Monoclonal and Bispecific Anti-BCMA Antibodies in Multiple Myeloma
Abstract
:1. Introduction
2. Biologic Role of BCMA in MM Cells
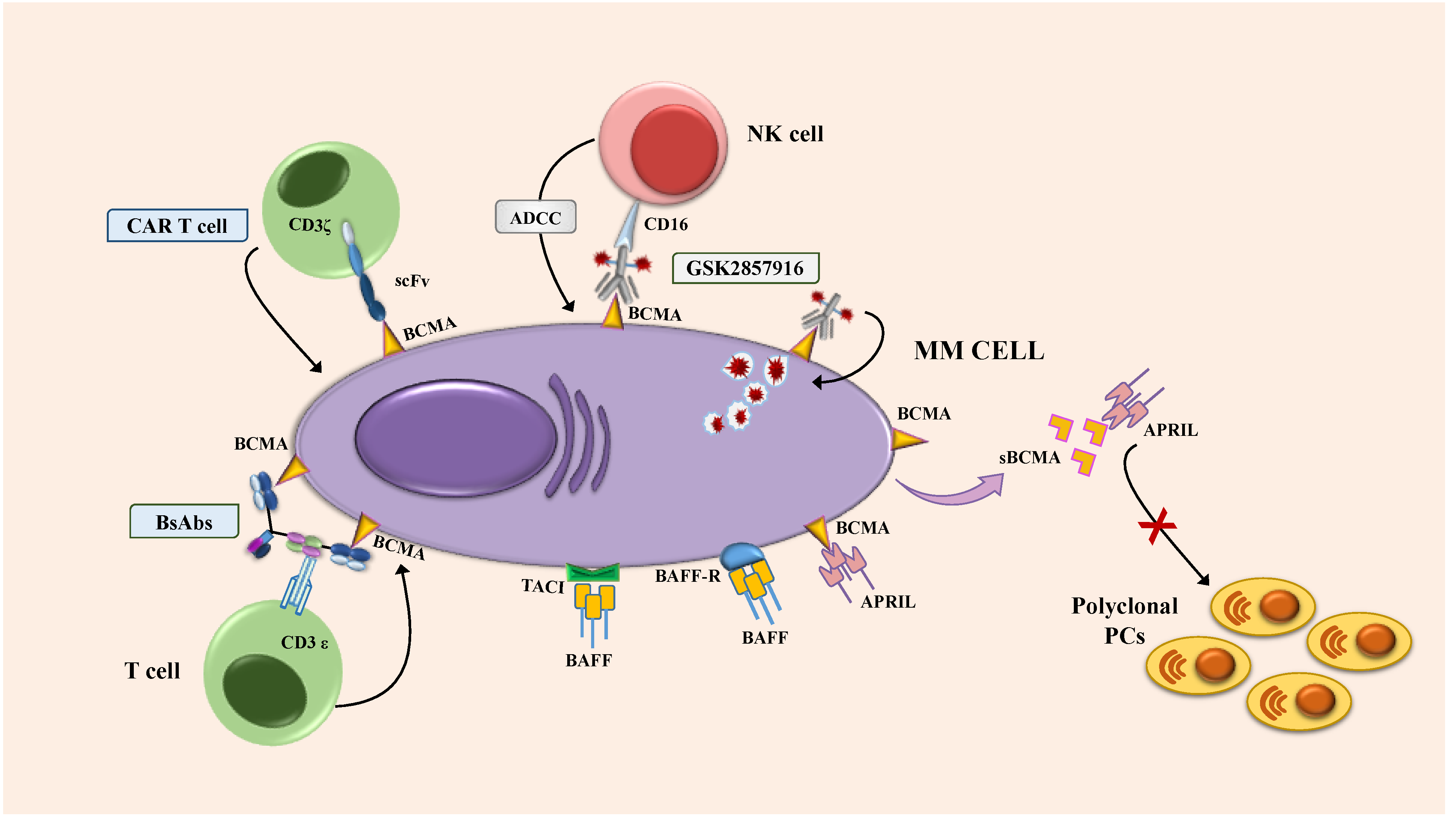
3. BCMA as a Therapeutic Target
4. Investigational Antibody-Based Approach Targeting BCMA
4.1. Antibody-Drug Conjugate: Mechanism and Preclinical Model
4.1.1. Clinical Trials, Patient Population and Efficacy
4.1.2. Toxicity
4.2. Bispecific Antibodies
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Cottini, F.; Anderson, K. Novel therapeutic targets in multiple myeloma. Clin. Adv. Hematol. Oncol. 2015, 13, 236–248. [Google Scholar] [PubMed]
- Neri, P.J.; Bahlis, N. Targeting of adhesion molecules as a therapeutic strategy in multiple myeloma. Curr. Cancer Drug Targets 2012, 12, 776–796. [Google Scholar] [CrossRef] [PubMed]
- Podar, K.; Jager, D. Targeting the immune niche within the bone marrow microenvironment: The rise of immunotherapy in multiple myeloma. Curr. Cancer Drug Targets 2017, 17, 782–805. [Google Scholar] [CrossRef] [PubMed]
- Giuliani, N.; Accardi, F.; Marchica, V.; Dalla Palma, B.; Storti, P.; Toscani, D.; Vicario, E.; Malavasi, F. Novel targets for the treatment of relapsing multiple myeloma. Expert Rev. Hematol. 2019, 12, 481–496. [Google Scholar] [CrossRef]
- Bonello, F.; Mina, R.; Boccadoro, M.; Gay, F. Therapeutic monoclonal antibodies and antibody products: Current practices and development in multiple myeloma. Cancers 2020, 12, 15. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Durer, C.; Durer, S.; Lee, S.; Chakraborty, R.; Malik, M.N.; Rafae, A.; Zar, M.A.; Kamal, A.; Rosko, N.; Samaras, C. Treatment of relapsed multiple myeloma: Evidence-based recommendations. Blood Rev. 2020, 39, 100616. [Google Scholar] [CrossRef] [PubMed]
- Zamagni, E.; Tacchetti, P.; Pantani, L.; Cavo, M. Anti-CD38 and anti-SLAMF7: The future of myeloma immunotherapy. Expert Rev. Hematol. 2018, 11, 423–435. [Google Scholar] [CrossRef]
- Dimopoulos, M.A.; Oriol, A.; Nahi, H.; San-Miguel, J.; Bahlis, N.J.; Usmani, S.Z.; Rabin, N.; Orlowski, R.Z.; Komarnicki, M.; Suzuki, K. Daratumumab, lenalidomide, and dexamethasone for multiple myeloma. N. Engl. J. Med. 2016, 375, 1319–1331. [Google Scholar] [CrossRef] [Green Version]
- Chari, A.; Suvannasankha, A.; Fay, J.W.; Arnulf, B.; Kaufman, J.L.; Ifthikharuddin, J.J.; Weiss, B.M.; Krishnan, A.; Lentzsch, S.; Comenzo, R. Daratumumab plus pomalidomide and dexamethasone in relapsed and/or refractory multiple myeloma. Blood 2017, 130, 974–981. [Google Scholar] [CrossRef]
- Lonial, S.; Dimopoulos, M.; Palumbo, A.; White, D.; Grosicki, S.; Spicka, I.; Walter-Croneck, A.; Moreau, P.; Mateos, M.-V.; Magen, H. Elotuzumab therapy for relapsed or refractory multiple myeloma. N. Engl. J. Med. 2015, 373, 621–631. [Google Scholar] [CrossRef] [Green Version]
