Biological Effects of Non-Ionizing Electromagnetic Fields at 27 GHz on Sperm Quality of Mytilus galloprovincialis
Abstract
:1. Introduction
1.1. 5G Technology
1.2. The Impact of 5G Technology to Health
1.3. The Impact of 5G Technology to Marine Coastal Marine Species
2. Materials and Methods
2.1. Study Design
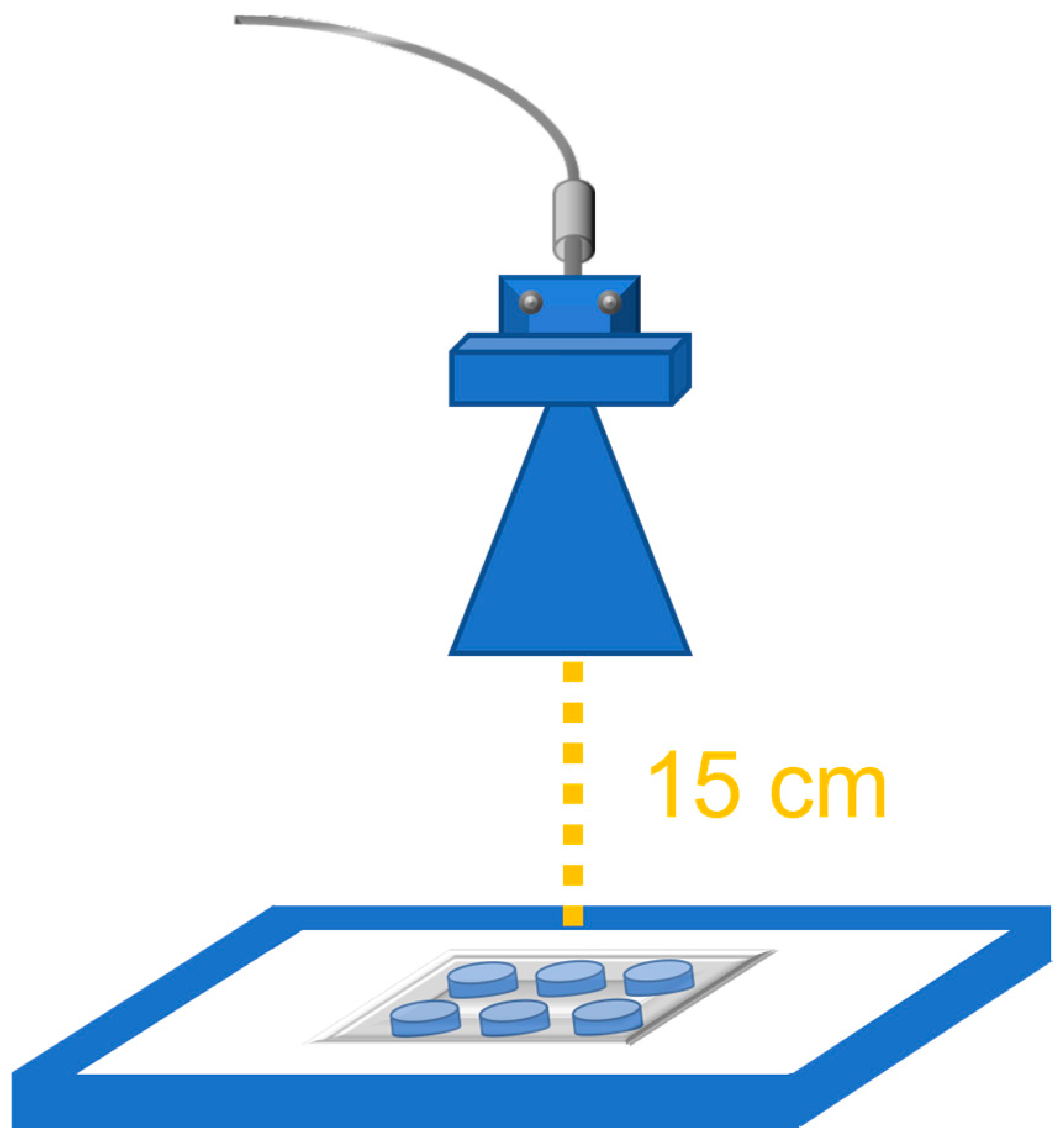
2.2. 27 GHz Antenna and Exposure Setup
2.3. Experimental Exposure Procedure
