First Record of Lepidodinium chlorophorum and the Associated Phytoplankton Community Responsible of the Green Tide South Western Mediterranean Sea (Hammam-Lif, Tunisia)
Abstract
1. Introduction
2. Materials and Methods
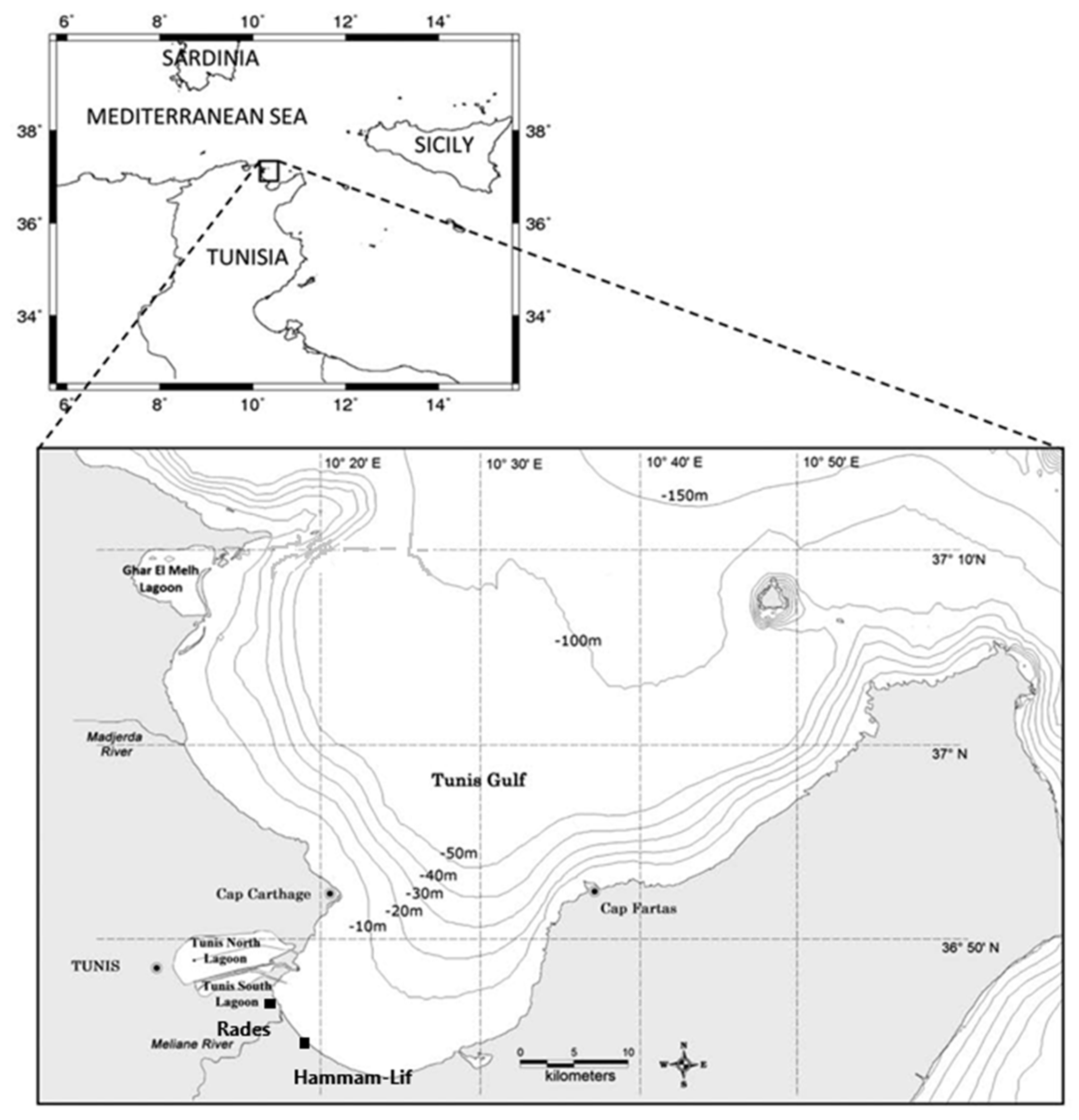
2.1. Study Area
2.2. Sampling Strategy and Physico-Chemical Parameters
2.3. Preparation of Nucleic Acids, PCR, Cloning, and Sequencing
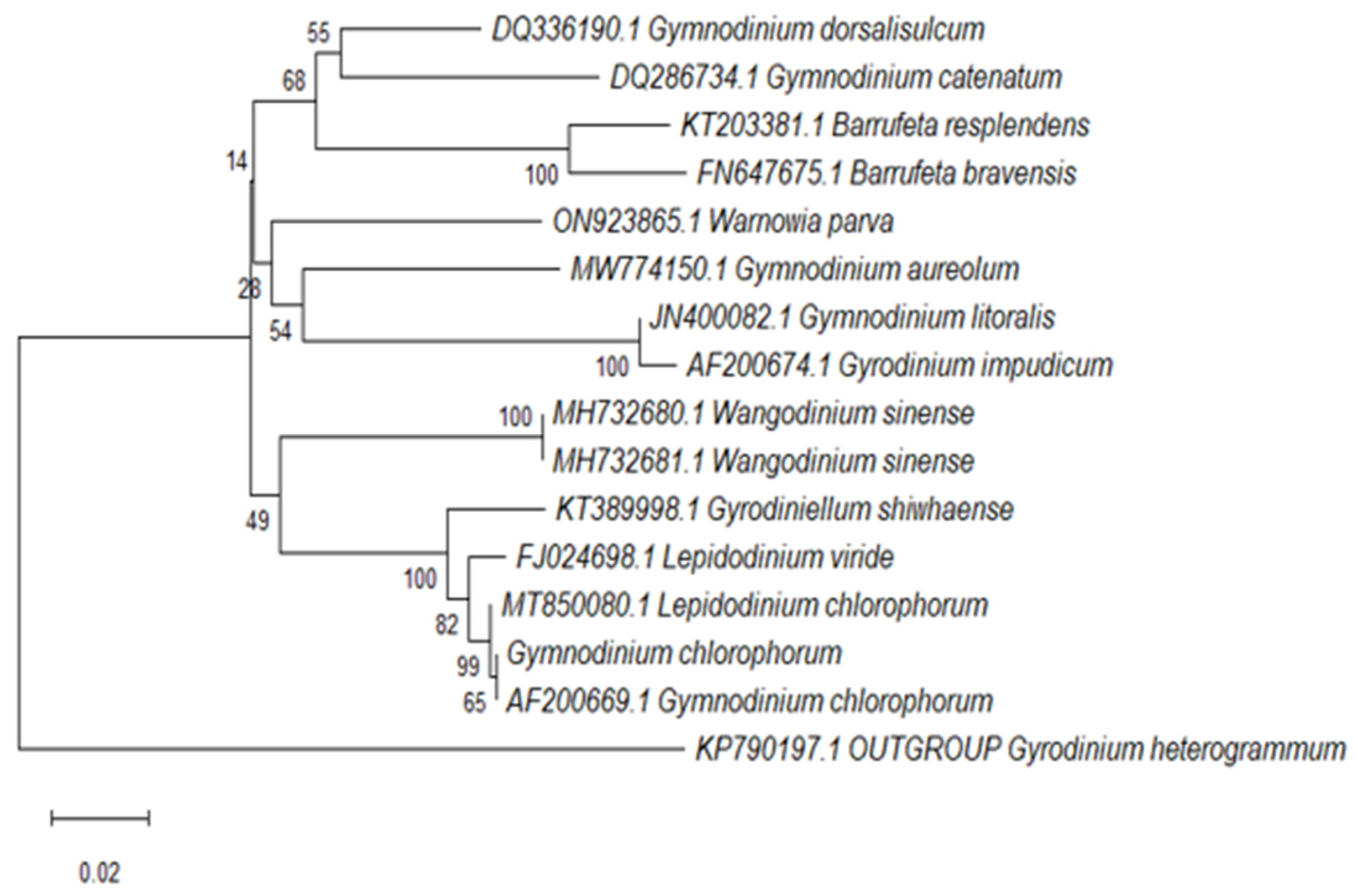
2.4. Sequence Alignment and Phylogenetic Analysis
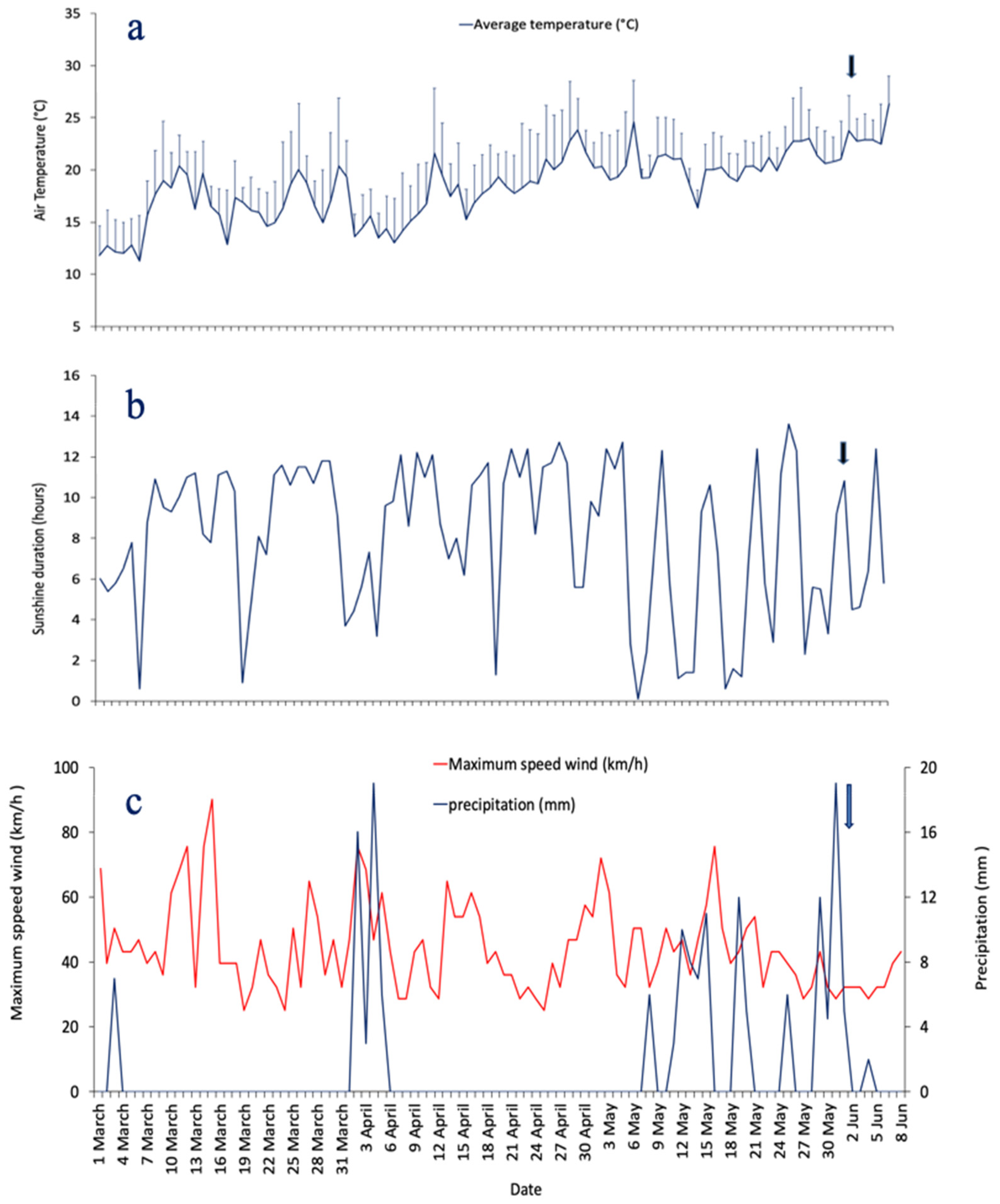
2.5. Meteorological and Hydrological Data Collection and Processing
2.6. Statistical Analysis
3. Results
3.1. Ribotyping
3.2. Meteorological and Hydrological Conditions During the Study Period
3.3. Chlorophyll a Concentration
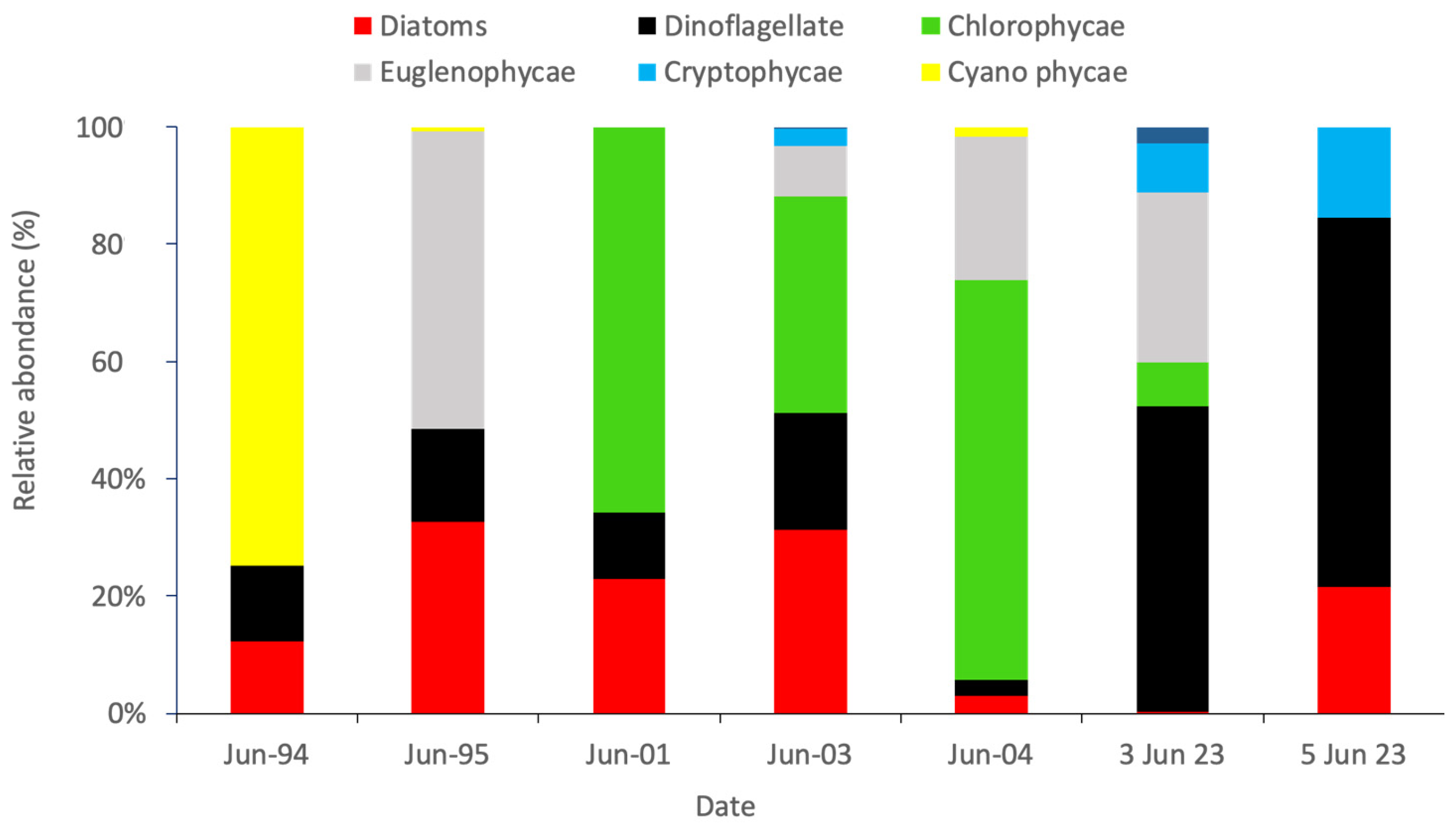
3.4. Phytoplankton Community Composition
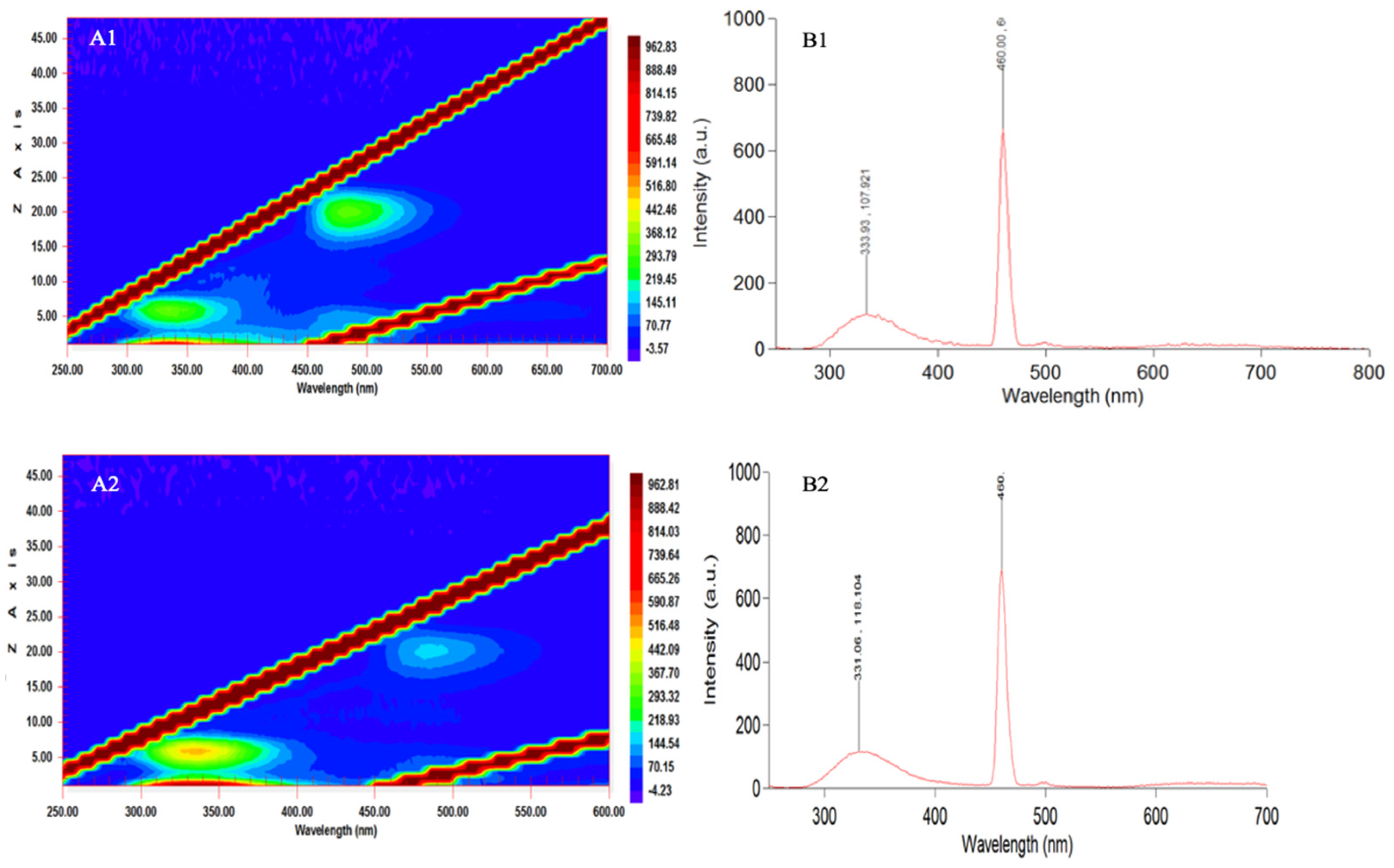
3.5. Fluorescence Spectra 15
3.6. Effect of Environmental Factors on the Green Algal Bloom Event (Ancillary Data from Rades Station)
4. Discussion
4.1. Occurrence of Gymnodinium chlorophorum and L. chlorophorum Blooms
4.2. Environmental Context of the Bloom in the Bay of Tunis
4.3. Potential Links Between Nutrient Loading and Phytoplankton Dynamics in the Bay of Tunis
4.4. Response to Sunlight (UV) on Phytoplankton Bloom
4.5. Response of the Phytoplanktonic Community to Hydrological Variability
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| L. chlorophrum | Lepidodinium chlorophorum |
| TEP | Transparent Exopolymer Particles |
| HABs | Harmful Algal Blooms |
| MAAs | Mycosporine-like amino acids |
Appendix A
| Years | Stations |
|---|---|
| 1994 | Rades |
| 1995 | Rades |
| 2001 | Rades |
| 2003 | Rades |
| 2004 | Rades |
| 2023 | Hammam-Lif |
| 2023 | Hammam-Lif |
Appendix B
| Period Investigated | 1994 | 1995 | 2001 | 2003 | 2004 |
|---|---|---|---|---|---|
| Temperature (°C) | 26.2 | 26.2 | 26 | 24 | - |
| Salinity (psu) | 38 | 37.9 | 37.7 | 37.1 | - |
| Phosphate (µmol·L−1) | 0.22 | 1.85 | 0.06 | 0.60 | - |
| Nitrate (µmol·L−1) | 1.62 | 1.42 | 1.33 | 3.45 | - |
| Nitrite (µmol·L−1) | 0.03 | 0.11 | 0.06 | 0.01 | - |
| Ammonium (µmol·L−1) | 1.22 | 1.85 | 16.80 | 5.467 | - |
| Silicate (µmol·L−1) | 1.89 | 2.26 | 2.30 | 1.3 | - |
| Diatoms (cells L−1) | 36,946 | 16,761 | 5090 | 29,040 | 47,520 |
| Dinoflagellates (cells L−1) | 39,780 | 6558 | 1900 | 18,480 | 36,960 |
| Chlorophycae (cells L−1) | 0 | 0 | 9420 | 34,400 | 90,816 |
| Cryptophycae (cells L−1) | 0 | 0 | 0 | 2640 | 0 |
| Euglenophycae (cells L−1) | 0 | 21,120 | 7920 | 324,720 | |
| Prymnesiophycae (cells L−1) | 0 | 0 | 0 | 240 | 0 |
| Cyanophycae (cells L−1) | 225,720 | 320 | 0 | 130 | 21,120 |
Appendix C
| Chlorophyll 0 | B. malleus | Licmophora spp. | Licmophora flabellata | Licmophora gracilis | Diatoms | P. micans | Pp.quinque Corne | Alex tamarense | Gym. chlorophorum | Dinoflagelates | Chlorophycea | Eutreptiella sp. | Euglena sp. | Euglenophyceae | Cryptophyceae | Cyanobacteria | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Sunshine | 0.91 | −0.254 | −0.254 | −0.254 | 0.909 | −0.431 | 0.909 | 0.909 | 0.909 | 0.91 | −0.372 | 0.909 | 0.909 | 0.909 | −0.258 | 0.909 | −0.372 |
| Wind | 0.634 | −0.254 | 0.623 | 0.335 | 0.779 | −0.431 | 0.623 | 0.335 | 0.335 | 0.335 | −0.372 | 0.623 | 0.335 | 0.335 | −0.258 | 0.335 | −0.372 |
| Max Temp | 0.664 | −0.254 | −0.254 | −0.254 | 0.634 | −0.431 | 0.623 | 0.623 | 0.623 | 0.623 | −0.372 | 0.623 | 0.623 | 0.634 | −0.258 | 0.335 | −0.372 |
| Min Temp | 0.625 | −0.254 | 0.623 | −0.254 | 1 | −0.431 | 1 | 1 | 1 | 1 | −0.372 | 0.997 | 1 | 1 | −0.258 | 1 | −0.372 |
| AvTemp | 0.654 | −0.454 | −0.254 | −0.254 | 0.159 | −0.431 | −0.26 | −0.263 | −0.263 | −0.26 | −0.372 | 0.887 | −0.26 | 0.877 | −0.258 | 0.877 | −0.372 |
| SST | 0.204 | 0.194 | 0.281 | 0.294 | 0.204 | −0.843 | 0.204 | 0.208 | 0.204 | 0.204 | −0.372 | 0.133 | 0.184 | 0.204 | −0.372 | 0.204 | 0.398 |
| Salinity | 0.999 | 0.262 | 0.281 | 0.999 | 0.159 | 0.999 | 0.999 | 0.999 | 0.999 | 0.159 | 0.9999 | 0.999 | 0.999 | 0.999 | −0.372 | 0.999 | 0.426 |
| pH | 0.588 | −0.254 | −0.254 | 0.588 | 0.588 | −0.431 | 0.588 | 0.588 | 0.588 | 0.588 | −0.372 | 0.588 | 0.588 | 0.779 | −0.258 | 0.779 | −0.442 |
