kralj, S.; Zajc, I.; Žiberna, L.; Ponikvar-Svet, M. NaYF4-based upconverting nanoparticles with optimized phosphonate coatings for chemical stability and viability of human endothelial cells. Methods Appl. Fluoresc. 2022, 10, 014001. [Google Scholar] [CrossRef]
- Neto, D.M.A.; da Costa, L.S.; de Menezes, F.L.; Fechine, L.M.U.D.; Freire, R.M.; Denardin, J.C.; Bañobre-López, M.; Vasconcelos, I.F.; Ribeiro, T.S.; Leal, L.K.A.M.; et al. A novel amino phosphonate-coated magnetic nanoparticle as MRI contrast agent. Appl. Surf. Sci. 2021, 543, 148824. [Google Scholar] [CrossRef]
- Cattalini, J.P.; Boccaccini, A.R.; Lucangioli, S.; Mouriño, V. Bisphosphonate-Based Strategies for Bone Tissue Engineering and Orthopedic Implants. Tissue Eng. Part B Rev. 2012, 18, 323–340. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ke, J.; Dou, H.; Zhang, X.; Uhagaze, D.S.; Ding, X.; Dong, Y. Determination of pKa values of alendronate sodium in aqueous solution by piecewise linear regression based on acid-base potentiometric titration. J. Pharm. Anal. 2016, 6, 404–409. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lisjak, L.D.; Arčon, I.; Poberžik, M.; Herrero-Saboya, G.; Tufani, A.; Mavrič, A.; Valant, M.; Bošt-jančič, H.P.; Mertelj, A.; Makovec, D.; et al. Interaction of phosphonic acids with barium hexaferrite nanoplatelets and their effect on magnetic properties. Nanoscale 2022, under Review. [Google Scholar]
