Tissue-Mimicking Geometrical Constraints Stimulate Tissue-Like Constitution and Activity of Mouse Neonatal and Human-Induced Pluripotent Stem Cell-Derived Cardiac Myocytes
Abstract
:1. Introduction
2. Experimental Section
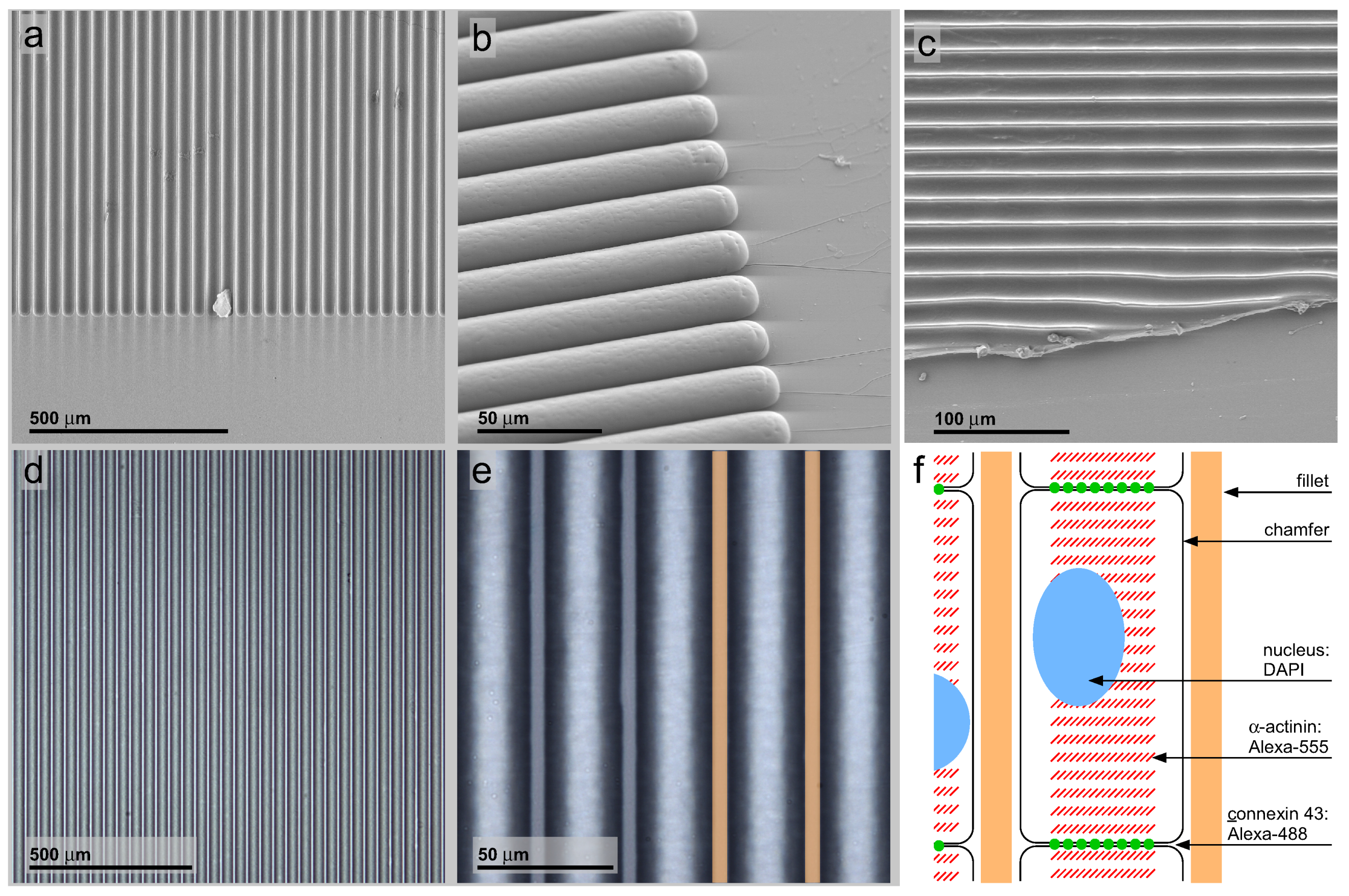
2.1. PDMS Micro-Patterning on Glass Cover Slides: Geometry-Patterned Surfaces
2.2. Cell Culture
2.3. Immunostaining, F-Actin and Nuclear Stain
2.4. Confocal Laser Scanning Microscopy
2.5. Scanning Electron Microscopy Preparation and Recording
2.6. Real-Time Recording of Cardiac Myocyte Contractions in Line Pattern-Organized Ensembles
3. Results and Discussion
3.1. PDMS Micro-Patterning on Glass Substrates, Surface Post-Processing and Cell Seeding
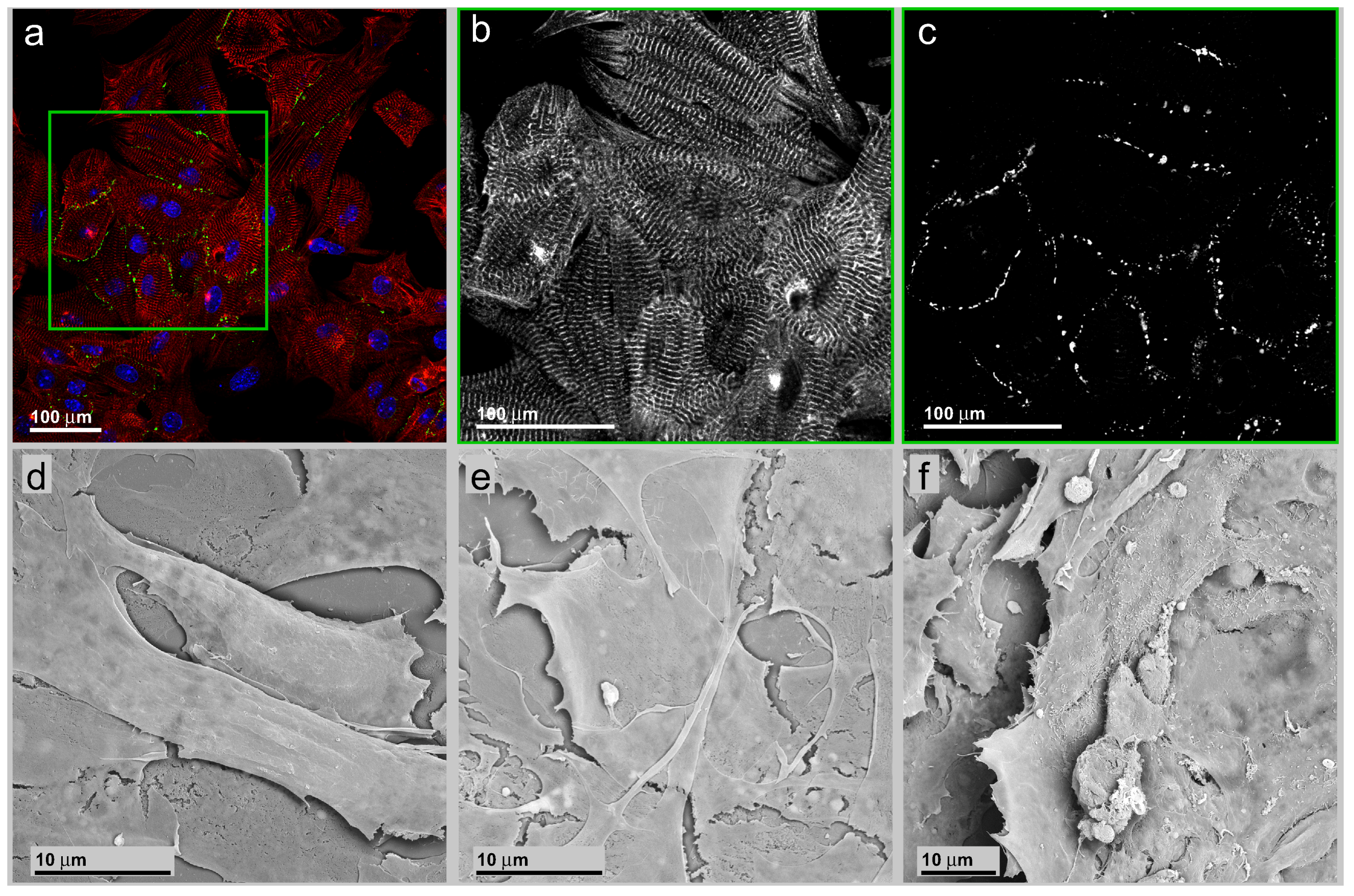
3.2. Neonatal Mouse Cardiomyocytes on Unstructured Surfaces
3.3. Neonatal Mouse Cardiomyocytes on Line-Patterned Surfaces
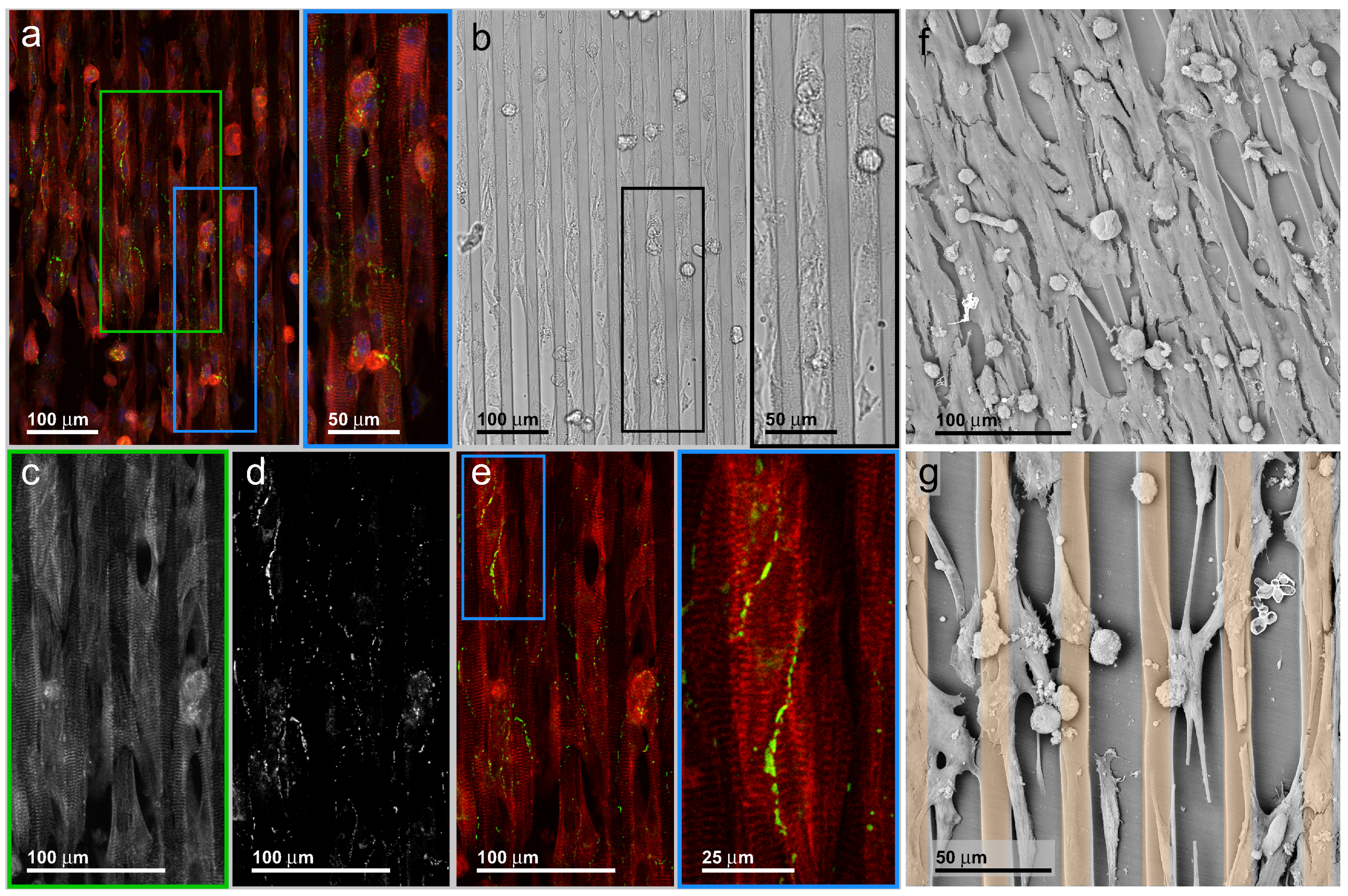
3.4. hIPSC-Derived Cardiac Myocytes on Line-Patterned Surfaces
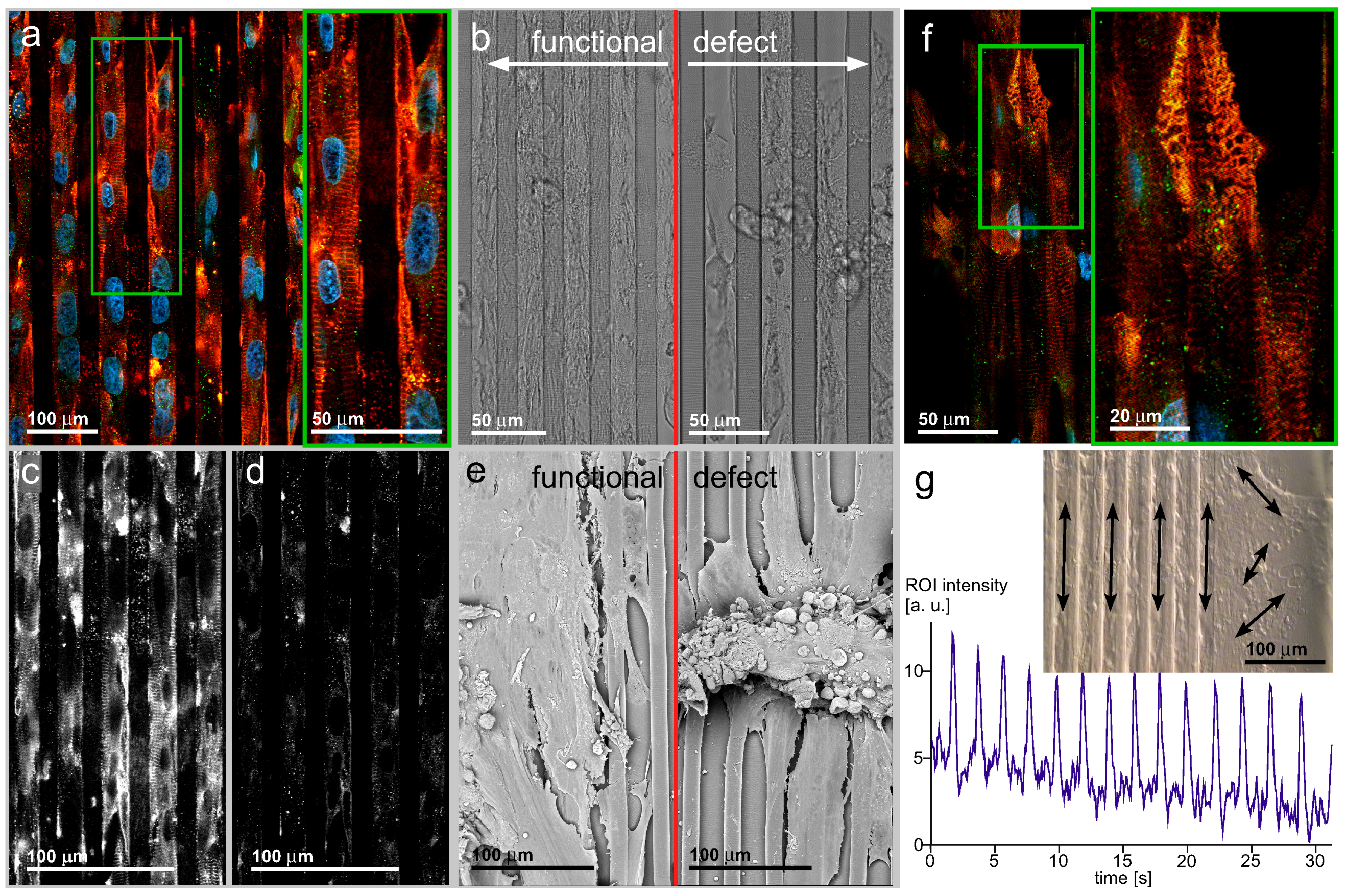
4. General Discussion
4.1. Statistical Aspects of the Parameters Observed
4.2. Line Patterns as Modular Elements in Cardiac Tissue Engineering
4.3. Pattern Geometry Mechanically Stimulates Cell Orientation via Interacting Adhesion and Contraction Behavior
4.4. hIPSC-Derived Cardiac Myocytes in Long-Term Culture as Targets for Either Apoptosis or Incomplete Maturation
4.5. Connexin-43-Containing Gap Junctions Require More Information for Correct Intercellular Localization than Patterned Surfaces Provide
4.6. Cell Organization by Line Patterns Influences Electrical Signal Spread via a Mechanism Different from Gap Junction-Based Depolarization Spread
4.7. Tissue Engineering and Pharmacology Drug Screening in an Organized Multicellular Environment
5. Conclusions
Supplementary Files
Supplementary File 1Acknowledgments
Author Contributions
Conflicts of Interest
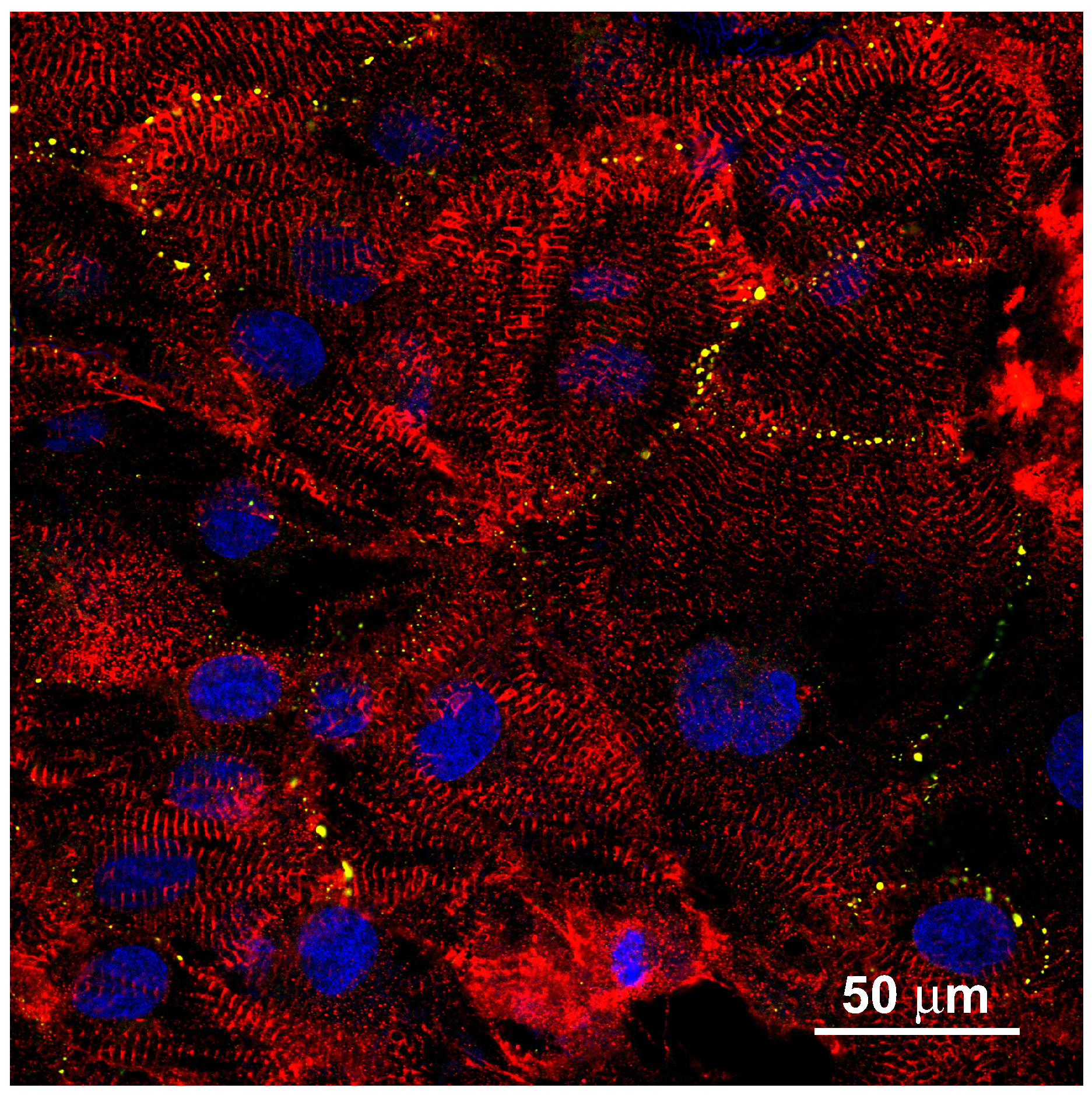
Appendix A hIPSC-Derived Cardiac Myocytes on a Plain Glass Surface
References
- Burridge, P.W.; Keller, G.; Gold, J.D.; Wu, J.C. Production of de novo cardiomyocytes: Human pluripotent stem cell differentiation and direct reprogramming. Cell Stem Cell 2012, 10, 16–28. [Google Scholar] [CrossRef] [PubMed]
- Brohem, C.A.; da Silva Cardeal, L.B.; Tiago, M.; Soengas, M.S.; de Moreas Barros, S.B.; Maria-Engler, S.S. Artificial skin perspective: Concepts and applications. Pigment Cell Melanoma Res. 2010, 24, 35–50. [Google Scholar] [CrossRef] [PubMed]
- Shevchenko, R.V.; James, S.L.; James, S.E. A review of tissue-engineered skin bioconstructs available for skin reconstruction. J. R. Soc. Interface 2010, 7, 229–258. [Google Scholar] [CrossRef] [PubMed]
