Field Study of the Effects of Two Different Environmental Conditions on Wheat Productivity and Chlorophyll Fluorescence Induction (OJIP) Parameters
Abstract
:1. Introduction
2. Materials and Methods
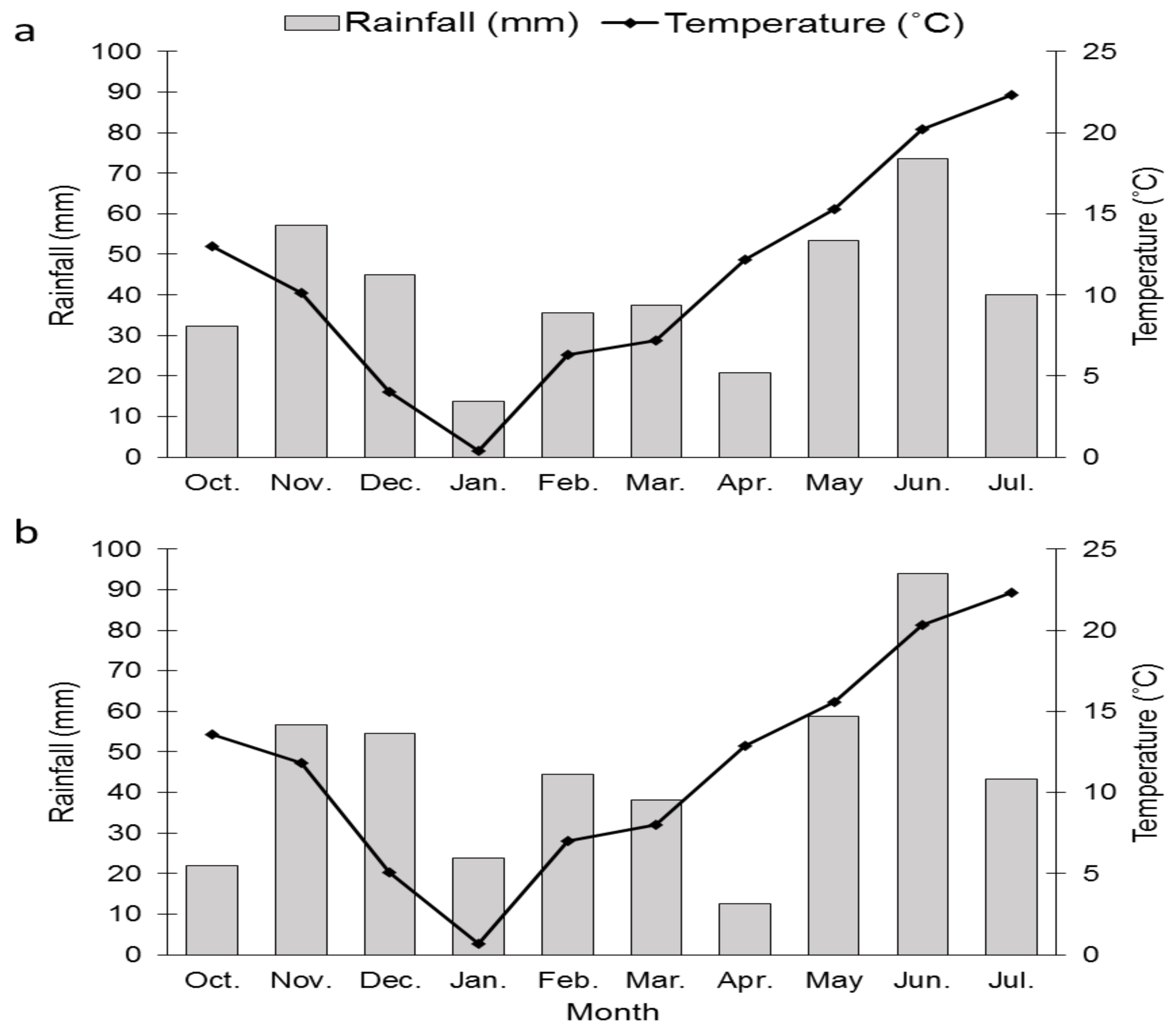
2.1. Field Experiments
2.2. Fast Chlorophyll a Fluorescence Measurements
2.3. Statistical Analysis
3. Results
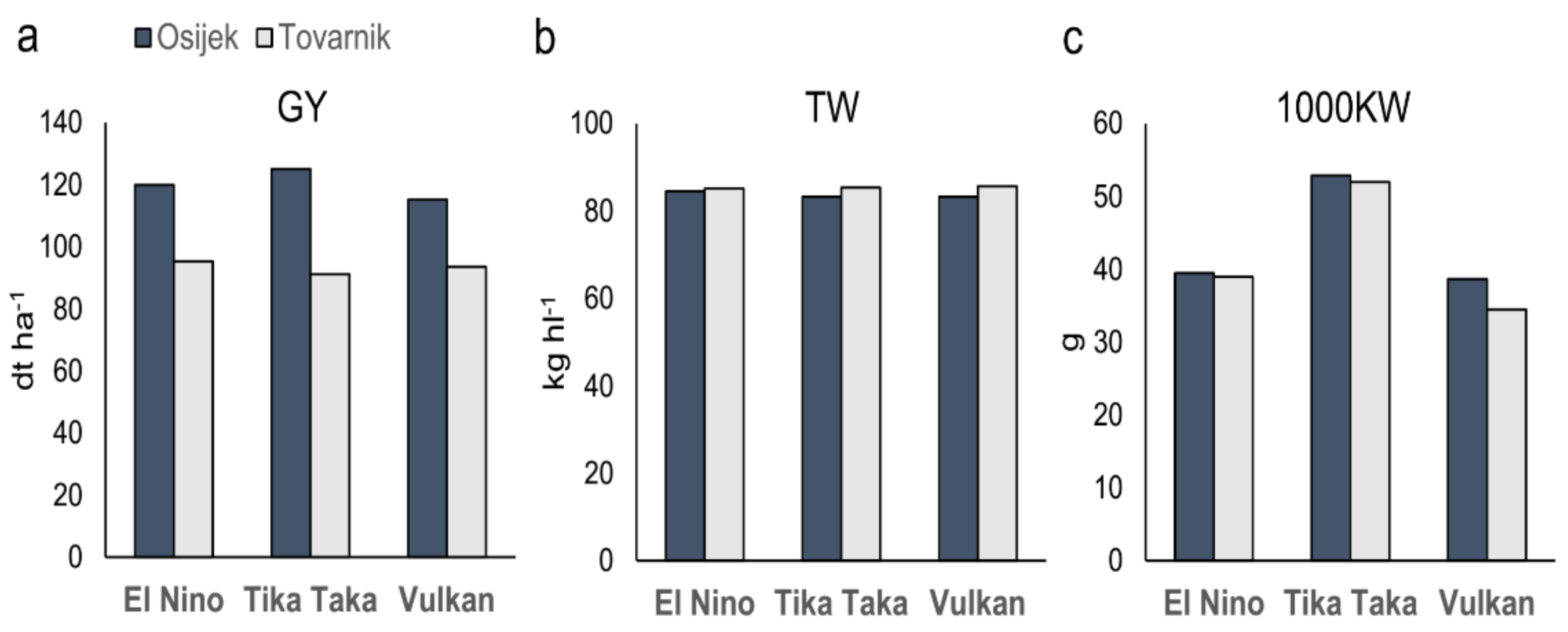
3.1. Grain Yield and Other Agronomical Traits
3.2. Chlorophyll a Fluorescence Measurements
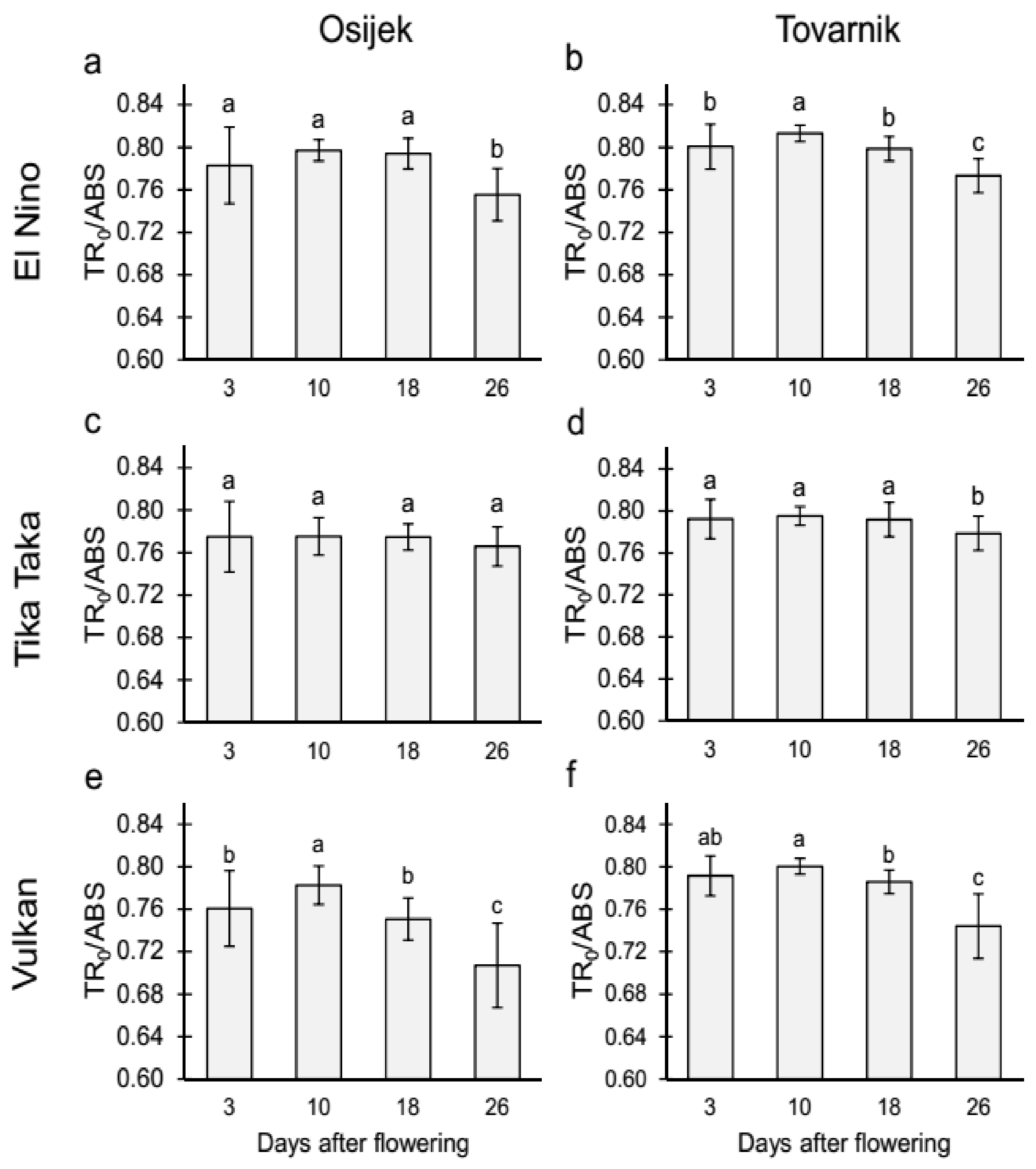
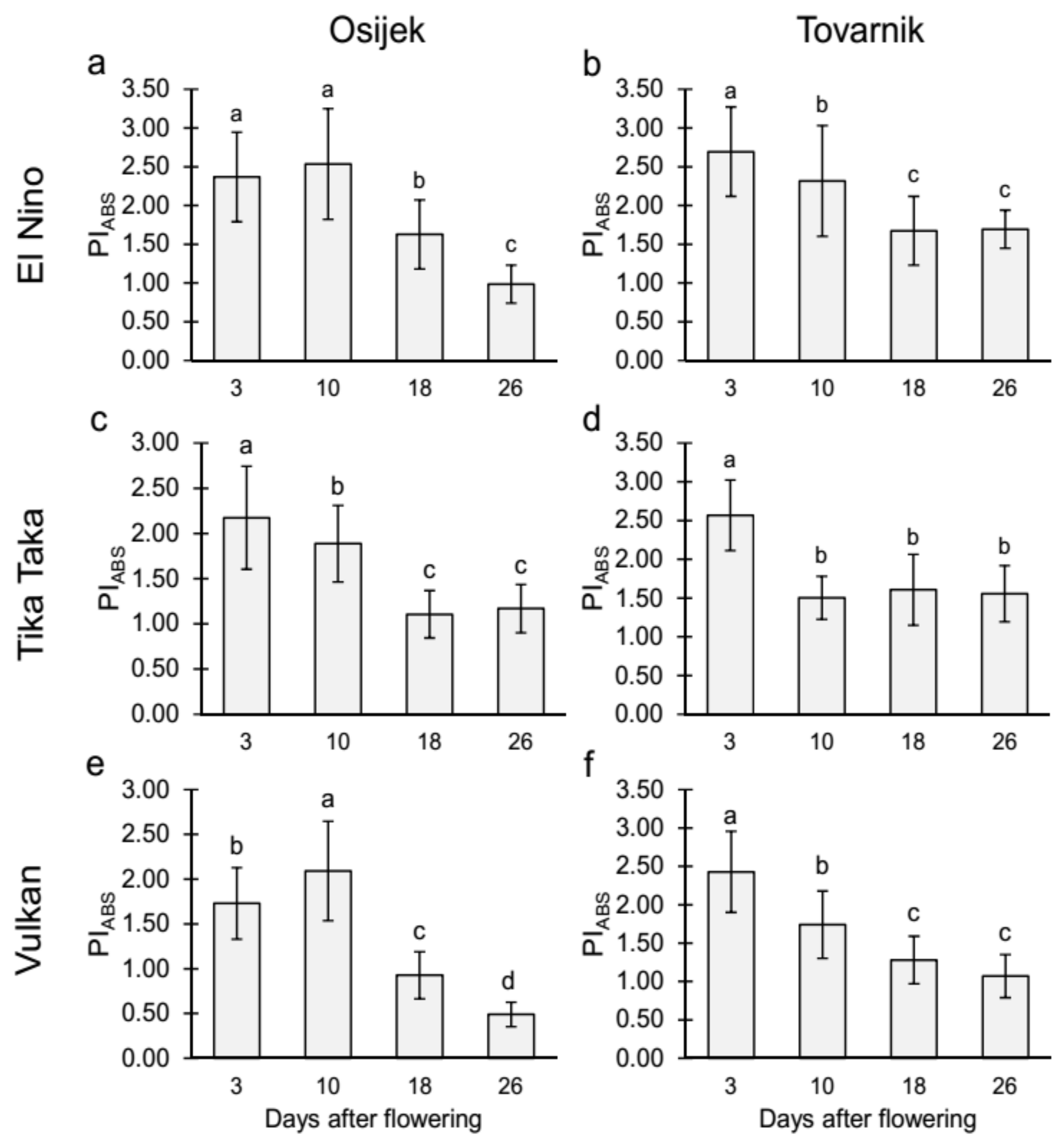
3.2.1. Maximum Quantum Yield of Primary Photochemistry (TR0/ABS) and Performance Index on Absorption Basis (PIabs) in the Flag Leaves
3.2.2. Maximum Quantum Yield of Primary Photochemistry (TR0/ABS) and Performance Index on Absorption Basis (PIabs) in the Glumes of the Heads
4. Discussion
4.1. Agronomical Traits
4.2. Chlorophyll (Chl) Fluorescence Parameters
4.3. Grain Productivity and Two Stress Indicators at Two Locations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Huang, Z.; Jiang, D.; Yang, Y.; Sun, J.; Jin, S. Effects of nitrogen deficiency on gas exchange, chlorophyll fluorescence, and antioxidant enzymes in leaves of rice plants. Photosynthetica 2004, 42, 357–364. [Google Scholar] [CrossRef]
- Zhang, M.P.; Zhang, C.J.; Yu, G.; Jiang, Y.Z.; Strasser, R.J.; Yuan, Z.Y.; Yang, X.S.; Chen, G.X. Changes in chloroplast ultrastructure, fatty acid components of thylakoid membrane and chlorophyll a fluorescence transient in flag leaves of a super-high-yield hybrid rice and its parents during the reproductive stage. J. Plant Physiol. 2010, 167, 277–285. [Google Scholar] [CrossRef]
- Wollmer, A.C.; Pitmann, K.; Mühling, K.H. Einfluss von temporärer Überstauung auf Wachstum, Nährstoffkonzentration und Ertrag von Weizen. In Kongressband 2016 Rostock. Vorträge zum Generalthema: Anforderungen an die Verwertung von Reststoffen in der Landwirtschaft; Ebertseder, T., Ed.; VDLUFA-Verlag VDLUFA-Schriftenreihe: Darmstadt, Germany, 2016; Volume 73, pp. 138–142. (In German) [Google Scholar]
