Effects of Agricultural Use on Endangered Plant Taxa in Spain
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Area
2.2. Studied Taxon
2.2.1. Conservation Status
2.2.2. Trait Data
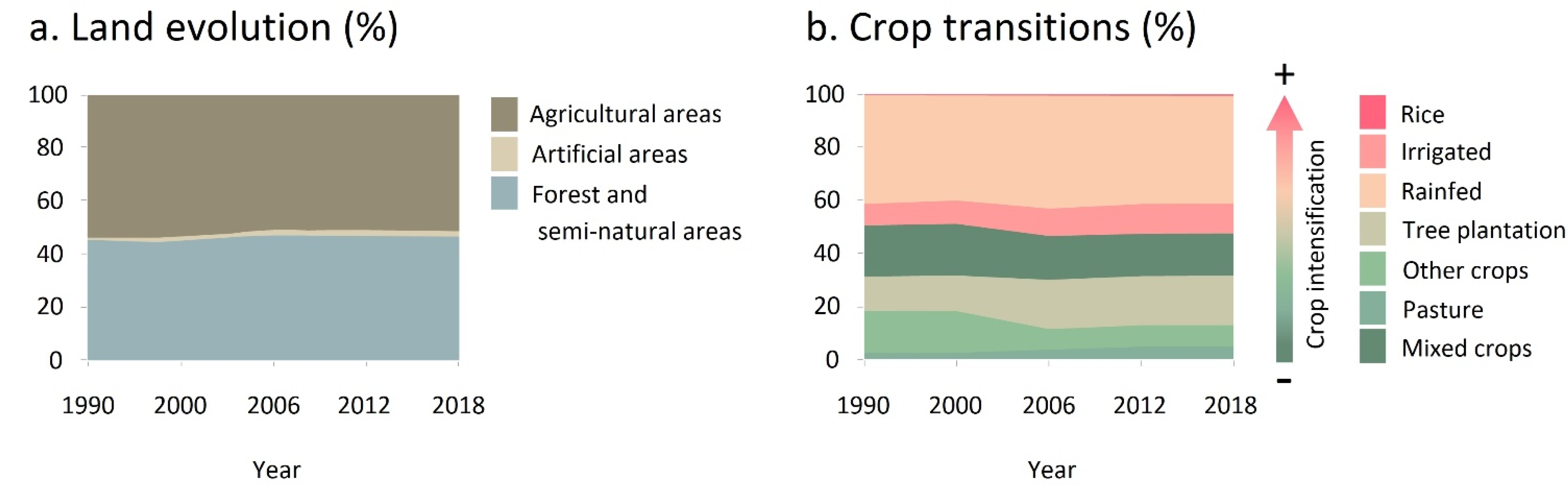
2.3. Agricultural Use Evolution
3. Results
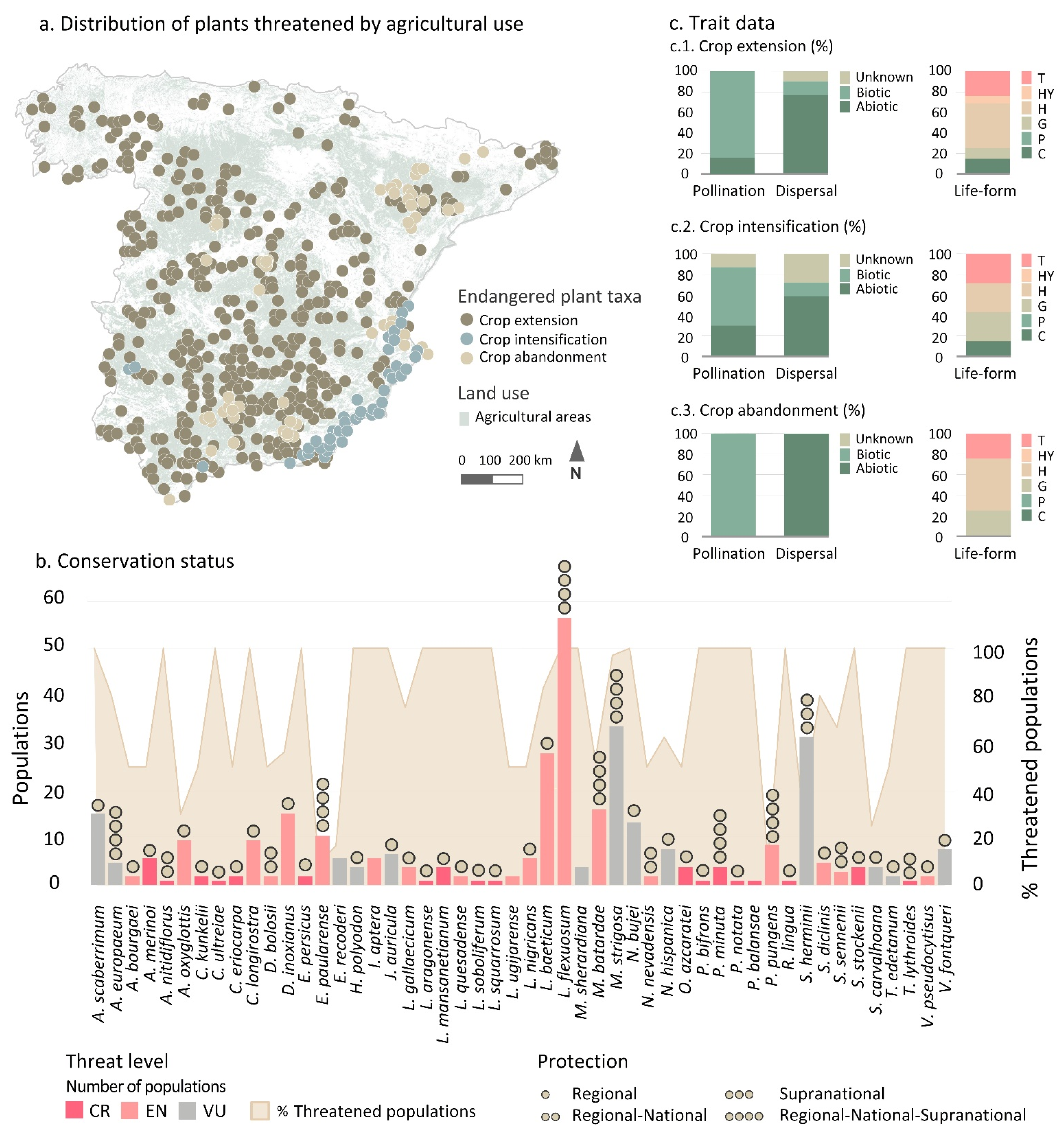
3.1. Threatened Plant Taxa and Level of Protection
3.2. Current State of Taxa Endangered by Agricultural Threat Categories and Trends
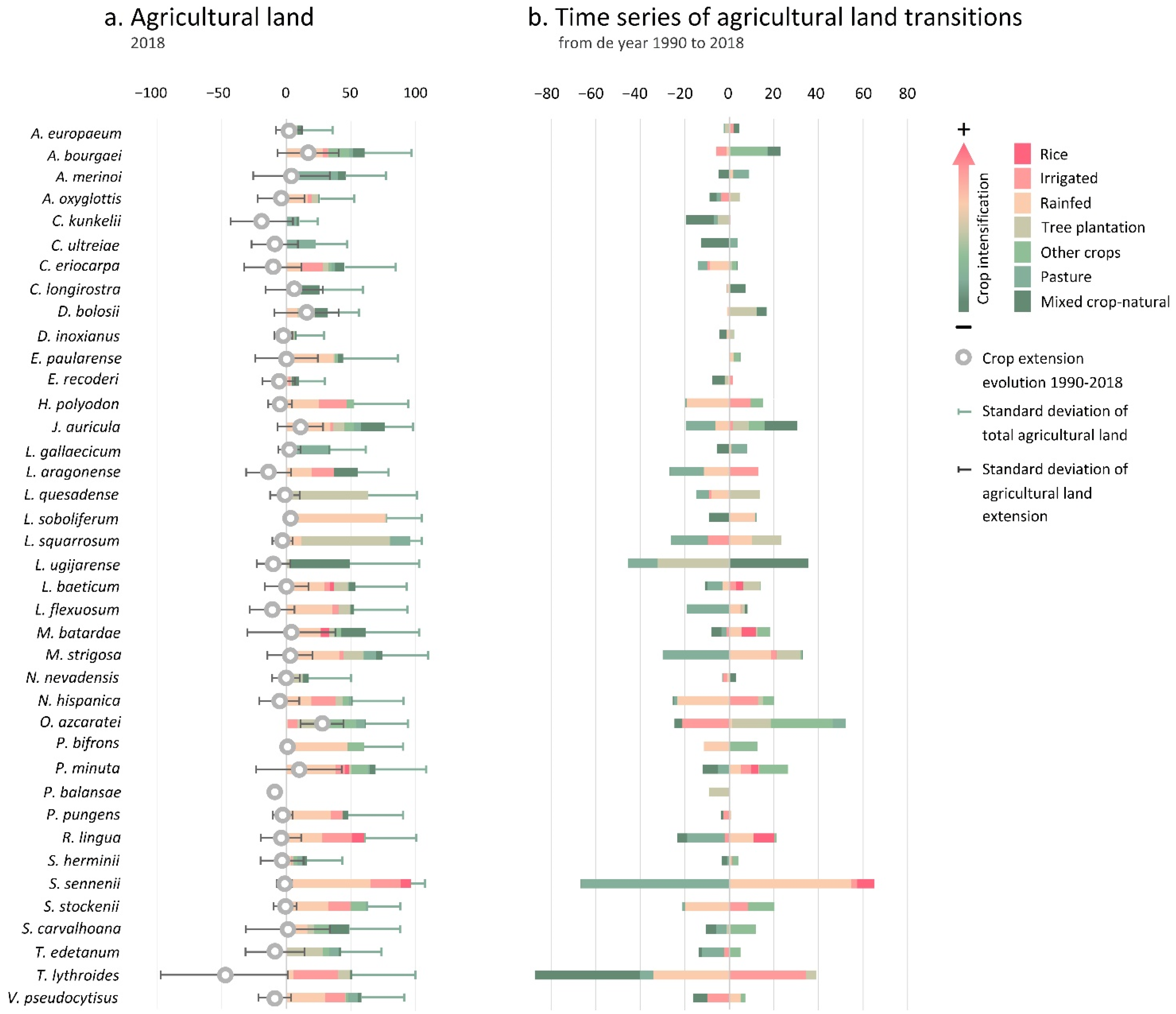
3.2.1. Taxa Endangered by Crop Extension
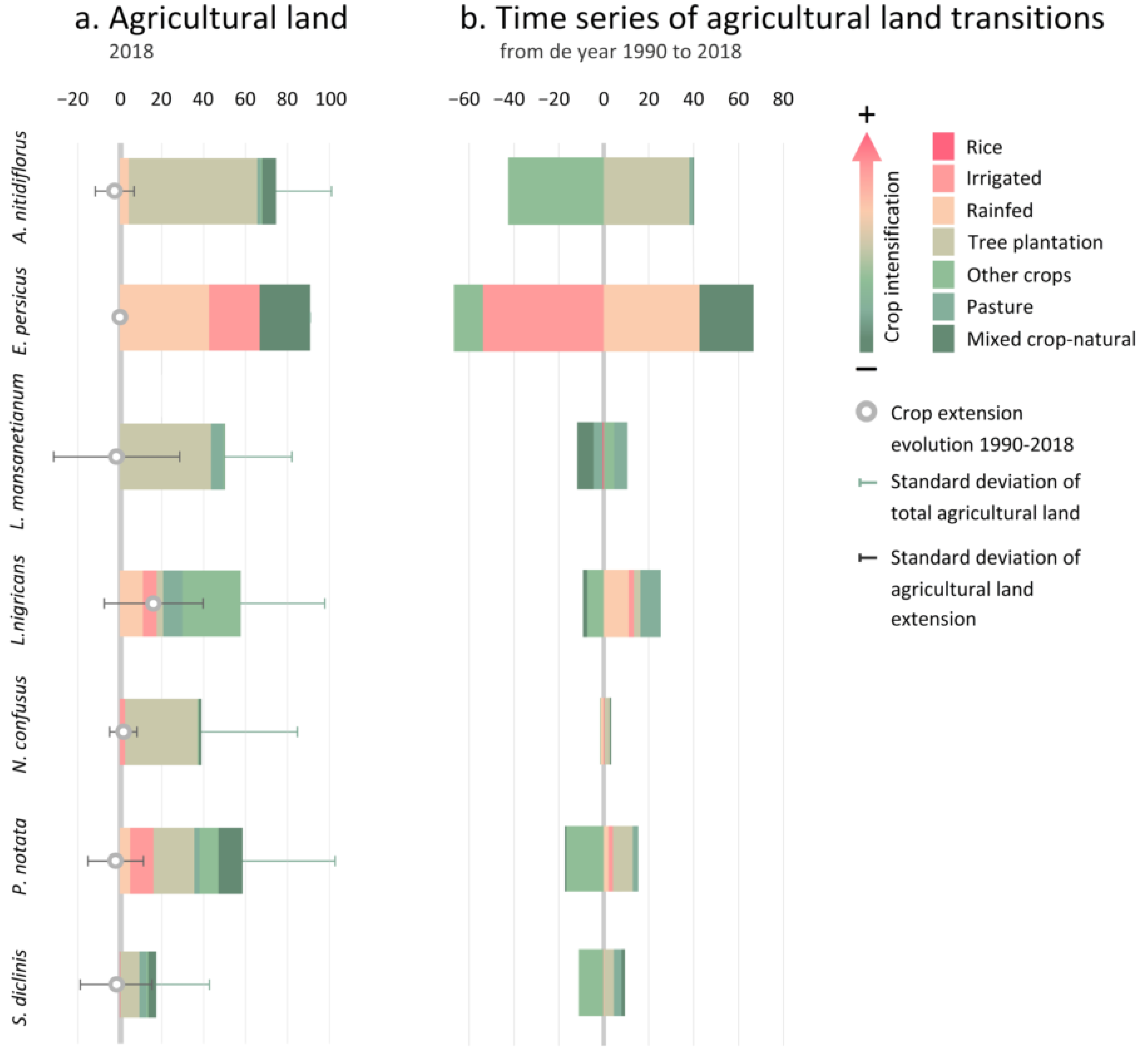
3.2.2. Taxa Endangered by Crop Intensification
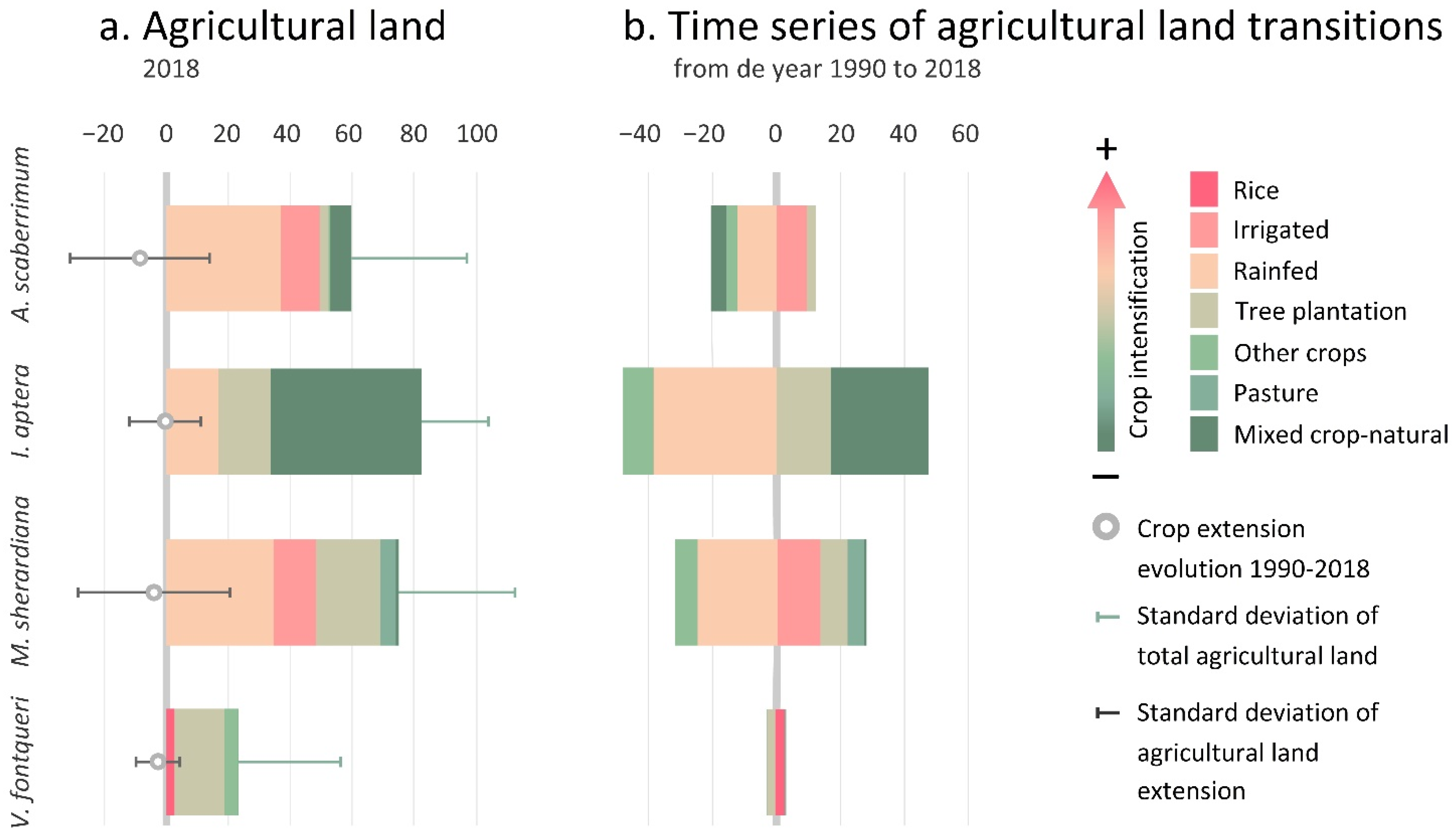
3.2.3. Taxa Endangered by Crop Abandonment
4. Discussion
4.1. Crop Extension
4.2. Crop Intensification
4.3. Crop Abandonment
4.4. Conservation Implications
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Ellis, E.C. Anthropogenic transformation of the terrestrial biosphere. Philos. Trans. R. Soc. A Math. Phys. Eng. Sci. 2011, 369, 1010–1035. [Google Scholar] [CrossRef]
- Foley, J.A.; DeFries, R.; Asner, G.P.; Barford, C.; Bonan, G.; Carpenter, S.R.; Chapin, F.S.; Coe, M.T.; Daily, G.C.; Gibbs, H.K.; et al. Global Consequences of Land Use. Science 2005, 309, 570–574. [Google Scholar] [CrossRef] [Green Version]
- Ellis, E.C. Anthromes. In Encyclopedia of the World’s Biomes; Elsevier: Amsterdam, The Netherlands, 2020; pp. 5–11. [Google Scholar]
- Tilman, D.; Fargione, J.; Wolff, B.; D’Antonio, C.; Dobson, A.; Howarth, R.; Schindler, D.; Schlesinger, W.H.; Simberloff, D.; Swackhamer, D. Forecasting Agriculturally Driven Global Environmental Change. Science 2001, 292, 281–284. [Google Scholar] [CrossRef] [Green Version]
- Steffen, W.; Richardson, K.; Rockström, J.; Cornell, S.E.; Fetzer, I.; Bennett, E.M.; Biggs, R.; Carpenter, S.R.; de Vries, W.; Wit, C.A.; et al. Planetary boundaries: Guiding human development on a changing planet. Science 2015, 347. [Google Scholar] [CrossRef] [Green Version]
- Ramankutty, N.; Evan, A.T.; Monfreda, C.; Foley, J.A. Farming the planet: 1. Geographic distribution of global agricultural lands in the year 2000. Global Biogeochem. Cycles 2008, 22, 1003. [Google Scholar] [CrossRef]
- Foley, J.A.; Ramankutty, N.; Brauman, K.A.; Cassidy, E.S.; Gerber, J.S.; Johnston, M.; Mueller, N.D.; O’Connell, C.; Ray, D.K.; West, P.C.; et al. Solutions for a cultivated planet. Nature 2011, 478, 337–342. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vitousek, P.M.; Mooney, H.A.; Lubchenco, J.; Melillo, J.M. Human Domination of Earth’s Ecosystems. Science 1997, 277, 494–499. [Google Scholar] [CrossRef] [Green Version]
- Liu, X.B.; Zhang, X.Y.; Wang, Y.X.; Sui1, Y.Y.; Zhang1, S.L.; Herbert, S.; Ding, G. Soil degradation: A problem threatening the sustainable development of agriculture in Northeast China. Plant Soil Environ. 2010, 56, 87–97. [Google Scholar] [CrossRef] [Green Version]
- Rodríguez, D. Greenhouse gas emissions of agriculture: A comparative analysis. In Environmental Chemistry and Recent Pollution Control Approaches; Saldarriaga-Noreña, H., Murillo-Tovar, M., Farooq, R., Dongre, R., Riaz, S., Eds.; IntechOpen: London, UK, 2019; pp. 21–40. ISBN 978-1-83968-063-2. [Google Scholar]
- Moss, B. Water pollution by agriculture. Philos. Trans. R. Soc. B Biol. Sci. 2007, 363, 659–666. [Google Scholar] [CrossRef] [Green Version]
- Peacock, E.; Haag, W.R.; Warren, M.L. Prehistoric Decline in Freshwater Mussels Coincident with the Advent of Maize Agriculture. Conserv. Biol. 2005, 19, 547–551. [Google Scholar] [CrossRef]
- Böhlke, J.-K. Groundwater recharge and agricultural contamination. Hydrogeol. J. 2002, 10, 153–179. [Google Scholar] [CrossRef]
- Carvalho, F.P. Agriculture, pesticides, food security and food safety. Environ. Sci. Policy 2006, 9, 685–692. [Google Scholar] [CrossRef]
- Kyrikou, I.; Briassoulis, D. Biodegradation of Agricultural Plastic Films: A Critical Review. J. Polym. Environ. 2007, 15, 125–150. [Google Scholar] [CrossRef]
- Baldock, D. Agriculture and Habitat Loss in Europe; WWF International: Gland, Switzerland, 1990; ISBN 2880850347. [Google Scholar]
- Campbell, B.M.; Beare, D.J.; Bennett, E.M.; Hall-Spencer, J.M.; Ingram, J.S.; Jaramillo, F. Agriculture production as a major driver of the Earth system exceeding planetary boundaries. Ecol. Soc. 2017, 22. [Google Scholar] [CrossRef]
