Migration and Diffusion of Heavy Metal Cu from the Interior of Sediment during Wave-Induced Sediment Liquefaction Process
Abstract
:1. Introduction
2. Materials and Methods
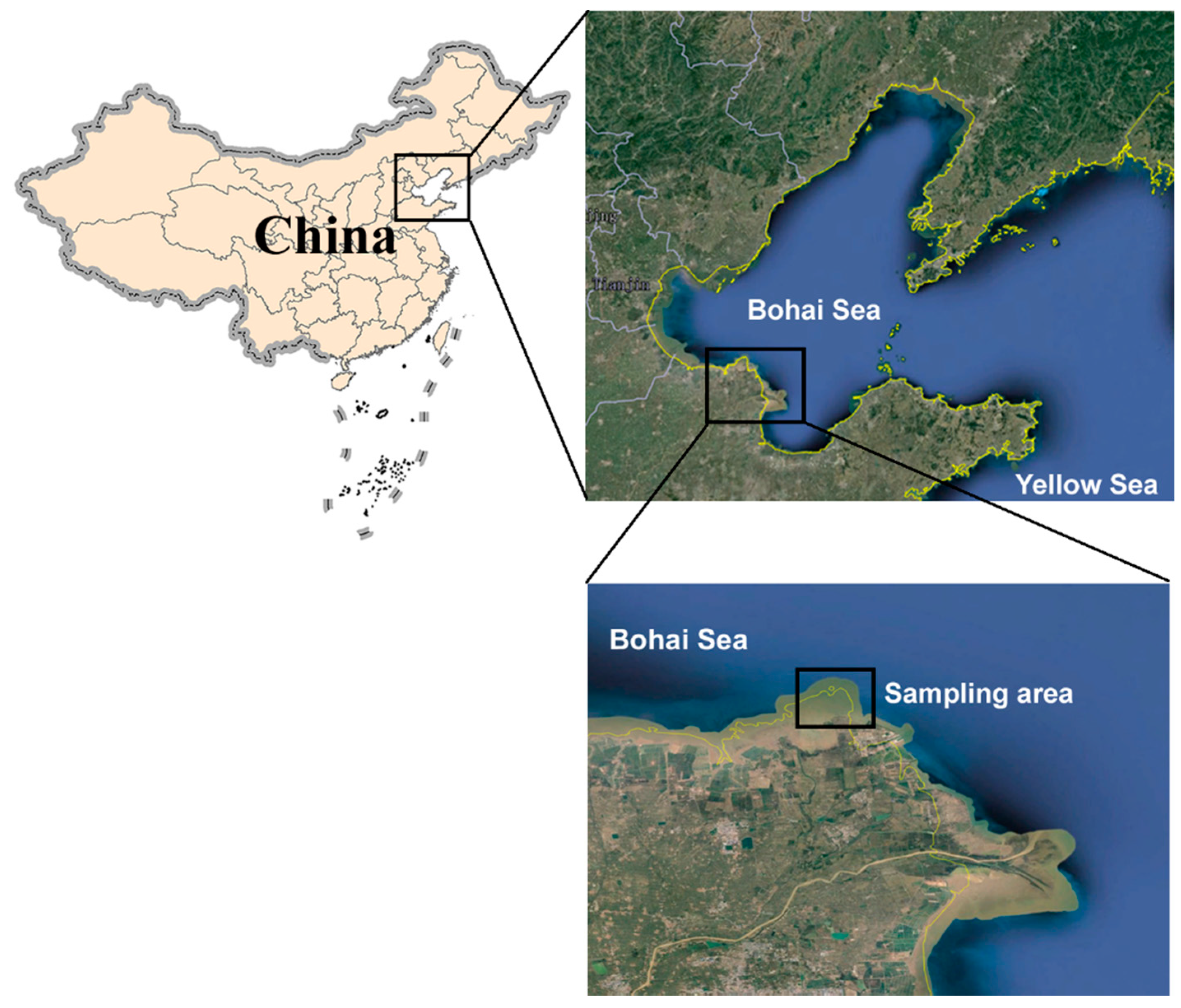
2.1. Study Area
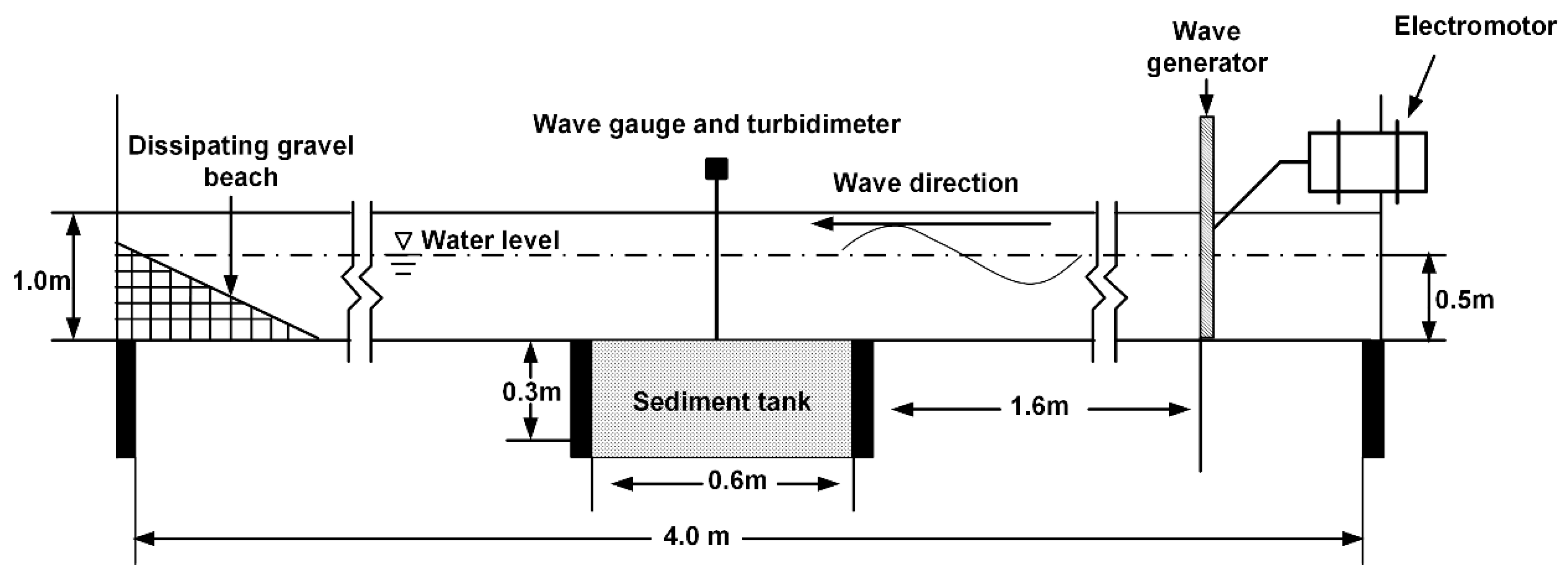
2.2. Experimental Facilities
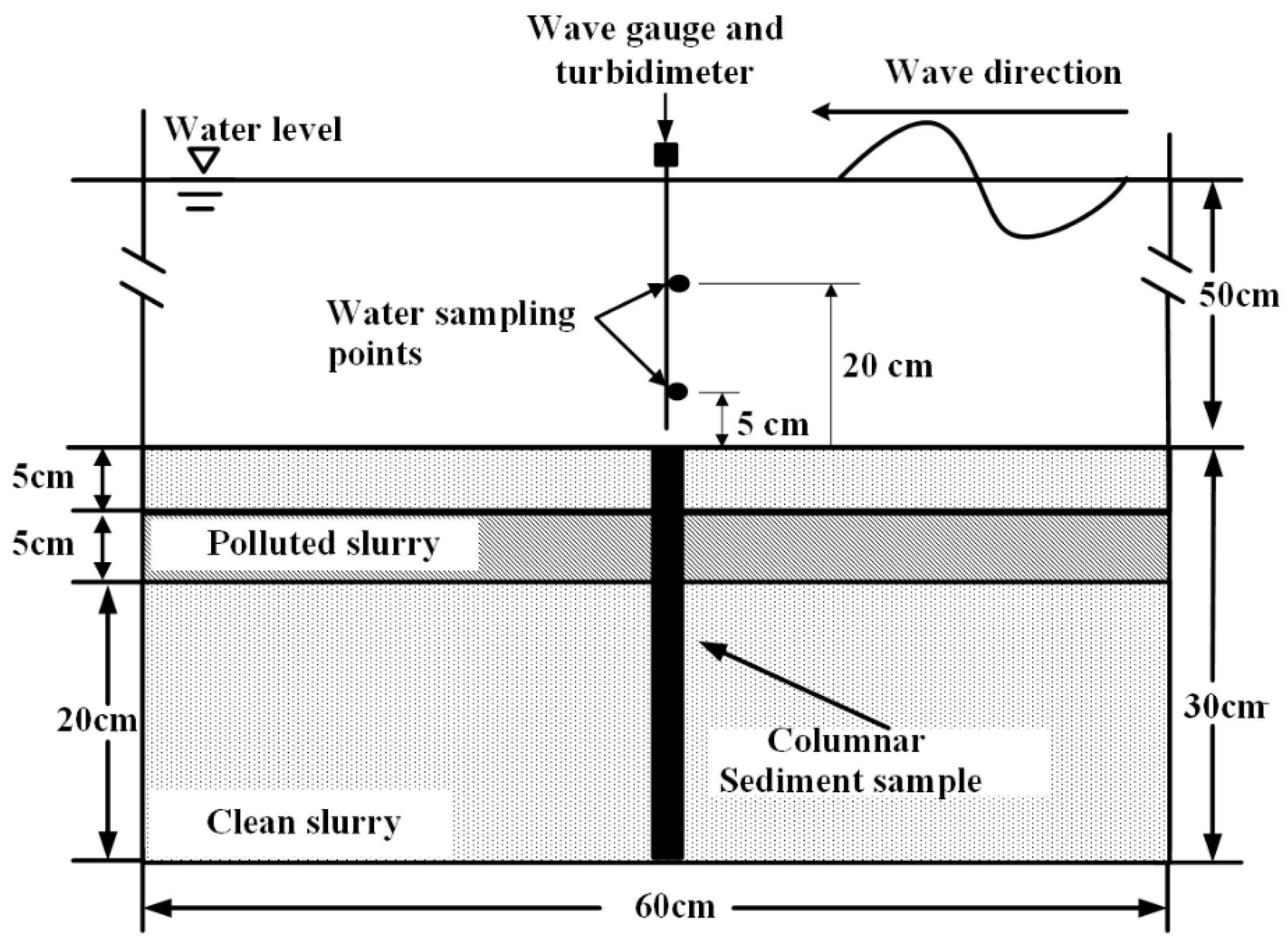
2.3. Experimental Preparation and Procedures
2.3.1. Sediment Preparation
2.3.2. Wave Flume Experiment Processes
2.4. Analytical Methods of Cu Concentrations in Water and Sediment Samples
2.5. Quality Control
3. Results
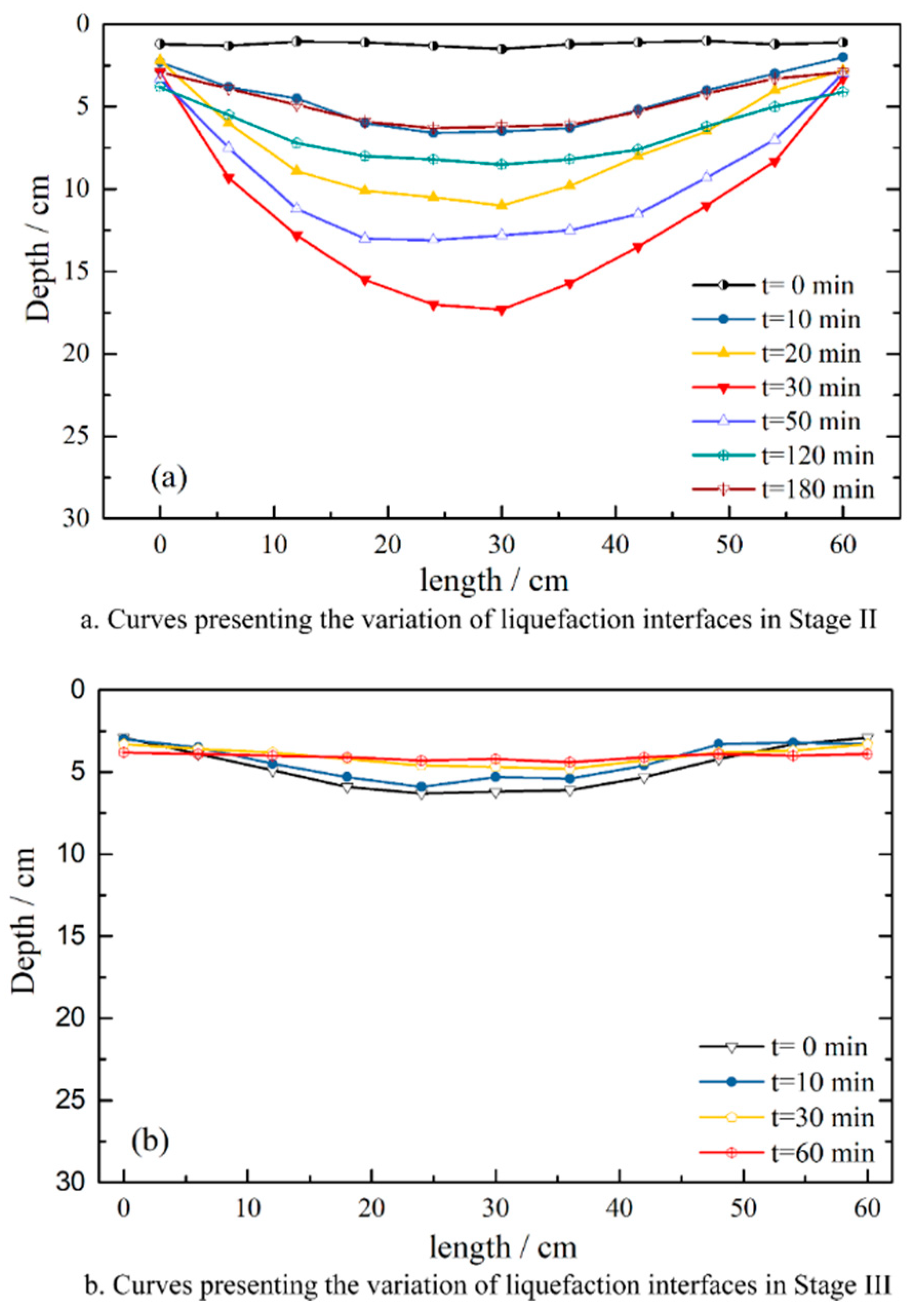
3.1. Sediment Liquefaction Process under Wave Action
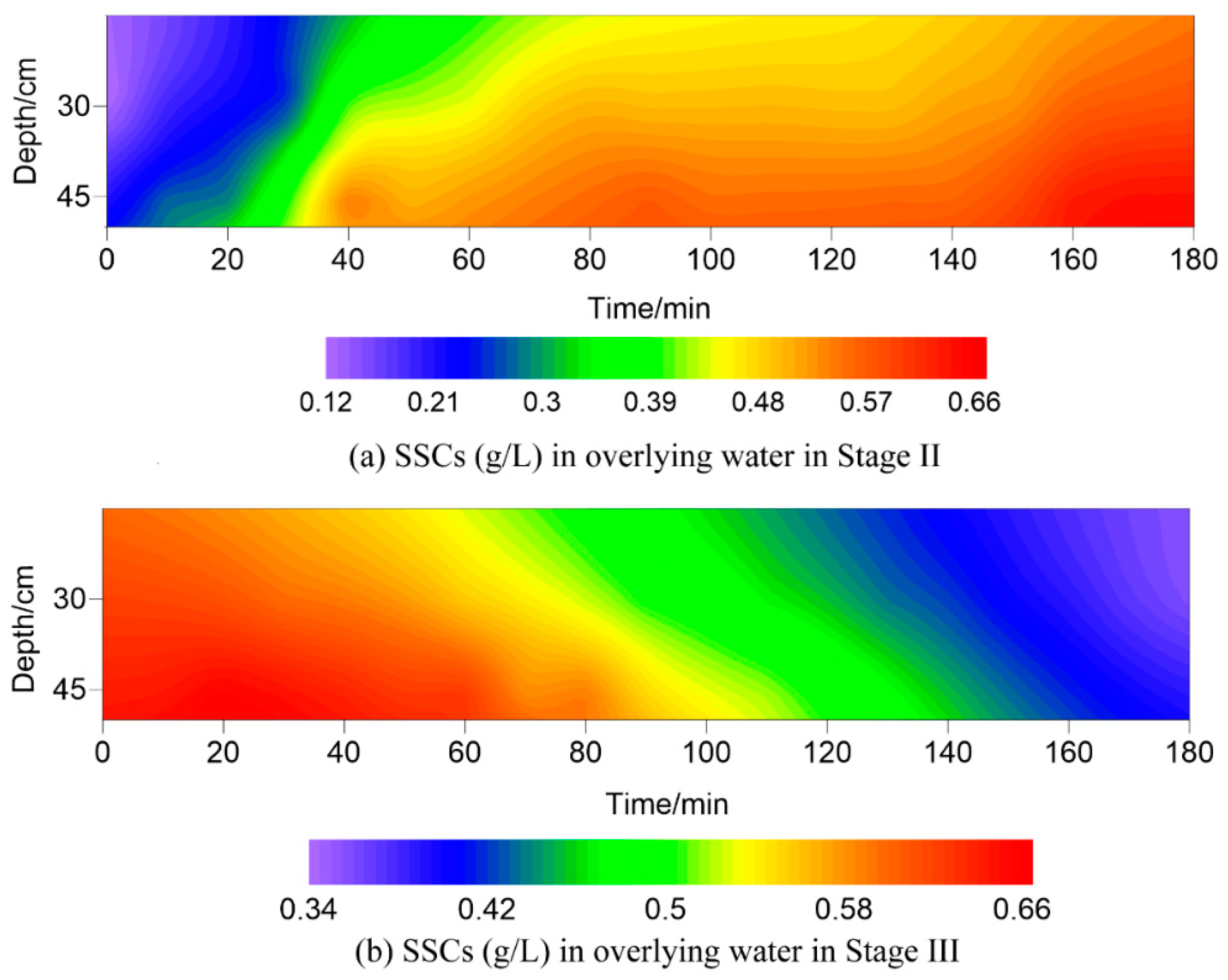
3.2. Variation of Suspended Sediments Concentrations (SSCs) in Overlying Water
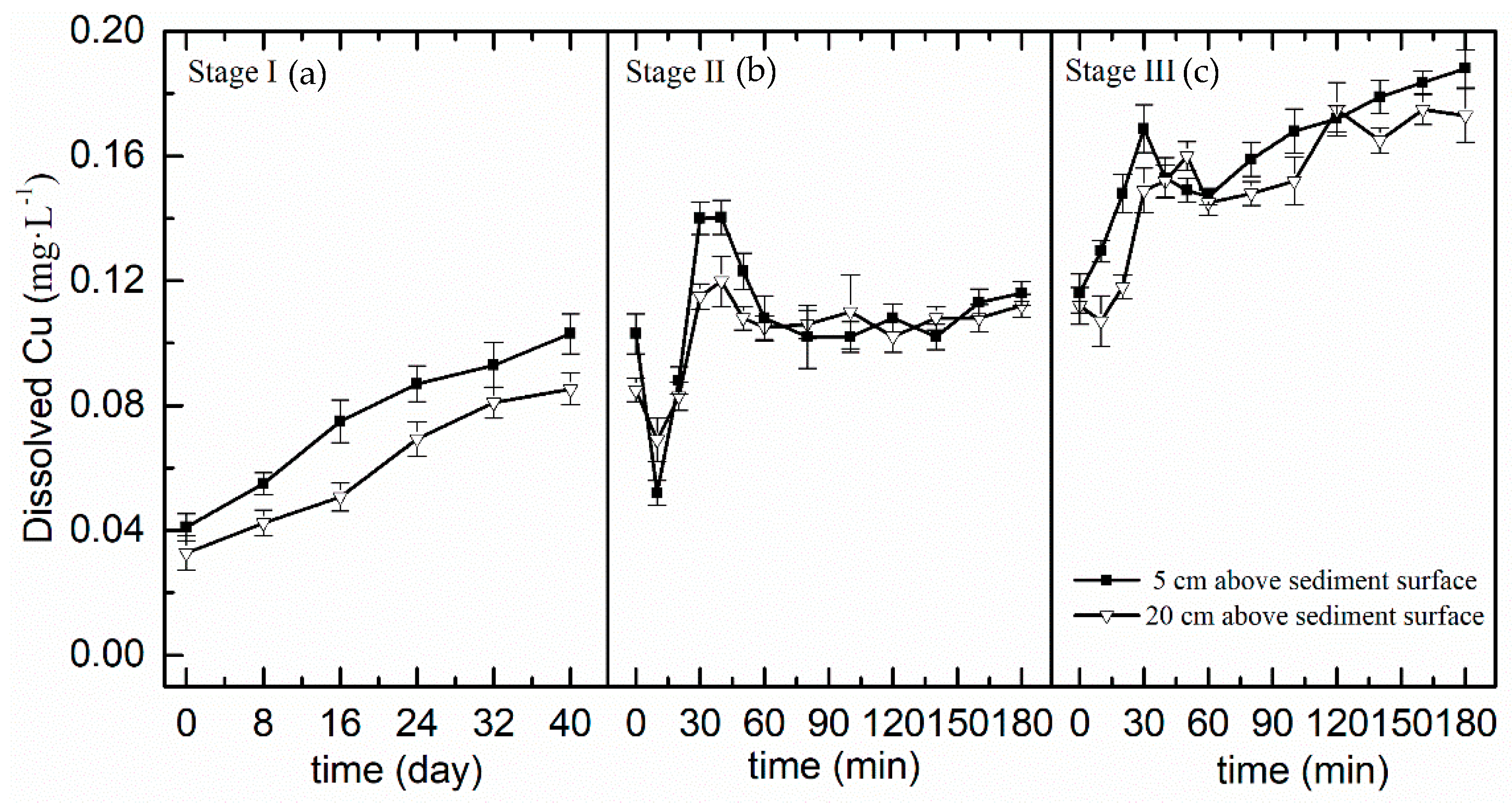
3.3. Variation of Dissolved Cu Concentration in the Overlying Water
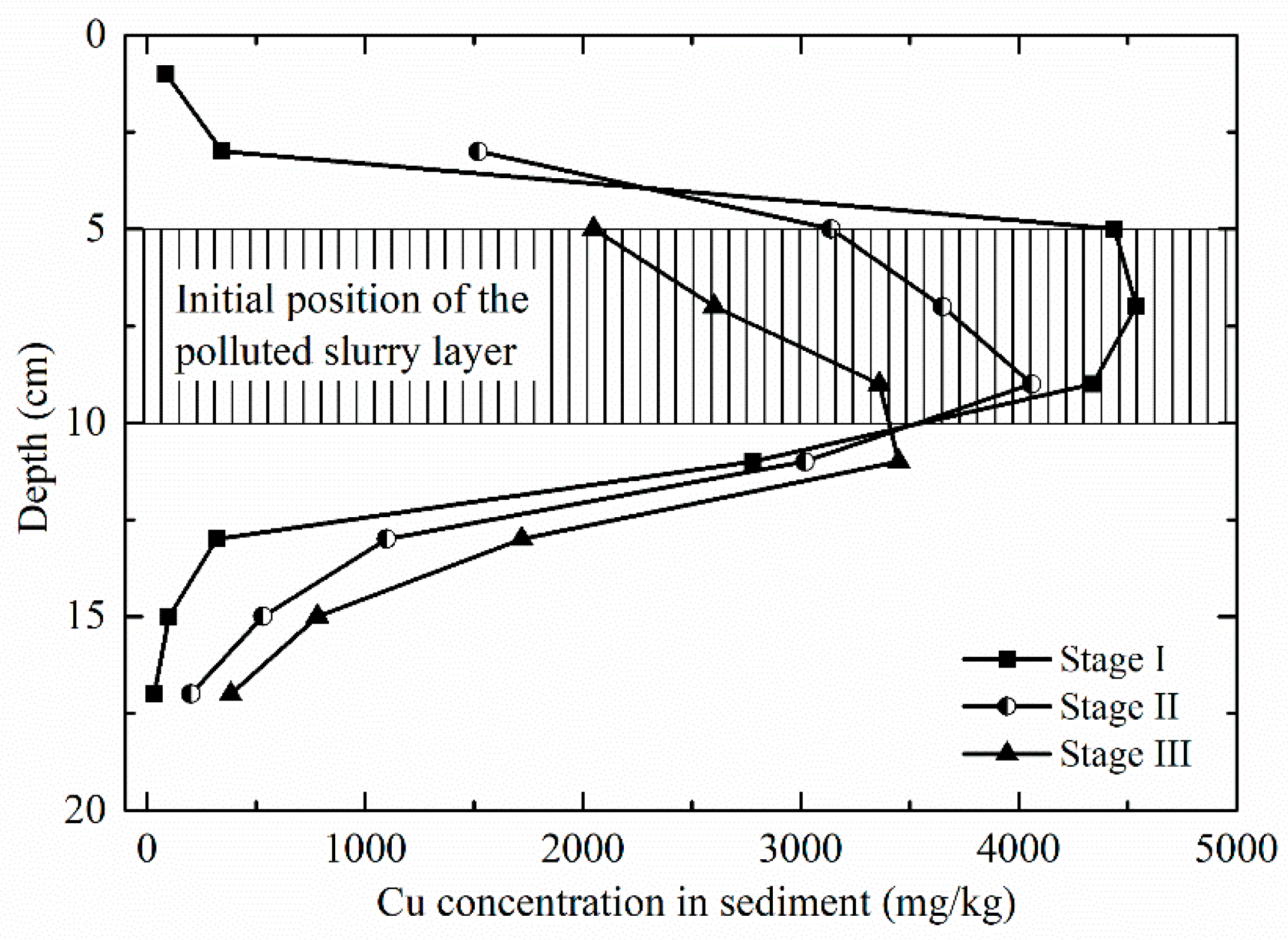
3.4. Vertical Distribution and Change of Cu Concentration in Sediment
4. Discussion
4.1. Variations of SSCs in the Overlying Water
4.2. Dissolved Cu Concentration Variations in the Overlying Water
4.3. Cu Concentration Profile in the Sediment after Each Wave Load
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Hu, C.; Dong, J.; Gao, L.; Yang, X.; Wang, Z.; Zhang, X. Macrobenthos functional trait responses to heavy metal pollution gradients in a temperate lagoon. Environ. Pollut. 2019, 253, 1107–1116. [Google Scholar] [CrossRef] [PubMed]