- Afzal, F.; Chaudhari, S.K.; Gul, A.; Farooq, A.; Ali, H.; Nisar, S.; Sarfraz, K.J.; Mujeeb-Kazi, A. Bread wheat (Triticum aestivum L.) under biotic and abiotic Stresses: An overview. In Crop Production and Global Environmental Issues; Hakeem, K., Ed.; Springer: Cham, Switzerland, 2015. [Google Scholar]
- FAO. Global agriculture towards 2050. In Proceedings of the Expert Meeting on How to Feed the World in 2050 Forum, Rome, Italy, 24–26 June 2009. [Google Scholar]
- Terletskaya, N.; Zobova, N.; Stupko, V.; Shuyskaya, E. Growth and photosynthetic reactions of different species of wheat seedlings under drought and salt stress. Period. Biol. 2017, 119, 37–45. [Google Scholar] [CrossRef]
- Makino, A. Photosynthesis, Grain Yield, and Nitrogen Utilization in Rice and Wheat. Plant Physiol. 2011, 155, 125–129. [Google Scholar] [CrossRef] [Green Version]
- Yang, H.; Dong, B.; Wang, Y.; Qiao, Y.; Shi, C.; Jin, L.; Li, M. Photosynthetic base of reduced grain yield by shading stress during the early reproductive stage of two wheat cultivars. Sci. Rep. 2020, 10, 14353. [Google Scholar] [CrossRef]
- Gaju, O.; de Silva, J.G.; Carvalho, P.; Hawkesford, M.J.; Griffiths, S.; Greenland, A.; Foulkes, M. Leaf photosynthesis and associations with grain yield, biomass and nitrogen-use efficiency in landraces, synthetic-derived lines and cultivars in wheat. Field Crops Res. 2016, 193, 1–15. [Google Scholar] [CrossRef]
- Maydup, M.L.; Antonietta, M.; Guiamet, J.J.; Graciano, C.; Lopez, J.R.; Tambussi, E.A. The contribution of ear photosynthesis to grain filling in bread wheat (Triticum aestivum L.). Field Crops Res. 2010, 119, 48–58. [Google Scholar] [CrossRef]
- Wang, Y.-q.; Xi, W.-x.; Wang, Z.-m.; Wang, B.; Xu, X.-x.; Han, M.-k.; Zhou, S.-l.; Zhang, Y.-h. Contribution of ear photosynthesis to grain yield under rainfed and irrigation conditions for winter wheat cultivars released in the past 30 years in North China Plain. J. Integr. Agric. 2016, 15, 2247–2256. [Google Scholar] [CrossRef] [Green Version]
