Different Floor Management Systems Affect Soil Properties and Initial Development of Apple Tree (Malus × domestica Borkh.) in an Orchard
Abstract
:1. Introduction
2. Materials and Methods
2.1. Experimental Site, Conditions, and Design
- (1)
- a herbicide strip (HS), which was made by applying glyphosate-containing agents twice per season (Roundup 360 SL in 4 L·ha−1 dose) at the beginning of June and in October, was used as a control;
- (2)
- mechanical cultivation (MC) was conducted using a rototiller-type tool mounted on a tractor that was run using a hydraulic system, and the soil was tilled depending on demands at least once per 4 weeks from April to October;
- (3)
- synthetic mulch (BC), for which the soil in a tree row was mulched with a black polypropylene cover material at a density of 100 g per m2;
- (4)
- Miscanthus × giganteus mulch I (MG1), for which the soil was mulched with straw obtained from shredded Miscanthus × giganteus plants;
- (5)
- Miscanthus × giganteus mulch II (MG2), for which the soil in planned tree rows was mulched with straw obtained from shredded Miscanthus × giganteus plants before tree planting, and it was thoroughly mixed with the soil, which was followed by mulching with the same material after the trees had been planted;
- (6)
- spent mushroom substrate I (SMS1), for which the soil was mulched with a spent mushroom substrate as an agricultural waste material derived from Agaricus bisporus production;
- (7)
- spent mushroom substrate II (SMS2), for which the soil in the planned tree rows was mulched with a spent mushroom substrate prior to tree planting, and it was thoroughly mixed with the soil, which was followed by mulching with the same material after the trees had been planted.
2.2. Soil Properties
2.3. The Nutrient Content in Leaves
2.4. Leaf Area and Tree Growth
2.5. Generative Tree Development and Yield Assessment
2.6. Statistical Analysis
3. Results
3.1. Physico-Chemical Soil Properties
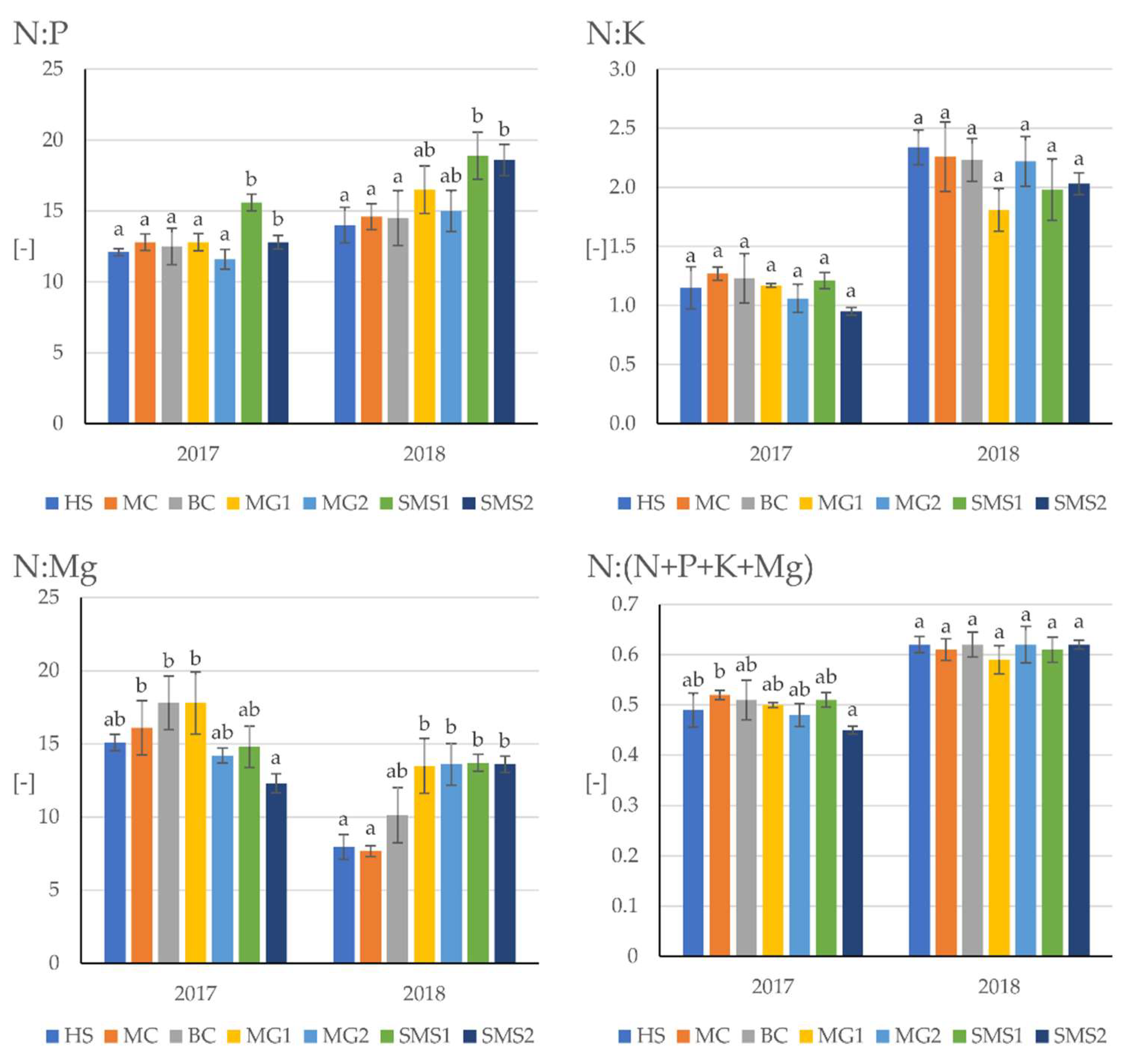
3.2. Nutritional Tree Status and Growth
3.3. Generative Tree Development
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Skroch, W.A.; Shribbs, J.M. Orchard Floor Management: An Overview. HortScience 1986, 21, 390–394. [Google Scholar] [CrossRef]
- Priori, S.; Pellegrini, S.; Vignozzi, N.; Costantini, E.A.C. Soil Physical-Hydrological Degradation in the Root-Zone of Tree Crops: Problems and Solutions. Agronomy 2021, 11, 68. [Google Scholar] [CrossRef]
- Markuszewski, B.; Kopytowski, J. Effects of some soil cultivation methods on the growth and yielding of apple trees grafted on semi-dwarf rootstocks and ‘Antonowka’ seedling with B 9 interstock. Zesz. Nauk. Inst. Sadow. 2008, 16, 21–34. [Google Scholar]
- Merwin, I.A.; Stiles, W.C. Orchard groundcover management impacts on apple tree growth and yield, and nutrient availability and uptake. J. Am. Soc. Hortic. Sci. 1994, 119, 209–215. [Google Scholar] [CrossRef] [Green Version]
