Morphological Characterization and Determination of Aflatoxigenic and Non-Aflatoxigenic Aspergillus flavus Isolated from Sweet Corn Kernels and Soil in Malaysia
Abstract
:1. Introduction
2. Materials and Methods
2.1. Description of the Study Site
2.2. Sample Collection
2.2.1. Sweet Corn Kernels
2.2.2. Soil Sampling
2.3. Isolation and Identification of Aspergillus flavus
2.3.1. Preparation of Growth Media
2.3.2. Aspergillus flavus from Sweet Corn Samples
2.3.3. Aspergillus flavus from Soil Samples
2.3.4. Morphological Confirmation of Aspergillus flavus
2.4. Determination of Aflatoxigenic Potential of Aspergillus flavus
3. Results
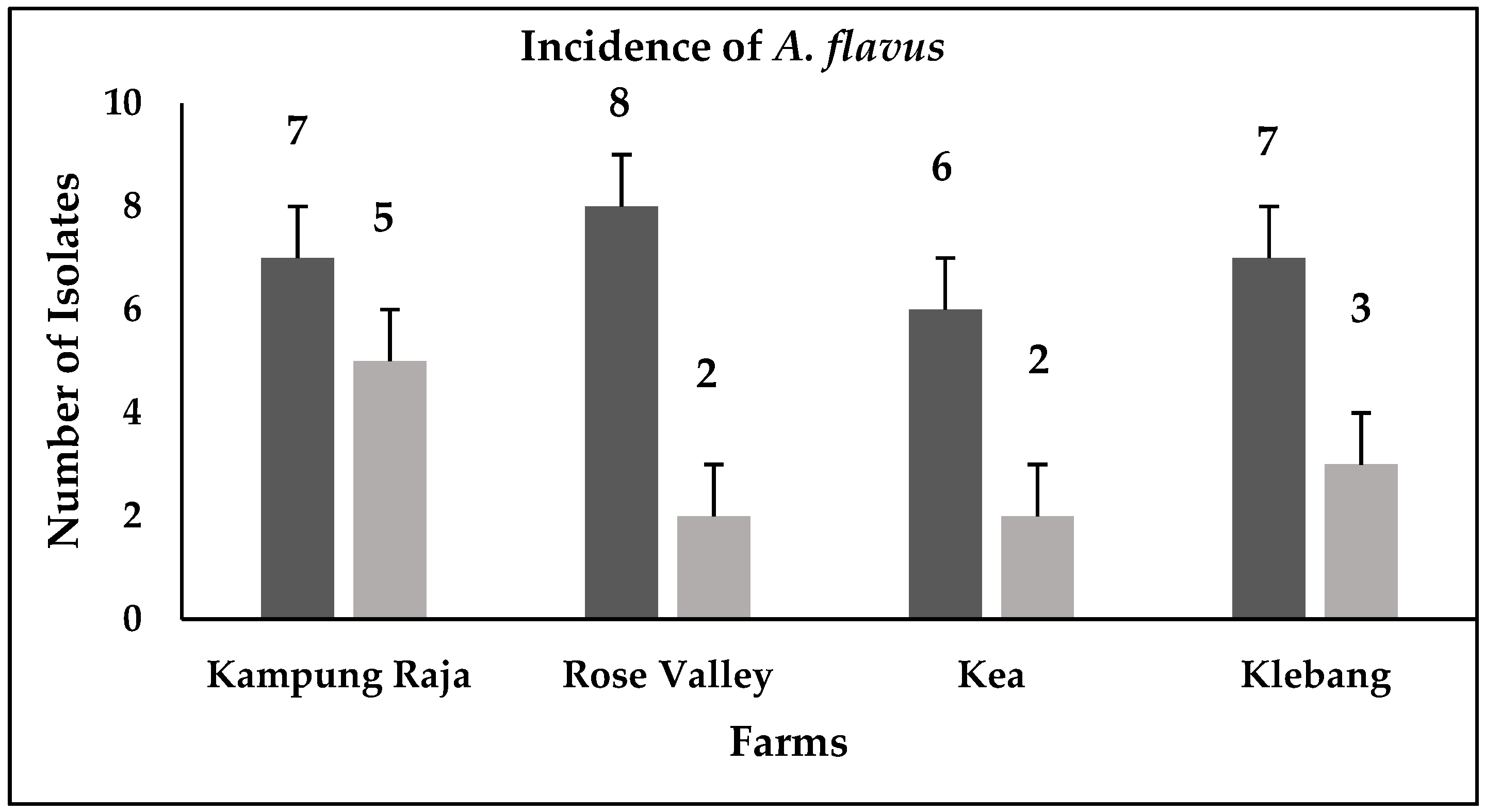
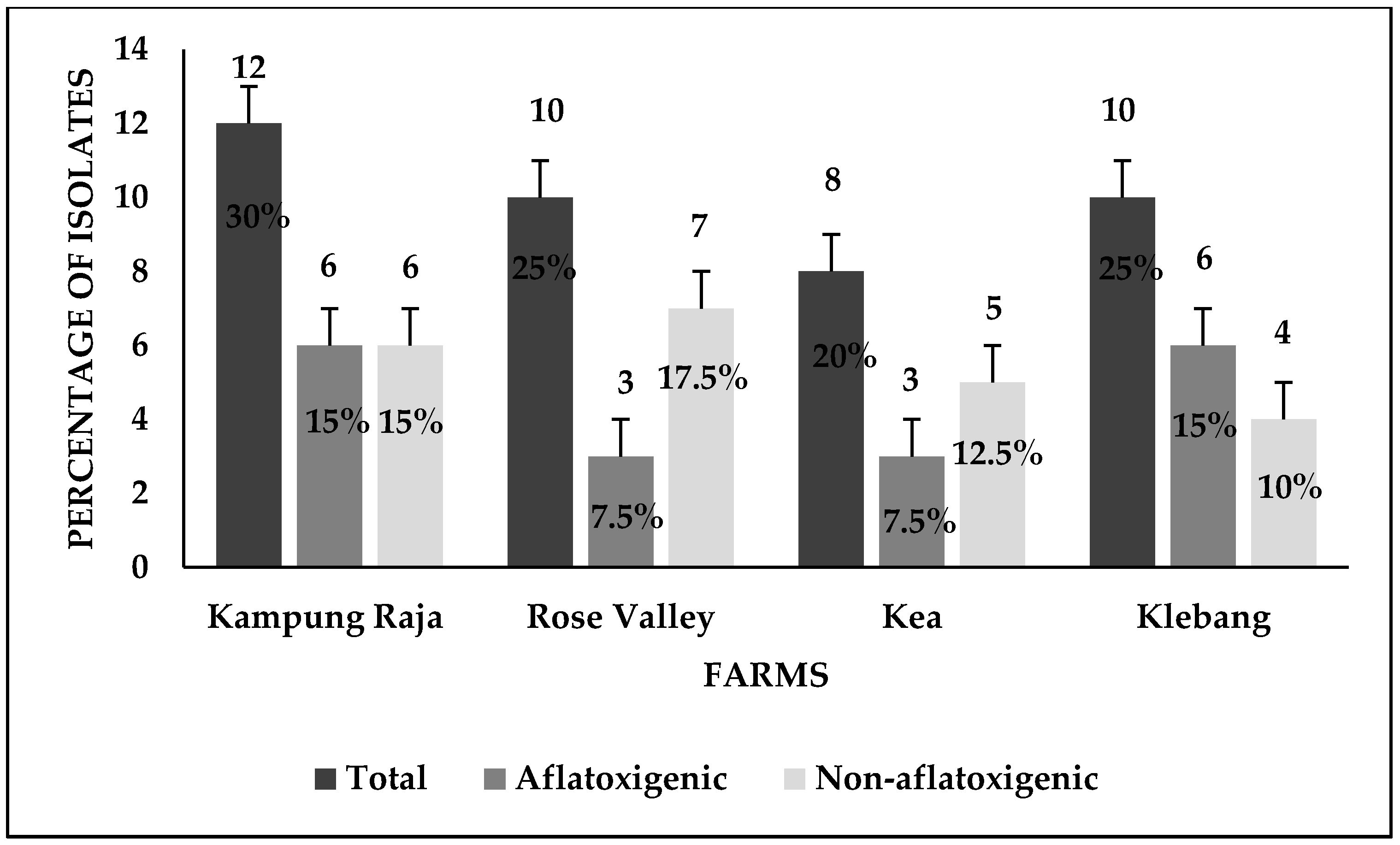
3.1. Detection of Aspergillus flavus
3.2. Morphological Characterization of Aspergillus flavus
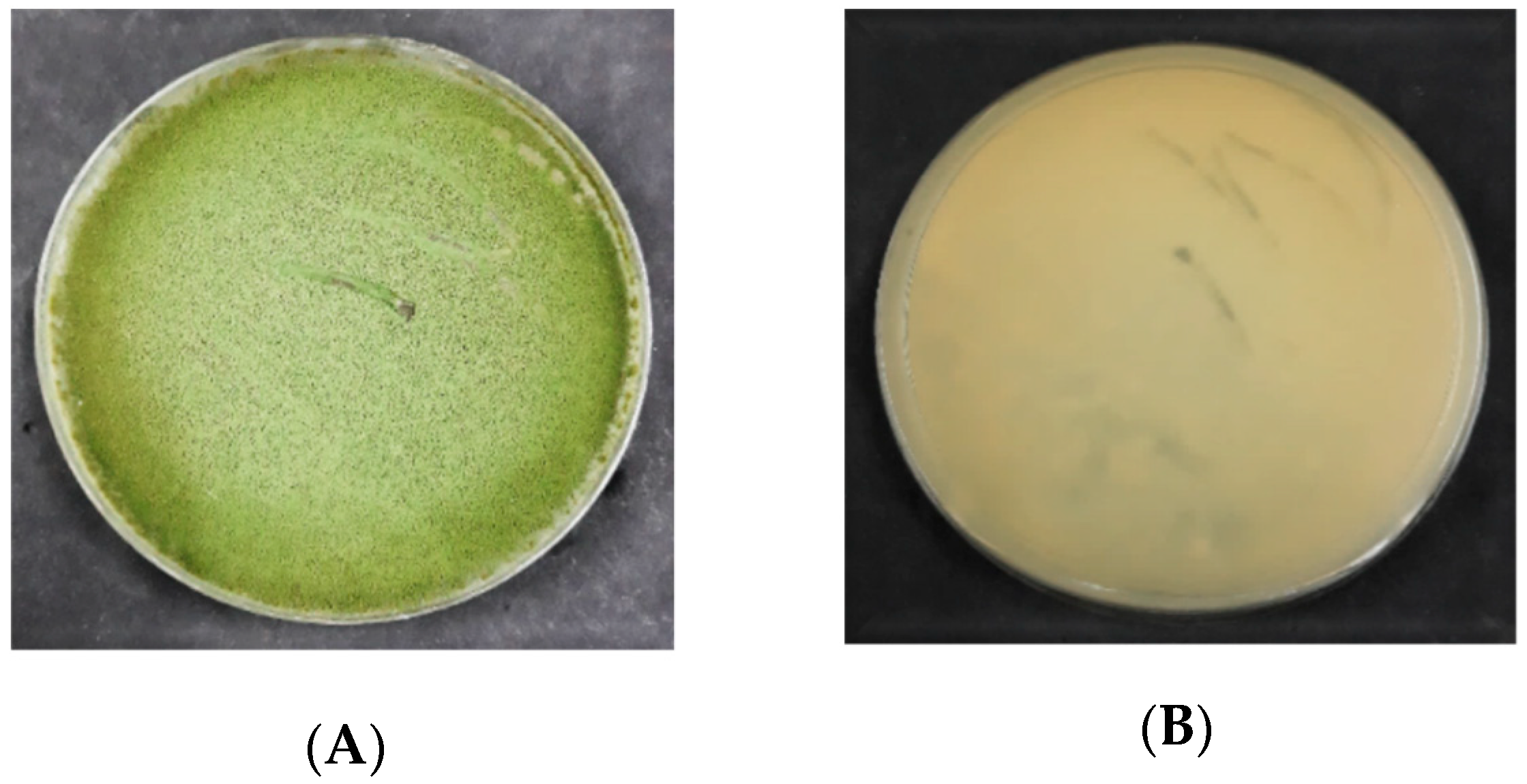
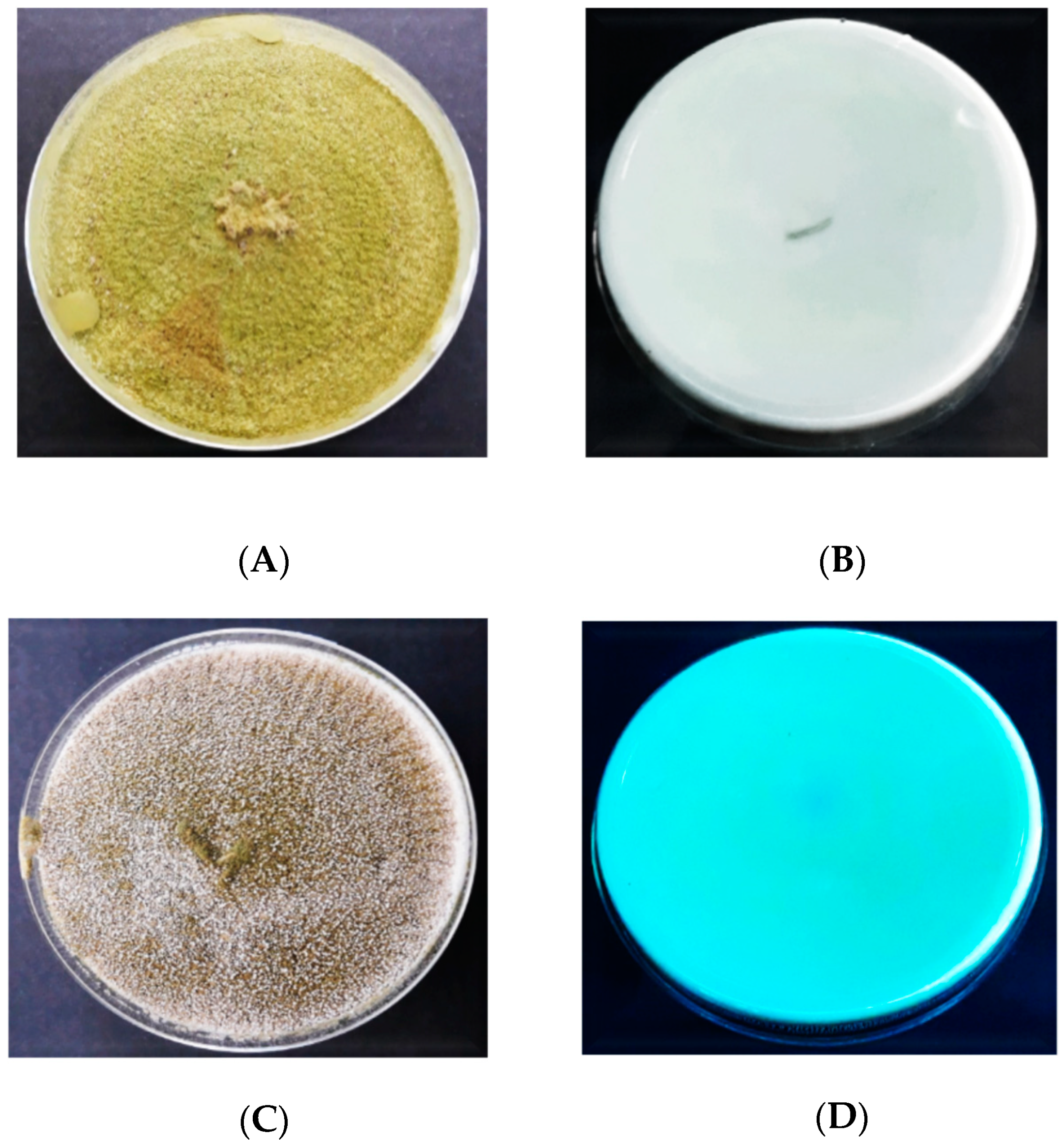
3.2.1. Macroscopic Characteristics of Aspergillus flavus on PDA
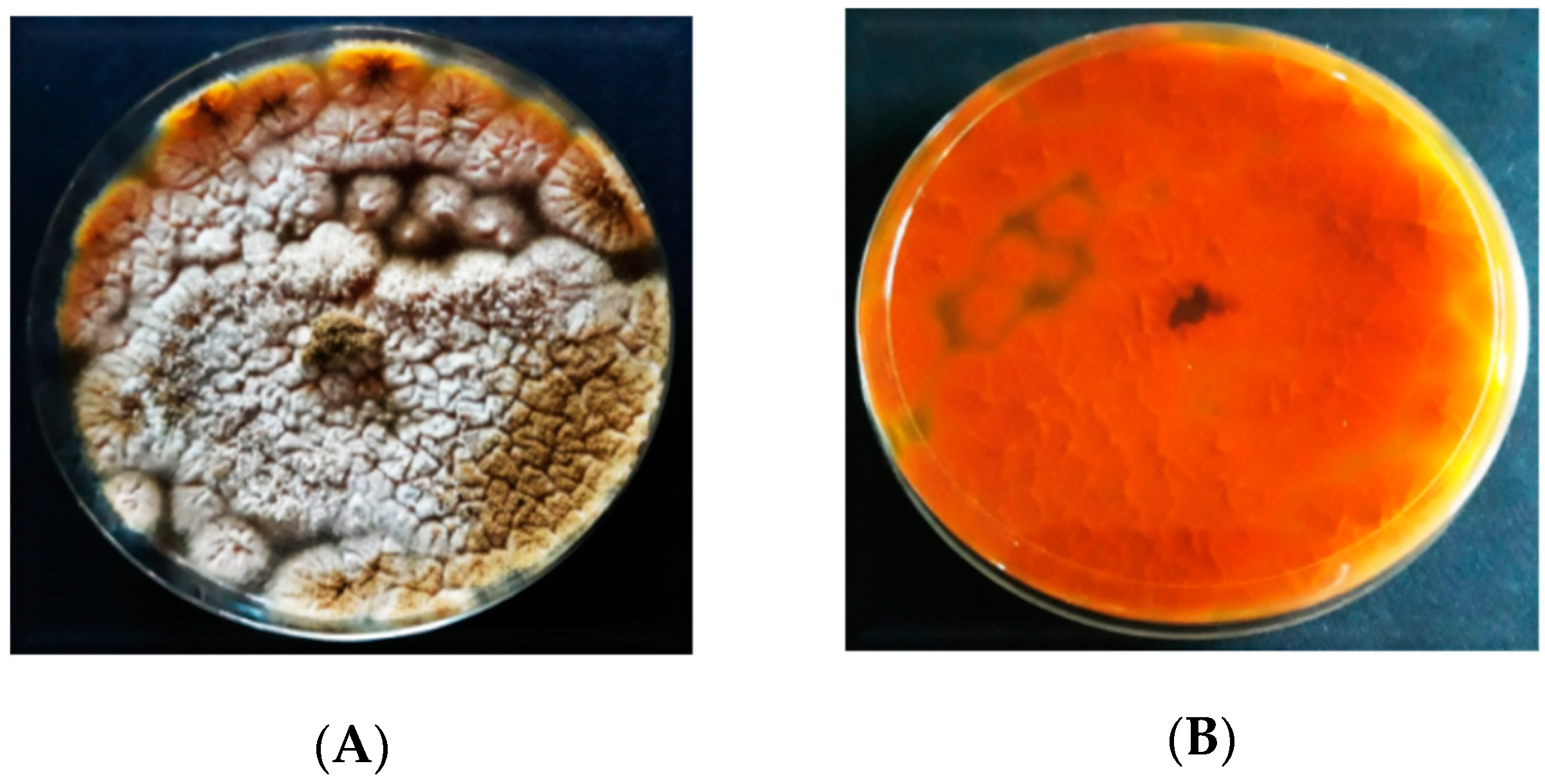
3.2.2. Macroscopic Characteristics of Aspergillus flavus on DRBC
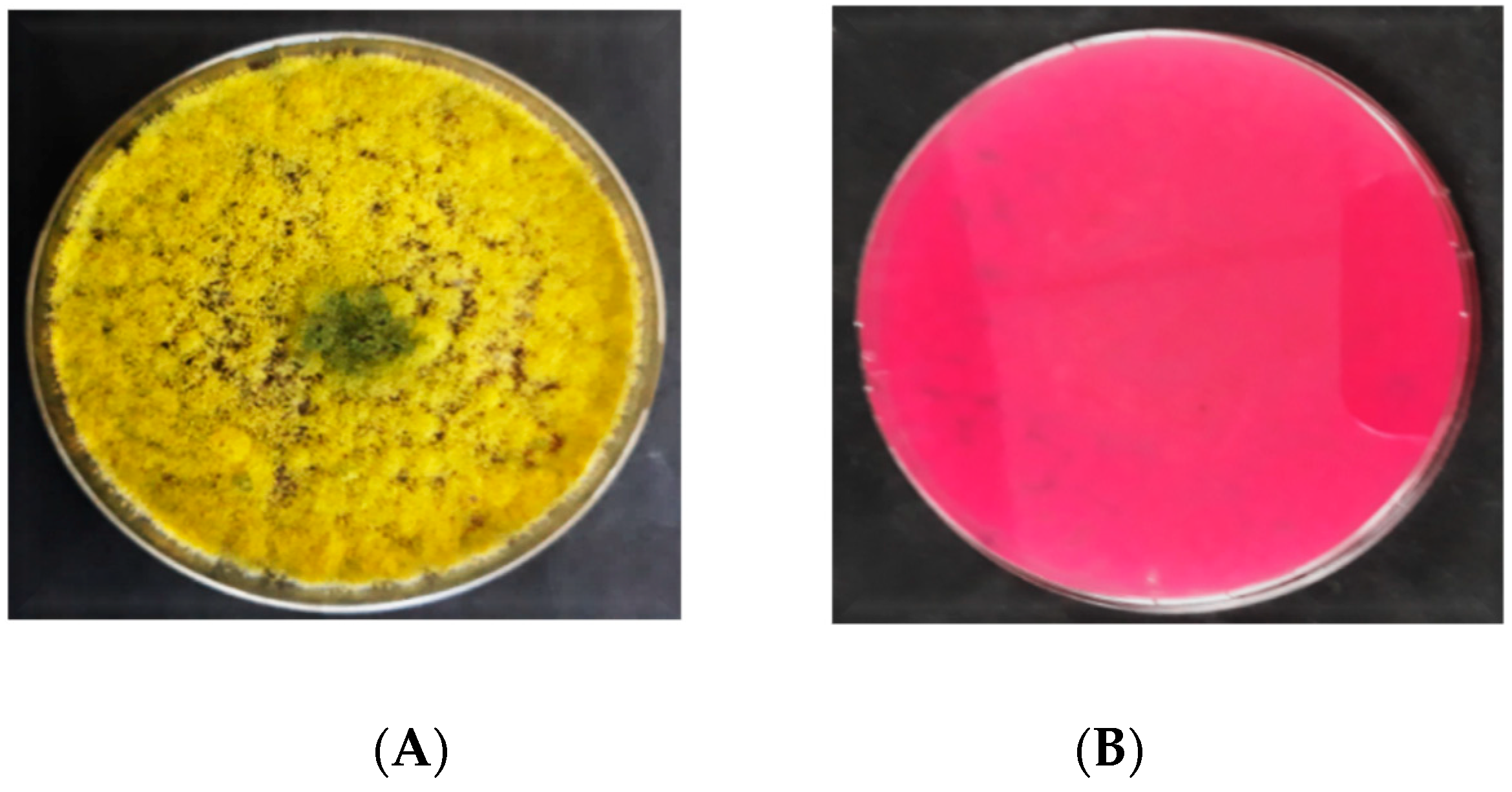
3.2.3. Macroscopic Characteristics of Aspergillus flavus on AFPA
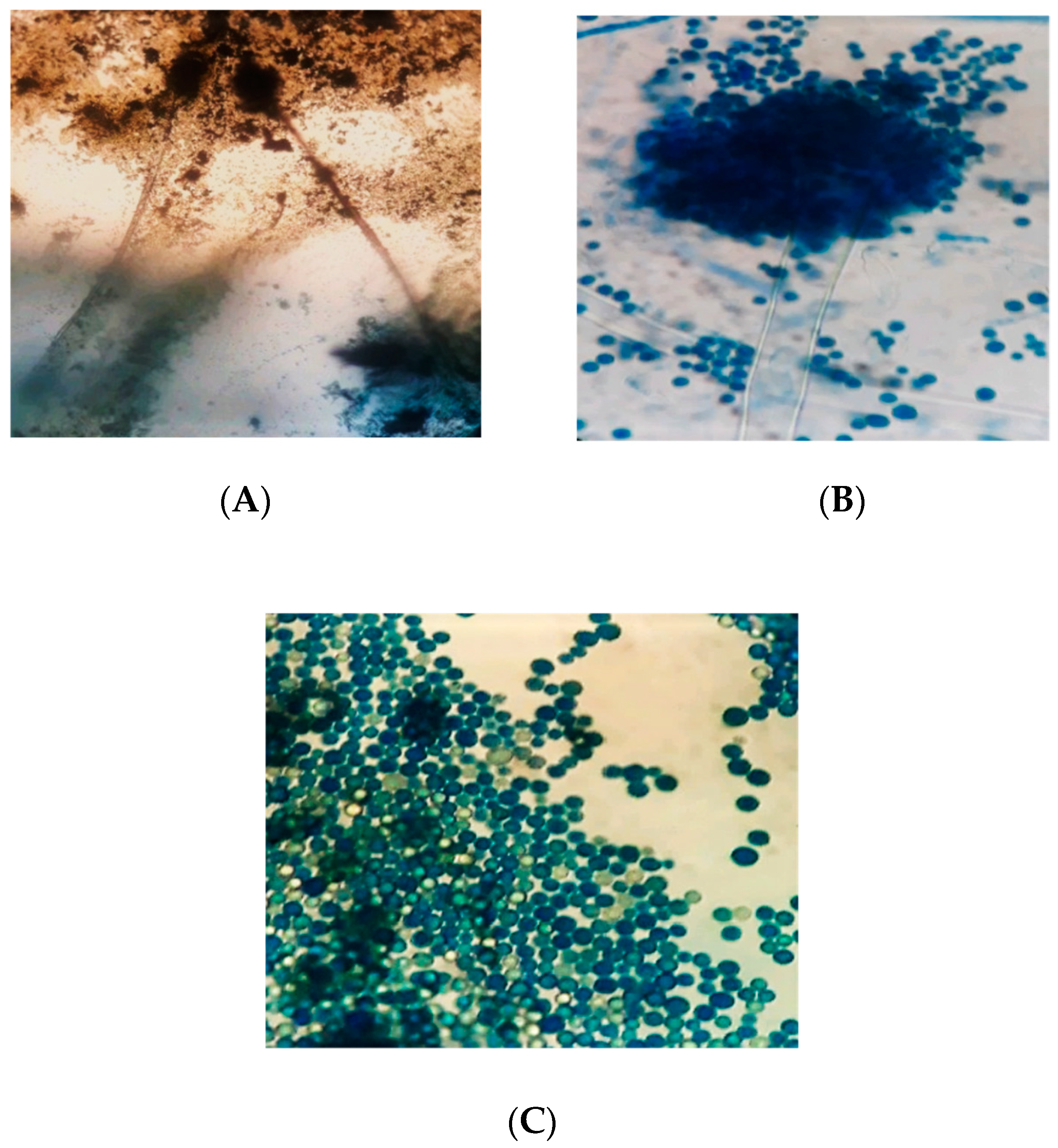
3.3. Microscopic Characteristics of Aspergillus flavus
3.4. Screening for Aflatoxin Production
4. Discussion
4.1. Prevalence of Aspergillus flavus across Sweet Corn Farms in Malaysia
4.2. Morphological Characterization of Aspergillus flavus
4.3. Discrimination between Aflatoxigenic and Non-aflatoxigenic Aspergillus flavus
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- IARC. Some Naturally Occurring Aromatic Amines. Mycotoxins; International Agency for Research on Cancer: Lyon, France, 1993. [Google Scholar]
- Richard, J.L. Some major mycotoxins and their mycotoxicoses-An overview. Int. J. Food Microbiol. 2007, 119, 3–10. [Google Scholar] [CrossRef]