- Wu, W.; Wu, P.; Yang, F.; Sun, D.-L.; Zhang, D.-X.; Zhou, Y.-K. Assessment of heavy metal pollution and human health risks in urban soils around an electronics manufacturing facility. Sci. Total Environ. 2018, 630, 53–61. [Google Scholar] [CrossRef] [PubMed]
- Xia, P.; Meng, X.; Feng, A.; Yin, P.; Zhang, J.; Wang, X. Geochemical characteristics of heavy metals in coastal sediments from the northern Beibu Gulf (SW China): The background levels and recent contamination. Environ. Earth Sci. 2012, 66, 1337–1344. [Google Scholar] [CrossRef]
- Barbieri, M.; Sappa, G.; Nigro, A. Soil pollution: Anthropogenic versus geogenic contributions over large areas of the Lazio region. J. Geochem. Explor. 2018, 195, 78–86. [Google Scholar] [CrossRef]
- Barbieri, M.; Sappa, G.; Vitale, S.; Parisse, B.; Battistel, M. Soil control of trace metals concentrations in landfills: A case study of the largest landfill in Europe, Malagrotta, Rome. J. Geochem. Explor. 2014, 143, 146–154. [Google Scholar] [CrossRef]
- Xia, P.; Meng, X.; Feng, A.; Yin, P.; Wang, X.; Zhang, J. 210Pb chronology and trace metal geochemistry in the intertidal sediment of Qinjiang River estuary, China. J. Ocean Univ. China 2012, 11, 165–173. [Google Scholar] [CrossRef]