- Mia, M.J.; Monaci, E.; Murri, G.; Massetani, F.; Facchi, J.; Neri, D. Soil nitrogen and weed biodiversity: An assessment under two orchard floor management practices in a nitrogen vulnerable zone in Italy. Horticulturae 2020, 6, 96. [Google Scholar] [CrossRef]
- Tadeo, J.L.; Sánchez-Brunete, C.; Pérez, R.A.; Fernández, M.D. Analysis of herbicide residues in cereals, fruits and vegetables. J. Chromatogr. A 2000, 882, 175–191. [Google Scholar] [CrossRef] [PubMed]
- Lisek, J. Possibilities and limitations of weed management in fruit crops of the temperate climate zone. J. Plant Prot. Res. 2014, 54, 318–326. [Google Scholar] [CrossRef]
- Neumann, G.; Kohls, S.; Landsberg, E.; Stock-Oliveira Souza, K.; Yamada, T.; Römheld, V. Relevance of glyphosate transfer to non-target plants via the rhizosphere. J. Plant Dis. Prot. 2006, 969, 963–969. [Google Scholar]
- Zobiole, L.H.S.; de Oliveira, R.S.; Huber, D.M.; Constantin, J.; de Castro, C.; de Oliveira, F.A.; de Oliveira, A. Glyphosate reduces shoot concentrations of mineral nutrients in glyphosate-resistant soybeans. Plant Soil 2010, 328, 57–69. [Google Scholar] [CrossRef]
- Bokszczanin, K.Ł.; Wrona, D.; Przybyłko, S. Influence of an Alternative Soil Management System to Herbicide Use on Tree Vigor, Yield, and Quality of Apple Fruit. Agronomy 2020, 11, 58. [Google Scholar] [CrossRef]
- Andersen, L.; Kühn, B.F.; Bertelsen, M.; Bruus, M.; Larsen, S.E.; Strandberg, M. Alternatives to herbicides in an apple orchard, effects on yield, earthworms and plant diversity. Agric. Ecosyst. Environ. 2013, 172, 1–5. [Google Scholar] [CrossRef]
- Rifai, M.N.; Lacko- Bartosova, M.; Brunclik, P. Alternative Methods of Weed Control in Apple Orchards. Pakistan J. Biol. Sci. 2000, 3, 933–938. [Google Scholar] [CrossRef]
- Webber, S.M.; Bailey, A.P.; Huxley, T.; Potts, S.G.; Lukac, M. Traditional and cover crop-derived mulches enhance soil ecosystem services in apple orchards. Appl. Soil Ecol. 2022, 178, 104569. [Google Scholar] [CrossRef]
- Mia, M.J.; Massetani, F.; Murri, G.; Neri, D. Sustainable alternatives to chemicals for weed control in the orchard—A Review. Hortic. Sci. 2020, 47, 1–12. [Google Scholar] [CrossRef] [Green Version]
- Mia, M.J.; Massetani, F.; Murri, G.; Facchi, J.; Monaci, E.; Amadio, L.; Neri, D. Integrated weed management in high density fruit orchards. Agronomy 2020, 10, 1492. [Google Scholar] [CrossRef]
- Lanauskas, J.; Kviklys, D.; Kviklienė, N.; Uselis, N.; Viškelis, P.; Rubauskis, E. Effect of soil management on tree nutrition and yield in apple organic orchards. Acta Hortic. 2014, 1058, 175–180. [Google Scholar] [CrossRef]
- Robinson, D.W.; O’Kennedy, N.D. The effect of overall herbicide systems of soil management on the growth and yield of apple trees ‘Golden Delicious’. Sci. Hortic. 1978, 9, 127–136. [Google Scholar] [CrossRef]
- Granatstein, D.; Sanchez, E. Research knowledge and needs for orchard floor management in organic tree fruit systems. Int. J. Fruit Sci. 2009, 9, 257–281. [Google Scholar] [CrossRef]