- Afsah-Hejri, L.; Jinap, S.; Arzandeh, S.; Mirhosseini, H. Optimization of HPLC conditions for quantitative analysis of aflatoxins in contaminated peanut. Food Control 2011, 22, 381–388. [Google Scholar] [CrossRef]
- Filazi, A.; Sireli, U.T. Occurrence of aflatoxins in food. In Aflatoxins: Recent Advances and Future Prospects; InTech: Rijeka, Croatia, 2013; pp. 70–143. [Google Scholar]
- Schmidt-Heydt, M.; Rüfer, C.E.; Abdel-Hadi, A.; Magan, N.; Geisen, R. The production of aflatoxin B1 or G1 by Aspergillus parasiticus at various combinations of temperature and water activity is related to the ratio of Afls, and Aflr expression. Mycotoxin Res. 2010, 26, 46–241. [Google Scholar] [CrossRef] [Green Version]
- Alpsoy, L. Inhibitory effect of essential oil on aflatoxin activities. Afr. J. Biotechnol. 2010, 9, 2474–2481. [Google Scholar]
- Giray, B.; Girgin, G.; Engin, A.B.; Aydın, S.; Sahin, G. Aflatoxin levels in wheat samples consumed in some regions of Turkey. Food Control 2007, 18, 23–29. [Google Scholar] [CrossRef]
- Park, J.W.; Choi, S.Y.; Hwang, H.J.; Kim, Y.B. Fungal mycoflora and mycotoxins in Korean polished rice destined for humans. Int. J. Food Microbiol. 2005, 103, 305–314. [Google Scholar] [CrossRef]
- Reddy, K.R.N.; Reddy, C.S.; Muralidharan, K. Detection of Aspergillus spp. and aflatoxin B1 in rice in India. Food Microbiol. 2009, 26, 27–31. [Google Scholar] [CrossRef]
- Toteja, G.S.; Mukherjee, A.; Diwakar, S.; Singh, P.; Saxena, B.N.; Sinha, K.K.; Sinha, A.K.; Kumar, N.; Nagaraja, K.V.; Bai, G.; et al. Aflatoxin B1 contamination of parboiled rice samples collected from different states of India: A multi-centre study. Food Addit. Contam. 2006, 23, 411–414. [Google Scholar] [CrossRef] [PubMed]
