Effect of Integration of Linseed and Vitamin E in Charolaise × Podolica Bulls’ Diet on Fatty Acids Profile, Beef Color and Lipid Stability
Abstract
:1. Introduction
2. Materials and Methods
2.1. Animals and Diets
2.2. Sample Collection and Animals Perfomances
2.3. Meat Chemical and Cholesterol Analysis
2.4. Meat Fatty Acids Analysis
2.5. Meat Color Determination
2.6. Lipid Oxidation
2.7. Statistical Analysis
3. Results
3.1. Animal Perfomances, Drip and Cooking Loss Analyses
3.2. Proximate and Cholesterol Analyses
3.3. Fatty Acids Analysis
3.4. Lipid Stability to Oxidation
3.5. Color Determinations
4. Discussion
4.1. Animal Performances, Drip and Cooking Loss
4.2. Proximate and Cholesterol Analyses
4.3. Fatty Acids Analysis
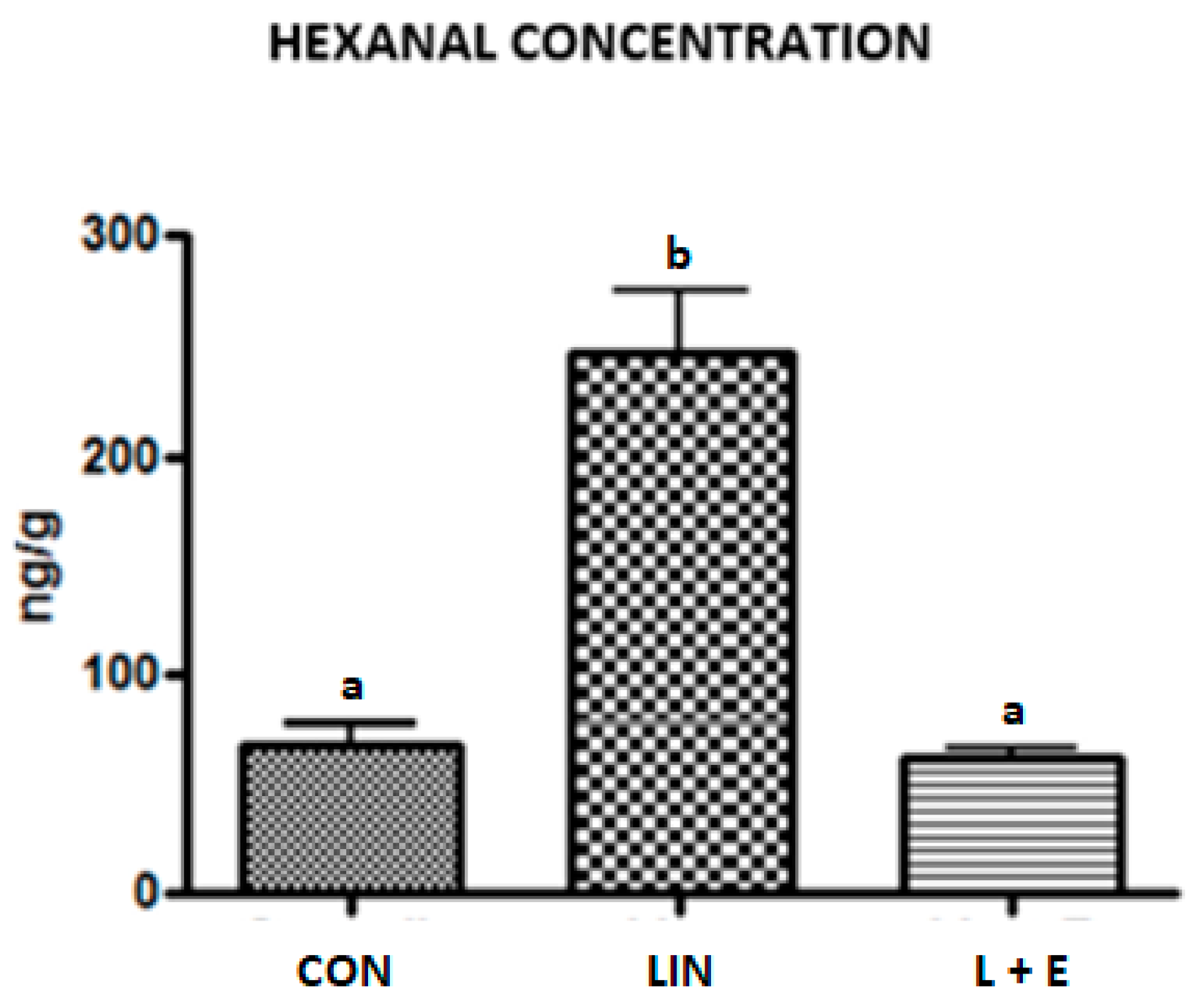
4.4. Lipid Stability to Oxidation
4.5. Color Determination
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Amine, E.K.; Baba, N.H.; Belhadj, M.; Deurenberg-Yap, M.; Djazayery, A.; Forrestre, T.; Galuska, D.A.; Herman, S.; James, W.P.T.; M’Buyamba Kabangu, J.R.; et al. Diet, nutrition and the prevention of chronic diseases. World Health Organ. Tech. Rep. Ser. 2003, 916, 1–49. [Google Scholar] [CrossRef] [Green Version]
- Maki, K.C.; Eren, F.; Cassens, M.E.; Dicklin, M.R.; Davidson, M.H. ω-6 Polyunsaturated Fatty Acids and Cardiometabolic Health: Current Evidence, Controversies, and Research Gaps. Adv. Nutr. 2018, 9, 688–700. [Google Scholar] [CrossRef]
- Corazzin, M.; Bovolenta, S.; Sepulcri, A.; Piasentier, E. Effect of whole linseed addition on meat production and quality of Italian Simmental and Holstein young bulls. Meat Sci. 2012, 90, 99–105. [Google Scholar] [CrossRef] [PubMed]
- EFSA Panel on Dietetic Products and Allergies (NDA), N. Scientific Opinion on Dietary Reference Values for fats, including saturated fatty acids, polyunsaturated fatty acids, monounsaturated fatty acids, trans fatty acids, and cholesterol. EFSA J. 2010, 8, 1461. [Google Scholar] [CrossRef] [Green Version]
- Renna, M.; Brugiapaglia, A.; Zanardi, E.; Prandini, A.; Moschini, M.; Sigolo, S. Fatty acid profile, meat quality and flavour acceptability of beef from double-muscled Piemontese young bulls fed ground flaxseed. Ital. J. Anim. Sci. 2019, 18, 355–365. [Google Scholar] [CrossRef] [Green Version]