- Hammermeister, A.M. Organic weed management in perennial fruits. Sci. Hortic. 2016, 208, 28–42. [Google Scholar] [CrossRef]
- Hogue, E.J.; Neilsen, G.H. Orchard Floor Vegetation Management. In Horticultural Reviews; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 1987; pp. 377–430. [Google Scholar] [CrossRef]
- Duncan, R.A.; Stapleton, J.J.; McKenry, M. V Establishment of orchards with black polyethylene film mulching: Effect on nematode and fungal pathogens, water conservation, and tree growth. J. Nematol. 1992, 24, 681–687. [Google Scholar]
- Huang, Z.; Xu, Z.; Chen, C. Effect of mulching on labile soil organic matter pools, microbial community functional diversity and nitrogen transformations in two hardwood plantations of subtropical Australia. Appl. Soil Ecol. 2008, 40, 229–239. [Google Scholar] [CrossRef]
- Steinmetz, Z.; Wollmann, C.; Schaefer, M.; Buchmann, C.; David, J.; Tröger, J.; Muñoz, K.; Frör, O.; Schaumann, G.E. Plastic mulching in agriculture. Trading short-term agronomic benefits for long-term soil degradation? Sci. Total Environ. 2016, 550, 690–705. [Google Scholar] [CrossRef] [PubMed]
- Bandopadhyay, S.; Martin-Closas, L.; Pelacho, A.M.; DeBruyn, J.M. Biodegradable Plastic Mulch Films: Impacts on Soil Microbial Communities and Ecosystem Functions. Front. Microbiol. 2018, 9, 819. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Merwin, I.A.; Stiles, W.C.; van Es, H.M. Orchard Groundcover Management Impacts on Soil Physical Properties. J. Am. Soc. Hortic. Sci. 1994, 119, 216–222. [Google Scholar] [CrossRef] [Green Version]
- Pickering, J.S.; Shepherd, A. Evaluation of organic landscape mulches: Composition and nutrient release characteristics. Arboric. J. 2000, 24, 175–187. [Google Scholar] [CrossRef]
- Stockmann, U.; Adams, M.A.; Crawford, J.W.; Field, D.J.; Henakaarchchi, N.; Jenkins, M.; Minasny, B.; McBratney, A.B.; de Courcelles, V.d.R.; Singh, K.; et al. The knowns, known unknowns and unknowns of sequestration of soil organic carbon. Agric. Ecosyst. Environ. 2013, 164, 80–99. [Google Scholar] [CrossRef]
- Singh, M.; Kukal, M.S.; Irmak, S.; Jhala, A.J. Water Use Characteristics of Weeds: A Global Review, Best Practices, and Future Directions. Front. Plant Sci. 2022, 12. [Google Scholar] [CrossRef]
- Russel, J.C. The Effect of Surface Cover on Soil Moisture Losses by Evaporation. Soil Sci. Soc. Am. J. 1940, 4, 65–70. [Google Scholar] [CrossRef] [Green Version]
- Iqbal, R.; Raza, M.A.S.; Valipour, M.; Saleem, M.F.; Zaheer, M.S.; Ahmad, S.; Toleikiene, M.; Haider, I.; Aslam, M.U.; Nazar, M.A. Potential agricultural and environmental benefits of mulches—A review. Bull. Natl. Res. Cent. 2020, 44, 75. [Google Scholar] [CrossRef]
- Oliveira, M.T.; Merwin, I.A. Soil physical conditions in a New York orchard after eight years under different groundcover management systems. Plant Soil 2001, 234, 233–237. [Google Scholar] [CrossRef]
- Merwin, I.A. Orchard floor management systems. In Apples: Botany, production and uses; Ferree, D.C., Warrington, I.J., Eds.; CABI: Wallingford, CT, USA, 2003; pp. 303–318. [Google Scholar]