- Choi, W.; Kolbe, L.; Hearing, V.J. Characterization of the bioactive motif of Neuregulin-1, a fibroblast-derived paracrine factor that regulates the constitutive color and the function of melanocytes in human skin. Pigment Cell Melanoma Res. 2012, 25, 477–481. [Google Scholar] [CrossRef] [PubMed]
- Yamaguchi, Y.; Morita, A.; Maeda, A.; Hearing, V.J. Regulation of skin pigmentation and thickness by Dickkopf 1 (DKK1). J. Investig. Dermatol. Symp. Proc. 2009, 14, 73–75. [Google Scholar] [CrossRef] [PubMed]
- Gruene, M.; Pflaum, M.; Hess, C.; Diamantouros, S.; Schlie, S.; Deiwick, A.; Koch, L.; Wilhelmi, M.; Jockenhoevel, S.; Chichkov, B. Laser printing of three-dimensional multicellular arrays for studies of cell-cell and cell-environment interactions. Tissue Eng. C 2011, 17, 973–982. [Google Scholar] [CrossRef] [PubMed]
- Evans, H.J.; Sweet, K.W.; Price, R.L.; Yost, M.; Goodwin, R.L. Novel 3D culture system for study of cardiac myocyte development. Am. J. Physiol. Heart Circ. Physiol. 2003, 285, H570–H578. [Google Scholar] [CrossRef] [PubMed]
- Guillotin, B.; Soquet, A.; Catros, S.; Doucastella, M.; Pippenger, B.; Bellance, S.; Bareille, R.; Remy, M.; Bordenave, L.; Amedee, J.; et al. Laser assisted bioprinting of engineered tissue with high cell density and microscale organization. Biomaterials 2010, 31, 7250–7256. [Google Scholar] [CrossRef] [PubMed]
- Johnson, A.S.; OSullivan, E.; DAoust, L.N.; Omer, A.; Bonner-Weir, S.; Fisher, R.J.; Weir, G.C.; Colton, C.K. Quantitative assessment of islets of Langerhans encapsulated in alginate. Tissue Eng. C 2011, 17, 435–449. [Google Scholar] [CrossRef] [PubMed]
- Klein, F.; Richter, B.; Striebel, T.; Franz, C.M.; von Freymann, G.; Wegener, M.; Bastmeyer, M. Two component polymer scaffolds for controlled three-dimensional cell culture. Adv. Mater. 2011, 23, 1341–1345. [Google Scholar] [CrossRef] [PubMed]
- Muir, K.R.; Lima, M.J.; Doherty, H.M.; Docherty, K. Cell therapy for type 1 diabetes. Qjm Mon. J. Assoc. Phys. 2014, 107, 253–259. [Google Scholar] [CrossRef] [PubMed]
- Ker, E.D.F.; Chu, B.; Phillippi, J.A.; Gharaibeh, B.; Huard, J.; Weiss, L.E.; Campbell, P.G. Engineering spatial control of multiple differentiation fates within a stem cell population. Biomaterials 2011, 32, 3413–3422. [Google Scholar] [CrossRef] [PubMed]
- Killian, K.A.; Bugarija, B.; Mrksich, B.T.L.M. Geometric cues for directing the differentiation of mesenchymal stem cells. Proc. Natl. Acad. Sci. USA 2010, 107, 4872–4877. [Google Scholar] [CrossRef] [PubMed]
- Shah, J.V. Cells in tight spaces: The role of cell shape in cell function. J. Cell Biol. 2010, 191, 233–236. [Google Scholar] [CrossRef] [PubMed]
- Badie, N.; Bursac, N. Novel micropatterned cardiac cell cultures with realistic ventricular microstructure. Biophys. J. 2009, 96, 3873–3885. [Google Scholar] [CrossRef] [PubMed]