- Wagacha, J.M.; Muthomi, J.W. Mycotoxin problem in Africa: Current status, implications to food safety and health and possible management strategies. Int. J. Food Microbiol. 2008, 124, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Lizárraga-Paulín, E.G.; Moreno-Martínez, E.; Miranda-Castro, S.P. Aflatoxins and their impact on human and animal health: An emerging problem. Aflatoxins Biochem. Mol. Biol. 2011, 13, 255–282. [Google Scholar]
- Williams, J.H.; Phillips, T.D.; Jolly, P.E.; Stiles, J.K.; Jolly, C.M.; Aggarwal, D. Human aflatoxicosis in developing countries: A review of toxicology, exposure, potential health consequences, and interventions. Am. J. Clin. Nutr. 2004, 80, 1106–1122. [Google Scholar] [CrossRef] [PubMed]
- Qian, G.S.; Ross, R.K.; Yu, M.C.; Yuan, J.M.; Gao, Y.T.; Henderson, B.E.; Wogan, G.N.; Groopman, J.D. A follow-up study of urinary markers of aflatoxin exposure and liver cancer risk in Shanghai, People’s Republic of China. Cancer Epidemiol. Prev. Biomark. 1994, 3, 3–10. [Google Scholar]
- Ross, R.K.; Yu, M.C.; Henderson, B.E.; Yuan, J.M.; Qian, G.S.; Tu, J.T.; Gao, Y.T.; Wogan, G.N.; Groopman, J.D. Urinary aflatoxin biomarkers and risk of hepatocellular carcinoma. Lancet 1992, 339, 943–946. [Google Scholar] [CrossRef]
- Wang, L.Y.; Hatch, M.; Chen, C.J.; Levin, B.; You, S.L.; Lu, S.N.; Wu, M.H.; Wu, W.P.; Wang, L.W.; Wang, Q.; et al. Aflatoxin exposure and risk of hepatocellular carcinoma in Taiwan. Int. J. Cancer 1996, 67, 620–625. [Google Scholar] [CrossRef]
- Gong, Y.; Hounsa, A.; Egal, S.; Turner, P.C.; Sutcliffe, A.E.; Hall, A.J.; Cardwell, K.; Wild, C.P. Postweaning exposure to aflatoxin results in impaired child growth: A longitudinal study in Benin, West Africa. Environ. Health Perspect. 2004, 112, 1334–1338. [Google Scholar] [CrossRef]