- Kader, M.A.; Singha, A.; Begum, M.A.; Jewel, A.; Khan, F.H.; Khan, N.I. Mulching as water-saving technique in dryland agriculture: Review article. Bull. Natl. Res. Cent. 2019, 43, 147. [Google Scholar] [CrossRef] [Green Version]
- Solomakhin, A.A.; Trunov, Y.V.; Blanke, M.; Noga, G. Organic mulch in apple tree rows as an alternative to herbicide and to improve fruit quality. Acta Hortic. 2012, 513–521. [Google Scholar] [CrossRef]
- Verdú, A.M.; Mas, M.T. Mulching as an alternative technique for weed management in mandarin orchard tree rows. Agron. Sustain. Dev. 2007, 27, 367–375. [Google Scholar] [CrossRef]
- Prandecki, K.; Wrzaszcz, W.; Zieliński, M. Environmental and Climate Challenges to Agriculture in Poland in the Context of Objectives Adopted in the European Green Deal Strategy. Sustainability 2021, 13, 10318. [Google Scholar] [CrossRef]
- Strašil, Z. Evaluation of Miscanthus grown for energy use. Res. Agric. Eng. 2016, 62, 92–97. [Google Scholar] [CrossRef] [Green Version]
- Mohd Hanafi, F.H.; Rezania, S.; Mat Taib, S.; Md Din, M.F.; Yamauchi, M.; Sakamoto, M.; Hara, H.; Park, J.; Ebrahimi, S.S. Environmentally sustainable applications of agro-based spent mushroom substrate (SMS): An overview. J. Mater. Cycles Waste Manag. 2018, 20, 1383–1396. [Google Scholar] [CrossRef]
- Komosa, A.; Stafecka, A. Comparison of phosphorus, potassium, and magnesium contents determined with the universal, Egner-Riehm and Schachtschabel methods in orchard soils. Folia Hortic. 2003, 15, 137–149. [Google Scholar]
- Łądkiewicz, K.; Jaśkiewicz, K.; Wszędyrówny-Nast, M. Porównanie różnych metod oznaczania zawartości substancji organicznej. Przegląd Nauk. Inż. Kształt. Środ. 2017, 26, 99–107. [Google Scholar] [CrossRef]
- Bilbao, B.; Giraldo, D.; Hevia, P. Quantitative determination of nitrogen content in plant tissue by a colorimetric method. Commun. Soil Sci. Plant Anal. 1999, 30, 1997–2005. [Google Scholar] [CrossRef]
- Galas, W.; Kita, J. Use of the ICP-AES method for multi-element analysis of tobacco leaves. Rocz. Państw. Zakł. Hig. 2019, 46, 53–57. [Google Scholar]
- Jordán, A.; Zavala, L.M.; Muñoz-Rojas, M. Mulching, Effects on Soil Physical Properties. In Encyclopedia of Agrophysics; Gliński, J., Horabik, J., Lipiec, J., Eds.; Springer: Dordrecht, The Netherlands, 2011; pp. 492–496. [Google Scholar]
- Van Dung, T.; Ngoc, N.P.; Van Dang, L.; Hung, N.N. Impact of cover crop and mulching on soil physical properties and soil nutrients in a citrus orchard. PeerJ 2022, 10, e14170. [Google Scholar] [CrossRef] [PubMed]
- Hosseini Bai, S.; Blumfield, T.J.; Reverchon, F. The impact of mulch type on soil organic carbon and nitrogen pools in a sloping site. Biol. Fertil. Soils 2014, 50, 37–44. [Google Scholar] [CrossRef] [Green Version]
- Han, X.; Li, Y.L.; Wang, L.F.; Liu, S.L.; Zhang, J.G. Effect of tree-row mulching on soil characteristics as well as growth and development of pear trees. Acta Hortic. 2015, 1094, 299–306. [Google Scholar] [CrossRef]