- Burney, J.A.; Davis, S.J.; Lobell, D.B. Greenhouse gas mitigation by agricultural intensification. Proc. Natl. Acad. Sci. USA 2010, 107, 12052–12057. [Google Scholar] [CrossRef] [Green Version]
- Tilman, D.; Balzer, C.; Hill, J.; Befort, B.L. Global food demand and the sustainable intensification of agriculture. Proc. Natl. Acad. Sci. USA 2011, 108, 20260–20264. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ut, T.T.; Kajisa, K. The impact of green revolution on rice production in Vietnam. Dev. Econ. 2006, 44, 167–189. [Google Scholar] [CrossRef]
- Evenson, R.E.; Gollin, D. Assessing the Impact of the Green Revolution, 1960 to 2000. Science 2003, 300, 758–762. [Google Scholar] [CrossRef] [Green Version]
- FAO. Construyendo una Visión Común Para la Agricultura y Alimentación Sostenibles. Principios y Enfoques; FAO: Rome, Italy, 2015. [Google Scholar]
- Matson, P.A.; Parton, W.J.; Power, A.G.; Swift, M.J. Agricultural Intensification and Ecosystem Properties. Science 1997, 277, 504–509. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pingali, P.L. Green Revolution: Impacts, limits, and the path ahead. Proc. Natl. Acad. Sci. USA 2012, 109, 12302–12308. [Google Scholar] [CrossRef] [Green Version]
- Eisenstein, M. Natural solutions for agricultural productivity. Nature 2020, 588, S58–S59. [Google Scholar] [CrossRef] [PubMed]
- McNeely, J. How traditional agro-ecosystems can contribute to conserving biodiversity. In Conserving Biodiversity Outside Protected Area the Role of Traditional Agro-Ecosystems; Halladay, P., Gilmour, D.A., Eds.; IUCN Publishing Unit: Cambridge, UK, 1995. [Google Scholar]
- Rey, P.J.; Manzaneda, A.J.; Valera, F.; Alcántara, J.M.; Tarifa, R.; Isla, J.; Molina-Pardo, J.L.; Calvo, G.; Salido, T.; Gutiérrez, J.E.; et al. Landscape-moderated biodiversity effects of ground herb cover in olive groves: Implications for regional biodiversity conservation. Agric. Ecosyst. Environ. 2019, 277, 61–73. [Google Scholar] [CrossRef]
- Krebs, J.R.; Wilson, J.D.; Bradbury, R.B.; Siriwardena, G.M. The second Silent Spring? Nature 1999, 400, 611–612. [Google Scholar] [CrossRef]
- Martínez-Valderrama, J.; Guirado, E.; Maestre, F.T. Unraveling Misunderstandings about Desertification: The Paradoxical Case of the Tabernas-Sorbas Basin in Southeast Spain. Land 2020, 9, 269. [Google Scholar] [CrossRef]
- Flynn, D.F.B.; Gogol-Prokurat, M.; Nogeire, T.; Molinari, N.; Richers, B.T.; Lin, B.B.; Simpson, N.; Mayfield, M.M.; DeClerck, F. Loss of functional diversity under land use intensification across multiple taxa. Ecol. Lett. 2009, 12, 22–33. [Google Scholar] [CrossRef]
- Wagner, D.L.; Grames, E.M.; Forister, M.L.; Berenbaum, M.R.; Stopak, D. Insect decline in the Anthropocene: Death by a thousand cuts. Proc. Natl. Acad. Sci. USA 2021, 118. [Google Scholar] [CrossRef]
- Bennett, E.M.; Baird, J.; Baulch, H.; Chaplin-Kramer, R.; Fraser, E.; Loring, P.; Morrison, P.; Parrott, L.; Sherren, K.; Winkler, K.J.; et al. Ecosystem services and the resilience of agricultural landscapes. Adv. Ecol. Res. 2021, 64, 1–43. [Google Scholar] [CrossRef]
- Martínez-Núñez, C.; Manzaneda, A.J.; Isla, J.; Tarifa, R.; Calvo, G.; Molina, J.L.; Salido, T.; Ruiz, C.; Gutiérrez, J.E.; Rey, P.J. Low-intensity management benefits solitary bees in olive groves. J. Appl. Ecol. 2020, 57, 111–120. [Google Scholar] [CrossRef]
- Martínez-Núñez, C.; Rey, P.J.; Manzaneda, A.J.; García, D.; Tarifa, R.; Molina, J.L. Insectivorous birds are not effective pest control agents in olive groves. Basic Appl. Ecol. 2021, 56, 270–280. [Google Scholar] [CrossRef]
- Dainese, M.; Martin, E.A.; Aizen, M.A.; Albrecht, M.; Bartomeus, I.; Bommarco, R.; Carvalheiro, L.G.; Chaplin-Kramer, R.; Gagic, V.; Garibaldi, L.A.; et al. A global synthesis reveals biodiversity-mediated benefits for crop production. Sci. Adv. 2019, 5, eaax0121. [Google Scholar] [CrossRef] [Green Version]
- Millard, J.; Outhwaite, C.L.; Kinnersley, R.; Freeman, R.; Gregory, R.D.; Adedoja, O.; Gavini, S.; Kioko, E.; Kuhlmann, M.; Ollerton, J.; et al. Global effects of land-use intensity on local pollinator biodiversity. Nat. Commun. 2021, 12, 1–11. [Google Scholar] [CrossRef] [PubMed]