- Shuaib, M.; Ouararhni, K.; Dimitrov, S.; Hamiche, A. HJURP binds CENP-A via a highly conserved N-terminal domain and mediates its deposition at centromeres. Proc. Natl. Acad. Sci. USA 2010, 107, 1349–1354. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Abbas, H.K.; Wilkinson, J.R.; Zablotowicz, R.M.; Accinelli, C.; Abel, C.A.; Bruns, H.A.; Weaver, M.A. Ecology of Aspergillus flavus, regulation of aflatoxin production, and management strategies to reduce aflatoxin contamination of corn. Toxin Rev. 2009, 28, 53–142. [Google Scholar] [CrossRef]
- Amaike, S.; Keller, N.P. Aspergillus flavus. Annu. Rev. Phytopathol. 2011, 49, 33–107. [Google Scholar] [CrossRef]
- Hell, K.; Mutegi, C. Aflatoxin control and prevention strategies in key crops of sub-Saharan Africa. Afr. J. Microbiol. Res. 2011, 5, 66–459. [Google Scholar]
- Yu, J. Current understanding of aflatoxin biosynthesis and future perspective in reducing aflatoxin contamination. Toxins 2012, 4, 57–1024. [Google Scholar] [CrossRef] [Green Version]
- Abdullah, N.; Nawawi, A.; Othman, I. Survey of fungal counts and natural occurrence of aflatoxins in Malaysian starch-based foods. Mycopathologia 1998, 143, 53–58. [Google Scholar] [CrossRef]
- Sulaiman, M.R.; Yee, C.F.; Hamid, A.; Yatim, A.M. The occurrence of aflatoxins in raw shelled peanut samples from three districts of Perak, Malaysia. Electron. J. Environ. Agric. Food Chem. 2007, 6, 2045–2052. [Google Scholar]
- Chan, N.W. Cameron Highlands: Issues and challenges in sustainable development. Malaysian J. Environ. Manage. 2009, 10, 89–114. [Google Scholar]
- Okoth, S.A.; Nyongesa, B.; Joutsjoki, V.; Korhonen, H.; Ayugi, V.; Kang’ethe, E.K. Sclerotia formation and toxin production in large sclerotial Aspergillus flavus isolates from Kenya. Adv. Microbiol. 2016, 6, 1–10. [Google Scholar] [CrossRef] [Green Version]