| Conductivit y | 0.623 | 0.262 | −0.254 | −0.254 | 0.623 | −0.431 | 0.623 | 0.262 | 0.261 | 0.623 | −0.372 | 0.261 | 0.623 | 0.623 | −0.258 | 0.261 | −0.442 |
| Oxygene | 0.999 | −0.254 | −0.254 | 1 | 0.204 | 0.999 | 0.999 | 1000 | 0.999 | 0.261 | 0.999 | 1 | 1 | 1 | −0.442 | 1 | −0.372 |
| N/P | −0.654 | −0.254 | 0.997 | 0.779 | −0.26 | −0.315 | −0.26 | 0.294 | −0.26 | −0.26 | −0.372 | −0.26 | −0.26 | −0.26 | −0.442 | −0.442 | 0.838 |
| N/Si | 0.959 | −0.254 | 0.958 | −0.854 | 0.958 | 0.588 | 0.958 | 0.958 | 0.958 | 0.958 | −0.426 | 0.958 | 0.958 | 0.958 | −0.26 | 0.958 | −0.372 |
| PO4 | 0.779 | 0.376 | −0.454 | −0.354 | 0.779 | −0.315 | 0.779 | 0.785 | 0.779 | 0.779 | −0.372 | 0.739 | 0.779 | 0.779 | 0.921 | 0.779 | 0.913 |
| NO3 | −0.754 | 0.904 | −0.254 | −0.254 | 0.862 | −0.015 | −0.372 | 0.779 | −0.372 | 0.91 | −0.149 | −0.418 | −0.372 | −0.372 | −0.622 | −0.372 | 0.492 |
| NO2 | −0.254 | −0.34 | 0.971 | 0.874 | −0.209 | −0.643 | −0.209 | −0.26 | −0.26 | −0.26 | −0.442 | −0.258 | −0.26 | −0.442 | −0.342 | −0.209 | 0.656 |
| NH4 | −0.072 | −0.167 | 0.937 | 0.934 | −0.007 | −0.383 | −0.072 | −0.26 | −0.26 | −0.26 | −0.442 | −0.108 | −0.372 | −0.442 | −0.442 | −0.072 | 0.899 |
| Si04 | −0.556 | 0.102 | 0.634 | 0.634 | −0.556 | −0.451 | −0.561 | −0.556 | −0.556 | −0.556 | −0.556 | −0.619 | −0.556 | −0.556 | −0.556 | −0.556 | 0.623 |
| Mann-Whitney Test for “Equal Medians” | ||
|---|---|---|
| 3 June 2023 | 5 June 2023 | |
| N | 29 | 29 |
| Mean rank | 17.164 | 12.336 |
| Mann-Whitey U | 280.5 | |
| Z | 2.2 | |
| P (same med) | 0.02 | |
References
- Caruana, A.M.; Amzil, Z. Microalgae and toxins. In Microalgae in Health and Disease Prevention; Levine, I.A., Fleurence, J., Eds.; Academic Press: Cambridge, MA, USA, 2018; pp. 263–305. [Google Scholar] [CrossRef]
- Granéli, E.; Turner, J.T. An introduction to harmful algae. In Ecology of Harmful Algae; Granéli, E., Turner, J.T., Eds.; Springer: Berlin/Heidelberg, Germany, 2006; pp. 3–7. [Google Scholar]
- Stanisiere, J.-Y.; Mazurie, J.; Bouget, J.-F.; Langlade, A.; Gabellec, R.; Retho, M.; Meidy-Deviarni, I.; Goubert, E.; Cochet, H.; Dreano, A.; et al. Les Risques Conchylicoles en Baie de Quiberon. Troisième Partie: Risque D’hypoxie pour L’huître Creuse Crassostrea gigas. Rapport Final du Projet RISCO 2010–2013; Ifremer: Brest, France, 2013; p. 72. [Google Scholar]
- Wells, M.L.; Trainer, V.L.; Smayda, T.J.; Karlson, B.S.O.; Trick, C.G.; Kudela, R.M.; Ishikawa, A.; Bernard, S.; Wulff, A.; Anderson, D.M.; et al. Harmful algal blooms and climate change: Learning from the past and present to forecast the future. Harmful Algae 2015, 49, 68–93. [Google Scholar] [CrossRef]
- Zhou, M.J.; Liu, D.Y.; Anderson, D.M.; Valiela, I. Introduction to the special issue on green tides in the Yellow Sea. Estuar. Coast. Shelf Sci. 2015, 163, 3–8. [Google Scholar] [CrossRef]
- Siano, R.; Chapelle, A.; Antoine, V.; Michel-Guillou, E.; Rigaut-Jalabert, F.; Guillou, L.; Curd, A. Citizen participation in monitoring phytoplankton seawater discolorations. Mar. Policy 2020, 117, 103039. [Google Scholar] [CrossRef]
- Roux, P. Propriétés Écologiques des Efflorescences de Lepidodinium Chlorophorum: De L’écophysiologie Cellulaire à L’impact sur L’écosystème. Ph.D. Thesis, Université de Bretagne Occidentale, Brest, France, 2022; p. 330. [Google Scholar]
- Elbrächter, M.; Schnepf, E. Gymnodinium chlorophorum, a new, green, bloom-forming dinoflagellate (Gymnodinales, Dinophyceae) with a vestigial prasinophyte endosymbiont. Phycologia 1996, 35, 381–393. [Google Scholar] [CrossRef]
- Hansen, G.; Botes, L.; De Salas, M. Ultrastructure and large subunit rDNA sequences of Lepidodinium viride reveal a close relationship to Lepidodinium chlorophorum comb. nov. (=Gymnodinium chlorophorum). Phycol. Res. 2007, 55, 25–41. [Google Scholar] [CrossRef]
- Zapata, M.; Fraga, S.; Rodríguez, F.; Garrido, J.L. Pigment-based chloroplast types in dinoflagellates. Mar. Ecol. Prog. Ser. 2012, 465, 33–52. [Google Scholar] [CrossRef]
- Karasiewicz, S.; Chapelle, A.; Bacher, C.; Soudant, D. Harmful algae niche responses to environmental and community variation along the French coast. Harmful Algae 2020, 93, 101785. [Google Scholar] [CrossRef]