- McKechnie, I.M.; Sargent, R.D. Do plant traits influence a species’ response to habitat disturbance? A meta-analysis. Biol. Conserv. 2013, 168, 69–77. [Google Scholar] [CrossRef]
- Martínez-Núñez, C.; Rey, P.J.; Salido, T.; Manzaneda, A.J.; Camacho, F.M.; Isla, J. Ant community potential for pest control in olive groves: Management and landscape effects. Agric. Ecosyst. Environ. 2021, 305, 107185. [Google Scholar] [CrossRef]
- Potts, S.G.; Imperatriz-Fonseca, V.; Ngo, H.T.; Aizen, M.A.; Biesmeijer, J.C.; Breeze, T.D.; Dicks, L.V.; Garibaldi, L.A.; Hill, R.; Settele, J.; et al. Safeguarding pollinators and their values to human well-being. Nature 2016, 540, 220–229. [Google Scholar] [CrossRef] [PubMed]
- Díaz, S.; Settele, J.; Brondízio, E.S.; Ngo, H.T.; Agard, J.; Arneth, A.; Balvanera, P.; Brauman, K.A.; Butchart, S.H.M.; Chan, K.M.A.; et al. Pervasive human-driven decline of life on Earth points to the need for transformative change. Science 2019, 366. [Google Scholar] [CrossRef] [Green Version]
- Chamberlain, D.E.; Fuller, R.J.; Bunce, R.G.H.; Duckworth, J.C.; Shrubb, M. Changes in the abundance of farmland birds in relation to the timing of agricultural intensification in England and Wales. J. Appl. Ecol. 2000, 37, 771–788. [Google Scholar] [CrossRef] [Green Version]
- Donald, P.F.; Green, R.E.; Heath, M.F. Agricultural intensification and the collapse of Europe’s farmland bird populations. Proc. R. Soc. London. Ser. B Biol. Sci. 2001, 268, 25–29. [Google Scholar] [CrossRef]
- Emmerson, M.; Morales, M.B.; Oñate, J.J.; Batáry, P.; Berendse, F.; Liira, J.; Aavik, T.; Guerrero, I.; Bommarco, R.; Eggers, S.; et al. How Agricultural Intensification Affects Biodiversity and Ecosystem Services. Adv. Ecol. Res. 2016, 55, 43–97. [Google Scholar] [CrossRef]
- Wright, H.L.; Lake, I.R.; Dolman, P.M. Agriculture—A key element for conservation in the developing world. Conserv. Lett. 2012, 5, 11–19. [Google Scholar] [CrossRef]
- Hobbs, P.R.; Sayre, K.; Gupta, R. The role of conservation agriculture in sustainable agriculture. Philos. Trans. R. Soc. B Biol. Sci. 2007, 363, 543–555. [Google Scholar] [CrossRef]
- Altieri, M. Agroecology: The Science of Sustainable Agriculture; CRC Press: Boca Raton, FL, USA, 2018. [Google Scholar]
- Thrupp, L.A. Linking Agricultural Biodiversity and Food Security: The Valuable Role of Agrobiodiversity for Sustainable Agriculture. Int. Aff. 2000, 76, 265–281. [Google Scholar] [CrossRef]
- Diacono, M.; Trinchera, A.; Montemurro, F. An Overview on Agroecology and Organic Agriculture Strategies for Sustainable Crop Production. Agron 2021, 11, 223. [Google Scholar] [CrossRef]
- Batáry, P.; Dicks, L.V.; Kleijn, D.; Sutherland, W.J. The role of agri-environment schemes in conservation and environmental management. Conserv. Biol. 2015, 29, 1006–1016. [Google Scholar] [CrossRef] [Green Version]
- Wade, M.R.; Gurr, G.M.; Wratten, S.D. Ecological restoration of farmland: Progress and prospects. Philos. Trans. R. Soc. B Biol. Sci. 2007, 363, 831–847. [Google Scholar] [CrossRef] [Green Version]
- Perrings, C.; Jackson, L.; Bawa, K.; Brussaard, L.; Brush, S.; Gavin, T.; Papa, R.; Pascual, U.; Ruiter, P. De Biodiversity in Agricultural Landscapes: Saving Natural Capital without Losing Interest. Conserv. Biol. 2006, 20, 263–264. [Google Scholar] [CrossRef] [PubMed]
- Fischer, J.; Lindenmayer, D.B.; Manning, A.D. Biodiversity, ecosystem function, and resilience: Ten guiding principles for commodity production landscapes. Front. Ecol. Environ. 2006, 4, 80–86. [Google Scholar] [CrossRef] [Green Version]
- Nabel, M.; Selig, C.; Gundlach, J.; Decken, H.; Klein, M. Biodiversity in agricultural used soils: Threats and options for its conservation in Germany and Europe. Soil Org. 2021, 93, 1–11. [Google Scholar] [CrossRef]
- Santos, J.S.; Dodonov, P.; Oshima, J.E.F.; Martello, F.; Santos de Jesus, A.; Eduardo Ferreira, M.; Silva-Neto, C.M.; Ribeiro, M.C.; Collevatti, R.G. Landscape ecology in the Anthropocene: An overview for integrating agroecosystems and biodiversity conservation. Perspect. Ecol. Conserv. 2021, 19, 21–32. [Google Scholar] [CrossRef]
- Singh, M.; Kumar, A.; Sharma, M. Conservation of Plant Diversity in Agroforestry Systems in a Biodiversity Hotspot Region of Northeast India. Agric. Res. 2021, 1–13. [Google Scholar] [CrossRef]