- Albertí, P.; Beriain, M.J.; Ripoll, G.; Sarriés, V.; Panea, B.; Mendizabal, J.A.; Purroy, A.; Olleta, J.L.; Sañudo, C. Effect of including linseed in a concentrate fed to young bulls on intramuscular fatty acids and beef color. Meat Sci. 2014, 96, 1258–1265. [Google Scholar] [CrossRef]
- Conte, G.; Serra, A.; Casarosa, L.; Ciucci, F.; Cappucci, A.; Bulleri, E.; Corrales-Retana, L.; Buccioni, A.; Mele, M. Effect of linseed supplementation on total longissimus muscle lipid composition and shelf-life of beef from young Maremmana bulls. Front. Vet. Sci. 2019, 5, 1–15. [Google Scholar] [CrossRef]
- Faustman, C.; Sun, Q.; Mancini, R.; Suman, S.P. Myoglobin and lipid oxidation interactions: Mechanistic bases and control. Meat Sci. 2010, 86, 86–94. [Google Scholar] [CrossRef] [PubMed]
- Amaral, A.B.; Da Solva, M.V.; Lannes, S.C.D.S. Lipid oxidation in meat: Mechanisms and protective factors—A review. Food Sci. Technol. 2018, 38, 1–15. [Google Scholar] [CrossRef] [Green Version]
- Wu, D.; Sun, D. Colour measurements by computer vision for food quality control—A review. Trends Food Sci. Technol. 2013, 29, 5–20. [Google Scholar] [CrossRef]
- Juárez, M.; Dugan, M.E.R.; Aalhus, J.L.; Aldai, N.; Basarab, J.A.; Baron, V.S.; McAllister, T.A. Effects of vitamin E and flaxseed on rumen-derived fatty acid intermediates in beef intramuscular fat. Meat Sci. 2011, 88, 434–440. [Google Scholar] [CrossRef]
- Tarricone, S.; Colonna, M.A.; Giannico, F.; Facciolongo, A.M.; Jambrenghi, A.C.; Ragni, M. Effects of dietary extruded linseed (Linum usitatissimum L.) on performance and meat quality in Podolian young bulls. S. Afr. J. Anim. Sci. 2019, 49, 781–789. [Google Scholar] [CrossRef] [Green Version]
- Sellick, J. Enhancing the protection of animals used for scientific purposes. Environ. Law Manag. 2011, 23, 75–82. [Google Scholar]
- European Council. European Council Regulation (EC) 1099/2009 of 24 September 2009 on the Protection of Animals at the Time of Killing. Official Journal of the European Union. L 303. 18 November 2009. Available online: https://eur-lex.europa.eu/legal-content/EN/TXT/?uri=OJ:L:2009:303:TOC (accessed on 8 September 2021).