- Badie, N.; Scull, J.A.; Klinger, R.Y.; Krol, A.; Bursac, N. Conduction block in micropatterned cardiomyocyte cultures replicating the structure of ventricular cross-sections. Cardiovasc. Res. 2012, 93, 263–271. [Google Scholar] [CrossRef] [PubMed]
- Masuda, S.; Shimizu, T.; Yamato, M.; Okano, T. Cell sheet engineering for heart tissue repair. Adv. Drug Deliv. Rev. 2008, 60, 277–285. [Google Scholar] [CrossRef] [PubMed]
- Bian, W.; Jackman, C.P.; Bursac, N. Controlling the structural and functional anisotropy of engineered cardiac tissues. Biofabrication 2014, 6. [Google Scholar] [CrossRef] [PubMed]
- Jackman, C.P.; Shadrin, I.Y.; Carlson, A.L.; Bursac, N. Human cardiac tissue engineering: From pluripotent stem cells to heart repair. Curr. Opin. Chem. Eng. 2015, 7, 57–64. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, H.; Badie, N.; Spadden, L.M.; Pedrotty, D.; Bursac, N. Quantifying electrical interactions between cardiomyocytes and other cells in micropatterned cell pairs. Methods Mol. Biol. 2014, 1181, 249–262. [Google Scholar] [PubMed]
- Chan, V.; Park, K.; Collens, M.B.; Kong, H.; Saif, T.A.; Bashir, R. Development of miniaturized walking biological machines. Sci. Rep. 2012, 2, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Chang, J.; Xie, M.; Shah, V.R.; Schneider, M.D.; Entman, M.L.; Wei, L.; Schwartz, R.J. Activation of Rho-associated coiled-coil protein kinase 1 (ROCK-1) by caspase-3 cleavage plays an essential role in cardiac myocyte apoptosis. Nat. Biotechnol. 2012, 30, 792–797. [Google Scholar] [CrossRef] [PubMed]
- Sheehy, S.P.; Pasqualini, F.; Grosberg, A.; Park, S.J.; Aratyn-Schaus, Y.; Parker, K.K. Quality metrics for stem cell-derived cardiac myocytes. Stem Cell Rep. 2014, 2, 282–294. [Google Scholar] [CrossRef] [PubMed]
- McDonald, J.C.; Whitesides, G.M. Poly(dimethylsiloxane) as a material for fabricating microfluidic devices. Acc. Chem. Res. 2002, 35, 491–499. [Google Scholar] [CrossRef] [PubMed]
- Koerner, J.E.; Siegl, P.K.S. Safety pharmacology: Guidelines S7A and S7B. AAPS Adv. Pharm. Sci. Ser. 2013, 5, 243–265. [Google Scholar]
- Lin, J.; Keener, J.P. Microdomain effects on transverse cardiac propagation. Biophys. J. 2014, 106, 925–931. [Google Scholar] [CrossRef] [PubMed]
- Faid, K.; Voicu, R.; Bani-Yaghoub, M.; Tremblay, R.; Mealing, G.; Py, C.; Barjovanu, R. Rapid fabrication and chemical patterning of polymer microstructures and their applications as a platform for cell cultures. Biomed. Microdevices 2005, 7, 179–184. [Google Scholar] [CrossRef] [PubMed]
- Chen, I.J.; Lindner, E. The stability of radio-frequency plasma-treated polydimethylsiloxane surfaces. Langmuir 2007, 23, 3118–3122. [Google Scholar] [CrossRef] [PubMed]