- Salhi, N. Perturbations Environnementales et Structuration du Peuplement Phytoplanctonique Côtier de la Baie de Tunis. Master’s Thesis, Université de Tunis, Tunis, Tunisia, 2009; p. 126. [Google Scholar]
- Roux, P.; Schapira, M.; Mertens, K.N.; André, C.; Terre-Terrillon, A.; Schmitt, A.; Siano, R. When phytoplankton do not bloom: The case of the dinoflagellate Lepidodinium chlorophorum in southern Brittany (France) assessed by environmental DNA. Prog. Oceanogr. 2023, 212, 102999. [Google Scholar] [CrossRef]
- Häder, D.P.; Richter, P.R.; Villafañe, V.E.; Helbling, E.W. Influence of light history on the photosynthetic and motility responses of Gymnodinium chlorophorum exposed to UVR and different temperatures. J. Photochem. Photobiol. B 2014, 138, 273–281. [Google Scholar] [CrossRef]
- Daly Yahia, M.-N. Dynamique Saisonnière du Zooplancton de la Baie de Tunis (Systématique, Écologie Numérique et Biogéographie Méditerranéenne). Ph.D. Thesis, Université de Tunis, Tunis, Tunisia, 1998; p. 237. [Google Scholar]
- Kouki, A. Contribution à L’étude de la Dynamique Sédimentaire dans le Petit Golfe de Tunis; Thèse de 3e cycle; Faculté des Sciences de Tunis: Tunis, Tunisia, 1984; p. 167. [Google Scholar]
- Daly Yahia-Kéfi, O.; Nézan, E.; Daly Yahia, M.N. Sur la présence du genre Alexandrium Halim (Dinoflagellés) dans la Baie de Tunis (Tunisie). Oceanol. Acta 2001, 24, 17–25. [Google Scholar] [CrossRef]
- Ben Lamine, Y.; Pringault, O.; Aissi, M.; Ensibi, C.; Mahmoudi, E.; Kefi, O.D.Y.; Yahia, M.N.D. Environmental controlling factors of copepod communities in the Gulf of Tunis (South Western Mediterranean Sea). Cah. Biol. Mar. 2015, 56, 213–229. [Google Scholar]
- Yahyaoui, A.; Amor, R.B.; Tissaoui, C.; Chouba, L. Répartition du mercure dans les sédiments de surface de l’oued Meliane et de la frange littorale Rades-Hammam-Lif, golfe de Tunis (Tunisie). INSTM Bull. Mar. Freshwater Sci. 2020, 47, 139–147. [Google Scholar] [CrossRef]
- Ennouri, R.; Zaaboub, N.; Fertouna-Bellakhal, M.; Chouba, L.; Aleya, L. Assessing trace metal pollution through high spatial resolution of surface sediments along the Tunis Gulf coast (Southwestern Mediterranean). Environ. Sci. Pollut. Res. 2016, 23, 5322–5334. [Google Scholar] [CrossRef] [PubMed]
- Lorenzen, C.J. Determination of chlorophylle and pheopigments: Spectrophotomètrie equations. Limnol. Oceanogr. 1967, 12, 343–346. [Google Scholar] [CrossRef]
- Aminot, A.; Kérouel, R. Hydrologie des Écosystèmes Marins. Paramètres et Analyses; Ifremer Éditions: Brest, France, 2004. [Google Scholar]
- Aminot, A.; Chaussepied, M. Manuel des Analyses Chimiques en Milieu Marin; Centre National pour l’Exploitation des Océans: Brest, France, 1983; p. 395. [Google Scholar]
- Parsons, T.R.; Maita, Y.; Lalli, C.M. A Manual of Chemical and Biological Methods for Seawater Analysis; Pergamon Press: Oxford, UK, 1984; p. 173. [Google Scholar]
- Utermöhl, H. Zur Vervollkommnung der quantitativen Phytoplankton-Methodik. Int. Ver. Theor. Angew. Limnol. 1958, 9, 1–38. [Google Scholar] [CrossRef]
- Trégouboff, G.; Rose, M. Manuel de Planctonologie Méditerranéenne; CNRS: Paris, France, 1978; Volume 1, p. 587. [Google Scholar]
- Dodge, J.D.; Hart-Jones, B. Marine Dinoflagellates of the British Isles; Her Majesty’s Stationery Office: London, UK, 1982. [Google Scholar]
- Sournia, A. Atlas du Phytoplancton Marin. Volume 1: Introduction, Cyanophycées, Dictyophycées, Raphidophycées; CNRS: Paris, France, 1986. [Google Scholar]
- Ricard, M. Atlas du Phytoplancton Marin. Volume 2: Diatomophycées; CNRS: Paris, France, 1987. [Google Scholar]
- Chrétiennot-Dinet, M.J. Atlas du Phytoplancton Marin. Volume 3: Chlorarachniophycées, Prasinophycées, Eugléniophycées, Chlorophycées; CNRS: Paris, France, 1990. [Google Scholar]
- Sournia, A.; Chrétiennot-Dinet, M.J.; Ricard, M. Marine phytoplankton: How many species? J. Plankton Res. 1991, 13, 1093–1099. [Google Scholar] [CrossRef]
- Saitou, N.; Nei, M. The neighbor-joining method: A new method for reconstructing phylogenetic trees. Mol. Biol. Evol. 1987, 4, 406–425. [Google Scholar] [CrossRef]
- Felsenstein, J. Confidence limits on phylogenies: An approach using the bootstrap. Evolution 1985, 39, 783–791. [Google Scholar] [CrossRef]