- White, L.D.; Tripp, C.P. Reaction of (3-Aminopropyl)dimethylethoxysilane with Amine Catalysts on Silica Surfaces. J. Colloid Interface Sci. 2000, 232, 400–407. [Google Scholar] [CrossRef] [PubMed]
- Bini, R.A.; Marques, R.F.C.; Santos, F.J.; Chaker, J.A.; Jafelicci, M. Synthesis and functionalization of magnetite nanoparticles with different amino-functional alkoxysilanes. J. Magn. Magn. Mater. 2012, 324, 534–539. [Google Scholar] [CrossRef] [Green Version]
- Pušnik, K.; Peterlin, M.; Cigić, I.K.; Marolt, G.; Kogej, K.; Mertelj, A.; Gyergyek, S.; Makovec, D. Adsorption of Amino Acids, Aspartic Acid, and Lysine onto Iron-Oxide Nanoparticles. J. Phys. Chem. C 2016, 120, 14372–14381. [Google Scholar] [CrossRef]
- Lalatonne, Y.; Paris, C.; Serfaty, J.M.; Weinmann, P.; Lecouvey, M.; Motte, L. Bis-phosphonates-ultra small superparamagnetic iron oxide nanoparticles: A platform towards diagnosis and therapy. Chem. Commun. 