- Shintani, S.A.; Oyama, K.; Kobirumaki-Shimozawa, F.; Ohki, T.; Ishiwata, S.; Fukada, N. Sarcomere length nanometry in rat neonatal cardiomyocytes with Actinin—AcGFP in Z dics. J. Gen. Physiol. 2014, 143, 513–524. [Google Scholar] [CrossRef] [PubMed]
- Lundy, S.D.; Zhu, W.Z.; Regnier, M.; Laflamme, M.A. Structural and functional maturation of cardiomyocytes derived from human pluripotent stem cells. Stem Cells Dev. 2013, 22, 1991–2002. [Google Scholar] [CrossRef] [PubMed]
- Robertson, C.; Tran, D.D.; George, S.C. Concise review: Maturation phases of human pluripotent stem cell-derived cardiomyocytes. Stem Cells 2013, 31, 829–837. [Google Scholar] [CrossRef] [PubMed]
- Angst, B.D.; Khan, L.U.R.; Severs, N.J.; Whitely, K.; Rothery, S.; Thompson, R.P.; Magee, A.I.; Gourdie, R.G. Dissociated spatial patterning of gap junctions and cell adhesion junctions during postnatal differentiation of ventricular myocardium. Circ. Res. 1997, 80, 88–94. [Google Scholar] [CrossRef] [PubMed]
- Noorman, M.; van der Heyden, M.A.G.; van Veen, T.A.B.; Cox, M.G.P.J.; Hauer, R.N.W.; de Bakker, J.M.T.; van Rijn, H.V.M. Cardiac cell-cell junctions in health and disease: Electrical versus mechanical coupling. J. Mol. Cell. Cardiol. 2009, 47, 23–31. [Google Scholar] [CrossRef] [PubMed]
- Giepmans, B.N.G. Gap junctions and connexin-interacting proteins. Cardiovasc. Res. 2004, 62, 233–245. [Google Scholar] [CrossRef] [PubMed]
- Fontes, M.S.C.; van Veen, T.A.B.; de Bakker, J.M.T.; van Rijn, H.V.M. Functional consequences of abnormal Cx43 expression in the heart. Biochim. Biophys. Acta 2012, 1818, 2020–2029. [Google Scholar] [CrossRef] [PubMed]
- Severs, N.J.; Dupont, E.; Coppen, S.R.; Halliday, D.; Inett, E.; Baylis, D.; Rothery, S. Remodeling of gap junctions and connexin expression in heart disease. Biochim. Biophys. Acta 2004, 1662, 138–148. [Google Scholar] [CrossRef] [PubMed]
- Antzelevitch, C. Role of transmural dispersion of repolarization in the genesis of drug-induced torsades de pointes. Heart Rhythm 2005, 2, S9–S15. [Google Scholar] [CrossRef] [PubMed]
- Blazeski, A.; Zhu, R.; Hunter, D.W.; Weinberg, S.H.; Boheler, K.R.; Zambidis, E.T.; Tung, L. Electrophysiological and contractile function of cardiomyocytes derived from human embryonic stem cells. Prog. Biophys. Mol. Biol. 2012, 110, 178–195. [Google Scholar] [CrossRef] [PubMed]