- Sinkevičienė, A.; Jodaugienė, D.; Pupalienė, R.; Urbonienė, M. The influence of organic mulches on soil properties and crop yield. Agron. Res. 2009, 7, 485–491. [Google Scholar]
- Billeaud, L.A.; Zajicek, J.M. Influence of Mulches on Weed Control, Soil pH, Soil Nitrogen Content, and Growth of Ligustrum japonicum. J. Environ. Hortic. 1989, 7, 155–157. [Google Scholar] [CrossRef]
- Kiczorowski, P.; Kopacki, M.; Kiczorowska, B. The response of Šampion trees growing on different rootstocks to applied organic mulches and mycorrhizal substrate in the orchard. Sci. Hortic. 2018, 241, 267–274. [Google Scholar] [CrossRef]
- Sønsteby, A.; Nes, A.; Måge, F. Effects of Bark Mulch and NPK Fertilizer on Yield, Leaf Nutrient Status and Soil Mineral Nitrogen during Three Years of Strawberry Production. Acta Agric. Scand. Sect. B Soil Plant Sci. 2007, 54, 4710. [Google Scholar] [CrossRef]
- Id, B.Q.; Liu, Y.; Sun, X.; Li, S.; Wang, X.; Xiong, K.; Yun, B.; Zhang, H. Effect of various mulches on soil physico-chemical properties and tree growth (Sophora japonica) in urban tree pits. PLoS ONE 2019, 14, e0210777. [Google Scholar]
- Atucha, A.; Merwin, I.A.; Brown, M.G. Long-term Effects of Four Groundcover Management Systems in an Apple Orchard. Am. Soc. Hortic. Sci. 2011, 46, 1176–1183. [Google Scholar] [CrossRef] [Green Version]
- Lazicki, P.; Geisseler, D.; Lloyd, M. Nitrogen mineralization from organic amendments is variable but predictable. J. Environ. Qual. 2020, 49, 483–495. [Google Scholar] [CrossRef]
- Wrona, D. Effect of nitrogen fertilization on growth, cropping and fruit quality of ‘Šampion’ apple trees during 9 years after planting. Folia Hortic. 2004, 16, 55–60. [Google Scholar]
- Wrona, D.; Sadowski, A. Effect of nitrogen fertilization and soil management on soil mineral nitrogen in the apple orchard. J. Fruit Ornam. Plant Res. 2004, 12, 191–199. [Google Scholar]
- Kowalczyk, W.; Wrona, D.; Przybyłko, S. Content of minerals in soil, apple tree leaves and fruits depending on nitrogen fertilization. J. Elem. 2017, 22, 67–77. [Google Scholar] [CrossRef]
- Przybyłko, S.; Kowalczyk, W.; Wrona, D. The Effect of Mycorrhizal Fungi and PGPR on Tree Nutritional Status and Growth in Organic Apple Production. Agronomy 2021, 11, 1402. [Google Scholar] [CrossRef]
- El-Beltagi, H.S.; Basit, A.; Mohamed, H.I.; Ali, I.; Ullah, S.; Kamel, E.A.R.; Shalaby, T.A.; Ramadan, K.M.A.; Alkhateeb, A.A.; Ghazzawy, H.S. Mulching as a Sustainable Water and Soil Saving Practice in Agriculture: A Review. Agronomy 2022, 12, 1881. [Google Scholar] [CrossRef]
- Chen, B.; Liu, E.; Tian, Q.; Yan, C.; Zhang, Y. Soil nitrogen dynamics and crop residues. A review. Agron. Sustain. Dev. 2014, 34, 429–442. [Google Scholar] [CrossRef] [Green Version]
- Mészáros, M.; Hnátková, H.; Čonka, P.; Náměstek, J. Linking mineral nutrition and fruit quality to growth intensity and crop load in apple. Agronomy 2021, 11, 506. [Google Scholar] [CrossRef]