- Košulič, O.; Michalko, R.; Hula, V. Recent artificial vineyard terraces as a refuge for rare and endangered spiders in a modern agricultural landscape. Ecol. Eng. 2014, 68, 133–142. [Google Scholar] [CrossRef]
- Blanca, G.; Cueto, M.; Fuentes, J.; Sáez, L.; Tarifa, R. Linaria qartobensis sp. nov. (Plantaginaceae) from the southern Iberian Peninsula. J. Bot. 2018, 36, e01914. [Google Scholar] [CrossRef]
- Storkey, J.; Meyer, S.; Still, K.S.; Leuschner, C. The impact of agricultural intensification and land-use change on the European arable flora. Proc. R. Soc. B Biol. Sci. 2012, 279, 1421–1429. [Google Scholar] [CrossRef]
- Lenzen, M.; Lane, A.; Widmer-Cooper, A.; Williams, M. Effects of Land Use on Threatened Species. Conserv. Biol. 2009, 23, 294–306. [Google Scholar] [CrossRef]
- Richner, N.; Holderegger, R.; Linder, H.P.; Walter, T. Reviewing change in the arable flora of Europe: A meta-analysis. Weed Res. 2015, 55, 1–13. [Google Scholar] [CrossRef]
- Osawa, T.; Kohyama, K.; Mitsuhashi, H. Areas of Increasing Agricultural Abandonment Overlap the Distribution of Previously Common, Currently Threatened Plant Species. PLoS ONE 2013, 8, e79978. [Google Scholar] [CrossRef] [Green Version]
- Bañares, A.; Blanca, G.; Guemes, J.; Moreno, J.; Ortiz, S. Atlas y Libro Rojo de la Flora Vascular Amenazada de España; Dirección General de Conservación de la Naturaleza: Madrid, Spain, 2004; ISBN 84-8014-521-8. [Google Scholar]
- Stoate, C.; Báldi, A.; Beja, P.; Boatman, N.D.; Herzon, I.; van Doorn, A.; de Snoo, G.R.; Rakosy, L.; Ramwell, C. Ecological impacts of early 21st century agricultural change in Europe–A review. J. Environ. Manag. 2009, 91, 22–46. [Google Scholar] [CrossRef]
- Alignier, A.; Solé-Senan, X.O.; Robleño, I.; Baraibar, B.; Fahrig, L.; Giralt, D.; Gross, N.; Martin, J.-L.; Recasens, J.; Sirami, C.; et al. Configurational crop heterogeneity increases within-field plant diversity. J. Appl. Ecol. 2020, 57, 654–663. [Google Scholar] [CrossRef]
- Fried, G.; Kazakou, E.; Gaba, S. Trajectories of weed communities explained by traits associated with species’ response to management practices. Agric. Ecosyst. Environ. 2012, 158, 147–155. [Google Scholar] [CrossRef]
- Zanin, G.; Otto, S.; Riello, L. Ecological interpretation of weed flora dynamics under different tillage systems. Agric. Ecosyst. Environ. 1997, 66, 77–188. [Google Scholar] [CrossRef]
- Cornelissen, J.H.C.; Lavorel, S.; Garnier, E.; Díaz, S.; Buchmann, N.; Gurvich, D.E.; Reich, P.B.; Ter Steege, H.; Morgan, H.D.; Van Der Heijden, M.G.A.; et al. A handbook of protocols for standardised and easy measurement of plant functional traits worldwide. Aust. J. Bot. 2003, 51, 335–380. [Google Scholar] [CrossRef] [Green Version]
- Renwick, A.; Jansson, T.; Verburg, P.H.; Revoredo-Giha, C.; Britz, W.; Gocht, A.; McCracken, D. Policy reform and agricultural land abandonment in the EU. Land Use Policy 2013, 30, 446–457. [Google Scholar] [CrossRef]
- Traba, J.; Morales, M.B. The decline of farmland birds in Spain is strongly associated to the loss of fallowland. Sci. Rep. 2019, 9, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Moreno-Saiz, J.C.; Albertos, B.; Ruiz-Molero, E.; Mateo, R.G. The European Union can afford greater ambition in the conservation of its threatened plants. Biol. Conserv. 2021, 261, 109231. [Google Scholar] [CrossRef]
- Bañares, A.; Blanca, G.; Güemes, J.; Moreno, C.; Ortiz, S. Atlas y Libro Rojo de la Flora Vascular Amenazada de España-Adenda 2006; Dirección General para la Biodiversidad-Sociedad Española de Biología de la Conservación de Plantas: Madrid, Spain, 2006; ISBN 978-84-8014-706-4. [Google Scholar]
- Bañares, A.; Blanca, G.; Güemes, J.; Moreno, C.; Ortiz, S. Atlas y Libro Rojo de la Flora Vascular Amenazada de España-Adenda 2008; Dirección General de Medio Natural y Política Forestal (Ministerio de Medio Ambiente, y Medio Rural y Marino)-Sociedad Española de Biología de la Conservación de Plantas: Madrid, Spain, 2009; ISBN 978-84-8014-741-5.
- Bañares, A.; Blanca, G.; Güemes, J.; Moreno, C.; Ortiz, S. Atlas y Libro Rojo de la Flora Vascular Amenazada de España-Adenda 2010; Dirección General de Medio Natural y Política Forestal (Ministerio de Medio Ambiente, y Medio Rural y Marino)-Sociedad Española de Biología de la Conservación de Plantas: Madrid, Spain, 2010.