- Tambussi, E.A.; Nogué, S.; Araus, J.L. Ear of durum wheat under water stress: Water relations and photosynthetic metabolism. Planta 2005, 221, 446–458. [Google Scholar] [CrossRef]
- Zhang, M.; Gao, Y.; Zhang, Y.; Fischer, T.; Zhao, Z.; Zhou, X.; Wang, Z.; Wang, E. The contribution of spike photosynthesis to wheat yield needs to be considered in process-based crop models. Field Crops Res. 2020, 257, 107931. [Google Scholar] [CrossRef]
- Davies, P.J.; Gan, S. Towards an integrated view of monocarpic plant senescence. Russ. J. Plant Physiol. 2012, 59, 467–478. [Google Scholar] [CrossRef]
- Feller, U.; Anders, I.; Mae, T. Rubiscolytics: Fate of Rubisco after its enzymatic function in a cell is terminated. J. Exp. Bot. 2008, 59, 1615–1624. [Google Scholar] [CrossRef] [Green Version]
- Distelfeld, A.; Avni, R.; Fischer, A.M. Senescence, nutrient remobilization, and yield in wheat and barley. J. Exp. Bot. 2014, 65, 3783–3798. [Google Scholar] [CrossRef] [Green Version]
- Gregersen, P.L.; Culetic, A.; Boschian, L.; Krupinska, K. Plant senescence and crop productivity. Plant Mol. Biol. 2013, 82, 603–622. [Google Scholar] [CrossRef]
- Waters, B.M.; Uauy, C.; Dubcovsky, J.; Grusak, M.A. Wheat (Triticum aestivum) NAM proteins regulate the translocation of iron, zinc, and nitrogen compounds from vegetative tissues to grain. J. Exp. Bot. 2009, 60, 4263–4274. [Google Scholar] [CrossRef] [Green Version]
- Serrago, R.A.; Alzueta, I.; Savin, R.; Slafer, G.A. Understanding grain yield responses to source–sink ratios during grain filling in wheat and barley under contrasting environments. Field Crops Res. 2013, 150, 42–51. [Google Scholar] [CrossRef] [Green Version]
- Yang, S.M.; Li, X.; Chen, W.Q.; Liu, T.G.; Zhong, S.F.; Ma, L.X.; Zhang, M.; Zhang, H.Y.; Yu, D.L.; Luo, P.G. Wheat Resistance to Fusarium Head Blight is Associated with Changes in Photosynthetic Parameters. Plant Dis. 2016, 100, 847–852. [Google Scholar] [CrossRef]