- Noziere, P.; Sauvant, D.; Delaby, L. INRA Feeding System for Ruminants; Wageningen Academic Publishers: Wageningen, The Netherlands, 2018; ISBN 9086862926. [Google Scholar]
- ASPA (Animal Science and Production Association). Metodiche per la Determinazione delle Caratteristiche Qualitative della Carne (Procedures for Meat Quality Evaluation); University of Perugia: Perugia, Italy, 1996. [Google Scholar]
- ASPA (Animal Science and Production Association). Metodologie Relative alla Macellazione degli Animali di Interesse Zootecnico ed alla Valutazione e Dissezione della Loro Carcassa (Procedures for Carcass Quality Evaluation); ISMEA: Roma, Italy, 1991. [Google Scholar]
- Latimer, G.W. AOAC International Official Methods of Analysis of AOAC International, 20th ed.; AOAC International: Rockville, MD, USA, 2016; Volume 3172. [Google Scholar]
- De Almeida, J.C.; Perassolo, M.S.; Camargo, J.L.; Bragagnolo, N.; Gross, J.L. Fatty acid composition and cholesterol content of beef and chicken meat in Southern Brazil. Rev. Bras. Cienc. Farm. J. Pharm. Sci. 2006, 42, 109–117. [Google Scholar] [CrossRef] [Green Version]
- Tapp, W.N.; Yancey, J.W.S.; Apple, J.K. How is the instrumental color of meat measured? Meat Sci. 2011, 89, 1–5. [Google Scholar] [CrossRef]
- Rentfrow, G.; Linville, M.L.; Stahl, C.A.; Olson, K.C.; Berg, E.P. The effects of the antioxidant lipoic acid on beef longissimus bloom time. J. Anim. Sci. 2004, 82, 3034–3037. [Google Scholar] [CrossRef]
- Insausti, K.; Beriain, M.J.; Purroy, A.; Alberti, P.; Lizaso, L.; Hernandez, B. Colour stability of beef from different Spanish native cattle breeds stored under vacuum and modified atmosphere. Meat Sci. 1999, 53, 241–249. [Google Scholar] [CrossRef]
- OIV Compendium Of International Analysis Of Methods -OIV Chromatic Characteristics Method OIV-MA-AS2-11 Determination of Chromatic Characteristics According to CIELab. 2006. Available online: https://www.oiv.int/public/medias/2478/oiv-ma-as2-11.pdf (accessed on 14 October 2021).