- Tamura, K.; Nei, M. Estimation of the number of nucleotide substitutions in the control region of mitochondrial DNA in humans and chimpanzees. Mol. Biol. Evol. 1993, 10, 512–526. [Google Scholar] [CrossRef]
- Kumar, S.; Stecher, G.; Li, M.; Knyaz, C.; Tamura, K. MEGA X: Molecular evolutionary genetics analysis across computing platforms. Mol. Biol. Evol. 2018, 35, 1547–1549. [Google Scholar] [CrossRef]
- Yahia-Kéfi, O.D.; Souissi, S.; Gomez, F.; Yahia, M.D. Spatio-temporal distribution of the dominant diatom and dinoflagellate species in the Bay of Tunis (SW Mediterranean Sea). Mediterr. Mar. Sci. 2005, 6, 17–34. [Google Scholar] [CrossRef]
- Hansen, G.; Larsen, J.; Moestrup, O. Phylogeny of some of the major genera of dinoflagellates based on ultrastructure and partial LSU rDNA sequence data, including the erection of three new genera of unarmoured dinoflagellates. Phycologia 2000, 39, 302–317. [Google Scholar] [CrossRef]
- Kofoid, C.A.; Swezy, O. The Free-Living Unarmored Dinoflagellata; Memoirs of the University of California: Berkeley, CA, USA, 1921; Volume 5, pp. i–viii, 1–562. [Google Scholar]
- Larsen, J. Unarmoured dinoflagellates from Australian waters I. The genus Gymnodinium (Gymnodiniales, Dinophyceae). Phycologia 1994, 33, 24–33. [Google Scholar] [CrossRef]
- Balech, E. El género Protoperidinium Bergh 1881 (Peridinium Ehrenberg 1831, partim). Rev. Mus. Argent. Cienc. Nat. Bernardino Rivadavia Inst. Nac. Invest. Cienc. Nat. (Hidrobiología) 1974, 4, 1–79. [Google Scholar]
- Abé, T.H. Report on the biological survey of Mutsu Bay. Sci. Rep. Tohoku Imp. Univ. 1927, 2, 383–438. [Google Scholar]
- Dodge, J.D. Prorocentrum Cordatum (Ostenfeld) J.D. Dodge, 1976; World Register of Marine Species: Oostende, Belgium, 1976. [Google Scholar]
- Ehrenberg, C.G. Dritter Beitrag zur Erkenntniss grosser Organisation in der Richtung des kleinsten Raumes. Abh. Königl. Akad. Wiss. Berlin 1833, 1833, 145–336. [Google Scholar]
- Schütt, F. Die Peridineen der Plankton-Expedition; Ergebnisse der Plankton-Expedition der Humboldt-Stiftung: Kiel, Germany, 1895; Volume 4, pp. 1–170. [Google Scholar]
- Schiller, J. Über neue Prorocentrum-und Exuviella-Arten aus der Adria. Arch. Protistenkd. 1918, 38, 250–262. [Google Scholar]
- Balech, E. The Genus Alexandrium Halim (Dinoflagellata); Sherkin Island Marine Station: Sherkin Island, Ireland, 1995; p. 151. [Google Scholar]
- Steidinger, K.A.; Balech, E. Scrippsiella subsalsa (Ostenfeld) comb. nov. (Dinophyceae) with a discussion on Scrippsiella. Phycologia 1977, 16, 69–73. [Google Scholar] [CrossRef]
- Honeywill, C. A study of British Licmophora species and a discussion of its morphological features. Diatom Res. 1998, 13, 221–271. [Google Scholar] [CrossRef]
- Van Heurck, H. A Treatise on the Diatomaceae; William Wesley & Son: London, UK, 1896; p. 558, 35. [Google Scholar]
- Castracane, F. Report on the diatomaceae collected by H.M.S. Challenger during the years 1873–1876. Rep. Sci. Results Voyag. H.M.S. Chall. 1886, 2, 1–178. [Google Scholar]
- Manguin, E. Les Diatomées de la Terre Adélie Campagne du Commandant Charcot 1949–1950. Ann. Sci. Nat. Bot. 1960, 12, 223–363. [Google Scholar]
- Schmidt, A. Die in den Grundproben der Nordseefahrt vom 21 Juli bis 9 Sept 1872 enthaltenen Diatomaceen. Erste Folge. Jahresber. Komm. Unters. Deutsch. Meer. 1874, 2, 81–95. [Google Scholar]
- Cleve, P.T.; Möller, J.D. Diatoms. Part IV, No. 169–216; Esaias Edquists Boktryckeri: Upsala, Sweden, 1879; No. 174. [Google Scholar]
- Riaux-Gobin, C.; Romero, O.E.; Coste, M.; Galzin, R. A new Cocconeis (Bacillariophyceae) from Moorea Island, Society Archipelago, South Pacific Ocean with distinctive valvocopula morphology and linking system. Bot. Mar. 2013, 56, 339–356. [Google Scholar] [CrossRef]
- Mereschkowsky, C. Liste des Diatomées de la mer Noire. Scripta Bot. (Botanisheskia Zapiski) 1902, 19, 51–88. [Google Scholar]
- Ehrenberg, C.G. Abhandlungen der Königlichen Akademie der Wissenschaften in Berlin; Verlag der Königlichen Akademie der Wissenschaften in Commission bei Georg Reimer: Berlin, Germany, 1875. [Google Scholar]