- Surricchio, G.; Pompilio, L.; Arizzi Novelli, A.; Scamosci, E.; Marinangeli, L.; Tonucci, L.; d’Alessandro, N.; Tangari, A.C. Evaluation of heavy metals background in the Adriatic Sea sediments of Abruzzo region, Italy. Sci. Total Environ. 2019, 684, 445–457. [Google Scholar] [CrossRef]
- Valdés, J.; Tapia, J.S. Spatial monitoring of metals and as in coastal sediments of northern Chile: An evaluation of background values for the analysis of local environmental conditions. Mar. Pollut. Bull. 2019, 145, 624–640. [Google Scholar] [CrossRef]
- Ruiz-Fernández, A.C.; Sanchez-Cabeza, J.A.; Pérez-Bernal, L.H.; Gracia, A. Spatial and temporal distribution of heavy metal concentrations and enrichment in the southern Gulf of Mexico. Sci. Total Environ. 2019, 651, 3174–3186. [Google Scholar] [CrossRef]
- Wang, J.; Ye, S.; Laws, E.A.; Yuan, H.; Ding, X.; Zhao, G. Surface sediment properties and heavy metal pollution assessment in the Shallow Sea Wetland of the Liaodong Bay, China. Mar. Pollut. Bull. 2017, 120, 347–354. [Google Scholar] [CrossRef]
- Zhuang, Q.; Li, G.; Liu, Z. Distribution, source and pollution level of heavy metals in river sediments from South China. CATENA 2018, 170, 386–396. [Google Scholar] [CrossRef]