- Hunt, M.C.; King, A.; Barbut, S.; Clause, J.; Cornforth, D.; Hanson, D.; Lindahl, G.; Mancini, R.; Milkowski, A.; Mohan, A. AMSA Meat Color Measurement Guidelines; American Meat Science Association: Champaign, IL, USA, 2012; Volume 61820, ISBN 8005172672. [Google Scholar]
- Krzywicki, K. Assessment of relative content of myoglobin, oxymyoglobin and metmyoglobin at the surface of beef. Meat Sci. 1979, 3, 1–10. [Google Scholar] [CrossRef]
- Panseri, S.; Soncin, S.; Chiesa, L.M.; Biondi, P.A. A headspace solid-phase microextraction gas-chromatographic mass-spectrometric method (HS-SPME-GC/MS) to quantify hexanal in butter during storage as marker of lipid oxidation. Food Chem. 2011, 127, 886–889. [Google Scholar] [CrossRef]
- Ragni, M.; Toteda, F.; Tufarelli, V.; Laudadio, V.; Facciolongo, A.; Dipalo, F.; Vicenti, A. Feeding of extruded flaxseed (linum usitatissimum L.) and pasture in podolica young bulls: Effects on growth traits, meat quality and fatty acid composition. Pak. J. Zool. 2014, 46, 1101–1109. [Google Scholar]
- Barton, L.; Marounek, M.; Kudrna, V.; Bures, D. MEAT Growth performance and fatty acid profiles of intramuscular and subcutaneous fat from Limousin and Charolais heifers fed extruded linseed. Meat Sci. 2007, 76, 517–523. [Google Scholar] [CrossRef] [PubMed]
- Mach, N.; Devant, M.; Díaz, I.; Font-Furnols, M.; Oliver, M.A.; García, J.A.; Bach, A. Increasing the amount of n-3 fatty acid in meat from young Holstein bulls through nutrition. J. Anim. Sci. 2006, 84, 3039–3048. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Carter, J.N.; Gill, D.R.; Krehbiel, C.R.; Confer, A.W.; Smith, R.A.; Lalman, D.L.; Claypool, P.L.; Mcdowell, L.R. Vitamin E supplementation of newly arrived feedlot calves 1, 2. J. Anim. Sci. 2005, 83, 1924–1932. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, S.K.; Panjono; Kang, S.M.; Kim, T.S.; Park, Y.S. The effects of dietary sulfur and Vitamin E supplementation on the quality of beef from the longissimus muscle of Hanwoo bulls. Asian-Australas. J. Anim. Sci. 2008, 21, 1059–1066. [Google Scholar] [CrossRef]
- Cuvelier, C.; Clinquart, A.; Hocquette, J.F.; Cabaraux, J.F.; Dufrasne, I.; Istasse, L.; Hornick, J.L. Comparison of composition and quality traits of meat from young finishing bulls from Belgian Blue, Limousin and Aberdeen Angus breeds. Meat Sci. 2006, 74, 522–531. [Google Scholar] [CrossRef] [PubMed]
- Grandin, T. The Effect of Stress on Livestock and Meat Quality Prior to and During Slaughter. Int. J. Study Anim. Probl. 1980, 1, 313–337. [Google Scholar]
- Dawson, L.E.R.; Fearon, A.M.; Moss, B.W.; Woods, V.B. Effects of substitution of a proportion of the concentrate in grass silage/concentrate-based diets with extruded linseed on performance and meat quality of dairy bulls. Anim. Feed Sci. Technol. 2010, 156, 10–18. [Google Scholar] [CrossRef]