- Kützing, F.T. Species Algarum; F.A. Brockhaus: Leipzig, Germany, 1849; pp. vi–922. [Google Scholar]
- Armi, Z.; Milandri, A.; Turki, S.; Hajjem, B. Alexandrium catenella and Alexandrium tamarense in the North Lake of Tunis: Bloom characteristics and the occurrence of paralytic shellfish toxin. Afr. J. Aquat. Sci. 2011, 36, 47–56. [Google Scholar] [CrossRef]
- Sahraoui, I.; Bouchouicha, D.; Mabrouk, H.H.; Hlaili, A.S. Driving factors of the potentially toxic and harmful species of Prorocentrum Ehrenberg in a semi-enclosed Mediterranean lagoon (Tunisia, SW Mediterranean). Mediterr. Mar. Sci. 2013, 14, 353–362. [Google Scholar] [CrossRef]
- Bouchouicha Smida, D.; Sahraoui, I.; Grami, B.; Hadj Mabrouk, H.; Sakka Hlaili, A. Population dynamics of potentially harmful algal blooms in Bizerte Lagoon, Tunisia. Afr. J. Aquat. Sci. 2014, 39, 177–188. [Google Scholar] [CrossRef]
- Illoul, H.; Masó, M.; Fortuño, J.M.; Cros, L.; Morales-Blake, A.; Seridji, R. Potentially harmful microalgae in coastal waters of the Algiers area (Southern Mediterranean Sea). Cryptogam. Algol. 2008, 29, 261. [Google Scholar]
- Roux, P.; Siano, R.; Souchu, P.; Collin, K.; Schmitt, A.; Manach, S.; Schapira, M. Spatio-temporal dynamics and biogeochemical properties of green seawater discolorations caused by the marine dinoflagellate Lepidodinium chlorophorum along the southern Brittany coast. Estuar. Coast. Shelf Sci. 2022, 275, 107950. [Google Scholar] [CrossRef]
- Roux, N.; Simon, N.; Sourisseau, M. Temporal and spatial distribution of Lepidodinium chlorophorum blooms in French coastal waters. Mar. Ecol. Prog. Ser. 2022, 686, 49–65. [Google Scholar]
- Honsell, G.; Talarico, L. First record of Gymnodinium chlorophorum (Dinophyceae) bloom in the Adriatic Sea identified by electron microscopy. Bot. Mar. 2004, 47, 50–56. [Google Scholar] [CrossRef]
- Baldrich, A.; Toro, J.E.; Navarro, J.M. Thermal stress and coastal eutrophication as drivers of Lepidodinium blooms in the Southern Hemisphere. Harmful Algae 2024, 130, 102597. [Google Scholar]
- Serre-Fredj, L.; Jacqueline, F.; Navon, M.; Izabel, G.; Chasselin, L.; Jolly, O.; Claquin, P. Coupling high frequency monitoring and bioassay experiments to investigate a harmful algal bloom in the Bay of Seine (French-English Channel). Mar. Pollut. Bull. 2021, 168, 112387. [Google Scholar] [CrossRef]
- Sourisseau, M.; Jegou, K.; Lunven, M.; Quere, J.; Gohin, F.; Bryere, P. Distribution and dynamics of two species of Dinophyceae producing high biomass blooms over the French Atlantic shelf. Harmful Algae 2016, 53, 53–63. [Google Scholar] [CrossRef]
- Sournia, A.; Belin, C.; Billard, C.; Catherine, M.; Fresnel, J.; Lassus, P.; Soulard, R. The repetitive and expanding occurrence of a green bloom-forming dinoflagellate (Dinophyceae) on the coasts of France. Cryptogam. Algol. 1992, 13, 1–13. [Google Scholar] [CrossRef]
- Paerl, H.W.; Xu, H.; McCarthy, M.J.; Zhu, G.; Qin, B.; Li, Y.; Gardner, W.S. Controlling harmful cyanobacterial blooms in a hyper-eutrophic lake (Lake Taihu, China): The need for a dual nutrient (N and P) management strategy. Water Res. 2011, 45, 1973–1983. [Google Scholar] [CrossRef]
- Xu, H.; Paerl, H.W.; Qin, B.; Zhu, G.; Gao, G. Nitrogen and phosphorus inputs control phytoplankton growth in eutrophic lake. Limnol. Oceanogr. 2010, 55, 420–432. [Google Scholar] [CrossRef]
- Liang, W.; Liu, Y.; Jiao, J.J.; Luo, X. The dynamics of dissolved inorganic nitrogen species mediated by fresh submarine groundwater discharge and their impact on phytoplankton community structure. Sci. Total Environ. 2020, 703, 134897. [Google Scholar] [CrossRef] [PubMed]
- Chislock, M.F.; Doster, E.; Zitomer, R.A.; Wilson, A.E. Eutrophication: Causes, consequences, and controls in aquatic ecosystems. Nat. Educ. Knowl. 2013, 4, 10. [Google Scholar]
- Gardner, W.S.; Newell, S.E.; McCarthy, M.J.; Hoffman, D.K.; Lu, K.; Lavrentyev, P.J.; Paerl, H.W. Community biological ammonium demand: A conceptual model for cyanobacteria blooms in eutrophic lakes. Environ. Sci. Technol. 2017, 51, 7785–7793. [Google Scholar] [CrossRef]
- Piranti, A.S.; Wibowo, D.N.; Rahayu, D.R. Nutrient determinant factor of causing algal bloom in tropical lake (case study in Telaga Menjer Wonosobo, Indonesia). J. Ecol. Eng. 2021, 22, 156–165. [Google Scholar] [CrossRef]