- Kim, Y.M.; Bouras, N.; Kav, N.N.V.; Strelkov, S.E. Inhibition of photosynthesis and modification of the wheat leaf proteome by Ptr ToxB: A host-specific toxin from the fungal pathogen Pyrenophora tritici-repentis. Proteomics 2010, 10, 2911–2926. [Google Scholar] [CrossRef]
- Fghire, R.; Anaya, F.; Ali, O.I.; Benlhabib, O.; Ragab, R.; Wahbi, S. Physiological and photosynthetic response of quinoa to drought stress. Chil. J. Agric. Res. 2015, 75, 174–183. [Google Scholar] [CrossRef] [Green Version]
- Wang, P.; Duan, W.; Takabayashi, A.; Endo, T.; Shikanai, T.; Ye, J.Y.; Mi, H.L. Chloroplastic NAD(P)H dehydrogenase in tobacco leaves functions in alleviation of oxidative damage caused by temperature stress. Plant Physiol. 2006, 141, 465–474. [Google Scholar] [CrossRef] [Green Version]
- Kalaji, M.H.; Carpentier, R.; Allakhverdiev, S.I.; Bosa, K. Fluorescence parameters as early indicators of light stress in barley. J. Photochem. Photobiol. B 2012, 112, 1–6. [Google Scholar] [CrossRef]
- Gupta, R. Tissue specific disruption of photosynthetic electron transport rate in pigeonpea (Cajanuscajan L.) under elevated temperature. Plant Signal. Behav. 2019, 14, 1601952. [Google Scholar] [CrossRef]
- Brestič, M.; Živčák, M.; Olšovská, K. Photosynthesis parameters may serve to better characterization and parametrization of wheat genotypes under climate change conditions. Acta Physiol. Plant. 2007, 29, S43. [Google Scholar]
- Sayed, O.H. Chlorophyll fluorescence as a tool in cereal crop research. Phytosynthetica 2003, 41, 321–330. [Google Scholar] [CrossRef]
- Sharma, A.; Kumar, V.; Shahzad, B.; Ramakrishnan, M.; Singh Sidhu, G.P.; Bali, A.S.; Handa, N.; Kapoor, D.; Yadav, P.; Khanna, K.; et al. Photosynthetic Response of Plants Under Different Abiotic Stresses: A Review. J. Plant Growth Regul. 2020, 39, 509–531. [Google Scholar] [CrossRef]
- Isoda, I.; Isoda, A. Adaptive responses of soybean and cotton to water stress. II. Changes in CO2 assimilation rate, chlorophyll fluorescence and photochemical reflectance index in relation to leaf temperature. Plant Prod. Sci. 2005, 8, 131–138. [Google Scholar]