- Otremba, M.M.; Dikeman, M.E.; Milliken, G.A.; Stroda, S.L.; Chambers Iv, E.; Chambers, D. Interrelationships between descriptive texture profile sensory panel and descriptive attribute sensory panel evaluations of beef Longissimus and Semitendinosus muscles. Meat Sci. 2000, 54, 325–332. [Google Scholar] [CrossRef]
- Hughes, J.M.; Oiseth, S.K.; Purslow, P.P.; Warner, R.D. A structural approach to understanding the interactions between colour, water-holding capacity and tenderness. Meat Sci. 2014, 98, 520–532. [Google Scholar] [CrossRef]
- Fernandez, M.L.; Calle, M. Revisiting dietary cholesterol recommendations: Does the evidence support a limit of 300 mg/d? Curr. Atheroscler. Rep. 2010, 12, 377–383. [Google Scholar] [CrossRef]
- Özer, N.K.; Şirikçi, Ö.; Taha, S.; Şan, T.; Moser, U.; Azzi, A. Effect of Vitamin E and Probucol on Dietary Cholesterol-Induced Atherosclerosis in Rabbits. Free Radic. Biol. Med. 1998, 24, 226–233. [Google Scholar] [CrossRef]
- Bozaykut, P.; Karademir, B.; Yazgan, B.; Sozen, E.; Siow, R.C.M.; Mann, G.E.; Ozer, N.K. Effects of vitamin e on peroxisome proliferator-activated receptor γ and nuclear factor-erythroid 2-related factor 2 in hypercholesterolemia-induced atherosclerosis. Free Radic. Biol. Med. 2014, 70, 174–181. [Google Scholar] [CrossRef] [PubMed]
- Yoshimura, E.H.; Santos, N.W.; Machado, E.; Agustinho, B.C.; Pereira, L.M.; De Aguiar, S.C.; Franzolin, R.; Gasparino, E.; Santos, G.T.; Zeoula, L.M. Effects of dairy cow diets supplied with flaxseed oil and propolis extract, with or without vitamin E, on the ruminal microbiota, biohydrogenation, and digestion. Anim. Feed Sci. Technol. 2018, 241, 163–172. [Google Scholar] [CrossRef]
- Marino, R.; Della Malva, A.; Caroprese, M.; De Palo, P.; Santillo, A.; Sevi, A.; Albenzio, M. Effects of whole linseed supplementation and treatment duration on fatty acid profile and endogenous bioactive compounds of beef muscle. Animal 2019, 13, 444–452. [Google Scholar] [CrossRef]
- Raes, K.; Haak, L.; Balcaen, A.; Claeys, E.; Demeyer, D.; De Smet, S. Effect of linseed feeding at similar linoleic acid levels on the fatty acid composition of double-muscled Belgian Blue young bulls. Meat Sci. 2004, 66, 307–315. [Google Scholar] [CrossRef]
- Givens, D.I.; Gibbs, R.A. Very long chain n-3 polyunsaturated fatty acids in the food chain in the UK and the potential of animal-derived foods to increase intake. Nutr. Bull. 2006, 31, 104–110. [Google Scholar] [CrossRef]
- Tudisco, R.; Chiofalo, B.; Lo Presti, V.; Morittu, V.M.; Moniello, G.; Grossi, M.; Musco, N.; Grazioli, R.; Mastellone, V.; Lombardi, P.; et al. Influence of feeding linseed on SCD activity in grazing goat mammary glands. Animals 2019, 9, 786. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Simopoulos, A.P. Essential fatty acids in health and chronic disease 1,2. Am. J. Clin. Nutr. 1999, 70, 560–569. [Google Scholar] [CrossRef] [Green Version]