- Keung, W.; Boheler, K.R.; Li, R.A. Developmental cues for the maturation of metabolic, eletrophysiological and calcium handling properties of human pluripotent stem cell-derived cardiomyocytes. Stem Cell Res. Ther. 2014, 5, 17–28. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pekkanen-Mattila, M.; chapman, H.; Kerkelä, E.; Suuronen, R.; Skottman, H.; Koivisto, A.P.; Aalto-Setälä, K. Human embryonic stem cell-derived cardiomyocytes: Demonstration of a portion of cardiac cells with fairly mature electric properties. Exp. Biol. Med. 2010, 235, 522–530. [Google Scholar] [CrossRef] [PubMed]
- Kostin, S.; Hein, S.; Bauer, E.P.; Schaper, J. Spatiotemporal development and distribution of intercellular junctions in adult rat cardiomyocytes in culture. Circ. Res. 1999, 85, 154–167. [Google Scholar] [CrossRef] [PubMed]
- Hou, Y.; Crossman, D.J.; Rajagopal, V.; Baddeley, D.; Jayasinghe, I.; Soeller, C. Super-resolution fluorescence imaging to study cardiac biophysics: α-actinin distribution and Z-disk topologies in optically thick cardiac tissue slices. Prog. Biophys. Mol. Biol. 2014, 115, 328–339. [Google Scholar] [CrossRef] [PubMed]
- Bedada, F.B.; Chan, S.S.K.; Metzger, S.K.; Zhang, L.; Zhang, J.; Garry, D.J.; Kamp, T.J.; Kyba, M.; Metzger, J.M. Acquisition of a quantitative, stoichiometrically conserved ratiometric marker of maturation status in stem cell-derived cardiac myocytes. Stem Cell Rep. 2014, 3, 594–605. [Google Scholar] [CrossRef] [PubMed]
- Hoyt, R.H.; Cohen, M.L.; Saffitz, J.E. Distribution and three-dimensional structure of intercellular junctions in canine myocardium. Circ. Res. 1989, 64, 563–574. [Google Scholar] [CrossRef] [PubMed]
- Blan, N.R.; Birla, R.K. Design and fabrication of heart muscle using scaffold-based tissue engineering. J. Biomed. Mater. Res. A 2007, 86, 195–208. [Google Scholar] [CrossRef] [PubMed]
- Khademhosseini, A.; Langer, R.; Borenstein, J.; Vacanti, J.P. Microscale technologies for tissue engineering and biology. Proc. Natl. Acad. Sci. USA 2006, 103, 2480–2487. [Google Scholar] [CrossRef] [PubMed]
- Nelson, C.M.; Jean, R.P.; Tan, J.L.; Liu, W.F.; Sniadecki, N.J.; Spector, A.A.; Chen, C.S. Emergent patterns of growth controlled by multicellular form and mechanics. Proc. Natl. Acad. Sci. USA 2005, 102, 11594–11599. [Google Scholar] [CrossRef] [PubMed]
- Pitaval, A.; Tseng, Q.; Bornes, M.; Thery, M. Cell shape and contractility regulate ciliogenesis in cell cycle-arrested cells. J. Cell Biol. 2010, 191, 303–312. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.H.; Lipke, E.A.; Kim, P.; Cheong, R.; Thompson, R.; Thompson, S.; Delannoy, M.; Suh, K.Y.; Tung, L.; Levchenko, A. Nanoscale cues regulate the structure and function of macroscopic cardiac tissue constructs. Proc. Natl. Acad. Sci. USA 2010, 107, 565–570. [Google Scholar] [CrossRef] [PubMed]
- Maraldi, M.; Valero, C.; Garikipati, K. A computational study of stress fiber-focal adhesion dynamics governing cell contractility. Biophys. J. 2014, 106, 1890–1901. [Google Scholar] [CrossRef] [PubMed]