- Strasser, R.J.; Srivastava, A.; Tsimilli-Michael, M. Analysis of chlorophyll a fluorescence transient. In Chlorophyll a Fluorescence: A Signature of Photosynthesis; Govindjee, G.C., Ed.; Springer: Dordrecht, The Netherlands, 2004; pp. 321–362. [Google Scholar]
- Strasser, R.J.; Srivastava, A.; Tsimilli-Michael, M. The fluorescent transient as a tool to characterise and screen photosynthetic samples. In Probing Photosynthesis: Mechanisms, Regulation and Adaptation; Yunus, M., Pathre, U., Mohanty, P., Eds.; CRC Press: New York, NY, USA, 2000; pp. 445–483. [Google Scholar]
- Carew, R.; Smith, E.G.; Grant, C. Factors influencing wheat yield and variability: Evidence from Manitoba, Canada. J. Agric. Appl. Econ. 2009, 41, 625–639. [Google Scholar] [CrossRef] [Green Version]
- Morris, M.L.; Belaid, A.; Byerlee, D. Wheat and barley production in rainfed marginal environments of the developing world. CIMMYT Econ. Work. Pap. (CIMMYT) 1990. [Google Scholar]
- Chen, C.C.; McCarl, B.A.; Schimmelpfennig, D.E. Yield variability as influenced by climate: A statistical investigation. Clim. Change 2004, 66, 239–261. [Google Scholar] [CrossRef] [Green Version]
- Gürsoy, S.; Sessiz, A.; Malhi, S.S. Short-term effects of tillage and residue management following cotton on grain yield and quality of wheat. Field Crops Res. 2010, 119, 260–268. [Google Scholar] [CrossRef]
- Kelly, J.; Bacon, R.; Gbur, E. Relationship of grain yield and test weight in soft red winter wheat. Cereal Res. Commun. 1995, 23, 53–57. [Google Scholar]
- Chen, J.; Liang, Y.; Hu, X.; Wang, X.; Tan, F.; Zhang, H.; Ren, Z.; Luo, P. Physiological characterization of ‘stay green’ wheat cultivars during the grain filling stage under field growing conditions. Acta Physiol. Plant. 2010, 32, 875–882. [Google Scholar] [CrossRef]