- Ho, W.C.; Ko, W.H. A simple method for obtaining single-spore isolates of fungi. Bot. Bull. Acad. Sin. 1997, 38, 41–44. [Google Scholar]
- Choi, Y.W.; Hyde, K.D.; Ho, W.H. Single spore isolation of fungi. Fungal Divers. 1999, 3, 29–88. [Google Scholar]
- Singh, K.P.; Kumar, D.; Bandyopadhyay, P. A new technique for single spore isolation of two predacious fungi forming constricting ring. Mycobiology 2004, 32, 197–198. [Google Scholar] [CrossRef]
- Raper, K.B.; Fennell, D.I. Aspergillus terreus group. In The genus Aspergillus; The Williams & Wilkins Co.: Baltimore, MD, USA, 1965; pp. 293–344. [Google Scholar]
- Samson, R.A.; Hoekstra, E.S.; Frisvad, J.C. Introduction to Food-and Airborne Fungi; Centraalbureau voor Schimmelcultures (CBS) Fungal Biodiversity Centre, Royal Netherlands Academy of Arts and Sciences: Utrecht, The Netherlands, 2004; Volume 7, pp. 6–389. [Google Scholar]
- Cotty, P.J. Comparison of Four Media for the Isolation of Aspergillus flavus Group Fungi. Mycopathologia 1994, 125, 62–157. [Google Scholar] [CrossRef] [PubMed]
- Klich, M.A. Identification of Common Aspergillus Species; Centraalbureau voor Schimmelcultures (CBS) Fungal Biodiversity Centre, Royal Netherlands Academy of Arts and Sciences: Utrecht, The Netherlands, 2002; pp. 426–432. [Google Scholar]
- Pitt, J.I.; Hocking, A.D.; Glenn, D.R. An Improved Medium for the Detection of Aspergillus flavus and A. parasiticus. J. Appl. Bacteriol. 1983, 54, 14–109. [Google Scholar] [CrossRef]
- Davis, N.D.; Iyer, S.K.; Diener, U.L. Improved method of screening for aflatoxin with a coconut agar medium. Appl. Environ. Microbiol. 1987, 53, 1593–1595. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fente, C.A.; Ordaz, J.J.; Vazquez, B.I.; Franco, C.M.; Cepeda, A. New additive for culture media for rapid identification of aflatoxin-producing Aspergillus strains. Appl. Environ. Microbiol. 2001, 67, 62–4858. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Clayton, Y.M. Medically Important Fungi: A Guide to Identification. Proc. R. Soc. Med. 1977, 70, 359. [Google Scholar]