2008, 22, 2553–2555. [Google Scholar] [CrossRef]
- Mutin, P.H.; Guerrero, G.; Vioux, A. Hybrid materials from organophosphorus coupling molecules. J. Mater. Chem. 2005, 15, 3761–3768. [Google Scholar] [CrossRef]
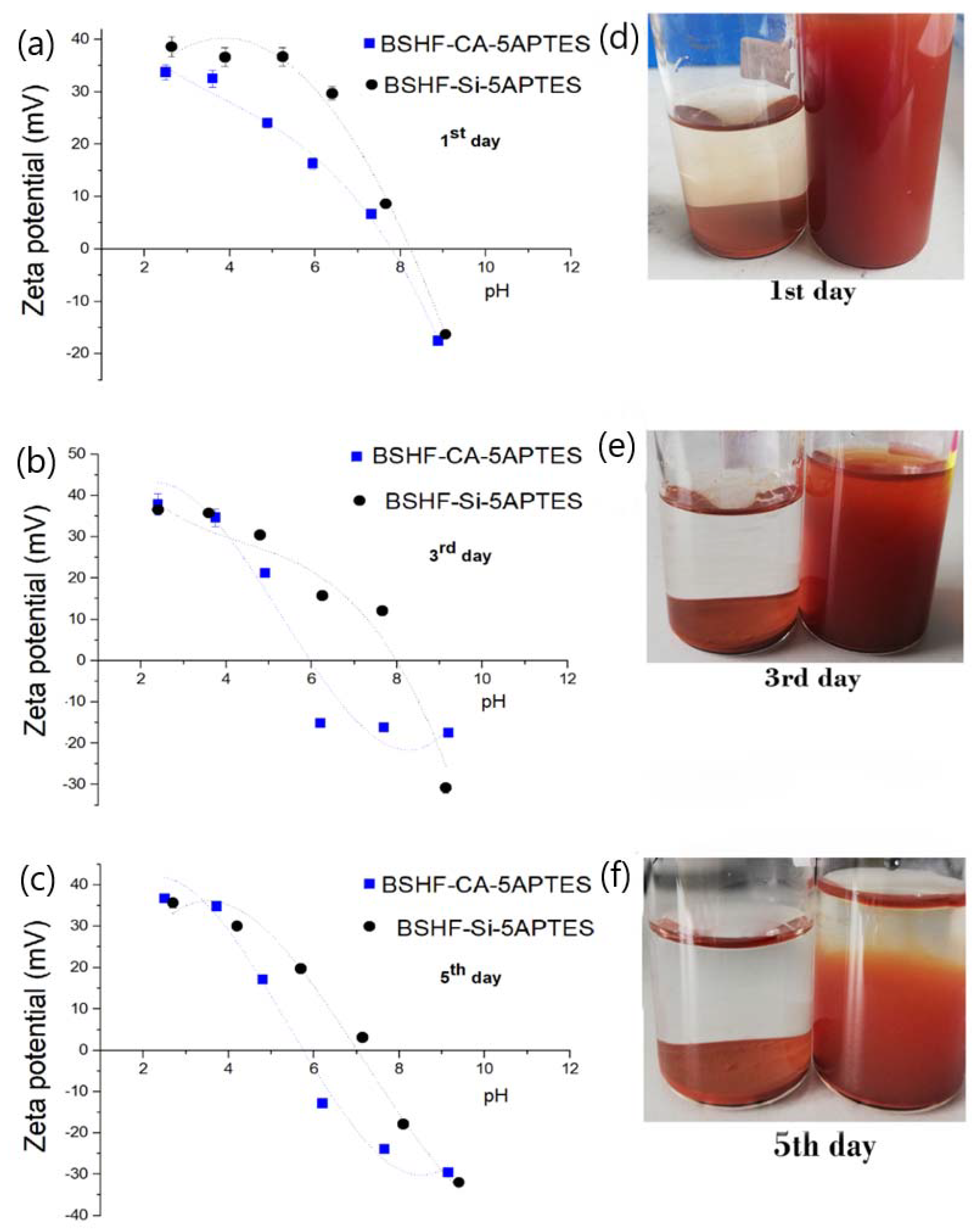
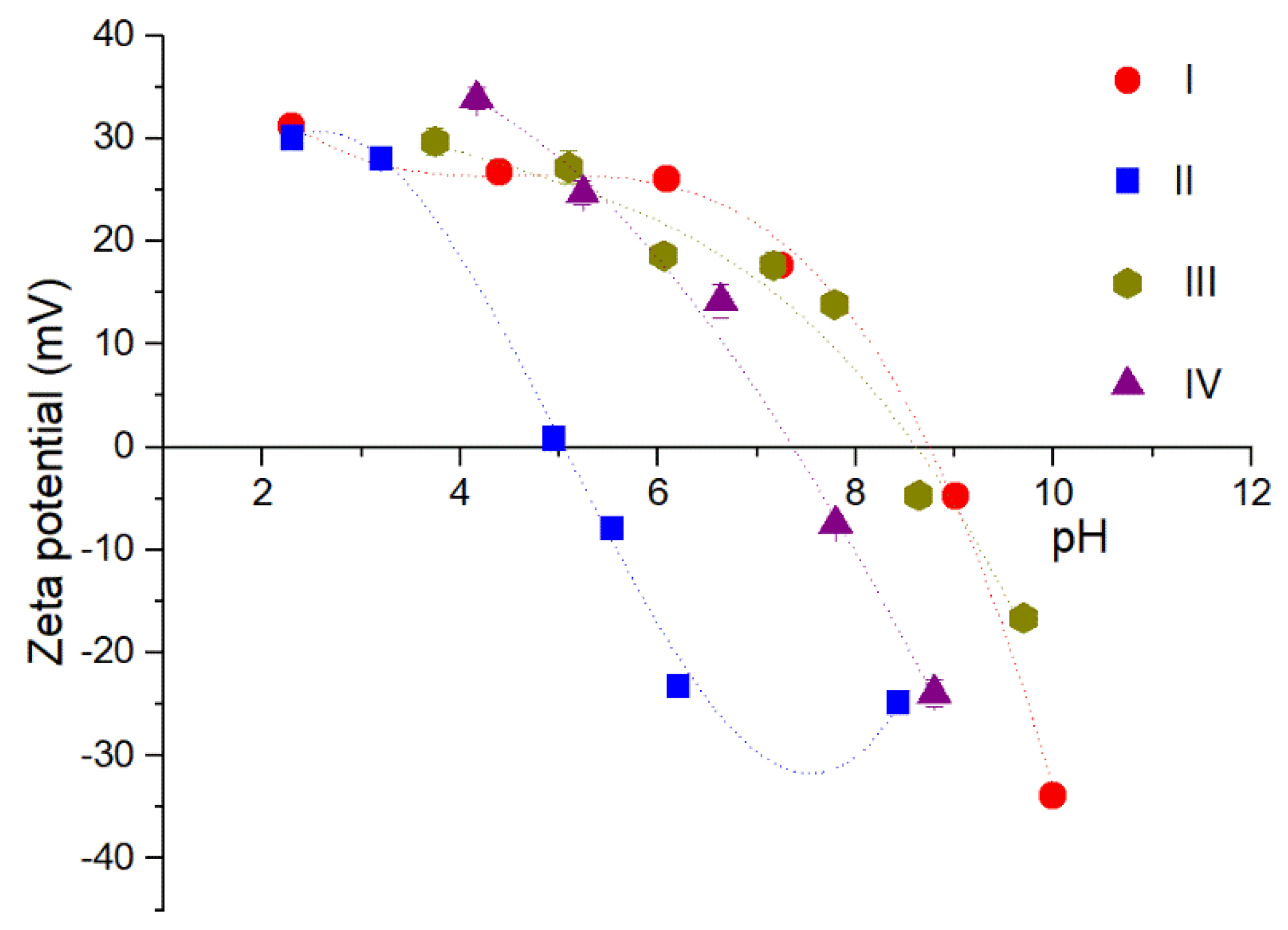
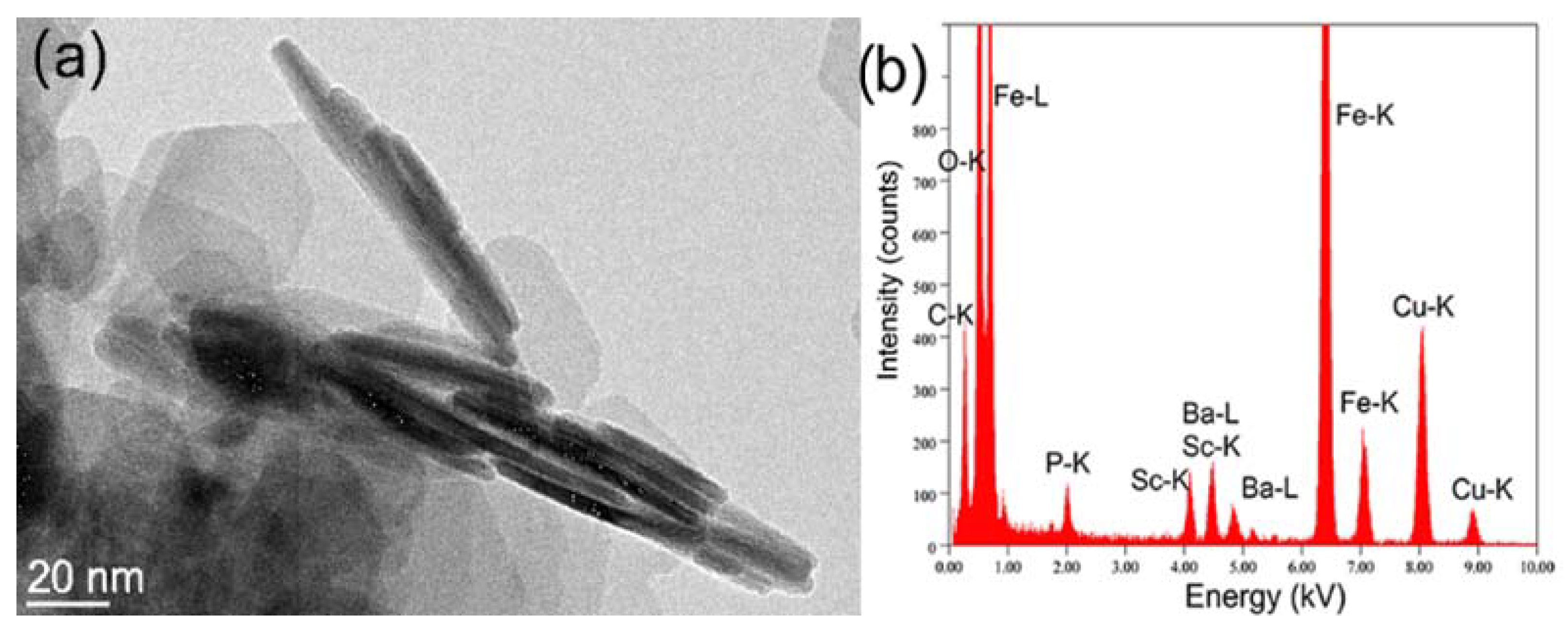
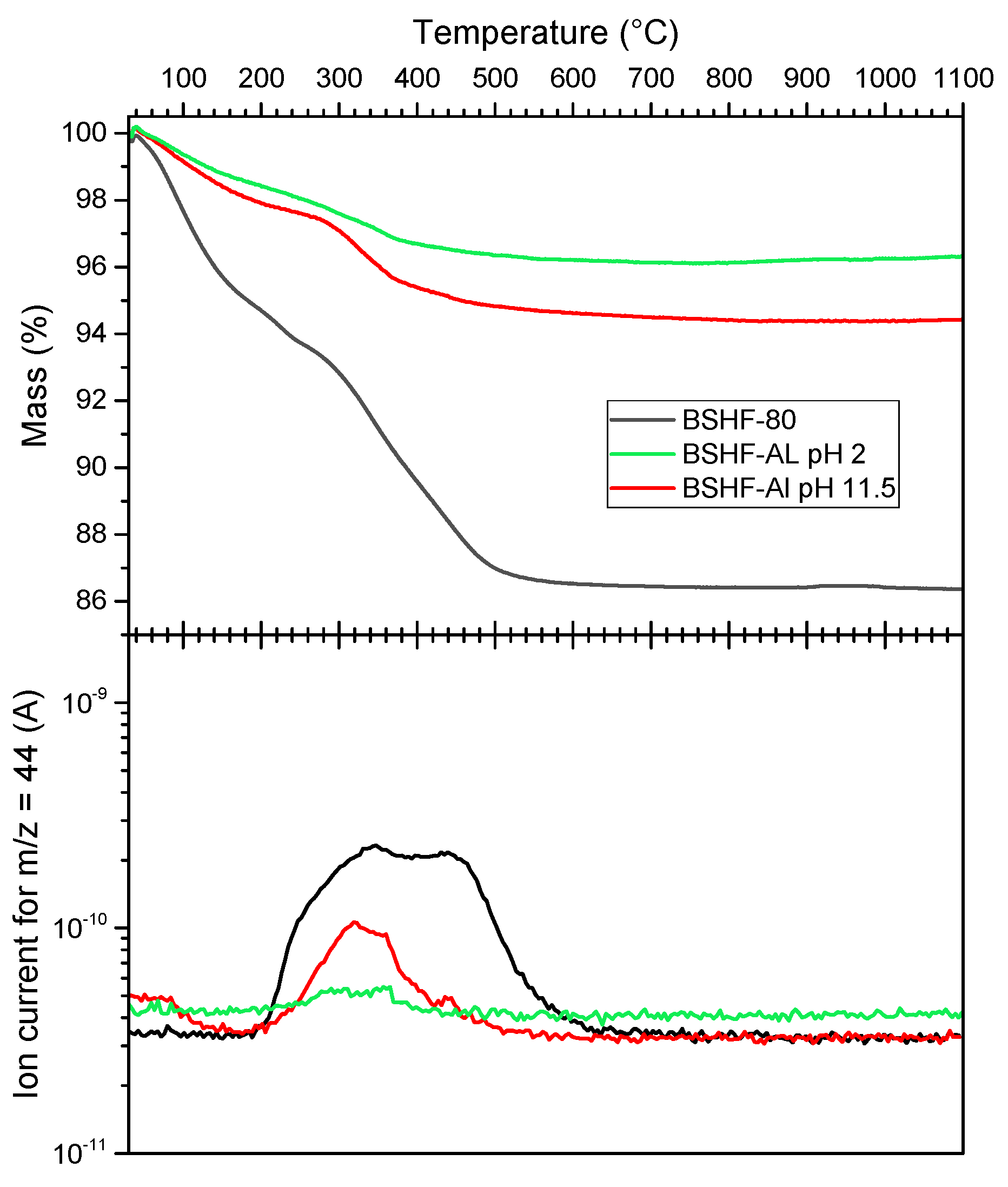
| Sample Name | Nominal Ligand Fraction (APTES/nm2) | pH | Washing Solvent |
|---|---|---|---|
| BSHF-CA-5APTES | 5 | 10 | Water + EtOH |
| BSHF-Si-5APTES | 5 | 10 | Water + EtOH |
| BSHF-Si-30APTES | 30 | 10 | Water + EtOH |
| Sample Name | Nominal Ligand Fraction (AL/nm2) | pH | Washing Solvent |