- Tataw, J.T.; Baier, F.; Krottenthaler, F.; Pachler, B.; Schwaiger, E.; Wyhlidal, S.; Formayer, H.; Hösch, J.; Baumgarten, A.; Zaller, J.G. Climate change induced rainfall patterns affect wheat productivity and agroecosystem functioning dependent on soil types. Ecol. Res. 2016, 31, 203–212. [Google Scholar] [CrossRef]
- Lawlor, D.W. Limitation to photosynthesis in water-stressed leaves: Stomata vs. metabolism and the role of ATP. Ann. Bot. 2002, 89, 871–885. [Google Scholar] [CrossRef]
- Tsimilli-Michael, M.; Strasser, R. In vivo assessment of stress impact on plants’ vitality: Applications in detecting and evaluating the beneficial role of Mycor-rhization on host plants. In Mycorrhiza: State of the Art, Genetics and Molecular Biology, Ecofunction, Biotechnology, Eco-Physiology, Structure and Systematics, 3rd ed.; Varma, A., Ed.; Springer: Berlin/Heidelberg, Germany, 2008; pp. 679–703. [Google Scholar]
- Rapacz, M. 2007. Chlorophyll a fluorescence transient during freezing and recovery in winter wheat. Photosynthetica 2007, 45, 409–418. [Google Scholar] [CrossRef]
- Lawlor, D.W.; Cornic, G. Photosynthetic carbon assimilation and associated metabolism in relation to water deficits in higher plants. Plant Cell Environ. 2002, 25, 275–294. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Maxwell, K.; Johnson, G.N. Chlorophyll fluorescence—A practical guide. J. Exp. Bot. 2000, 51, 659–668. [Google Scholar] [CrossRef]
- Murchie, E.H.; Lawson, T. Chlorophyll fluorescence analysis: A guide to good practice and understanding some new applications. J. Exp. Bot. 2013, 64, 3983–3998. [Google Scholar] [CrossRef] [Green Version]
- Sharma, D.K.; Andersen, S.B.; Ottosen, C.O.; Rosenqvist, E. Wheat cultivars selected for high Fv/Fm under heat stress maintain high photosynthesis, total chlorophyll, stomatal conductance, transpiration and dry matter. Physiol. Plant. 2015, 153, 284–298. [Google Scholar] [CrossRef]
- Long, S.P.; Humphries, S.; Falkowski, P.G. Photoinhibition of photosynthesis in nature. Annu. Rev. Plant Physiol. Plant. Mol. Biol. 1994, 45, 633–662. [Google Scholar] [CrossRef]