- Lucas, L.V.; Koseff, J.R.; Monismith, S.G.; Cloern, J.E.; Thompson, J.K. Processes governing phytoplankton blooms in estuaries. II: The role of horizontal transport. Mar. Ecol. Prog. Ser. 1999, 187, 17–30. [Google Scholar] [CrossRef]
- KM, G.S.; Ons, K.D.Y.; Raja, B.; Nejib, D.Y.M. Phytoplankton and zooplankton diversity and community dynamics in connected coastal wetlands’ ecosystems under anthropogenic pressure (SW Mediterranean Sea). Estuar. Coast. Shelf Sci. 2025, 314, 109147. [Google Scholar]
- Iriarte, J.L.; Quiñones, R.A.; González, R.R. Relationship between biomass and enzymatic activity of a bloom-forming dinoflagellate (Dinophyceae) in southern Chile (41° S): A field approach. J. Plankton Res. 2005, 27, 159–166. [Google Scholar] [CrossRef]
- Litchman, E.; Klausmeier, C.A.; Yoshiyama, K. Contrasting size evolution in marine and freshwater diatoms. Proc. Natl. Acad. Sci. USA 2009, 106, 2665–2670. [Google Scholar] [CrossRef]
- Gobler, C.J.; Doherty, O.M.; Hattenrath-Lehmann, T.K.; Griffith, A.W.; Kang, Y.; Litaker, R.W. Ocean warming since 1982 has expanded the niche of toxic algal blooms in the North Atlantic and North Pacific oceans. Proc. Natl. Acad. Sci. USA 2017, 114, 4975–4980. [Google Scholar] [CrossRef]
- Macias, D.; Garcia-Gorriz, E.; Stips, A. Understanding the causes of recent warming of Mediterranean waters. How much could be attributed to climate change? PLoS ONE 2013, 8, e81591. [Google Scholar] [CrossRef]
- Winder, M.; Sommer, U. Phytoplankton response to a changing climate. Hydrobiologia 2012, 698, 5–16. [Google Scholar] [CrossRef]
- Yang, Y.; Shi, J.; Jia, Y.; Zhang, Y.; Li, Y.; Wang, X. Unveiling the impact of glycerol phosphate (DOP) in the dinoflagellate Peridinium bipes by physiological and transcriptomic analysis. Environ. Sci. Eur. 2020, 32, 38. [Google Scholar] [CrossRef]
- Luo, H.; Lin, X.; Li, L.; Lin, L.; Zhang, C.; Lin, S. Transcriptomic and physiological analyses of the dinoflagellate Karenia mikimotoi reveal non-alkaline phosphatase-based molecular machinery of ATP utilization. Environ. Microbiol. 2017, 19, 4506–4518. [Google Scholar] [CrossRef]
- Smalley, G.W.; Coats, D.W.; Stoecker, D.K. Feeding the mixotrophic dinoflagellate Ceratium furca is influenced by intracellular nutrient concentrations. Mar. Ecol. Prog. Ser. 2003, 262, 137–151. [Google Scholar] [CrossRef]
- Meunier, C.L.; Alvarez-Fernandez, S.; Cunha-Dupont, A.; Geisen, C.; Malzahn, A.M.; Boersma, M.; Wiltshire, K.H. The craving for phosphorus in heterotrophic dinoflagellates and its potential implications for biogeochemical cycles. Limnol. Oceanogr. 2018, 63, 1774–1784. [Google Scholar] [CrossRef]
- Banaszak, A.T. Photoprotective physiological and biochemical responses of aquatic organisms. In UV Effects in Aquatic Organisms and Ecosystems; Helbling, E.W., Zagarese, H.E., Eds.; Springer: Dordrecht, The Netherlands, 2003; pp. 329–356. [Google Scholar] [CrossRef]
- Cai, X.; Hutchins, D.A.; Fu, F.; Gao, K. Effects of ultraviolet radiation on photosynthetic performance and N2 fixation in Trichodesmium erythraeum IMS101. Biogeosciences 2017, 14, 4455–4466. [Google Scholar] [CrossRef]
- Lewellyn, C.A.; Airs, R.L. Distribution and abundance of MAAs in 33 species of microalgae across 13 classes. Mar. Drugs 2010, 8, 1273–1291. [Google Scholar] [CrossRef]
- Garcia-Pichel, F.; Castenholz, R.W. Occurrence of UV-absorbing, mycosporine-like compounds among cyanobacterial isolates and an estimate of their screening capacity. Appl. Environ. Microbiol. 1993, 59, 163–169. [Google Scholar] [CrossRef]
- Stengel, D.B.; Connan, S.; Popper, Z.A. Algal chemodiversity and bioactivity: Sources of natural variability and implications for commercial application. Biotechnol. Adv. 2011, 29, 483–501. [Google Scholar] [CrossRef]
- Zhang, Y.; Li, K.; Zhou, Q.; Chen, L.; Yang, X.; Zhang, H. Phytoplankton responses to solar UVR and its combination with nutrient enrichment in a plateau oligotrophic Lake Fuxian: A mesocosm experiment. Environ. Sci. Pollut. Res. 2021, 28, 23271–23285. [Google Scholar] [CrossRef]