- Da Gloria, M.E. Aflatoxin contamination distribution among grains and nuts. In Aflatoxins–Detection, Measurement and Control; Torres-Pacheco, I., Ed.; InTech: Rijeka, Croatia, 2011; pp. 75–90. [Google Scholar]
- Thathana, M.G.; Murage, H.; Abia, A.L.K.; Pillay, M. Morphological characterization and determination of aflatoxin-production potentials of Aspergillus flavus isolated from maize and soil in Kenya. Agriculture 2017, 7, 80. [Google Scholar] [CrossRef] [Green Version]
- Cotty, P.J.; Jaime-Garcia, R. Influences of climate on aflatoxin producing fungi and aflatoxin contamination. Int. J. Food Microbiol. 2007, 119, 109–115. [Google Scholar] [CrossRef]
- Rodrigues, P.; Soares, C.; Kozakiewicz, Z.; Paterson, R.; Lima, N.; Venâncio, A. Identification and characterization of Aspergillus flavus and aflatoxins. In Microbiology Book Series–Communicating, Current Research and Educational Topics and Trends in Applied, Microbiology; Méndez-Vilas, A., Ed.; Formatex: Badajoz, Spain, 2007; Volume 2, pp. 527–534. ISBN 13: 978-84-611-9423-0. [Google Scholar]
- Diba, K.; Kordbacheh, P.; Mirhendi, S.H.; Rezaie, S.; Mahmoudi, M. Identification of Aspergillus Species Using Morphological Characteristics. Pak. J. Med. Sci. 2007, 23, 867–872. [Google Scholar]
- Gao, J.; Liu, Z.; Yu, J. Identification of Aspergillus Section Flavi in Maize in Northeastern China. Mycopathologia 2007, 164, 91–95. [Google Scholar] [CrossRef]
- Mohammadi, A.H.; Banihashemi, Z.; Haghdel, M. Identification and prevalence of aspergillus species in soils of Fars and Kerman provinces of Iran and evaluation of their aflatoxin production. Rostanilha 2009, 23, 49–50. [Google Scholar]