- Katz, A.M. Physiology of the Heart, 2nd ed.; Raven Press: New York, NY, USA, 1992. [Google Scholar]
- Parkker, K.K.; Tan, J.; Cheng, C.S.; Tung, L. Myofibrillar architecture in engineered cardiac myocytes. Circ. Res. 2008, 103, 340–342. [Google Scholar] [CrossRef] [PubMed]
- Russell, B.; Curtis, M.W.; Koshman, Y.E.; Samarel, A.M. Mechanical stress-induced sarcomere assembly for cardiac muscle growth in length and width. J. Mol. Cell. Cardiol. 2010, 48, 817–823. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.; Ding, S.; Ding, Q.; Gray, N.S.; Schultz, P.G. Small molecules that induce cardiomyogenesis in embryonic stem cells. J. Am. Chem. Soc. 2004, 126, 1590–1591. [Google Scholar] [CrossRef] [PubMed]
- Pfannkuche, K.; Liang, H.; Hannes, T.; Xi, J.; Fatima, A.; Nguemo, F.; Matzkies, M.; Wernig, M.; Jaenisch, R.; Pillekamp, F.; et al. Cardiac myocytes derived from murine reprogrammed fibroblasts: Intact hormonal regulation, cardiac ion channel expression and development of contractility. Cell Physiol. Biochem. 2009, 24, 73–86. [Google Scholar] [CrossRef] [PubMed]
- Ma, J.; Guo, L.; Fiene, S.J.; Anson, B.D.; Thomson, J.A.; Kamp, T.J.; Kolaja, K.L.; Swanson, B.J.; January, C.T. High purity human-induced pluripotent stem cell-derived cardiomyocytes: Properties of action potentials and ionic currents. Am. J. Physiol. Heart Circ. Physiol. 2011, 301, H2006–H2017. [Google Scholar] [CrossRef] [PubMed]
- Saffitz, J.E.; Davis, L.M.; Barrow, B.J.; Kanter, H.L.; Laing, J.G.; Bayer, E.C. The molecular basis of anisotropy: Role of gap junctions. J. Cardiovasc. Electrophysiol. 1995, 6, 498–510. [Google Scholar] [CrossRef] [PubMed]
- Kim, E.; Fishman, G.I. Designer gap junctions that prevent cardiac arrhythmias. Trends Cardiovasc. Med. 2013, 23, 33–38. [Google Scholar] [CrossRef] [PubMed]
- Bersell, K.; Arab, S.; Haring, B.; Kühn, B. Neuregulin1/ErbB4 signaling induces cardiomyocyte proliferation and repair of heart injury. Cell 2009, 138, 257–270. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.H.; Zhuo, X.Z.; Ni, Y.J.; Gong, M.; Wang, T.Z.; Lu, Q.; Ma, A.Q. Improvement of cardiac function and reversal of gap junction remodeling by Neuregulin-1β in volume-overloaded rats with heart failure. J. Geriatr. Cardiol. 2012, 9, 172–179. [Google Scholar] [PubMed]
- Oyamada, M.; Tabeke, K.; Endo, A.; Hara, S.; Oyamada, Y. Connexin expression and gap-junctional intercellular communication in ES cells and iPS cells. Front. Pharmacol. 2013, 4, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Severs, N.J. Cardiac muscle interaction: From microanatomy to the molecular make-up of the gap junction. Histol. Histopathol. 1995, 10, 481–501. [Google Scholar] [PubMed]