2.4. Motility Analysis
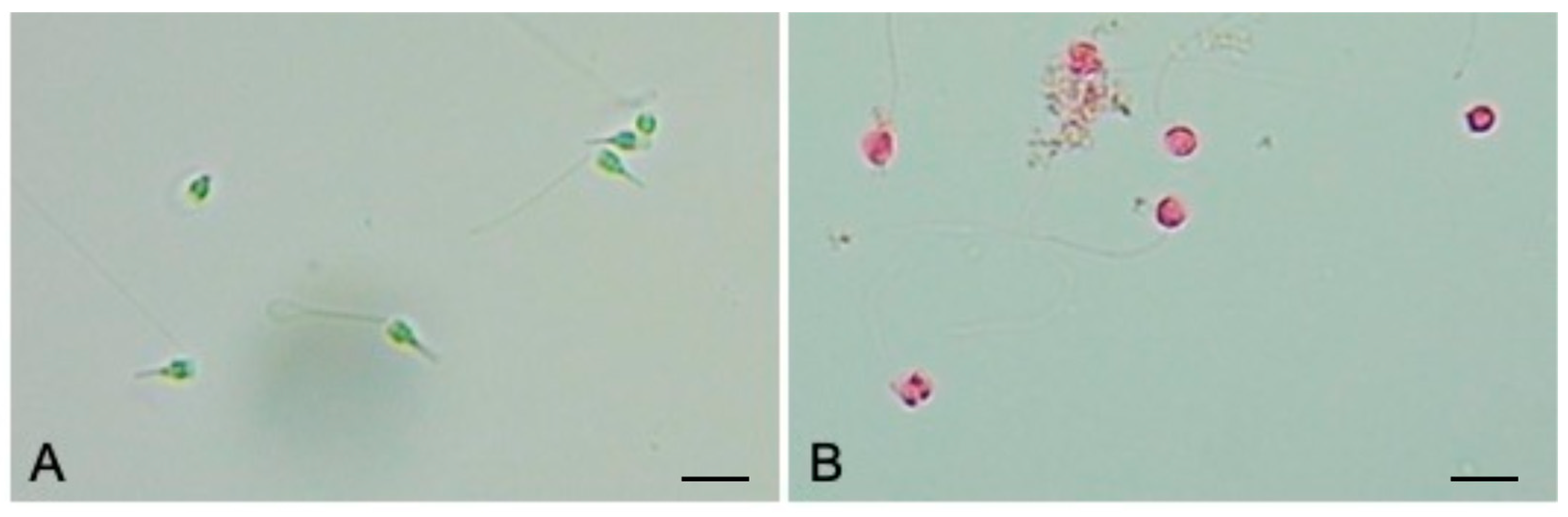
2.5. Vitality Analysis
2.6. Statistical Analyses
3. Results
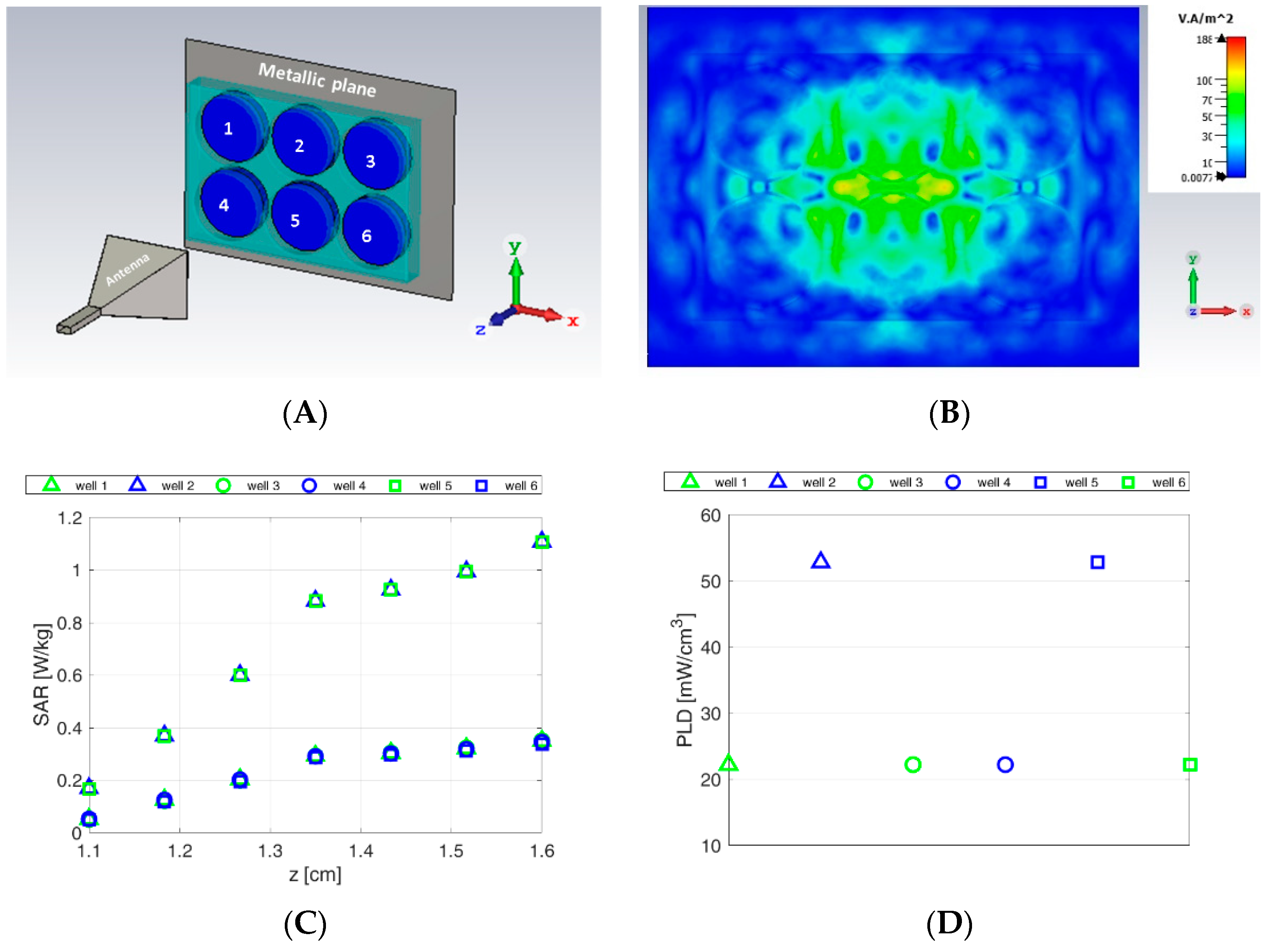
3.1. Numerical Dosimetry Analyses
- -
- density power of the incident field [W/m2];
- -
- the local specific absorption rate (point SAR) [W/Kg];
- -
- power loss density (PLD) deposited into the exposed aqueous samples [W/m3].
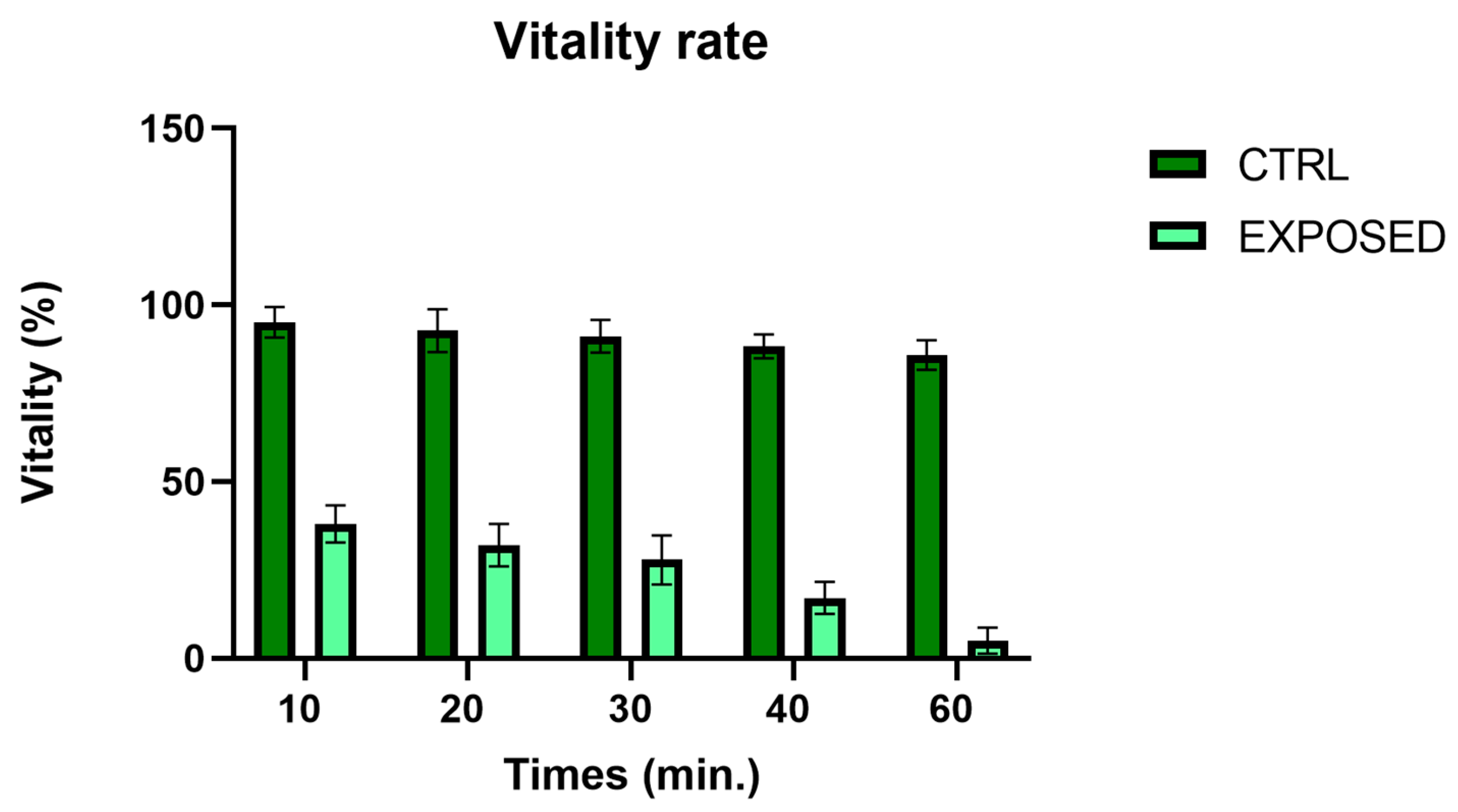