- Moreno, J.C.; Iriondo, J.M.; Martínez, F.; Martínez, J.; Salazar, C. Atlas y Libro Rojo de la Flora Vascular Amenazada de España-Adenda 2017; Ministerio para la Transición Ecológica-Sociedad Española de Biología de la Conservación de Plantas: Madrid, Spain, 2017. [Google Scholar]
- Martínez, I.; Carreño, F.; Escudero, A.; Rubio, A. Are threatened lichen species well-protected in Spain? Effectiveness of a protected areas network. Biol. Conserv. 2006, 133, 500–511. [Google Scholar] [CrossRef]
- Médail, F.; Quézel, P. Hot-spots analysis for conservation of plant biodiversity in the Mediterranean Basin. Ann. Missouri Bot. Gard. 1997, 84, 112–127. [Google Scholar] [CrossRef]
- Myers, N.; Mittermeier, R.A.; Mittermeier, C.G.; da Fonseca, G.A.B.; Kent, J. Biodiversity hotspots for conservation priorities. Nature 2000, 403, 853–858. [Google Scholar] [CrossRef]
- Benayas, R.; José, M.; Scheiner, S.M. Plant diversity, biogeography and environment in Iberia: Patterns and possible causal factors. J. Veg. Sci. 2002, 13, 245–258. [Google Scholar] [CrossRef] [Green Version]
- Underwood, E.C.; Viers, J.H.; Klausmeyer, K.R.; Cox, R.L.; Shaw, M.R. Threats and biodiversity in the mediterranean biome. Divers. Distrib. 2009, 15, 188–197. [Google Scholar] [CrossRef]
- Fernández-González, F.; Loidi, J.; Moreno, C.J.; Del Arco, M.; Fernández-Cancio, A. Impactos sobre la biodiversidad vegetal. In Evaluación Preliminar de los Impactos en España por Efecto del Cambio Climático; Centro de Publicaciones. Secretaría General Técnica, Ministerio de Medio Ambiente: Madrid, Spain, 2005; pp. 18–247. ISBN 84-8320-303-0. [Google Scholar]
- Moreno Saiz, J.C. La diversidad florística vascular española. In Biodiversidad: Aproximación a la Diversidad Botánica y Zoológica de España. Memorias de la Real Sociedad Española de Historia Natural; Real Sociedad Española de Historia Natural: Madrid, Spain, 2011; Volume 9, pp. 75–107. ISBN 978-84-936677-6-4. [Google Scholar]
- Moreno, J.C. Lista Roja 2008 de la Flora Vascular Española; Dirección General de Medio Natural y Política Forestal (Ministerio de Medio Ambiente, y Medio Rural y Marino, y Sociedad Española de Biología de la Conservación de Plantas): Madrid, Spain, 2008; ISBN 978-84-691-7375-6.
- Algarra, J.; Blanca, G.; Cueto, M.; Phytotaxa, J.F.-U. New data on daffodils of the Narcissus nevadensis complex (Amaryllidaceae) in SE Spain: N. nevadensis subsp. herrerae subsp. nov., and N. nevadensis. Phytotaxa 2018, 371, 133–139. [Google Scholar] [CrossRef]
- Molina, J.; Michaud, H.; Tison, J.; Fernandez Zamudio, R.; Véla, E. Allium scaberrimum. The IUCN Red List of Threatened Species. 2018. Available online: https://dx.doi.org/10.2305/IUCN.UK.2018-1.RLTS.T110805790A87775132.en (accessed on 27 August 2021).
- Gutiérrez-Larruscain, D.; Santos-Vicente, M.; Anderberg, A.A.; Rico, E.; Martínez-Ortega, M.M. Phylogeny of the Inula group (Asteraceae: Inuleae): Evidence from nuclear and plastid genomes and a recircumscription of Pentanema. Taxon 2018, 67, 149–164. [Google Scholar] [CrossRef]
- Pelser, P.B.; Veldkamp, J.F.; Van Der Meijden, R. New combinations in Jacobaea Mill. (Asteraceae, Senecioneae). Compo News 2006, 44, 1–11. [Google Scholar]
- Al-Shehbaz, I.A. A generic and tribal synopsis of the Brassicaceae (Cruciferae). Taxon 2012, 61, 931–954. [Google Scholar] [CrossRef]
- Maldonado, C.; Molina, C.I.; Zizka, A.; Persson, C.; Taylor, C.M.; Albán, J.; Chilquillo, E.; Rønsted, N.; Antonelli, A. Estimating species diversity and distribution in the era of Big Data: To what extent can we trust public databases? Glob. Ecol. Biogeogr. 2015, 24, 973–984. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tarifa, R.; Martínez-Núñez, C.; Valera, F.; González-Varo, J.P.; Salido, T.; Rey, P.J. Agricultural intensification erodes taxonomic and functional diversity in Mediterranean olive groves by filtering out rare species. J. Appl. Ecol. 2021, 58, 2266–2276. [Google Scholar] [CrossRef]
- Raunkiaer, C. The Life Forms of Plants and Statistical Plant Geography; Being the Collected Papers of C. raunkiaer; Claredon Press: Oxford, UK, 1934. [Google Scholar]
- Green, R.E.; Cornell, S.J.; Scharlemann, J.P.W.; Balmford, A. Farming and the Fate of Wild Nature. Science 2005, 307, 550–555. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pinke, G.; Király, G.; Barina, Z.; Mesterházy, A.; Balogh, L.; Csiky, J.; Schmotzer, A.; Molnár, A.V.; Pál, R.W. Assessment of endangered synanthropic plants of Hungary with special attention to arable weeds. Plant Biosyst. 2011, 145, 426–435. [Google Scholar] [CrossRef]
- Bergmeier, E.; Strid, A. Regional diversity, population trends and threat assessment of the weeds of traditional agriculture in Greece. Bot. J. Linn. Soc. 2014, 175, 607–623. [Google Scholar] [CrossRef] [Green Version]
- Lozano, F.D.; Atkins, K.J.; Moreno Sáiz, J.C.; Sims, A.E.; Dixon, K. The nature of threat category changes in three Mediterranean biodiversity hotspots. Biol. Conserv. 2013, 157, 21–30. [Google Scholar] [CrossRef]
- Carman, K.; Jenkins, D.G. Comparing diversity to flower-bee interaction networks reveals unsuccessful foraging of native bees in disturbed habitats. Biol. Conserv. 2016, 202, 110–118. [Google Scholar] [CrossRef]
- Erfanian, M.B.; Ejtehadi, H.; Vaezi, J.; Moazzeni, H. Plant community responses to multiple disturbances in an arid region of northeast Iran. L. Degrad. Dev. 2019, 30, 1554–1563. [Google Scholar] [CrossRef]
- Basu, P.; Bhattacharya, R.; Ianetta, P. A decline in pollinator dependent vegetable crop productivity in India indicates pollination limitation and consequent agro-economic crises. Nat. Preced. 2011. [Google Scholar] [CrossRef]
- Gallai, N.; Salles, J.M.; Settele, J.; Vaissière, B.E. Economic valuation of the vulnerability of world agriculture confronted with pollinator decline. Ecol. Econ. 2009, 68, 810–821. [Google Scholar] [CrossRef]
- Klein, A.-M.; Vaissire, B.E.; Cane, J.H.; Steffan-Dewenter, I.; Cunningham, S.A.; Kremen, C.; Tscharntke, T. Importance of pollinators in changing landscapes for world crops. Proc. R. Soc. B Biol. Sci. 2006, 274, 303–313. [Google Scholar] [CrossRef] [Green Version]
- Velthuis, H.H.W.; Doorn, A. van A century of advances in bumblebee domestication and the economic and environmental aspects of its commercialization for pollination. Apidologie 2006, 37, 421–451. [Google Scholar] [CrossRef] [Green Version]
- Carreck, N.L.; Williams, I.H.; Little, D.J. The movement of honey bee colonies for crop pollination and honey production by beekeepers in Great Britain. Bee World 2015, 78, 67–77. [Google Scholar] [CrossRef]
- Németh, M.B.; Smith-Huerta, N.L. Pollen Deposition, Pollen Tube Growth, Seed Production, and Seedling Performance in Natural Populations of Clarkia unguiculata (Onagraceae). Int. J. Plant Sci. 2015, 164, 153–164. [Google Scholar] [CrossRef]
- Kalla, S.E.; Ashman, T.L. The effects of pollen competition on progeny vigor in Fragaria virginiana (Rosaceae) depend on progeny growth environment. Int. J. Plant Sci. 2002, 163, 335–340. [Google Scholar] [CrossRef]
- Brown, E.; Kephart, S. Variability in pollen load: Implications for reproduction and seedling vigor in a rare plant, Silene douglasii var. Oraria. Int. J. Plant Sci. 1999, 160, 1145–1152. [Google Scholar] [CrossRef] [PubMed]
- Bertin, R.I. Effects of pollination intensity in Campsis radicans. Am. J. Bot. 1990, 77, 178–187. [Google Scholar] [CrossRef] [PubMed]
- Chautá-Mellizo, A.; Campbell, S.A.; Bonilla, M.A.; Thaler, J.S.; Poveda, K. Effects of natural and artificial pollination on fruit and offspring quality. Basic Appl. Ecol. 2012, 13, 524–532. [Google Scholar] [CrossRef]
- Brittain, C.; Potts, S.G. The potential impacts of insecticides on the life-history traits of bees and the consequences for pollination. Basic Appl. Ecol. 2011, 12, 321–331. [Google Scholar] [CrossRef]
- Franzon, R.C.; Castro, C.M.; Raseira, M. do C.B. Genetic variability in surinam cherry populations originated from self-pollination and cross pollination, estimated by AFLP. Rev. Bras. Frutic. 2010, 32, 240–250. [Google Scholar] [CrossRef] [Green Version]
- Poschlod, P.; Bakker, J.; Bonn, S.; Fischer, S. Dispersal of Plants in Fragmented Landscapes. In Species Survival in Fragmented Landscapes; Settele, C.J., Margules, P., Poschlod, K., Eds.; Henle: Dordrecht, The Netherlands, 1996; pp. 123–127. ISBN 978-94-009-0343-2. [Google Scholar]
- Poschlod, P.; Bonn, S. Changing dispersal processes in the central European landscape since the last ice age: An explanation for the actual decrease of plant species richness in different habitats? Acta Bot. Neerl. 1998, 47, 27–44. [Google Scholar]
- Cain, M.L.; Milligan, B.G.; Strand, A.E. Long-distance seed dispersal in plant populations. Am. J. Bot. 2000, 87, 1217–1227. [Google Scholar] [CrossRef] [Green Version]
- Vittoz, P.; Engler, R. Seed dispersal distances: A typology based on dispersal modes and plant traits. Bot. Helv. 2007, 117, 109–124. [Google Scholar] [CrossRef] [Green Version]
- Valdivia, A.; Wolf, S.; Suckling, K. Marine mammals and sea turtles listed under the U.S. Endangered Species Act are recovering. PLoS ONE 2019, 14, e0210164. [Google Scholar] [CrossRef] [Green Version]
- Evans, D.; Che-Castaldo, J.; Crouse, D.; Davis, F.; Epanchin-Niell, R.; Flather, C.; Frohlich, R.; Goble, D.; Li, Y.; Male, T.; et al. Species recovery in the united states: Increasing the effectiveness of the Endangered Species Act. Issues Ecol. 2016, 20, 1–28. [Google Scholar]
- Antonelli, A.; Smith, R.J.; Fry, C.; Simmonds, M.S.J.; Kersey, P.J.; Pritchard, H.W.; Abbo, M.S.; Acedo, C.; Adams, J. State of the World’s Plants and Fung 2020; Royal Botanic Gardens: London, UK, 2020.