- Guidi, L.; Lo, P.E.; Landi, M. Chlorophyll Fluorescence, Photoinhibition and Abiotic Stress: Does it Make Any Difference the Fact to Be a C3 or C4 Species? Front. Plant Sci. 2019, 10, 174. [Google Scholar] [CrossRef] [PubMed]
- Jing, Y.X.; Li, G.L.; Gu, B.H.; Yang, D.J.; Xiao, L.; Liu, R.X.; Peng, C.L. Leaf gas exchange, chlorophyll fluorescence and growth responses of Melaleuca alternifolia seedlings to flooding and subsequent recovery. Photosynthetica 2009, 47, 595–601. [Google Scholar] [CrossRef]
- Strauss, A.J.; Krüger, G.H.J.; Strasser, R.J.; Van Herdeen, P.D.R. Ranking of dark chilling tolerance in soybean genotypes probed by the chlorophyll a fluorescence transient O-J-I-P. Environ. Exp. Bot. 2006, 56, 147–157. [Google Scholar] [CrossRef]
- Falqueto, A.R.; Cassol, D.; Magalhães Junior, A.M.; Oliveira, A.C.; Bacarin, M.A. Physiological analysis of leaf senescence of two rice cultivars with different yield potential. Pesquisa Agropecuária Brasileira 2009, 44, 695–700. [Google Scholar] [CrossRef]
- Frankel, O. Floral Initiation in Wheat. Proc. R. Soc. Lond. 1976, 192, 273–298. [Google Scholar]
- Fischer, R.A. Number of kernels in wheat crops and the influence of solarradiation and temperature. J. Agric. Sci. 1985, 105, 447–461. [Google Scholar] [CrossRef]
- Zhang, C.J.; Chen, G.X.; Gao, X.X.; Chu, C.J. Photosynthetic decline in flag leaves of two field-grown spring wheat cultivars with different senescence properties. S. Afr. J. Sci. 2006, 72, 15–23. [Google Scholar] [CrossRef] [Green Version]
- Carmo-Silva, E.; Andralojc, P.J.; Scales, J.C.; Driever, S.M.; Mead, A.; Lawson, T.; Raines, C.A.; Parry, M.A.J. Phenotyping of field-grown wheat in the UK highlights contribution of light response of photosynthesis and flag leaf longevity to grain yield. J. Exp. Bot. 2017, 68, 3473–3486. [Google Scholar] [CrossRef]
- Wang, H.; Mc Caig, T.N.; De Pauw, R.M.; Clarke, J.M. Flag leaf physiological traits in two high-yielding Canada Western red spring wheat cultivars. Can. J. Plant Sci. 2008, 88, 35–42. [Google Scholar] [CrossRef]