- Yu, M.; Gao, Q.; Wang, Y.; Zhang, W.; Li, L.; Wang, Y.; Dai, Y. Unbalanced omega-6/omega-3 ratio in red meat products in China. J. Biomed. Res. 2013, 27, 366–371. [Google Scholar] [CrossRef] [Green Version]
- Wood, J.D.; Enser, M.; Fisher, A.V.; Nute, G.R.; Sheard, P.R.; Richardson, R.I.; Hughes, S.I.; Whittington, F.M. Fat deposition, fatty acid composition and meat quality: A review. Meat Sci. 2008, 78, 343–358. [Google Scholar] [CrossRef]
- Tudisco, R.; Calabrò, S.; Cutrignelli, M.I.; Moniello, G.; Grossi, M.; Gonzalez, O.J.; Piccolo, V.; Infascelli, F. Influence of organic systems on Stearoyl-CoA desaturase in goat milk. Small Rumin. Res. 2012, 106, 37–42. [Google Scholar] [CrossRef]
- Kalač, P.; Samková, E. The effects of feeding various forages on fatty acid composition of bovine milk fat: A review. Czech J. Anim. Sci. 2010, 55, 521–537. [Google Scholar] [CrossRef] [Green Version]
- Fernández, J.; Pérez-Álvarez, J.A.; Fernández-López, J.A. Thiobarbituric acid test for monitoring lipid oxidation in meat. Food Chem. 1997, 59, 345–353. [Google Scholar] [CrossRef]
- García-Llatas, G.; Lagarda, M.J.; Romero, F.; Abellán, P.; Farré, R. A headspace solid-phase microextraction method of use in monitoring hexanal and pentane during storage: Application to liquid infant foods and powdered infant formulas. Food Chem. 2007, 101, 1078–1086. [Google Scholar] [CrossRef] [Green Version]
- Trout, G.R.; Dale, S. Prevention of warmed-over flavor in cooked beef: Effect of phosphate type, phosphate concentration, a lemon juice/phosphate blend, and beef extract. J. Agric. Food Chem. 1990, 38, 665–669. [Google Scholar] [CrossRef]
- Ahn, J.; Grün, I.U.; Fernando, L.N. Antioxidant properties of natural plant extracts containing polyphenolic compounds in cooked ground beef. J. Food Sci. 2002, 67, 1364–1369. [Google Scholar] [CrossRef]
- Scollan, N.; Hocquette, J.F.; Nuernberg, K.; Dannenberger, D.; Richardson, I.; Moloney, A. Innovations in beef production systems that enhance the nutritional and health value of beef lipids and their relationship with meat quality. Meat Sci. 2006, 74, 17–33. [Google Scholar] [CrossRef]
- Yang, A.; Brewster, M.J.; Beilken, S.L.; Lanari, M.C.; Taylor, D.G.; Tume, R.K. Warmed-Over Flavor and Lipid Stability of Beef: Effects of Prior Nutrition. J. Food Sci. 2002, 67, 3309–3313. [Google Scholar] [CrossRef]
- King, D.A.; Shackelford, S.D.; Kuehn, L.A.; Kemp, C.M.; Rodriguez, A.B.; Thallman, R.M.; Wheeler, T.L. Contribution of genetic influences to animal-to-animal variation in myoglobin content and beef lean color stability. J. Anim. Sci. 2010, 88, 1160–1167. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, Q.; Lanari, M.C.; Schaefer, D.M. A review of dietary vitamin E supplementation for improvement of beef quality. J. Anim. Sci. 1995, 73, 3131–3140. [Google Scholar] [CrossRef] [PubMed]