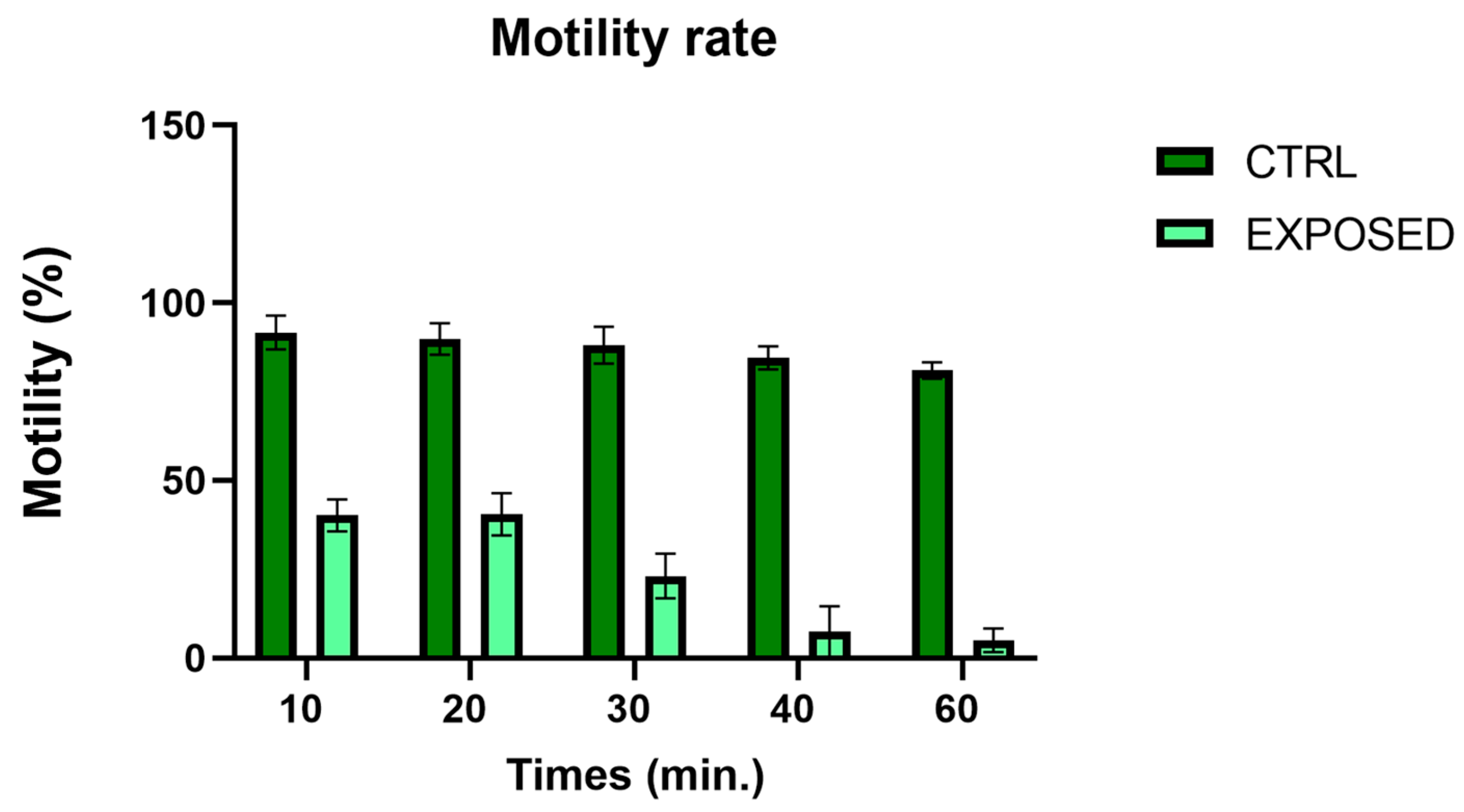
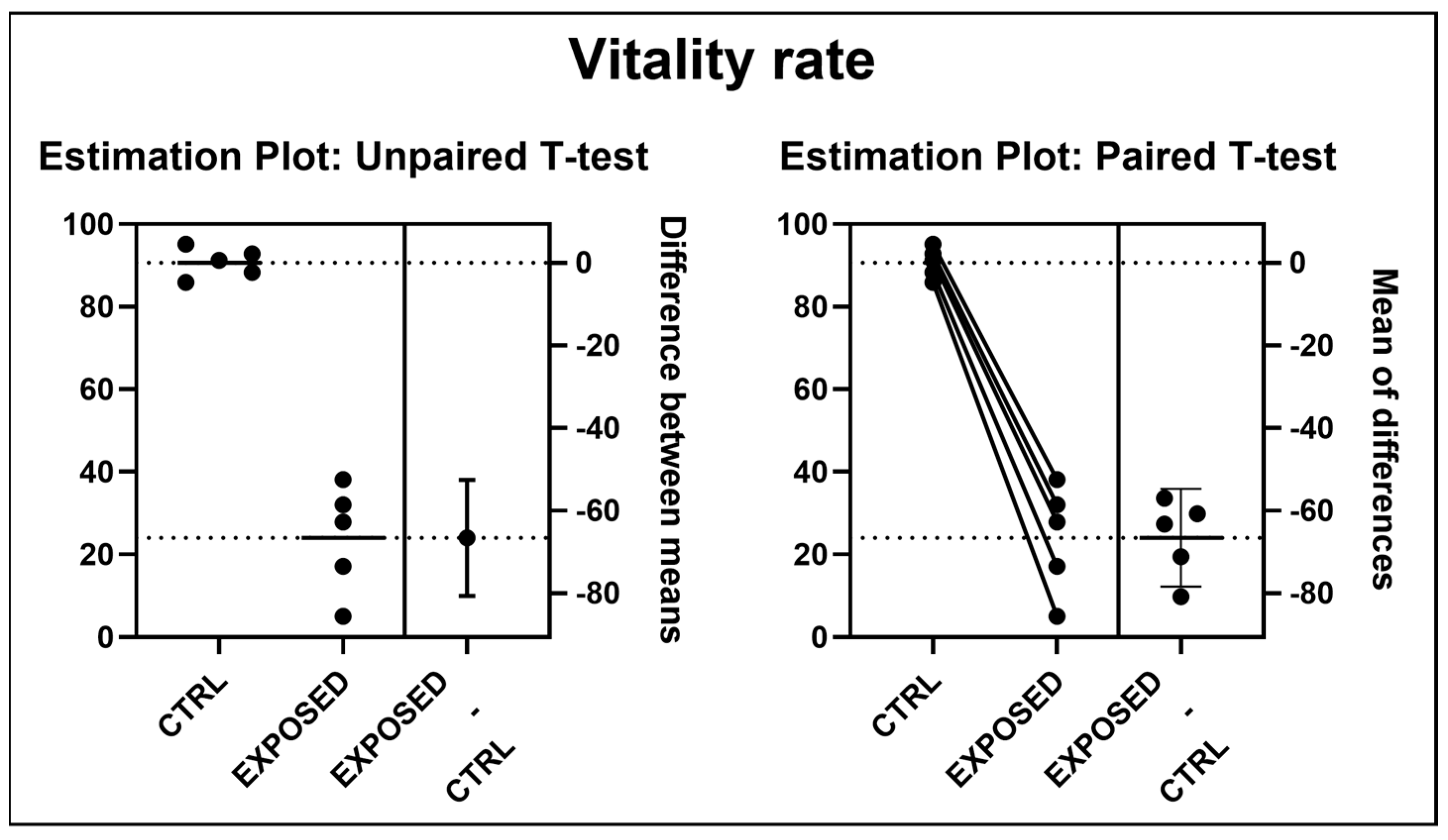
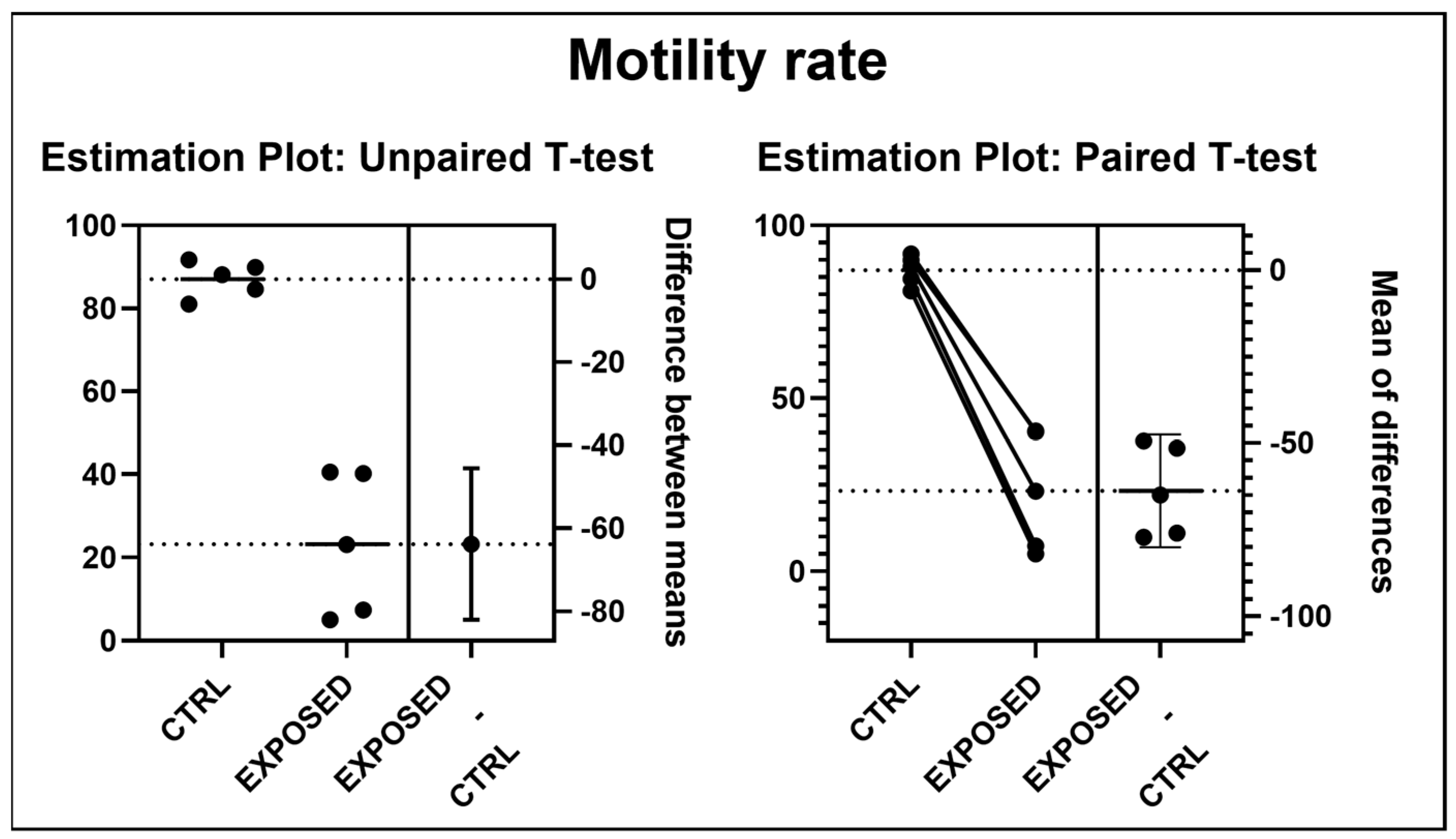
3.2. Sperms Vitality and Motility Analyses
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Attaran, M. The impact of 5G on the evolution of intelligent automation and industry digitization. J. Ambient. Intell. Hum. Comput. 2021, 1–17. [Google Scholar] [CrossRef] [PubMed]