| Source of Variation | DF | MS | ||
|---|---|---|---|---|
| GY | TW | TKW | ||
| Variety | 2 | 15.7 | 0.26 | 287.74 *** |
| Location | 1 | 2131.3 *** | 8.84 *** | 10.70 |
| Replication | 1 | 439.1 ** | 0.01 | 0.04 |
| Error | 7 | 27.40 | 0.37 | 3.47 |
| Location | GY (dt ha−1) | TW (kg hl−1) | TKW (g) |
|---|---|---|---|
| Osijek | 119.98a | 83.67b | 43.66a |
| Tovarnik | 93.33b | 85.38a | 41.77a |
| Source of Variation | DF | MS | |
|---|---|---|---|
| TR0/ABS | PIabs | ||
| PT | 1 | 0.3790 *** | 203.460 *** |
| L | 1 | 0.0320 *** | 12.802 *** |
| V | 2 | 0.0194 *** | 15.273 *** |
| MT | 3 | 0.0400 *** | 54.942 *** |
| PT*L | 1 | 0.0177 *** | 0.073 |
| PT*V | 2 | 0.0064 *** | 0.535 |
| L*V | 2 | 0.0020 *** | 0.003 |
| PT*MT | 3 | 0.0049 *** | 3.594 *** |
| L*MT | 3 | 0.0005 | 2.027 *** |
| V*MT | 6 | 0.0030 *** | 0.843 *** |
| PT*L*V | 2 | 0.0013 *** | 0.350 |
| PT*L*MT | 3 | 0.0008 * | 3.492 *** |
| PT*V*MT | 6 | 0.0023 *** | 0.986 *** |
| L*V*MT | 6 | 0.0002 | 0.302 |
| PT*L*V*MT | 6 | 0.0005 | 0.522 * |
| Error | 913 | 0.0003 | 0.223 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Spanic, V.; Mlinaric, S.; Zdunic, Z.; Katanic, Z. Field Study of the Effects of Two Different Environmental Conditions on Wheat Productivity and Chlorophyll Fluorescence Induction (OJIP) Parameters. Agriculture 2021, 11, 1154. https://doi.org/10.3390/agriculture11111154
Spanic V, Mlinaric S, Zdunic Z, Katanic Z. Field Study of the Effects of Two Different Environmental Conditions on Wheat Productivity and Chlorophyll Fluorescence Induction (OJIP) Parameters. Agriculture. 2021; 11(11):1154. https://doi.org/10.3390/agriculture11111154
Chicago/Turabian StyleSpanic, Valentina, Selma Mlinaric, Zvonimir Zdunic, and Zorana Katanic. 2021. "Field Study of the Effects of Two Different Environmental Conditions on Wheat Productivity and Chlorophyll Fluorescence Induction (OJIP) Parameters" Agriculture 11, no. 11: 1154. https://doi.org/10.3390/agriculture11111154