- Albertí, P.; Campo, M.M.; Beriain, M.J.; Ripoll, G.; Sañudo, C. Effect of including whole linseed and vitamin E in the diet of young bulls slaughtered at two fat covers on the sensory quality of beef packaged in two different packaging systems. J. Sci. Food Agric. 2017, 97, 753–760. [Google Scholar] [CrossRef] [Green Version]
- MacDougall, D.B. Changes in the colour and opacity of meat. Food Chem. 1982, 9, 75–88. [Google Scholar] [CrossRef]
- Van den Oord, A.H.A.; Wesdorp, J.J. Analysis of pigments in intact beef samples: A simple method for the determination of oxymyoglobin and ferric myoglobin in intact beef samples using reflectance spectrophotometry. Int. J. Food Sci. Technol. 1971, 6, 1–13. [Google Scholar] [CrossRef]
- Chan, W.K.M.; Hakkarainen, K.; Faustman, C.; Schaefer, D.M.; Scheller, K.K.; Liu, Q. Dietary Vitamin E Effect on Color Stability and Sensory Assessment of Spoilage in Three Beef Muscles. Meat Sci. 1996, 42, 387–399. [Google Scholar] [CrossRef]
| Concentrate | Extruded Linseed | Wheat Straw | |
|---|---|---|---|
| Chemical composition (% DM) | |||
| Crude Protein | 17.2 | 22.0 | 3.8 |
| Ether extract | 5.3 | 35.0 | 3.1 |
| Crude fiber | 7.5 | 7.5 | 38.2 |
| Ash | 7.1 | 4.5 | 5.9 |
| NEF, MJ/kg DM | 8.4 | 10.3 | 2.5 |
| Fatty acid profile (% total FA) | |||
| C16:0 | 17.0 | 14.0 | - |
| C18:0 | 6.4 | 4.1 | - |
| C18:1n-9 | 20.96 | 18.5 | - |
| C18:2n-6 | 42.22 | 11.9 | - |
| C18:3n-3 | 0.5 | 42.5 | - |
| Diet | |||||
|---|---|---|---|---|---|
| Item | CON | LIN | L+E | SEM | p-Value |
| Initial live weight (kg) | 400 | 419 | 370 | 24.98 | 0.093 |
| Slaughter weight (kg) | 522 | 531 | 480 | 25.67 | 0.171 |
| Daily weight gain (kg/d) | 1.36 | 1.25 | 1.23 | 0.07 | 0.270 |
| Hot carcass weight (kg) | 299 | 307 | 262 | 20.20 | 0.151 |
| Hot dressing (%) | 57.03 | 57.57 | 54.41 | 1.29 | 0.192 |
| Shrink loss (%) | 0.98 | 1.00 | 0.98 | 0.06 | 0.929 |
| pH0 | 6.49 | 6.61 | 6.58 | 0.09 | 0.645 |
| pH24 | 5.68 | 5.73 | 5.77 | 0.08 | 0.665 |
| Drip loss (%) | 1.78 | 1.23 | 1.46 | 0.53 | 0.708 |
| Cooking loss (%) | 21.68 | 23.52 | 24.96 | 1.59 | 0.161 |
| Diet | |||||
|---|---|---|---|---|---|
| Item | CON | LIN | L+E | SEM | p-Value |
| Dry matter (%) | 27.37 | 26.53 | 26.38 | 0.86 | 0.561 |
| Crude ash (%) | 1.08 | 1.01 | 1.01 | 0.07 | 0.542 |
| Crude Protein (%) | 21.82 | 22.24 | 21.65 | 0.39 | 0.474 |
| Ether extract (%) | 2.92 | 3.73 | 2.80 | 0.33 | 0.050 |
| Cholesterol (mg/100 g) | 45.93a | 49.81a | 28.63b | 5.47 | 0.010 |
| Diet | |||||
|---|---|---|---|---|---|
| Item | CON | LIN | L+E | SEM | p-Value |
| SFA | 52.22a | 48.66b | 48.02b | 0.84 | 0.025 |
| MUFA | 35.81a | 34.15ab | 33.53b | 0,99 | 0.006 |
| PUFA | 11.97b | 17.19a | 20.69a | 1.22 | 0.001 |
| n−6 | 09.89b | 14.32ab | 17.14a | 1.84 | 0.008 |
| n−3 | 1.37c | 2.49b | 3.15a | 0.239 | ** |
| n−6 to n−3 ratio | 7.21a | 5.75b | 5.43b | 0.57 | 0.001 |
| CLA * | 0.31b | 0.40a | 0.40a | 0.03 | 0.009 |