- Ogunlaja, A.; Ogunlaja, O.O.; Okewole, D.M.; Morenikeji, O.A. Risk assessment and source identification of heavy metal contamination by multivariate and hazard index analyses of a pipeline vandalised area in Lagos State, Nigeria. Sci. Total Environ. 2019, 651, 2943–2952. [Google Scholar] [CrossRef] [PubMed]
- Lu, F. Eco-Environmental Impact of Shengli Chengdao Offshore Oil Exploration and Development. Master’s Thesis, Ocean University of China, Qingdao, China, 2010. [Google Scholar]
- Dou, Y.; Li, J.; Zhao, J.; Hu, B.; Yang, S. Distribution, enrichment and source of heavy metals in surface sediments of the eastern Beibu Bay, South China Sea. Mar. Pollut. Bull. 2013, 67, 137–145. [Google Scholar] [CrossRef] [PubMed]
- Unda-Calvo, J.; Ruiz-Romera, E.; Fdez-Ortiz de Vallejuelo, S.; Martínez-Santos, M.; Gredilla, A. Evaluating the role of particle size on urban environmental geochemistry of metals in surface sediments. Sci. Total Environ. 2019, 646, 121–133. [Google Scholar] [CrossRef] [PubMed]
- Whipple, A.C.; Luettich, R.A.; Reynolds-Fleming, J.V.; Neve, R.H. Spatial differences in wind-driven sediment resuspension in a shallow, coastal estuary. Estuar. Coast. Shelf Sci. 2018, 213, 49–60. [Google Scholar] [CrossRef]
- Jalil, A.; Li, Y.; Zhang, K.; Gao, X.; Wang, W.; Khan, H.O.S.; Pan, B.; Ali, S.; Acharya, K. Wind-induced hydrodynamic changes impact on sediment resuspension for large, shallow Lake Taihu, China. Int. J. Sediment Res. 2019, 34, 205–215. [Google Scholar] [CrossRef]