- Marcoval, M.A.; Villafañe, V.E.; Helbling, E.W. Interactive effects of ultraviolet radiation and nutrient addition on growth and photosynthesis performance of four species of marine phytoplankton. J. Photochem. Photobiol. B Biol. 2007, 89, 78–87. [Google Scholar] [CrossRef]
- Zurlini, G.; Zattera, A.; Bruschi, A. Structural analysis of phytoplankton community’s variation in the Archipelago of La Maddalena (North Sardinian coast): A canonical correlation approach. J. Exp. Mar. Biol. Ecol. 1983, 70, 227–248. [Google Scholar] [CrossRef]
- Feki-Sahnoun, W.; Njah, H.; Barraj, N.; Mahfoudhi, M.; Akrout, F.; Rebai, A.; Bel Hassen, M.; Hamza, A. Influence of phosphorus-contaminated sediments on the abundance of potentially toxic phytoplankton along the Sfax coasts (Gulf of Gabes, Tunisia). J. Sediment. Environ. 2019, 4, 458–470. [Google Scholar] [CrossRef]
- Mabrouk, L.; Ben Brahim, M.; Hamza, A.; Mahfoudhi, M.; Bradai, M.N. A comparison of abundance and diversity of epiphytic microalgal assemblages on the leaves of the seagrasses Posidonia oceanica (L.) and Cymodocea nodosa (Ucria) Asch in Eastern Tunisia. J. Mar. Biol. 2014, 2014, 275305. [Google Scholar] [CrossRef]



| Sampling Date | 3 June 2023 | 5 June 2023 |
|---|---|---|
| Temperature (°C) | 24.2 | 22.5 |
| Salinity | 34.1 | 38.1 |
| pH | 6.9 | 7.6 |
| Conductivity (mS·cm−1) | 54.9 | 56.1 |
| Dissolved Oxygen (mg·L−1) | 2.2 | 7.3 |
| Chlorophyll a (mg·L−1) | 980.42 | 2.32 |
| Nitrite (µmol·L−1) | 0.016 | 0.014 |
| Nitrate (µmol·L−1) | 0.5 | 0.5 |
| Nitrogen-Ammonia (µmol·L−1) | 4.80 | 1.06 |
| Silicate (µmol·L−1) | <0.05 | <0.05 |
| Phosphate (µmol·L−1) | 0.88 | 0.23 |
| Species | Authors (Taxonomic Authority) | Cells Densities (Cells·L−1) 3 June 2023 | Cells Densities (Cells·L−1) 5 June 2023 |
|---|---|---|---|
| Karenia mikimotoi | [37] | 1.9 × 106 | 0 |
| Lepidodinium chlorophorum (=Gymnodinium chlorophorum) | [8,38] | 2.3 × 107 | 240 |
| Gyrodinium spirale | [39] | 4.2 × 106 | 0 |
| Gymnodinium galeatum | [39] | 2.1 × 105 | 0 |
| Gymnodinium impudicum | [37] | 1.3 × 107 | 80 |
| Protoperidinium steinii | [40] | 2.1 × 105 | 0 |
| Blixaea quinquecornis | [41] | 6.4 × 105 | 360 |
| Prorocentrum cordatum | [42] | 1.1 × 105 | 40 |
| Prorocentrum micans | [43] | 4.2 × 105 | 560 |
| Prorocentrum gracile | [44] | 1.5 × 106 | 0 |
| Prorocentrum triestinum | [45] | 5.8 × 106 | 40 |
| Alexandrium tamarense | [46] | 6.4 × 105 | 80 |
| Scrippsiella trochoidea | [47] | 0 | 240 |
| Licmophora gracilis | [48] | 2.1 × 105 | 0 |
| Licmophora ehrenbergii | [49] | 0 | 40 |
| Amphora sp. | [50] | 0 | 200 |
| Nitzschia sp. | [51] | 0 | 480 |
| Navicula distans | [52] | 0 | 40 |
| Navicula sp. | [49] | 0 | 480 |
| Nitzschia palea | [53] | 0 | 320 |
| Cocconeis sp. | [54] | 0 | 40 |
| Cylindrotheca closterium | [55] | 0 | 40 |
| Thalassionema nitzschioides | [55] | 0 | 40 |
| Euglena spp. | [56] | 1.4 × 107 | 440 |
| Eutreptiella sp. | [56] | 1.5 × 107 | 200 |
| Chlorophycae | - | 7.8 × 106 | 200 |
| Cryptophycae | - | 8.6 × 106 | 400 |
| Prasinophycae | - | 3 × 106 | 0 |
| Merismopedia elegans | [57] | 0 | 1560 |
| Peak No. | Pigment | Wavelengths (Minimum–Maximum) (nm) | Wavelength of Maximum Peak Signal (nm) | Reference |
|---|---|---|---|---|
| 1 | Unknown carotenoid | - | 465 | [10] |
| 2 | Unknown carotenoid | - | 463 | [10] |
| 3 | Unknown carotenoid from Lepidodinium chlorophorum | 420–472 | 443 | [10] |
| 4 | Chlorophyll b | - | 461 | [10] |
| 5 | Pigment type 1 | 450–470 | 460 | This study |
| 6 | MAA compounds | 300–350 | 331/333 | This study |
| Species | Identification Method | Maximum Concentrations During the Bloom (cells·L−1) | Location | Nuisance Effects | Key Environmental Factors Influencing the Blooms | Reference |
|---|---|---|---|---|---|---|
| L. chlorophorum | Inverted microscope and molocular analysis ribotyping, Fluorescence | (2.3 × 107 cells·L−1) | Coastal waters of Hammam-Lif, Tunisia’s capital (South Western Mediterranean Sea) | Low oxygen concentration (2.2 mg·L−1) Stranding of bivalve shells | -High solar radiation ultraviolet radiation (UVR) ammonia accumulated nutrients from river discharges rainfall, low wind water stratification due to calm conditions | Present work |