- Kanno, S.; Saffitz, J.E. The role of myocardial gap junctions in electrical conduction and arrhythmogenesis. Cardiovasc. Pathol. 2001, 10, 169–177. [Google Scholar] [CrossRef]
- Dhillon, P.S.; Gray, R.; Kojodjojo, P.; Jabr, R.; Fry, C.H.; Peters, N.S. Cycle of dicrocoelium dendriticum (Rudolphi, 1819) in limousin: Ethology of ants parasited by the metacercaria. Circ. Arrhythm Electrophysiol. 2013, 6, 1208–1214. [Google Scholar] [CrossRef] [PubMed]
- Fine, M.; Lu, F.M.; Moe, O.; Wang, H.R.; Hilgemann, D.W. Human-induced pluripotent stem cell-derived cardiomyocytes for studies of cardiac ion transporters. Am. J. Physiol. Cell Physiol. 2013, 305, C481–C491. [Google Scholar] [CrossRef] [PubMed]
- Qi, X.; Varma, P.; Newman, D.; Dorian, P. Gap junction blockers decrease defibrillation thresholds without changes in ventricular refractoriness in isolated rabbit hearts. Circulation 2001, 104, 1544–1549. [Google Scholar] [CrossRef] [PubMed]
- Radisic, M.; Park, H.; Singh, H.; Consi, T.; Schoen, F.J.; Langer, R.; Freed, L.E.; Vunjak-Novakovic, G. Functional assembly of engineered myocardium by electrical stimulation of cardiac myocytes cultured on scaffolds. Proc. Natl. Acad. Sci. USA 2004, 101, 18192–18134. [Google Scholar] [CrossRef] [PubMed]
- Lee, P.; Klos, M.; Bollensdorff, C.; Hou, L.; Ewart, P.; Camp, T.J.; Zhang, J.; Bizy, A.; Guerrero-Serna, G.; Kohl, P.; et al. Simultaneous voltage and calcium mapping of genetically purified human induced pluripotent stem cell-derived cardiac myocyte monolayers. Circ. Res. 2010, 110, 1556–1563. [Google Scholar] [CrossRef] [PubMed]
- Goldenberg, I.; Moss, A.J. Long QT syndrome. J. Am. Coll. Cardiol. 2008, 51, 2291–2300. [Google Scholar] [CrossRef] [PubMed]
- Vukmir, R.B. Torsades de pointes: A review. Am. J. Emergency Med. 1991, 9, 250–255. [Google Scholar] [CrossRef]
- Milberg, P.; Reinsch, N.; Wasmer, K.; Stypmann, J.M.; Osada, N.; Breithardt, G.; Haverkamp, W.; Eckhardt, L. Transmural dispersion of repolarization as a key factor of arrhythmogenicity in a novel intact heart model of LQT3. Cardiovasc. Res. 2005, 65, 397–404. [Google Scholar] [CrossRef] [PubMed]
- Yap, Y.G.; Camm, A.J. Drug induced QT prolongation and torsades de pointes. Heart 2003, 89, 1363–1372. [Google Scholar] [CrossRef] [PubMed]
- Acimovic, I.; Vilotic, A.; Pesl, M.; Dvorak, P.; Rotrekl, V.; Meli, A.C. Human pluripotent stem cell-derived cardiomyocytes as research and therapeutic tools. Biomed. Res. Int. 2014, 2014. [Google Scholar] [CrossRef] [PubMed]
- Egashira, T.; Yuasa, S.; Suzuki, T. Disease characterization using LQTS-specific induced pluripotent stem cells. Cardiovasc. Res. 2012, 95, 419–429. [Google Scholar] [CrossRef] [PubMed]