- Lipecki, J.; Jacyna, T.; Lipa, T.; Szot, I. the Quality of Apple Nursery Trees of Knip-Boom Type As Affected By the Methods of Propagation. Acta Sci. Pol. Hortorum Cultus 2013, 12, 157–165. [Google Scholar]
- Sosna, I.; Gudarowska, E. Early performance of ‘Mutsu’ apple trees on different rootstocks in the lower Silesia region. Acta Sci. Pol. Hortorum Cultus 2013, 12, 137–146. [Google Scholar]
- Jacyna, T. Factors influencing lateral-branch formation in woody plants. Acta Agrobot. 2013, 55, 5–25. [Google Scholar] [CrossRef]
- Łysiak, P.G.; Pacholak, E. Effects of 13 years soil fertilisation on storage quality of ‘Cortland’ apples. Acta Hortic. 1999, 485, 265–272. [Google Scholar] [CrossRef]
- Brunetto, G.; de Melo, G.W.B.; Toselli, M.; Quartieri, M.; Tagliavini, M. The role of mineral nutrition on yields and fruit quality in grapevine, pear and apple. Rev. Bras. Frutic. 2015, 37, 1089–1104. [Google Scholar] [CrossRef] [Green Version]
- Zhao, S.; Zhang, Q.; Liu, M.; Zhou, H.; Ma, C.; Wang, P. Regulation of plant responses to salt stress. Int. J. Mol. Sci. 2021, 22, 4609. [Google Scholar] [CrossRef]
- Singh, A.; Sharma, D.K.; Kumar, R.; Kumar, A. Soil Salinity Management in Fruit Crops: A Review of Options and Challenges. Eng. Pract. Manag. Soil Salin. 2020, 81–128. [Google Scholar] [CrossRef]
- Owens, C.L.; Stover, E. Vegetative growth and flowering of young apple trees in response to prohexadione-calcium. HortScience 1999, 34, 1194–1196. [Google Scholar] [CrossRef] [Green Version]
- Atay, E.; Crété, X.; Loubet, D.; Lauri, P.E. Effects of Different Crop Loads on Physiological, Yield and Fruit Quality of ‘JoyaTM’ Apple Trees: High Crop Load Decreases Maximum Daily Trunk Diameter and Does Not Affect Stem Water Potential. Int. J. Fruit Sci. 2021, 21, 955–969. [Google Scholar] [CrossRef]

| Mulch Type | Contents of Macro-Elements [% f.w.] | Salinity [g NaCl·L−1] | Organic Matter Content [% d.m.] | Volumetric Mass Density [kg·m−3] | ||
|---|---|---|---|---|---|---|
| P | K | Mg | ||||
| Spent mushroom substrate | 0.36 | 0.76 | 0.49 | 8.49 | 65.1 | 341 |
| Miscanthus × giganteus straw | <0.1 | 0.22 | <0.1 | 0.50 | 2.36 | 33 |
| Depth [cm] | pHKCl | Available Macro-Elements in Soil [mg⋅100 g−1] | K/Mg Ratio | Organic Matter [% d.m.] | Salinity [g NaCl·L−1] | ||
|---|---|---|---|---|---|---|---|
| P | K | Mg | |||||
| 0–20 | 5.5 | 2.76 | 23.8 | 15.2 | 1.57 | 2.57 | 0.20 |
| 21–40 | 5.9 | 2.63 | 11.2 | 16.1 | 0.70 | - | 0.18 |
| Depth [cm] | System | pHKCl | Available Macro-Elements in Soil [mg·100−1 g] | K:Mg Ratio | Salinity [g NaCl·L−1] | Organic Matter Content [% d.m.] | ||
|---|---|---|---|---|---|---|---|---|
| P | K | Mg | ||||||
| 0–20 | HS | 5.1 ± 0.5 a | 2.80 ± 0.40 a | 22.3 ± 4.0 a | 14.4 ± 1.6 ab | 1.30 ± 0.33 a | 0.19 ± 0.01 a | 2.77 ± 0.23 a |
| MC | 5.1 ± 0.3 a | 2.63 ± 0.50 a | 18.7 ± 3.1 a | 15.8 ± 0.5 ab | 1.10 ± 0.25 a | 0.21 ± 0.03 a | 2.79 ± 0.14 a | |