| L. chlorophorum | Inverted microscope | (>105 cells·L−1) | Chilean Patagonia from Southern Chiloé Island to the northern coasts of Aysén province | Hypoxia DO concentration of 2.2 mg·L−1 and 3.2 mg·L−1 | -River discharge -Rise in sea surface temperature (SST) and air temperature (SST of 25.4 °C) Increase in surface Dissolved inorganic phosphorus concentration Wind speed < 15 km·h−1 | [65] |
| L. chlorophorum | Microscopy-based monitoring/Analysing environmental DNA | (8.9 × 106 cells·L−1) | Southern Brittany | Mass mortalities of shellfish Production of Transparent Exopolymer Particles (TEP) Hypoxia | -Freshwater inputs and thermal stratification of the water column Tidal currents Ammonium inputs | [7] |
| L. chlorophorum | Light microscopy high-resolution satellite remote sensing | (5.0 × 105 cells·L−1) | Southern Brittany (NE-Atlantic, France) Vilaine Bay (NE Atlantic, France) | Green seawater discolorations Hypoxic conditions -Fauna mortalities Transparent exopolymer particles (TEP) | -Tidal currents Stratification waters High concentrations of phosphate and ammonium | [62] |
| L. chlorophorum | High frequency monitoring buoy equipped with sensors | - | Bay of Seine (France) | Oxygen depletion | Increase of the N/P ratio | [66] |
| L. chlorophorum | Light microscopy | (2.90 × 106 cells·L−1) | Coastal waters of Brittany (France) | Harmful Algal Blooms, HABs Bivalve mortality Fish mortality, Anoxic conditions | [6] | |
| L. chlorophorum | Microscopic observations | (>106 cells·L−1) | Loire and Vilaine river plumes Eastern Brittany area From Brest to Nantes along the Brittany coast. | High concentration of Suspended Particulate Matters (SPM) | -High concentrations of organic matter supported by ammonium Tide Wind-induced circulation | [67] |
| L. chlorophorum | Light microscopy HPLC pigment analysis | - | - | - | - | [10] |
| L. chlorophorum | Inverted microscope | (2.6 × 105 cells·L−1) | Algerian coastal waters | [61] | ||
| G. chlorophorum and L. chlorophorum | Olympus BHS Microscope/ Phylogeny analysis | Culturing | From the River Derwent, Tasmania | [9] | ||
| G. chlorophorum | Scanning and transmission electron microscopy | Coasts of the Northern Adriatic Sea (Mediterranean Sea) | - | - | [64] | |
| G. chlorophorum | Helgoland (North Sea) | - | - | [8] | ||
| G. chlorophorum | Microscope equipped for epifluorescence -HPLC for pigment analysis | Atlantic and English Channel coasts of France | Mortalities of marine organisms (mussels, oysters, sorne smaller motluscs, shrimps, crabs, and smaller crustaceans) Lowering of the oxygen -Tourists and swimmers suffered from skin irritation No toxicity reported | -Significant freshwater inputs After heavy rains | [68] | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Salhi, N.; Pagano, M.; Felix, C.; Hafferssas, A.; Laadouze, I.; Laabir, M.; Saidi, N. First Record of Lepidodinium chlorophorum and the Associated Phytoplankton Community Responsible of the Green Tide South Western Mediterranean Sea (Hammam-Lif, Tunisia). J. Mar. Sci. Eng. 2025, 13, 1982. https://doi.org/10.3390/jmse13101982
Salhi N, Pagano M, Felix C, Hafferssas A, Laadouze I, Laabir M, Saidi N. First Record of Lepidodinium chlorophorum and the Associated Phytoplankton Community Responsible of the Green Tide South Western Mediterranean Sea (Hammam-Lif, Tunisia). Journal of Marine Science and Engineering. 2025; 13(10):1982. https://doi.org/10.3390/jmse13101982
Chicago/Turabian StyleSalhi, Noussaiba, Marc Pagano, Christine Felix, Aziz Hafferssas, Imen Laadouze, Mohamed Laabir, and Neila Saidi. 2025. "First Record of Lepidodinium chlorophorum and the Associated Phytoplankton Community Responsible of the Green Tide South Western Mediterranean Sea (Hammam-Lif, Tunisia)" Journal of Marine Science and Engineering 13, no. 10: 1982. https://doi.org/10.3390/jmse13101982
APA StyleSalhi, N., Pagano, M., Felix, C., Hafferssas, A., Laadouze, I., Laabir, M., & Saidi, N. (2025). First Record of Lepidodinium chlorophorum and the Associated Phytoplankton Community Responsible of the Green Tide South Western Mediterranean Sea (Hammam-Lif, Tunisia). Journal of Marine Science and Engineering, 13(10), 1982. https://doi.org/10.3390/jmse13101982