- Schoellhamer, D.H. Sediment Resuspension Mechanisms in Old Tampa Bay, Florida. Estuar. Coast. Shelf Sci. 1995, 40, 603–620. [Google Scholar] [CrossRef]
- Zheng, S.-S.; Wang, P.-F.; Wang, C.; Hou, J. Sediment resuspension under action of wind in Taihu Lake, China. Int. J. Sediment Res. 2015, 30, 48–62. [Google Scholar] [CrossRef]
- Bolaños, R.; Thorne, P.D.; Wolf, J. Comparison of measurements and models of bed stress, bedforms and suspended sediments under combined currents and waves. Coast. Eng. 2012, 62, 19–30. [Google Scholar] [CrossRef] [Green Version]
- Wolanski, E.; Spagnol, S. Dynamics of the turbidity maximum in King Sound, tropical Western Australia. Estuar. Coast. Shelf Sci. 2003, 56, 877–890. [Google Scholar] [CrossRef]
- Jia, Y.; Zhang, L.; Zheng, J.; Liu, X.; Jeng, D.-S.; Shan, H. Effects of wave-induced seabed liquefaction on sediment re-suspension in the Yellow River Delta. Ocean Eng. 2014, 89, 146–156. [Google Scholar] [CrossRef]
- Tzang, S.-Y.; Ou, S.-H.; Hsu, T.-W. Laboratory flume studies on monochromatic wave-fine sandy bed interactions Part 2. Sediment suspensions. Coast. Eng. 2009, 56, 230–243. [Google Scholar] [CrossRef]
- Zhang, S.T.; Jia, Y.G.; Wang, Z.H.; Wen, M.Z.; Lu, F.; Zhang, Y.Q.; Liu, X.L.; Shan, H.X. Wave flume experiments on the contribution of seabed fluidization to sediment resuspension. Acta Oceanol. Sin. 2018, 37, 80–87. [Google Scholar] [CrossRef]