| BC | 5.7 ± 0.3 ab | 3.27 ± 0.15 a | 20.2 ± 5.8 a | 14.0 ± 0.1 a | 1.13 ± 0.36 a | 0.18 ± 0.02 a | 2.32 ± 0.46 a | |
| MG1 | 5.5 ± 0.5 ab | 3.06 ± 0.50 a | 24.3 ± 5.5 a | 14.7 ± 0.9 ab | 1.33 ± 0.34 a | 0.26 ± 0.09 a | 2.69 ± 0.29 a | |
| MG2 | 5.3 ± 0.7 ab | 4.27 ± 0.70 a | 25.2 ± 2.9 a | 16.0 ± 2.1 ab | 1.36 ± 0.28 a | 0.23 ± 0.08 a | 3.48 ± 0.67 ab | |
| SMS1 | 5.7 ± 0.1 ab | 14.9 ± 2.95 ab | 63.3 ± 4.8 b | 15.1 ± 1.5 ab | 2.93 ± 0.36 b | 1.26 ± 0.37 b | 3.52 ± 0.75 ab | |
| SMS2 | 6.3 ± 0.3 b | 22.6 ± 4.21 b | 66.3 ± 6.2 b | 18.8 ± 3.2 b | 3.46 ± 0.19 b | 2.08 ± 0.25 c | 4.30 ± 0.25 b | |
| 21–40 | HS | 5.4 ± 0.7 a | 3.33 ± 0.90 a | 16.1 ± 5.9 a | 16.1 ± 2.5 a | 0.86 ± 0.53 a | 0.19 ± 0.03 a | n.a. * |
| MC | 5.3 ± 0.4 a | 2.97 ± 0.53 a | 15.3 ± 3.3 a | 15.3 ± 1.5 a | 0.76 ± 0.20 a | 0.24 ± 0.03 a | ||
| BC | 6.0 ± 0.7 a | 1.80 ± 0.50 a | 14.9 ± 3.5 a | 14.9 ± 0.9 a | 0.63 ± 0.31 a | 0.17 ± 0.04 a | ||
| MG1 | 5.6 ± 0.5 a | 3.56 ± 0.87 a | 15.9 ± 2.4 a | 15.9 ± 0.6 a | 1.13 ± 0.62 a | 0.23 ± 0.01 a | ||
| MG2 | 6.0 ± 0.9 a | 3.20 ± 0.98 a | 15.6 ± 1.9 a | 15.6 ± 1.9 a | 0.86 ± 0.66 a | 0.26 ± 0.09 a | ||
| SMS1 | 5.6 ± 0.2 a | 7.80 ± 1.99 a | 17.2 ± 4.3 a | 17.2 ± 2.0 a | 1.33 ± 0.41 a | 1.26 ± 0.04 b | ||
| SMS2 | 6.3 ± 0.3 a | 23.8 ± 2.12 b | 18.5 ± 3.6 a | 18.5 ± 3.0 a | 2.46 ± 0.85 b | 2.04 ± 0.25 c | ||
| Year | System | Macroelement Concentrations in Leaves [% d.m.] | Leaf Area [cm2] | |||
|---|---|---|---|---|---|---|
| N | P | K | Mg | |||
| 2017 | HS | 2.31 ± 0.11 a | 0.16 ± 0.01 ab | 2.13 ± 0.24 a | 0.20 ± 0.01 a | 36.9 ± 3.76 ab |
| MC | 2.35 ± 0.01 a | 0.14 ± 0.01 a | 2.02 ± 0.09 a | 0.20 ± 0.02 a | 43.6 ± 1.59 b | |
| BC | 2.47 ± 0.20 a | 0.15 ± 0.01 a | 1.92 ± 0.19 a | 0.19 ± 0.01 a | 35.9 ± 2.35 ab | |
| MG1 | 2.83 ± 0.12 b | 0.14 ± 0.01 a | 2.05 ± 0.07 a | 0.19 ± 0.02 a | 41.3 ± 3.79 b | |
| MG2 | 2.87 ± 0.09 b | 0.16 ± 0.01 ab | 2.13 ± 0.15 a | 0.19 ± 0.01 a | 42.6 ± 3.41 b | |
| SMS1 | 2.97 ± 0.10 b | 0.16 ± 0.01 ab | 2.10 ± 0.04 a | 0.18 ± 0.01 a | 32.4 ± 1.79 a | |
| SMS2 | 2.46 ± 0.03 a | 0.20 ± 0.01 b | 2.58 ± 0.06 b | 0.19 ± 0.01 a | 31.5 ± 4.01 a | |
| 2018 | HS | 2.46 ± 0.03 bc | 0.17 ± 0.02 a | 1.12 ± 0.05 a | 0.27 ± 0.03 a | 36.4 ± 3.70 ab |
| MC | 2.36 ± 0.08 b | 0.18 ± 0.02 a | 1.27 ± 0.15 a | 0.22 ± 0.01 a | 43.8 ± 1.76 b | |
| BC | 2.47 ± 0.37 bc | 0.17 ± 0.01 a | 1.08 ± 0.16 a | 0.29 ± 0.03 a | 36.0 ± 2.43 ab | |
| MG1 | 2.45 ± 0.26 bc | 0.16 ± 0.01 a | 1.39 ± 0.06 a | 0.22 ± 0.04 a | 41.0 ± 5.51 ab | |
| MG2 | 2.21 ± 0.11 a | 0.18 ± 0.01 a | 1.28 ± 0.22 a | 0.22 ± 0.05 a | 42.5 ± 3.52 b | |
| SMS1 | 2.51 ± 0.09 c | 0.16 ± 0.01 a | 1.48 ± 0.18 a | 0.22 ± 0.02 a | 32.2 ± 2.26 a | |
| SMS2 | 2.47 ± 0.14 bc | 0.16 ± 0.01 a | 1.45 ± 0.06 a | 0.22 ± 0.01 a | 31.7 ± 4.19 a | |
| System | Initial TCSA [cm2] | Autumn 2017 | Autumn 2018 | TCSA Increment 2017–2018 [cm2] | Cumulative Shoot Length 2017–2018 [cm] | ||||
|---|---|---|---|---|---|---|---|---|---|
| TCSA [cm2] | Mean Shoot Length [cm] | Cumulative Shoot Length [cm] | TCSA [cm2] | Mean Shoot Length [cm] | Cumulative Shoot Length [cm] | ||||