- Afzal, H.; Shazad, S.; Qamar, S.; Nisa, U. morphological identification of Aspergillus species from the soil of Larkana District (Sindh, Pakistan). Asian J. Agric. Biotechnol. 2013, 1, 17–105. [Google Scholar]
- Cotty, P.J.; Mellon, J.E. Ecology of aflatoxin producing fungi and biocontrol of aflatoxin contamination. Mycotoxin Res. 2006, 22, 17–110. [Google Scholar] [CrossRef]
- Abbas, H.K.; Zablotowicz, R.M.; Weaver, M.A.; Horn, B.W.; Xie, W.; Shier, W.T. Cultural methods for aflatoxin detection. J. Toxicol. Toxin Rev. 2004, 23, 295–315. [Google Scholar]
- Baquião, A.C.; De Oliveira, M.M.M.; Reis, T.A.; Zorzete, P.; Diniz, A.D.; Correa, B. Polyphasic approach to the identification of Aspergillus Section Flavi isolated from Brazil Nuts. Food Chem. 2013, 139, 32–1127. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Giorni, P.; Magan, N.; Pietri, A.; Bertuzzi, T.; Battilani, P. Studies on Aspergillus Section Flavi isolated from maize in northern Italy. Int. J. Food Microbiol. 2007, 113, 38–330. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, H.; Kamimura, H.; Ichinoe, M. Distribution of aflatoxin-producing Aspergillus flavus and Aspergillus parasiticus in sugarcane fields in the southernmost islands of Japan. J. Food Prot. 2004, 67, 90–95. [Google Scholar] [CrossRef]
- Pildain, M.B.; Frisvad, J.C.; Vaamonde, G.; Cabral, D.; Varga, J.; Samson, R.A. Two novel aflatoxin-producing Aspergillus species from Argentinean peanuts. Int. J. Syst. Evol. Microbiol. 2008, 58, 35–725. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Varga, J.; Frisvad, J.C.; Samson, R.A. Two new aflatoxin producing species, and an overview of Aspergillus section Flavi. Stud. Mycol. 2011, 69, 57–80. [Google Scholar] [CrossRef]