- Foster, K.R.; Vijayalaxmi. Needed: More Reliable Bioeffects Studies at “High Band” 5G Frequencies. Front. Commun. Netw. 2021, 2, 721925. [Google Scholar] [CrossRef]
- Hardell, L.; Carlberg, M. Health risks from radiofrequency radiation, including 5G, should be assessed by experts with no conflicts of interest. Oncol. Lett. 2020, 20, 15. [Google Scholar] [CrossRef] [PubMed]
- Simkó, M.; Mattsson, M.O. 5G Wireless Communication and Health Effects—A Pragmatic Review Based on Available Studies Regarding 6 to 100 GHz. Int. J. Environ. Res. Public Health 2019, 16, 3406. [Google Scholar] [CrossRef] [Green Version]
- Mattsson, M.O.; Simkó, M.; Foster, K.R. 5G New Radio Requires the Best Possible Risk Assessment Studies: Perspective and Recommended Guidelines. Front. Commun. Netw. 2021, 2, 724772. [Google Scholar] [CrossRef]
- Agence nationale de sécurité sanitaire de l’alimentationde l’environnement et du travail (ANSES). Expositions aux champs électromagnétiques liées au déploiement de la technologie de communication « 5G » et effets sanitaires éventuels associés. Rapport d’expertise collective. 2021. Available online: https://www.vie-publique.fr/rapport/279567-deploiement-de-la-technologie-de-communication-5g-et-effets-sanitaires (accessed on 1 March 2022).