| HS | 1.15 ± 0.08 a | 2.78 ± 0.12 bc | 20.7 ± 0.8 b | 428 ± 33 c | 4.39 ± 0.22 b | 25.6 ± 1.1 a | 802 ± 79 c | 3.25 ± 0.24 b | 1230 ± 104 c |
| MC | 1.17 ± 0.12 a | 2.67 ± 0.13 bc | 18.9 ± 1.9 ab | 600 ± 96 d | 4.89 ± 0.19 b | 27.4 ± 3.5 a | 915 ± 47 d | 3.61 ± 0.14 b | 1515 ± 144 d |
| BC | 1.28 ± 0.05 a | 3.09 ± 0.28 c | 24.3 ± 2.4 b | 532 ± 59 cd | 4.19 ± 0.51 b | 26.7 ± 2.6 a | 609 ± 48 b | 3.02 ± 0.46 b | 1142 ± 86 c |
| MG1 | 1.17 ± 0.18 a | 2.88 ± 0.39 bc | 29.1 ± 3.9 c | 526 ± 34 cd | 4.58 ± 0.55 b | 25.8 ± 2.5 a | 744 ± 55 c | 3.41 ± 0.37 b | 1269 ± 54 c |
| MG2 | 1.16 ± 0.07 a | 2.81 ± 0.02 bc | 37.9 ± 2.8 d | 495 ± 67 cd | 4.46 ± 0.07 b | 33.0 ± 1.8 b | 1077 ± 59 d | 3.30 ± 0.14 b | 1573 ± 104 d |
| SMS1 | 1.30 ± 0.20 a | 2.37 ± 0.31 ab | 15.0 ± 2.6 ab | 272 ± 21 b | 3.47 ± 0.49 a | 23.9 ± 3.6 a | 567 ± 35 ab | 2.16 ± 0.40 a | 838.9 ± 35 b |
| SMS2 | 1.11 ± 0.09 a | 2.03 ± 0.08 a | 11.4 ± 2.2 a | 153 ± 19 a | 3.02 ± 0.11 a | 23.6 ± 0.6 a | 480 ± 46 a | 1.91 ± 0.04 a | 633.4 ± 60 a |
| System | No. of Flower Clusters [pcs·Tree−1] | Flower Cluster Density [pcs·m−1] | Blooming Efficiency Index (BEI) [pcs·cm−2] | Fruit Set [pcs·100−1 Flower Buds] | Initial Yield [kg·tree−1] | Cropping Efficiency Index (CEI) [kg·cm−2] |
|---|---|---|---|---|---|---|
| HS | 47.7 ± 3.2 a | 3.82 ± 0.55 a | 17.2 ± 2.2 ab | 75.0 ± 1.9 b | 7.44 ± 0.77 b | 1.70 ± 0.19 a |
| MC | 51.0 ± 5.7 ab | 2.93 ± 0.35 a | 19.0 ± 1.7 b | 68.5 ± 5.7 b | 6.21 ± 0.78 ab | 1.48 ± 0.15 a |
| BC | 61.3 ± 8.8 b | 5.97 ± 0.80 b | 19.9 ± 0.8 b | 60.4 ± 1.7 a | 8.90 ± 0.48 c | 1.84 ± 0.29 a |
| MG1 | 47.3 ± 6.3 a | 3.73 ± 0.65 a | 16.4 ± 0.8 ab | 86.7 ± 13.3 c | 9.12 ± 0.40 c | 2.00 ± 0.16 a |
| MG2 | 37.7 ± 3.2 a | 2.41 ± 0.35 a | 13.4 ± 1.2 a | 74.2 ± 6.8 b | 7.48 ± 0.24 b | 1.68 ± 0.03 a |
| SMS1 | 51.0 ± 5.1 ab | 7.37 ± 0.85 c | 26.2 ± 2.2 c | 52.7 ± 6.3 a | 6.73 ± 0.73 ab | 1.97 ± 0.41 a |
| SMS2 | 61.7 ± 3.6 b | 8.11 ± 1.01 c | 25.2 ± 2.8 c | 53.0 ± 2.7 a | 5.65 ± 0.53 a | 2.00 ± 0.24 a |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Przybyłko, S.; Szpadzik, E.; Marszał, J.; Kowalczyk, W.; Wrona, D. Different Floor Management Systems Affect Soil Properties and Initial Development of Apple Tree (Malus × domestica Borkh.) in an Orchard. Agriculture 2022, 12, 2070. https://doi.org/10.3390/agriculture12122070
Przybyłko S, Szpadzik E, Marszał J, Kowalczyk W, Wrona D. Different Floor Management Systems Affect Soil Properties and Initial Development of Apple Tree (Malus × domestica Borkh.) in an Orchard. Agriculture. 2022; 12(12):2070. https://doi.org/10.3390/agriculture12122070
Chicago/Turabian StylePrzybyłko, Sebastian, Ewa Szpadzik, Jacek Marszał, Wojciech Kowalczyk, and Dariusz Wrona. 2022. "Different Floor Management Systems Affect Soil Properties and Initial Development of Apple Tree (Malus × domestica Borkh.) in an Orchard" Agriculture 12, no. 12: 2070. https://doi.org/10.3390/agriculture12122070