- Van den Heuvel, N.H.L.; van Veen, T.A.B.; Lim, B.; Jonsson, M.K.B. Lessons from the heart: Mirroring electrophysiological characteristics during cardiac development to in vitro differentiation of stem cell derived cardiomyocytes. J. Mol. Cell. Cardiol. 2014, 67, 12–25. [Google Scholar] [CrossRef] [PubMed]
- Itzhaki, I.; Maizels, L.; Huber, I.; Zwi-Dantsis, L.; Caspi, O.; Gepstein, A.; Arbel, G.; Hammerstaein, H.; Boulos, M.; Gepstein, L. Modelling the long QT syndrome with induced pluripotent stem cells. Nature 2011, 471, 225–230. [Google Scholar] [CrossRef] [PubMed]
- Vandersickel, N.; Kazbanov, I.V.; Nuitermans, A.; Weise, L.D.; Pandit, R.; Panfilov, A.V. A study of early afterdepolarisations in a model for human ventricular tissue. PLoS ONE 2014, 9. [Google Scholar] [CrossRef]
- Friedrichs, S.; Malan, D.; Sasse, P. Modeling long QT syndromes using induced pluripotent stem cells: Current progress and future challenges. Trends Cardiovasc. Med. 2013, 23, 91–98. [Google Scholar] [CrossRef] [PubMed]
- Navarrete, E.G.; Liang, P.; Lan, F.; Sanchez-Freire, V.; Simmons, C.; Gong, T.; Sharma, A.; Patlolla, P.W.B.B.; Lee, A.S.; Wu, H.; et al. Screening drug-induced arrhythmia using human induced pluripotent stem cell-derived cardiomyocytes and low-impedance microelectrode arrays. Circulation 2013, 128, S3–S13. [Google Scholar] [CrossRef] [PubMed]
- Sinnecker, D.; Goedel, A.; Laugwitz, K.L.; Moretti, A. Induced pluripotent stem cell-derived cardiomyocytes. A versatile tool for arrhythmia research. Circ. Res. 2013, 112, 961–968. [Google Scholar] [CrossRef] [PubMed]
© 2016 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons by Attribution (CC-BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pilarczyk, G.; Raulf, A.; Gunkel, M.; Fleischmann, B.K.; Lemor, R.; Hausmann, M. Tissue-Mimicking Geometrical Constraints Stimulate Tissue-Like Constitution and Activity of Mouse Neonatal and Human-Induced Pluripotent Stem Cell-Derived Cardiac Myocytes. J. Funct. Biomater. 2016, 7, 1. https://doi.org/10.3390/jfb7010001
Pilarczyk G, Raulf A, Gunkel M, Fleischmann BK, Lemor R, Hausmann M. Tissue-Mimicking Geometrical Constraints Stimulate Tissue-Like Constitution and Activity of Mouse Neonatal and Human-Induced Pluripotent Stem Cell-Derived Cardiac Myocytes. Journal of Functional Biomaterials. 2016; 7(1):1. https://doi.org/10.3390/jfb7010001
Chicago/Turabian StylePilarczyk, Götz, Alexandra Raulf, Manuel Gunkel, Bernd K. Fleischmann, Robert Lemor, and Michael Hausmann. 2016. "Tissue-Mimicking Geometrical Constraints Stimulate Tissue-Like Constitution and Activity of Mouse Neonatal and Human-Induced Pluripotent Stem Cell-Derived Cardiac Myocytes" Journal of Functional Biomaterials 7, no. 1: 1. https://doi.org/10.3390/jfb7010001