- International Commission on Non-Ionizing Radiation Protection (ICNIRP). Guidelines for limiting exposure to time-varying electric, magnetic and electromagnetic fields (up to 300 GHz). Health Phys. 1998, 74, 494–522. [Google Scholar]
- Dreujou, E.; Desroy, N.; Carrière, J.; Tréau de Coeli, L.; McKindsey, C.W.; Archambault, P. Determining the Ecological Status of Benthic Coastal Communities: A Case in an Anthropized Sub-Arctic Area. Front. Mar. Sci. 2021, 8, 637546. [Google Scholar] [CrossRef]
- Klimley, A.; Wyman, M.; Kavet, R. Assessment of Potential Impact of Electromagnetic Fields from Undersea Cable on Migratory Fish Behaviour (No. FINAL REPORT, DOE-EPRI-Ee0006382 OCS Study BOEM 2016-041); Electric Power Research Institute (EPRI): Palo Alto, CA, USA, 2016. [Google Scholar]
- Ohman, M.; Sigray, P.; Westerberg, H. Offshore windmills and the effects of electromagnetic fields on fish. Ambio 2007, 36, 630–633. [Google Scholar] [CrossRef]
- Tricas, T.; Gill, A. Effects of EMFs from Undersea Power Cables on Elasmobranchs and Other Marine Species (No. OCS Study BOEMRE 2011-09); U.S. Department of the Interior, Bureau of Ocean Energy Management (BOEM): Camarillo, CA, USA, 2011.
- Wildt, D.E.; Comizzoli, P.; Pukazhenthi, B.; Songsasen, N. Lessons from biodiversity—The value of nontraditional species to advance reproductive science, conservation, and human health. Mol. Reprod. Dev. 2010, 77, 397–409. [Google Scholar] [CrossRef] [Green Version]
- Boncel, S.; Kyzioł-Komosińska, J.; Krzyżewska, I.; Czupioł, J. Interactions of carbon nanotubes with aqueous/aquatic media containing organic/inorganic contaminants and selected organisms of aquatic ecosystems—A review. Chemosphere 2015, 136, 211–221. [Google Scholar] [CrossRef]
- Maresca, V.; Fusaro, L.; Sorbo, S.; Siciliano, A.; Loppi, S.; Paoli, L.; Monaci, F.; Asadi karma, E.; Piscopo, M.; Guida, M.; et al. Functional and structural biomarkers to monitor heavy metal pollution of one of the most contaminated freshwater sites in Southern Europe. Ecotoxicol. Environ. Saf. 2018, 163, 665–673. [Google Scholar] [CrossRef] [PubMed]
- Piscopo, M.; Notariale, R.; Rabbito, D.; Ausi, J.; Olanrewaju, O.S.; Guerriero, G. Mytilus galloprovincialis (Lamarck, 1819) spermatozoa: Hsp70 expression and protamine-like protein property studies. Environ. Sci. Pollut. Res. 2018, 25, 12957–12966. [Google Scholar] [CrossRef] [PubMed]
- Reitsema, R.E.; Meire, P.; Schoelynck, J. The future of freshwater macrophytes in a changing world: Dissolved organic carbon quantity and quality and its interactions with macrophytes. Front. Plant Sci. 2018, 9, 629. [Google Scholar] [CrossRef] [PubMed]
- Scalici, M.; Traversetti, L.; Spani, F.; Malafoglia, V.; Colamartino, M.; Persichini, T.; Cappello, S.; Mancini, G.; Guerriero, G.; Colasanti, M. Shell fluctuating asymmetry in the sea-dwelling benthic bivalve Mytilus galloprovincialis (Lamarck, 1819) as morphological markers to detect environmental chemical contamination. Ecotoxicology 2017, 26, 396–404. [Google Scholar] [CrossRef]
- Vassalli, Q.A.; Caccavale, F.; Avagnano, S.; Murolo, A.; Guerriero, G.; Fucci, L.; Ausió, J.; Piscopo, M. New insights into protamine-like component organization in Mytilus galloprovincialis’ sperm chromatin. DNA Cell Biol. 2015, 34, 162–169. [Google Scholar] [CrossRef]
- Ozlem Nisbet, H.; Nisbet, C.; Akar, A.; Cevik, M.; Karayigit, M.O. Effects of exposure to electromagnetic field (1.8/0.9 GHz) on testicular function and structure in growing rats. Res. Vet. Sci. 2012, 93, 1001–1005. [Google Scholar] [CrossRef] [Green Version]
- Bin-Meferij, M.M.; El-Kott, O.F. The neuroprotective effects of Moringa oleifera against mobile phone electromagnetic radiation-induced infertility in rats. Int. J. Clin. Exp. Med. 2015, 8, 12487–12497. [Google Scholar]
- Bilgici, B.; Gun, S.; Avci, B.; Akar, A.; Engiz, B. What is adverse effect of wireless local area network, using 2.45 GHz, on the reproductive system? Int. J. Radiat. Biol. 2018, 94, 1054–1061. [Google Scholar] [CrossRef]
- Saygin, M.; Asci, H.; Ozmen, O.; Cankara, F.N.; Dincoglu, D.; Ilhan, I. Impact of 2.45 GHz microwave radiation on the testicular inflammatory pathway biomarkers in young rats: The role of gallic acid. Environ. Toxicol. 2016, 31, 1771–1784. [Google Scholar] [CrossRef]