- Liu, X.-L.; Jia, Y.-G.; Zheng, J.-W.; Hou, W.; Zhang, L.; Zhang, L.-P.; Shan, H.-X. Experimental evidence of wave-induced inhomogeneity in the strength of silty seabed sediments: Yellow River Delta, China. Ocean Eng. 2013, 59, 120–128. [Google Scholar] [CrossRef]
- Song, Y.; Sun, Y.; Du, X.; Cao, C.; Shuling, L. Monitoring of silt pore pressure responding precess to wave action. Mar. Geol. Quat. Geol. 2018, 38, 208–214. [Google Scholar]
- Wang, H.; Zhao, Y.; Wang, X.; Liang, D. Fluctuations in Cd release from surface-deposited sediment in a river-connected lake following dredging. J. Geochem. Explor. 2017, 172, 184–194. [Google Scholar] [CrossRef]
- Ciffroy, P.; Monnin, L.; Garnier, J.-M.; Ambrosi, J.-P.; Radakovitch, O. Modelling geochemical and kinetic processes involved in lead (Pb) remobilization during resuspension events of contaminated sediments. Sci. Total Environ. 2019, 679, 159–171. [Google Scholar] [CrossRef]
- Lu, J.; Jiang, J.; Li, A.; Ma, X. Impact of Typhoon Chan-hom on the marine environment and sediment dynamics on the inner shelf of the East China Sea: In-situ seafloor observations. Mar. Geol. 2018, 406, 72–83. [Google Scholar] [CrossRef]