- Wilson, P.; Aebischer, N.J. The distribution of dicotyledonous arable weeds in relation to distance from the field edge. J. Appl. Ecol. 1995, 295–310. [Google Scholar] [CrossRef]
- Mota, J.F.; Peñas, J.; Castro, H.; Cabello, J.; Guirado, J.S. Agricultural development vs biodiversity conservation: The Mediterranean semiarid vegetation in El Ejido (Almería, southeastern Spain). Biodivers. Conserv. 1996, 5, 1597–1617. [Google Scholar] [CrossRef]
- Wada, Y.; Beek, L.P.H.; Bierkens, M.F.P. Nonsustainable groundwater sustaining irrigation: A global assessment. Water Resour. Res. 2012, 48, 6. [Google Scholar] [CrossRef]
- Gherardi, F. Crayfish invading Europe: The case study of Procambarus clarkii. Mar. Freshw. Behav. Physiol. 2007, 39, 175–191. [Google Scholar] [CrossRef]
- Cruz, M.J.; Rebelo, R. Colonization of freshwater habitats by an introduced crayfish, Procambarus clarkii, in Southwest Iberian Peninsula. Hydrobiology 2006, 575, 191–201. [Google Scholar] [CrossRef]
- Gherardi, F.; Barbaresi, S. Invasive crayfish: Activity patterns of Procambarus clarkii in the rice fields of the Lower Guadalquivir (Spain). Arch. Hydrobiol. 2000, 153–168. [Google Scholar] [CrossRef]
- Gherardi, F.; Acquistapace, P. Invasive crayfish in Europe: The impact of Procambarus clarkii on the littoral community of a Mediterranean lake. Freshw. Biol. 2007, 52, 1249–1259. [Google Scholar] [CrossRef] [Green Version]
- Cruz, M.J.; Rebelo, R.; Crespo, E.G. Effects of an introduced crayfish, Procambarus clarkii, on the distribution of south-western Iberian amphibians in their breeding habitats. Ecography (Cop.) 2006, 29, 329–338. [Google Scholar] [CrossRef]
- Kelemen, K.; Kriván, A.; Standovár, T. Effects of land-use history and current management on ancient woodland herbs in Western Hungary. J. Veg. Sci. 2014, 25, 172–183. [Google Scholar] [CrossRef]
- Gilmour, D.; Halladay, P. Conserving Biodiversity Outside Protected Areas. The Role of Traditional Agro-Ecosystems; Gilmour, D., Halladay, P., Eds.; IUCN: Gland, Switzerland; Cambridge, UK, 1995. [Google Scholar]
- Tissier, M.L.; Kletty, F.; Handrich, Y.; Habold, C. Monocultural sowing in mesocosms decreases the species richness of weeds and invertebrates and critically reduces the fitness of the endangered European hamster. Oecologia 2017, 186, 589–599. [Google Scholar] [CrossRef]
- Marshall, E.J.P.; Brown, V.K.; Boatman, N.D.; Lutman, P.J.W.; Squire, G.R.; Ward, L.K. The role of weeds in supporting biological diversity within crop fields. Weed Res. 2003, 43, 77–89. [Google Scholar] [CrossRef] [Green Version]
- Kleijn, D.; Rundlöf, M.; Scheper, J.; Smith, H.G.; Tscharntke, T. Does conservation on farmland contribute to halting the biodiversity decline? Trends Ecol. Evol. 2011, 26, 474–481. [Google Scholar] [CrossRef]
- Sutcliffe, O.L.; Kay, Q.O.N. Changes in the arable flora of central southern England since the 1960’s. Biol. Conserv. 2000, 93, 1–8. [Google Scholar] [CrossRef]
- José-María, L.; Research, F.S. Weed seedbanks in arable fields: Effects of management practices and surrounding landscape. Weed Res. 2011, 51, 631–640. [Google Scholar] [CrossRef]
- Chauvel, B.; Gasquez, J.; Darmency, H. Changes of weed seed bank parameters according to species, time and environment. Weed Res. 1989, 29, 213–219. [Google Scholar] [CrossRef]
- Levassor, C.; Ortega, M.; Peco, B. Seed bank dynamics of Mediterranean pastures subjected to mechanical disturbance. J. Veg. Sci. 1990, 1, 339–344. [Google Scholar] [CrossRef]
- Peñas, J.; Benito, B.; Lorite, J.; Ballesteros, M.; Cañadas, E.M.; Martinez-Ortega, M. Habitat Fragmentation in Arid Zones: A Case Study of Linaria nigricans Under Land Use Changes (SE Spain). Environ. Manag. 2011, 48, 168–176. [Google Scholar] [CrossRef] [PubMed]
- Druckenbrod, D.L.; Dale, V.H. Experimental response of understory plants to mechanized disturbance in an oak-pine forest. Ecol. Indic. 2012, 15, 181–187. [Google Scholar] [CrossRef]
- Thompson, K.; Bakker, J.P.; Bekker, R.M.; Hodgson, J.G. Ecological correlates of seed persistence in soil in the north-west European flora. J. Ecol. 1998, 86, 163–169. [Google Scholar] [CrossRef]
- Martínez-Núñez, C.; Manzaneda, A.J.; Rey, P.J. Plant-solitary bee networks have stable cores but variable peripheries under differing agricultural management: Bioindicator nodes unveiled. Ecol. Indic. 2020, 115, 106422. [Google Scholar] [CrossRef]
- Forup, M.L.; Henson, K.S.E.; Craze, P.G.; Memmott, J. The restoration of ecological interactions: Plant–pollinator networks on ancient and restored heathlands. J. Appl. Ecol. 2008, 45, 742–752. [Google Scholar] [CrossRef] [Green Version]
- Redbo-Torstensson, P.; Telenius, A. Primary and secondary seed dispersal by wind and water in Spergularia salina. Ecography 1995, 18, 230–237. [Google Scholar] [CrossRef]
- Jiao, J.; Zou, H.; Jia, Y.; Wang, N. Research progress on the effects of soil erosion on vegetation. Acta Ecol. Sin. 2009, 29, 85–91. [Google Scholar] [CrossRef]
- Johnson, E.A.; Fryer, G.I. Physical Characterization of Seed Microsites—Movement on the Ground. J. Ecol. 1992, 80, 823. [Google Scholar] [CrossRef]
- Foucher, A.; Salvador-Blanes, S.; Evrard, O.; Simonneau, A.; Chapron, E.; Courp, T.; Cerdan, O.; Lefèvre, I.; Adriaensen, H.; Lecompte, F.; et al. Increase in soil erosion after agricultural intensification: Evidence from a lowland basin in France. Anthropocene 2014, 7, 30–41. [Google Scholar] [CrossRef] [Green Version]
- Watkinson, A.R. The Demography of a Sand Dune Annual: Vulpia Fasciculata: III. The Dispersal of Seeds. J. Ecol. 1978, 66, 483. [Google Scholar] [CrossRef]
- Fischer, J.; Hartel, T.; Kuemmerle, T. Conservation policy in traditional farming landscapes. Conserv. Lett. 2012, 5, 167–175. [Google Scholar] [CrossRef] [Green Version]
- Kleijn, D.; Baquero, R.; Clough, Y.; Díaz, M.; De Esteban, J.; Fernández, F.; Gabriel, D.; Herzog, F.; Holzschuh, A.; Jöhl, R.; et al. Mixed biodiversity benefits of agri-environment schemes in five European countries. Ecol. Lett. 2006, 9, 243–254. [Google Scholar] [CrossRef] [PubMed]
- Radović, A.; Nikolov, S.C.; Tepić, N.; Mikulić, K.; Jelaska, S.D.; Budinski, I. The influence of land abandonment on farmland bird communities: A case study from a floodplain landscape in Continental Croatia. J. Vertebr. Biol. 2013, 62, 269–281. [Google Scholar] [CrossRef]
- Carlesi, S.; Bocci, G.; Moonen, A.C.; Frumento, P.; Bàrberi, P. Urban sprawl and land abandonment affect the functional response traits of maize weed communities in a heterogeneous landscape. Agric. Ecosyst. Environ. 2013, 166, 76–85. [Google Scholar] [CrossRef]
- Soons, M.B.; Heil, G.W.; Nathan, R.; Katul, G.G. Determinants of Long-Distance Seed Dispersal By Wind In Grasslands. Ecology 2004, 85, 3056–3068. [Google Scholar] [CrossRef] [Green Version]
- Kaligarič, M.; Čuš, J.; Škornik, S.; Ivajnšič, D. The failure of agri-environment measures to promote and conserve grassland biodiversity in Slovenia. Land Use Policy 2019, 80, 127–134. [Google Scholar] [CrossRef]
- Pe’er, G.; Dicks, L.V.; Visconti, P.; Arlettaz, R.; Báldi, A.; Benton, T.G.; Collins, S.; Dieterich, M.; Gregory, R.D.; Hartig, F.; et al. EU agricultural reform fails on biodiversity. Science. 2014, 344, 1090–1092. [Google Scholar] [CrossRef]
- Baker, D.J.; Freeman, S.N.; Grice, P.V.; Siriwardena, G.M. Landscape-scale responses of birds to agri-environment management: A test of the English Environmental Stewardship scheme. J. Appl. Ecol. 2012, 49, 871–882. [Google Scholar] [CrossRef] [Green Version]