|---|---|---|---|
| BSHF-AL | 10 | 2 or 11.5 | water |
| 10 | 2 or 11.5 | NaOH + water | |
| BSHF-AL1.5 | 1.5 | 2 | water |
| 1.5 | 2 | NaOH + water | |
| BSHF-AL1.5-slow | 1.5 | 2 | water |
| HT-BSHF-AL | 10 10 | 5 or 12 5 | water NaOH + water |
| BSHF-80 | / | 2 | water |
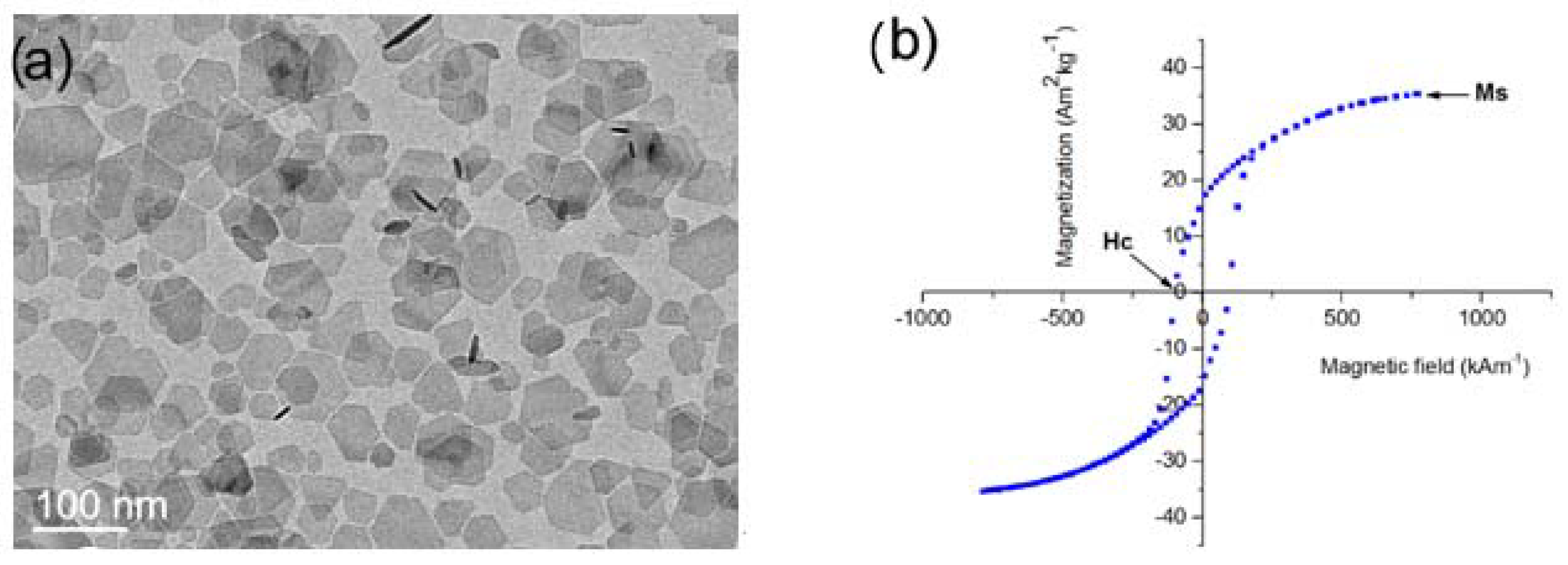
| NPLs | ωnonmagnetic (%) | ωligand (%) | Msmeas (Am2/kg) | Msexp (Am2/kg) |
|---|---|---|---|---|
| BSHF core | 14.35 | ~9 (nitrate) | 28.6 ± 1.0 | 33 (=Mscor) |
| BSHF-80 | 13.6 | >10 (carbonate) | 32 ± 8 | 29 |
| BSHF-AL, pH 2 | 8.18 | 7.21 (AL) | 27.9 ± 1.5 | 30 |
| BSHF-AL, pH 11.5 | 5.55 | ~3 (carbonate) | 33.3 ± 2.9 | 31 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Papan Djaniš, J.; Prinčič, G.G.; Mavrič, A.; Mertelj, A.; Iskra, J.; Lisjak, D. New Insights into Amino-Functionalization of Magnetic Nanoplatelets with Silanes and Phosphonates. Nanomaterials 2022, 12, 2123. https://doi.org/10.3390/nano12122123
Papan Djaniš J, Prinčič GG, Mavrič A, Mertelj A, Iskra J, Lisjak D. New Insights into Amino-Functionalization of Magnetic Nanoplatelets with Silanes and Phosphonates. Nanomaterials. 2022; 12(12):2123. https://doi.org/10.3390/nano12122123
Chicago/Turabian StylePapan Djaniš, Jelena, Griša Grigorij Prinčič, Andraž Mavrič, Alenka Mertelj, Jernej Iskra, and Darja Lisjak. 2022. "New Insights into Amino-Functionalization of Magnetic Nanoplatelets with Silanes and Phosphonates" Nanomaterials 12, no. 12: 2123. https://doi.org/10.3390/nano12122123