- Superville, P.-J.; Prygiel, E.; Magnier, A.; Lesven, L.; Gao, Y.; Baeyens, W.; Ouddane, B.; Dumoulin, D.; Billon, G. Daily variations of Zn and Pb concentrations in the Deûle River in relation to the resuspension of heavily polluted sediments. Sci. Total Environ. 2014, 470–471, 600–607. [Google Scholar] [CrossRef]
- Cantwell, M.G.; Burgess, R.M.; King, J.W. Resuspension of contaminated field and formulated reference sediments Part I: Evaluation of metal release under controlled laboratory conditions. Chemosphere 2008, 73, 1824–1831. [Google Scholar] [CrossRef]
- Zheng, S.; Wang, P.; Wang, C.; Hou, J.; Qian, J. Distribution of metals in water and suspended particulate matter during the resuspension processes in Taihu Lake sediment, China. Quat. Int. 2013, 286, 94–102. [Google Scholar] [CrossRef]
- Huang, J.; Ge, X.; Wang, D. Distribution of heavy metals in the water column, suspended particulate matters and the sediment under hydrodynamic conditions using an annular flume. J. Environ. Sci. 2012, 24, 2051–2059. [Google Scholar] [CrossRef]
- Sun, Z.; Xu, G.; Hao, T.; Huang, Z.; Fang, H.; Wang, G. Release of heavy metals from sediment bed under wave-induced liquefaction. Mar. Pollut. Bull. 2015, 97, 209–216. [Google Scholar] [CrossRef] [PubMed]
- State Oceanic Administration. Pretreatment Guideline of Heavy Metals Analysis in the Marine Sediments and Organisms-Microwave Assisted Acid Digestion (HY/T 132-2010); China Standards Press: Beijing, China, 2010.