- Ozguner, M.; Koyu, A.; Cesur, G.; Ural, M.; Ozguner, F.; Gokcimen, A.; Delibas, N. Biological and morphological effects on the reproductive organ of rats after exposure to electromagnetic field. Saudi Med. J. 2005, 26, 405–410. [Google Scholar]
- Sambucci, M.; Laudisi, F.; Nasta, F.; Pinto, R.; Lodato, R.; Lopresto, V.; Altavista, P.; Marino, C.; Pioli, C. Early life exposure to 2.45 GHz WiFi-like signals: Effects on development and maturation of the immune system. Prog. Biophys. Mol. Biol. 2011, 107, 393–398. [Google Scholar] [CrossRef] [PubMed]
- Guo, L.; Lin, J.J.; Xue, Y.Z.; An, G.Z.; Zhang, J.P.; Zhang, K.Y.; He, W.; Wang, H.; Li, W.; Ding, G.R. Effects of 220 MHz pulsed modulated radiofrequency field on the sperm quality in rats. Int. J. Environ. Res. Public Health 2019, 16, 1286. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Albert, L.; Deschamps, F.; Jolivet, A.; Olivier, F.; Chauvaud, L.; Chauvaud, S. A current synthesis on the effects of electric and magnetic fields emitted by submarine power cables on invertebrates. Mar. Environ. Res. 2020, 159, 104958. [Google Scholar] [CrossRef]
- Levin, M.; Ernst, S. Applied DC magnetic fields cause alterations in the time of cell divisions and developmental abnormalities in early sea-urchin embryos. Bioelectromagnetics 1997, 18, 255–263. [Google Scholar] [CrossRef]
- Zimmerman, S.; Zimmerman, A.; Winters, W.; Cameron, I. Influence of 60-Hz magnetic fields on sea urchin development. Bioelectromagnetics 1990, 11, 37–45. [Google Scholar] [CrossRef] [PubMed]
- Bochert, R.; Zettler, M. Long-term exposure of several marine benthic animals to static magnetic fields. Bioelectromagnetics 2004, 25, 498–502. [Google Scholar] [CrossRef]
- D’Agostino, S.; Della Monica, C.; Palizzi, E.; Di Pietrantonio, F.; Benetti, M.; Cannatà, D.; Cavagnaro, M.; Sardari, D.; Stano, P.; Ramundo-Orlando, A. Extremely high frequency electromagnetic fields facilitate electrical signal propagation by increasing transmembrane potassium efflux in an artificial axon model. Sci. Rep. 2018, 8, 9299. [Google Scholar] [CrossRef] [PubMed]
- Cosentino, K.; Beneduci, A.; Ramundo-Orlando, A.; Chidichimo, G. The influence of millimeter waves on the physical properties of large and giant unilamellar vesicles. J. Biol. Phys. 2013, 39, 395–410. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Di Donato, L.; Cataldo, M.; Stano, P.; Massa, R.; Ramundo-Orlando, A. Permeability changes of cationic liposomes loaded with carbonic anhydrase induced by millimeter waves radiation. Radiat. Res. 2012, 178, 437–446. [Google Scholar] [CrossRef] [Green Version]
| Component | Material | Dielectric Constant | Loss Tangent | Mass Density |
|---|---|---|---|---|
| Horn antenna | Perfect electric conductor (good metal) | - | ∞ | - |
| Aqueous sample | Salt water (30‰ salinity) | 23.64 | 1.27 | 1029 [Kg/m3] |
| 6-weel microplates | Polystyrene | 2.5 | 0 | - |
| Ground plane | Perfect electric conductor (good metal) | - | ∞ | - |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pecoraro, R.; Pavone, S.C.; Scalisi, E.M.; Sica, C.; Ignoto, S.; Contino, M.; Salvaggio, A.; Marmara, D.; Sorbello, G.; Di Donato, L.; et al. Biological Effects of Non-Ionizing Electromagnetic Fields at 27 GHz on Sperm Quality of Mytilus galloprovincialis. J. Mar. Sci. Eng. 2022, 10, 521. https://doi.org/10.3390/jmse10040521
Pecoraro R, Pavone SC, Scalisi EM, Sica C, Ignoto S, Contino M, Salvaggio A, Marmara D, Sorbello G, Di Donato L, et al. Biological Effects of Non-Ionizing Electromagnetic Fields at 27 GHz on Sperm Quality of Mytilus galloprovincialis. Journal of Marine Science and Engineering. 2022; 10(4):521. https://doi.org/10.3390/jmse10040521
Chicago/Turabian StylePecoraro, Roberta, Santi Concetto Pavone, Elena Maria Scalisi, Carmen Sica, Sara Ignoto, Martina Contino, Antonio Salvaggio, Dimitra Marmara, Gino Sorbello, Loreto Di Donato, and et al. 2022. "Biological Effects of Non-Ionizing Electromagnetic Fields at 27 GHz on Sperm Quality of Mytilus galloprovincialis" Journal of Marine Science and Engineering 10, no. 4: 521. https://doi.org/10.3390/jmse10040521