| Isolate | No. | CCA |
|---|---|---|
| AKR1 | 1 | + |
| AKR2 | 2 | - |
| AKR3 | 3 | + |
| AKR4 | 4 | + |
| AKR5 | 5 | + |
| AKR6 | 6 | - |
| AKR7 | 7 | - |
| AKR8 | 8 | + |
| AKR9 | 9 | + |
| AKR10 | 10 | - |
| AKR11 | 11 | - |
| AKR12 | 12 | - |
| ARV13 | 13 | - |
| ARV14 | 14 | + |
| ARV15 | 15 | - |
| ARV16 | 16 | - |
| ARV17 | 17 | + |
| ARV18 | 18 | + |
| ARV19 | 19 | - |
| ARV20 | 20 | - |
| ARV21 | 21 | - |
| ARV22 | 22 | - |
| AK23 | 23 | + |
| AK24 | 24 | + |
| AK25 | 25 | - |
| AK26 | 26 | - |
| AK27 | 27 | - |
| AK28 | 28 | + |
| AK29 | 29 | - |
| AK30 | 30 | - |
| AKL31 | 31 | + |
| AKL32 | 32 | - |
| AKL33 | 33 | - |
| AKL34 | 34 | + |
| AKL35 | 35 | + |
| AKL36 | 36 | - |
| AKL37 | 37 | + |
| AKL38 | 38 | + |
| AKL39 | 39 | - |
| AKL40 | 40 | + |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Khan, R.; Mohamad Ghazali, F.; Mahyudin, N.A.; Samsudin, N.I.P. Morphological Characterization and Determination of Aflatoxigenic and Non-Aflatoxigenic Aspergillus flavus Isolated from Sweet Corn Kernels and Soil in Malaysia. Agriculture 2020, 10, 450. https://doi.org/10.3390/agriculture10100450
Khan R, Mohamad Ghazali F, Mahyudin NA, Samsudin NIP. Morphological Characterization and Determination of Aflatoxigenic and Non-Aflatoxigenic Aspergillus flavus Isolated from Sweet Corn Kernels and Soil in Malaysia. Agriculture. 2020; 10(10):450. https://doi.org/10.3390/agriculture10100450
Chicago/Turabian StyleKhan, Rahim, Farinazleen Mohamad Ghazali, Nor Ainy Mahyudin, and Nik Iskandar Putra Samsudin. 2020. "Morphological Characterization and Determination of Aflatoxigenic and Non-Aflatoxigenic Aspergillus flavus Isolated from Sweet Corn Kernels and Soil in Malaysia" Agriculture 10, no. 10: 450. https://doi.org/10.3390/agriculture10100450