| C14:0 | 2.95 | 2.23 | 2.45 | 0.29 | 0.113 |
| C16:0 | 27.98 | 28.22 | 27.22 | 0.47 | 0.201 |
| C18:0 | 20.00 | 17.67 | 18.54 | 0.90 | 0.095 |
| Total trans C18:1 | 3.18a | 2.084b | 1.99b | 0.32 | 0.009 |
| C18:1 n-9 cis | 29.50a | 29.69ab | 26.58b | 0.99 | 0.036 |
| C18:2 n-6 cis | 7.86b | 11.72a | 13.86a | 1.54 | 0.003 |
| C18:3 n-3 | 0.94b | 1.26b | 1.85a | 0.16 | ** |
| CLA 9–11 | 0.29b | 0.39a | 0.40a | 0.03 | 0.003 |
| C20:2 n-6 | 0.04 | 0.07 | 0.06 | 0.02 | 0.204 |
| C20:3 n-6 | 0.33 | 0.37 | 0.47 | 0.08 | 0.275 |
| C22:4 n-6 | 1.66b | 1.90ab | 2.62a | 0.29 | 0.017 |
| C20:5 n-3 | 0.12b | 0.38ab | 0.68a | 0.05 | ** |
| C22:5 n-3 | 0.28b | 0.59a | 0.54a | 0.06 | ** |
| C22:6 n-3 | 0.02b | 0.10a | 0.10a | 0.02 | 0.003 |
| Diet | Day | p-Value | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Item | CON | LIN | L+E | 1 | 2 | 3 | 4 | 5 | 6 | SEM | Diet | Day | Diet x Day |
| L* | 44.16 | 43.59 | 44.69 | 40.84b | 44.95a | 44.91a | 44.82a | 44.81a | 44.54a | 0.50 | 0.326 | ** | 0.078 |
| a* | 22.51a | 19.31c | 20.67b | 20.26cd | 22.63a | 21.55b | 20.94bc | 19.98d | 19.66cd | 1.06 | 0.007 | ** | 0.905 |
| b* | 17.16a | 15.48b | 16.58ab | 13.51d | 17.61a | 17.22ab | 16.99ac | 16.44c | 16.58c | 0.70 | 0.045 | ** | 0.592 |
| H° | 37.30b | 38.65a | 38.68a | 33.57e | 37.94d | 38.68c | 39.12b | 39.52a | 40.28a | 0.75 | ** | ** | 0.0004 |
| C* | 28.31a | 24.78c | 26.52b | 24.36d | 28.69a | 27.59b | 26.97bc | 25.88cd | 25.73bd | 1.18 | 0.014 | ** | 0.975 |
| OMb% | 70.63 | 63.56 | 64.07 | 57.94fe | 75.81a | 70.32b | 66.56c | 63.87d | 62.73cde | 4.01 | 0.054 | ** | 0.203 |
| DMb% | 12.29 | 16.17 | 14.54 | 34.32a | 9.19c | 9.84c | 10.64bc | 11.38b | 10.56b | 4.19 | 0.067 | ** | ** |
| MMb% | 17.08 | 20.27 | 21.4 | 7.74f | 14.99e | 19.84d | 22.81c | 24.75ac | 26.71ab | 2.21 | 0.140 | ** | 0.146 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Morittu, V.M.; Spina, A.A.; Iommelli, P.; Poerio, A.; Oliverio, F.V.; Britti, D.; Tudisco, R. Effect of Integration of Linseed and Vitamin E in Charolaise × Podolica Bulls’ Diet on Fatty Acids Profile, Beef Color and Lipid Stability. Agriculture 2021, 11, 1032. https://doi.org/10.3390/agriculture11111032
Morittu VM, Spina AA, Iommelli P, Poerio A, Oliverio FV, Britti D, Tudisco R. Effect of Integration of Linseed and Vitamin E in Charolaise × Podolica Bulls’ Diet on Fatty Acids Profile, Beef Color and Lipid Stability. Agriculture. 2021; 11(11):1032. https://doi.org/10.3390/agriculture11111032
Chicago/Turabian StyleMorittu, Valeria Maria, Anna Antonella Spina, Piera Iommelli, Anselmo Poerio, Francesco Vincenzo Oliverio, Domenico Britti, and Raffaella Tudisco. 2021. "Effect of Integration of Linseed and Vitamin E in Charolaise × Podolica Bulls’ Diet on Fatty Acids Profile, Beef Color and Lipid Stability" Agriculture 11, no. 11: 1032. https://doi.org/10.3390/agriculture11111032