- Kong, Y.-Z.; Zhu, C.-F. Experimental study on vertical sediment mixing coefficient under waves. J. East China Norm. Univ. (Nat. Sci.) 2008, 6, 9–13. (In Chinese) [Google Scholar]
- Liu, X.-L.; Cui, H.-N.; Jeng, D.-S.; Zhao, H.-Y. A coupled mathematical model for accumulation of wave-induced pore water pressure and its application. Coast. Eng. 2019, 154, 103577. [Google Scholar] [CrossRef]
- Sumer, B.M.; Kirca, V.S.O.; Fredsøe, J. Experimental Validation of a Mathematical Model for Seabed Liquefaction under Waves. Int. J. Offshore Polar Eng. 2011, 22, 133–141. [Google Scholar] [CrossRef]
- Zhang, S.T.; Jia, Y.G.; Zhang, Y.Q.; Liu, X.L.; Shan, H.X. In situ observations of wave pumping of sediments in the Yellow River Delta with a newly developed benthic chamber. Mar. Geophys. Res. 2018, 39, 463–474. [Google Scholar] [CrossRef]
- Tzang, S.-Y.; Ou, S.-H. Laboratory flume studies on monochromatic wave-fine sandy bed interactions: Part 1. Soil fluidization. Coast. Eng. 2006, 53, 965–982. [Google Scholar] [CrossRef]
- Dang, D.H.; Lenoble, V.; Durrieu, G.; Omanović, D.; Mullot, J.U.; Mounier, S.; Garnier, C. Seasonal variations of coastal sedimentary trace metals cycling: Insight on the effect of manganese and iron (oxy)hydroxides, sulphide and organic matter. Mar. Pollut. Bull. 2015, 92, 113–124. [Google Scholar] [CrossRef]
- Bi, C.-J.; Chen, Z.-L.; Shen, J.; Sun, W.-W. Transfer and release of Hg from the Yangtze Estuarine Sediment during Sediment Resuspension Event. Environ. Sci. 2009, 30, 3256–3261. (In Chinese) [Google Scholar]
- Hao, T.; Guo-Hui, X.U.; Wang, G.; Zhang, C.Y. Laboratory Flume Study on Heavy Metal Release from Liquefied Bed Sediments. Period. Ocean Univ. China 2013, 8, 92–98. [Google Scholar]
- Villaescusa-Celaya, J.A.; Gutiérrez-Galindo, E.A.; Flores-Muñoz, G. Heavy metals in the fine fraction of coastal sediments from Baja California (Mexico) and California (USA). Environ. Pollut. 2000, 108, 453–462. [Google Scholar] [CrossRef]
- Xu, G.H.; Sun, Y.F.; Yu, Y.Q.; Lin, L.; Hu, G.H.; Zhao, Q.P.; Guo, X.J. Discussion on storm-induced liquefaction of the superficial stratum in the Yellow River subaqueous delta. Mar. Sci. Bull. 2012, 1, 80–89. [Google Scholar]
- Li, M.; Bi, C.; Zhang, J.; Lv, J.; Chen, Z. Laboratory simulation study on the influence of resuspension on the release of Mercury from Yangtze Estuarine Tidal Flat. Environ. Sci. 2011, 32, 3318–3326. [Google Scholar] [CrossRef]
| Stages | Wave Height/cm | Wave Period/s | Duration | Sampling Intervals |
|---|---|---|---|---|
| Static diffusion (Stage I) | - | - | 40 h | 8 h |
| 7 cm wave height (Stage II) | 7.0 | 2.3 | 180 min | every 10 min for the first 60 min and every 20 min for the last 120 min |
| 13.5 cm wave height (Stage III) | 13.5 | 1.2 | 180 min |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lu, F.; Zhang, H.; Jia, Y.; Liu, W.; Wang, H. Migration and Diffusion of Heavy Metal Cu from the Interior of Sediment during Wave-Induced Sediment Liquefaction Process. J. Mar. Sci. Eng. 2019, 7, 449. https://doi.org/10.3390/jmse7120449
Lu F, Zhang H, Jia Y, Liu W, Wang H. Migration and Diffusion of Heavy Metal Cu from the Interior of Sediment during Wave-Induced Sediment Liquefaction Process. Journal of Marine Science and Engineering. 2019; 7(12):449. https://doi.org/10.3390/jmse7120449
Chicago/Turabian StyleLu, Fang, Haoqing Zhang, Yonggang Jia, Wenquan Liu, and Hui Wang. 2019. "Migration and Diffusion of Heavy Metal Cu from the Interior of Sediment during Wave-Induced Sediment Liquefaction Process" Journal of Marine Science and Engineering 7, no. 12: 449. https://doi.org/10.3390/jmse7120449