- Concepción, E.; Díaz, M. Medidas agroambientales y conservación de la biodiversidad: Limitaciones y perspectivas de futuro. Ecosistemas 2013, 22, 44–49. [Google Scholar] [CrossRef]
- Lázaro, A.; Ecosistemas, C.T. Los cambios de uso del suelo como responsables del declive de polinizadores. Ecosistemas 2018, 27, 23–33. [Google Scholar] [CrossRef] [Green Version]
- Gonthier, D.J.; Ennis, K.K.; Farinas, S.; Hsieh, H.-Y.; Iverson, A.L.; Batáry, P.; Rudolphi, J.; Tscharntke, T.; Cardinale, B.J.; Perfecto, I. Biodiversity conservation in agriculture requires a multi-scale approach. Proc. R. Soc. B Biol. Sci. 2014, 281, 20141358. [Google Scholar] [CrossRef] [Green Version]
- Sirami, C.; Gross, N.; Baillod, A.B.; Bertrand, C.; Carrié, R.; Hass, A.; Henckel, L.; Miguet, P.; Vuillot, C.; Alignier, A.; et al. Increasing crop heterogeneity enhances multitrophic diversity across agricultural regions. Proc. Natl. Acad. Sci. USA 2019, 116, 16442–16447. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pacheco, R.; Vasconcelos, H.L.; Groc, S.; Camacho, G.P.; Frizzo, T.L.M. The importance of remnants of natural vegetation for maintaining ant diversity in Brazilian agricultural landscapes. Biodivers. Conserv. 2013, 22, 983–997. [Google Scholar] [CrossRef]
- Wuczyński, A.; Dajdok, Z.; Wierzcholska, S.; Kujawa, K. Applying red lists to the evaluation of agricultural habitat: Regular occurrence of threatened birds, vascular plants, and bryophytes in field margins of Poland. Biodivers. Conserv. 2014, 23, 999–1017. [Google Scholar] [CrossRef] [Green Version]
- Cole, L.J.; Brocklehurst, S.; Robertson, D.; Harrison, W.; McCracken, D.I. Exploring the interactions between resource availability and the utilisation of semi-natural habitats by insect pollinators in an intensive agricultural landscape. Agric. Ecosyst. Environ. 2017, 246, 157–167. [Google Scholar] [CrossRef]
- Thiere, G.; Milenkovski, S.; Lindgren, P.E.; Sahlén, G.; Berglund, O.; Weisner, S.E.B. Wetland creation in agricultural landscapes: Biodiversity benefits on local and regional scales. Biol. Conserv. 2009, 142, 964–973. [Google Scholar] [CrossRef]
- Hobbs, R.J. Can revegetation assist in the conservation of biodiversity in agricultural areas? Pacific Conserv. Biol. 1994, 1, 29–38. [Google Scholar] [CrossRef]
- Suárez-Seoane, S.; Osborne, P.E.; Baudry, J. Responses of birds of different biogeographic origins and habitat requirements to agricultural land abandonment in northern Spain. Biol. Conserv. 2002, 105, 333–344. [Google Scholar] [CrossRef]
- Moreno Saiz, J.C.; Domínguez Lozano, F.; Sainz Ollero, H. Recent progress in conservation of threatened Spanish vascular flora: A critical review. Biol. Conserv. 2003, 113, 419–431. [Google Scholar] [CrossRef]
- Directrices de Uso de la Gestión ex Situ para la Conservación de Especies de la Comisión de Supervivencia de Especies de la UICN|IUCN Library System. Available online: https://portals.iucn.org/library/node/45186 (accessed on 7 September 2021).
- Bowles, M.L.; Betz, R.F.; DeMauro, M.M. Propagation of Rare Plants from Historic Seed Collections: Implications for Species Restoration and Herbarium Management. Restor. Ecol. 1993, 1, 101–106. [Google Scholar] [CrossRef]
- Nakahama, N.; Hirasawa, Y.; Minato, T.; Hasegawa, M.; Isagi, Y.; Shiga, T. Recovery of genetic diversity in threatened plants through use of germinated seeds from herbarium specimens. Plant Ecol. 2015, 216, 1635–1647. [Google Scholar] [CrossRef] [Green Version]
- Willis, F.; Moat, J.; Paton, A. Defining a role for herbarium data in Red List assessments: A case study of Plectranthus from eastern and southern tropical Africa. Biodivers. Conserv. 2003, 12, 1537–1552. [Google Scholar] [CrossRef]
- Lughadha, E.N.; Walker, B.E.; Canteiro, C.; Chadburn, H.; Davis, A.P.; Hargreaves, S.; Lucas, E.J.; Schuiteman, A.; Williams, E.; Bachman, S.P.; et al. The use and misuse of herbarium specimens in evaluating plant extinction risks. Philos. Trans. R. Soc. B 2019, 374, 20170402. [Google Scholar] [CrossRef]
- Nualart, N.; Ibáñez, N.; Luque, P.; Pedrol, J.; Vilar, L.; Guàrdia, R. Dataset of herbarium specimens of threatened vascular plants in Catalonia. PhytoKeys 2017, 77, 41. [Google Scholar] [CrossRef] [Green Version]
- Suarez, A.; Tsutsui, N. The Value of Museum Collections for Research and Society. Bioscience 2004, 54, 66–74. [Google Scholar] [CrossRef]
- Dalton, R. Natural history collections in crisis as funding is slashed. Nature 2003, 423, 575. [Google Scholar] [CrossRef]
- Piñeiro, V.; Arias, J.; Dürr, J.; Elverdin, P.; Ibáñez, A.M.; Kinengyere, A.; Opazo, C.M.; Owoo, N.; Page, J.R.; Prager, S.D.; et al. A scoping review on incentives for adoption of sustainable agricultural practices and their outcomes. Nat. Sustain. 2020, 3, 809–820. [Google Scholar] [CrossRef]
- Phalan, B.; Balmford, A.; Green, R.E.; Scharlemann, J.P.W. Minimising the harm to biodiversity of producing more food globally. Food Policy 2011, 36, S62–S71. [Google Scholar] [CrossRef]
- Fischer, J.; Manning, A.D.; Steffen, W.; Rose, D.B.; Daniell, K.; Felton, A.; Garnett, S.; Gilna, B.; Heinsohn, R.; Lindenmayer, D.B.; et al. Mind the sustainability gap. Trends Ecol. Evol. 2007, 22, 621–624. [Google Scholar] [CrossRef]
- Williams, D.R.; Clark, M.; Buchanan, G.M.; Ficetola, G.F.; Rondinini, C.; Tilman, D. Proactive conservation to prevent habitat losses to agricultural expansion. Nat. Sustain. 2020, 4, 314–322. [Google Scholar] [CrossRef]
- FAO. Global Food Losses and Food Waste: Extent, Causes and Prevention; FAO: Rome, Italy, 2011. [Google Scholar]
- Martínez-Valderrama, J.; Guirado, E.; Maestre, F.T. Discarded food and resource depletion. Nat. Food 2020, 1, 660–662. [Google Scholar] [CrossRef]
- Abson, D.J.; Fischer, J.; Leventon, J.; Newig, J.; Schomerus, T.; Vilsmaier, U.; von Wehrden, H.; Abernethy, P.; Ives, C.D.; Jager, N.W.; et al. Leverage points for sustainability transformation. Ambio 2016, 46, 30–39. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Folke, C.; Jansson, Å.; Rockström, J.; Olsson, P.; Carpenter, S.R.; Chapin, F.S.; Crépin, A.-S.; Daily, G.; Danell, K.; Ebbesson, J.; et al. Reconnecting to the Biosphere. Ambio 2011, 40, 719–738. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zylstra, M.J.; Knight, A.T.; Esler, K.J.; Le Grange, L.L.L. Connectedness as a Core Conservation Concern: An Interdisciplinary Review of Theory and a Call for Practice. Springer Sci. Rev. 2014, 2, 119–143. [Google Scholar] [CrossRef] [Green Version]
- Pérez-Ramírez, I.; García-Llorente, M.; Portilla, C.S.; Benito, A.; Castro, A.J. Participatory collective farming as a leverage point for fostering human-nature connectedness. Ecosyst. People 2021, 17, 222–234. [Google Scholar] [CrossRef]
- Wandersee, J.H.; Schussler, E.E. Preventing Plant Blindness. Am. Biol. Teach. 1999, 61, 82–86. [Google Scholar] [CrossRef]
- Ives, C.D.; Abson, D.J.; von Wehrden, H.; Dorninger, C.; Klaniecki, K.; Fischer, J. Reconnecting with nature for sustainability. Sustain. Sci. 2018, 13, 1389–1397. [Google Scholar] [CrossRef] [Green Version]
- Jose, S.B.; Wu, C.-H.; Kamoun, S. Overcoming plant blindness in science, education, and society. Plants People Planet 2019, 1, 169–172. [Google Scholar] [CrossRef]
- López-Rodríguez, M.D.; Castro, A.J.; Castro, H.; Jorreto, S.; Cabello, J. Science–policy interface for addressing environmental problems in arid Spain. Environ. Sci. Policy 2015, 50, 1–14. [Google Scholar] [CrossRef]
- Van Ittersum, M.K.; Rabbinge, R.; Van Latesteijn, H.C. Exploratory land use studies and their role in strategic policy making. Agric. Syst. 1998, 58, 309–330. [Google Scholar] [CrossRef]
| Family | Taxon | Threat | P | % TP | IUCN | PR |
|---|---|---|---|---|---|---|
| Alliaceae | Allium scaberrimum M.Serres [84] | CA | 16 | 100 | VU | R |
| Amaryllidaceae | Narcissus nevadensis Pugsley subsp. nevadensis [83] | CE | 2 | 50 | EN | RN |
| Amaryllidaceae | Narcissus bujei (Fern. Casas) Fern. Casas | CI | 14 | 100 | VU | R |
| Caryophyllaceae | Dianthus inoxianus Gallego | CE | 16 | 56 | EN | R |
| Caryophyllaceae | Silene sennenii Pau | CE | 3 | 67 | EN | RN |
| Caryophyllaceae | Silene stockenii Chater | CE | 4 | 100 | CR | R |
| Caryophyllaceae | Silene diclinis (Lag.) M. Laínz | CI | 5 | 80 | EN | R |
| Colchicaceae | Androcymbium europaeum (Lange) K. Richt. | CE | 5 | 80 | VU | RNS |
| Compositae | Anthemis bourgaei Boiss. & Reut. | CE | 2 | 50 | EN | R |
| Compositae | Centaurea kunkelii N. García | CE | 2 | 50 | CR | R |
| Compositae | Centaurea ultreiae Silva Pando | CE | 1 | 100 | CR | R |
| Compositae | Jacobaea auricula (Coss.) Pelser [86] | CE | 7 | 100 | VU | R |
| Compositae | Leucanthemum gallaecicum Rodr. Oubiña & S. Ortiz | CE | 4 | 75 | EN | R |
| Compositae | Pentanema bifrons (L.) D. Gut. Larr. Santos-Vicente, Anderb., E. Rico & M.M. Mart. Ort. [85] | CE | 1 | 100 | CR | R |
| Cruciferae | Clypeola eriocarpa Cav. | CE | 2 | 50 | CR | R |
| Cruciferae | Coincya longirostra (Boiss.) Greuter & Burdet | CE | 10 | 100 | EN | R |
| Cruciferae | Vella pseudocytisus L. subsp. pseudocytisus | CE | 2 | 100 | EN | R |
| Cruciferae | Isatis aptera (Boiss. & Heldr.) Al-Shehbaz, Moazzeni & Mumm. [87] | CA | 6 | 100 | EN | - |
| Dipsacaceae | Succisella carvalhoana (Mariz) Baksay | CE | 4 | 25 | VU | R |
| Geraniaceae | Erodium paularense Fern. Gonz. & Izco | CE | 11 | 9 | EN | RNS |
| Geraniaceae | Erodium recoderi Auriault & Guitt. | CE | 6 | 17 | VU | - |
| Gramineae | Puccinellia pungens (Pau) Paunero | CE | 9 | 11 | EN | RNS |
| Gramineae | Enneapogon persicus Boiss. | CI | 2 | 100 | CR | R |
| Labiatae | Nepeta hispanica Boiss. & Reut. | CE | 8 | 62.5 | VU | R |
| Labiatae | Teucrium edetanum M.B. Crespo, Mateo & T. Navarro | CE | 2 | 50 | VU | R |
| Leguminosae | Astragalus oxyglottis M. Bieb. | CE | 10 | 30 | EN | R |
| Leguminosae | Ononis azcaratei Devesa | CE | 4 | 50 | CR | R |
| Leguminosae | Astragalus nitidiflorus Jiménez Mun. & Pau | CI | 1 | 100 | CR | RN |
| Lythraceae | Lythrum baeticum Gonz. Albo | CE | 24 | 83 | EN | R |
| Lythraceae | Lythrum flexuosum Lag. | CE | 57 | 100 | EN | RNS |
| Malvaceae | Malvella sherardiana (L.) Jaub. & Spach | CA | 4 | 100 | VU | - |
| Marsileaceae | Marsilea batardae Launert | CE | 17 | 53 | EN | RNS |
| Marsileaceae | Marsilea strigosa Willd. | CE | 33 | 97 | VU | RNS |
| Marsileaceae | Pilularia minuta Durieu | CE | 4 | 100 | CR | RNS |
| Plantaginaceae | Plantago notata Lag. | CI | 1 | 100 | CR | R |
| Plumbaginaceae | Armeria merinoi (Bernis) Nieto Fel. & Silva Pando | CE | 6 | 50 | CR | R |
| Plumbaginaceae | Limonium aragonense (Debeaux) Font Quer | CE | 1 | 100 | CR | R |
| Plumbaginaceae | Limonium quesadense Erben | CE | 2 | 100 | EN | R |
| Plumbaginaceae | Limonium soboliferum Erben | CE | 1 | 100 | CR | R |
| Plumbaginaceae | Limonium squarrosum Erben | CE | 1 | 100 | CR | R |
| Plumbaginaceae | Limonium ugijarense Erben | CE | 2 | 50 | EN | - |
| Plumbaginaceae | Limonium mansanetianum M.B. Crespo & Lledó | CI | 4 | 100 | CR | R |
| Polygalaceae | Polygaloides balansae (Coss.) O. Schwarz | CE | 1 | 100 | CR | - |
| Ranunculaceae | Delphinium bolosii C. Blanché & Molero | CE | 2 | 50 | EN | RN |
| Ranunculaceae | Ranunculus lingua L. | CE | 1 | 100 | CR | R |
| Scrophulariaceae | Scrophularia herminii Hoffmanns. & Link | CE | 31 | 32 | EN | S |
| Scrophulariaceae | Linaria nigricans Lange | CI | 6 | 50 | EN | R |
| Scrophulariaceae | Verbascum fontqueri Benedí & J.M. Monts. | CA | 8 | 100 | VU | R |
| Thymelaeaceae | Thymelaea lythroides Barratte & Murb. | CE | 1 | 100 | CR | RN |
| Umbelliferae | Hohenackeria polyodon Coss. & Durieu | CE | 4 | 100 | VU | R |
| Threat | Taxon | Life Form | Pollination Mode | Dispersal Mode |
|---|---|---|---|---|
| Crop extension | Androcymbium europaeum | G | Biotic | Abiotic |
| Anthemis bourgaei | T | Biotic | Abiotic | |
| Armeria merinoi | H | Biotic | Abiotic | |
| Astragalus oxyglottis | T | Biotic | Abiotic | |
| Centaurea kunkelii | H | Biotic | Abiotic | |
| Centaurea ultreiae | H | Biotic | Biotic | |
| Clypeola eriocarpa | T | Biotic | Abiotic | |
| Coincya longirostra | H | Biotic | Abiotic | |
| Delphinium bolosii | G | Biotic | Abiotic | |
| Dianthus inoxianus | C | Biotic | Abiotic | |
| Erodium paularense | C | Biotic | Abiotic | |
| Erodium recoderi | T | Biotic | Abiotic | |
| Hohenackeria polyodon | T | Abiotic | Abiotic | |
| Jacobaea auricula | H | Biotic | Abiotic | |
| Leucanthemum gallaecicum | H | Biotic | Biotic | |
| Limonium aragonense | H | Biotic | Abiotic | |
| Limonium quesadense | H | Biotic | Abiotic | |
| Limonium soboliferum | H | Abiotic | Biotic | |
| Limonium squarrosum | H | Biotic | Abiotic | |
| Limonium ugijarense | H | Biotic | Abiotic | |
| Lythrum baeticum | T | Biotic | - | |
| Lythrum flexuosum | T | Biotic | - | |
| Marsilea batardae | Hy | Abiotic | Abiotic | |
| Marsilea strigosa | Hy | Abiotic | Biotic | |
| Narcissus nevadensis nevadensis | G | Biotic | Abiotic | |
| Nepeta hispanica | G | Biotic | Abiotic | |
| Ononis azcaratei | T | Biotic | Abiotic | |
| Pentanema bifrons | H | Biotic | Abiotic | |
| Pilularia minuta | H | Abiotic | Abiotic | |
| Polygaloides balansae | P | Biotic | Abiotic | |
| Puccinellia pungens | H | Abiotic | Abiotic | |
| Ranunculus lingua | Hy | Biotic | - | |
| Scrophularia herminii | H | Biotic | - | |
| Silene sennenii | C | Biotic | Abiotic | |
| Silene stockenii | T | Biotic | Abiotic | |
| Succisella carvalhoana | H | Biotic | Abiotic | |
| Teucrium edetanum | H | Biotic | Abiotic | |
| Thymelaea lythroides | P | Biotic | Biotic | |
| Vella pseudocytisus pseudocytisus | P | Biotic | Abiotic | |
| Agricultural intensification | Astragalus nitidiflorus | H | Biotic | - |
| Enneapogon persicus | G | Abiotic | Abiotic | |
| Limonium mansanetianum | H | - | - | |
| Linaria nigricans | T | Biotic | Abiotic | |
| Narcissus bujei | G | Biotic | Abiotic | |
| Plantago notata | T | Abiotic | Biotic | |
| Silene diclinis | C | Biotic | Abiotic | |
| Crop abandonment | Allium scaberrimum | G | Biotic | Abiotic |
| Isatis aptera | T | Biotic | Abiotic | |
| Malvella sherardiana | H | Biotic | Abiotic | |
| Verbascum fontqueri | H | Biotic | Abiotic |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Molina-Pardo, J.L.; Rodríguez-Caballero, E.; Cueto, M.; Barranco, P.; Sánchez-Robles, M.; Laguía-Allué, A.; Giménez-Luque, E. Effects of Agricultural Use on Endangered Plant Taxa in Spain. Agriculture 2021, 11, 1097. https://doi.org/10.3390/agriculture11111097
Molina-Pardo JL, Rodríguez-Caballero E, Cueto M, Barranco P, Sánchez-Robles M, Laguía-Allué A, Giménez-Luque E. Effects of Agricultural Use on Endangered Plant Taxa in Spain. Agriculture. 2021; 11(11):1097. https://doi.org/10.3390/agriculture11111097
Chicago/Turabian StyleMolina-Pardo, José Luis, Emilio Rodríguez-Caballero, Miguel Cueto, Pablo Barranco, Manuel Sánchez-Robles, Azucena Laguía-Allué, and Esther Giménez-Luque. 2021. "Effects of Agricultural Use on Endangered Plant Taxa in Spain" Agriculture 11, no. 11: 1097. https://doi.org/10.3390/agriculture11111097