- Palumbo, A.; Chanan-Khan, A.; Weisel, K.; Nooka, A.K.; Masszi, T.; Beksac, M.; Spicka, I.; Hungria, V.; Munder, M.; Mateos, M.V. Daratumumab, bortezomib, and dexamethasone for multiple myeloma. N. Engl. J. Med. 2016, 375, 754–766. [Google Scholar] [CrossRef] [PubMed]
- Chari, A.; Martinez-Lopez, J.; Mateos, M.V.; Bladé, J.; Benboubker, L.; Oriol, A.; Arnulf, B.; Rodriguez-Otero, P.; Pineiro, L.; Jakubowiak, A. Daratumumab plus carfilzomib and dexamethasone in patients with relapsed or refractory multiple myeloma. Blood 2019, 134, 421–431. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mateos, M.-V.; Dimopoulos, M.A.; Cavo, M.; Suzuki, K.; Jakubowiak, A.; Knop, S.; Doyen, C.; Lucio, P.; Nagy, Z.; Kaplan, P. Daratumumab plus bortezomib, melphalan, and prednisone for untreated myeloma. N. Engl. J. Med. 2018, 378, 518–528. [Google Scholar] [CrossRef] [PubMed]
- Facon, T.; Kumar, S.; Plesner, T.; Orlowski, R.Z.; Moreau, P.; Bahlis, N.; Basu, S.; Nahi, H.; Hulin, C.; Quach, H. Daratumumab plus lenalidomide and dexamethasone for untreated myeloma. N. Engl. J. Med. 2019, 380, 2104–2115. [Google Scholar] [CrossRef] [PubMed]
- Tai, Y.-T.; Anderson, K.C. B cell maturation antigen (BCMA)-based immunotherapy for multiple myeloma. Expert Opin. Biol. Ther. 2019, 19, 1143–1156. [Google Scholar] [CrossRef] [PubMed]
- Shah, N.; Chari, A.; Scott, E.; Mezzi, K.; Usmani, S.Z. B-cell maturation antigen (BCMA) in multiple myeloma: Rationale for targeting and current therapeutic approaches. Leukemia 2020, 34, 1–21. [Google Scholar] [CrossRef]
- Novak, A.J.; Darce, J.R.; Arendt, B.K.; Harder, B.; Henderson, K.; Kindsvogel, W.; Gross, J.A.; Greipp, P.R.; Jelinek, D.F. Expression of BCMA, TACI, and BAFF-R in multiple myeloma: A mechanism for growth and survival. Blood 2004, 103, 689–694. [Google Scholar] [CrossRef] [Green Version]
- Moreaux, J.; Hose, D.; Jourdan, M.; Reme, T.; Hundemer, M.; Moos, M.; Robert, N.; Moine, P.; De Vos, J.; Goldschmidt, H. TACI expression is associated with a mature bone marrow plasma cell signature and C-MAF overexpression in human myeloma cell lines. Haematologica 2007, 92, 803–811. [Google Scholar] [CrossRef]
- Tai, Y.-T.; Li, X.F.; Breitkreutz, I.; Song, W.; Neri, P.; Catley, L.; Podar, K.; Hideshima, T.; Chauhan, D.; Raje, N. Role of B-cell–activating factor in adhesion and growth of human multiple myeloma cells in the bone marrow microenvironment. Cancer Res. 2006, 66, 6675–6682. [Google Scholar] [CrossRef] [Green Version]
- Moreaux, J.; Sprynski, A.C.; Dillon, S.R.; Mahtouk, K.; Jourdan, M.; Ythier, A.; Moine, P.; Robert, N.; Jourdan, E.; Rossi, J.F. APRIL and TACI interact with syndecan-1 on the surface of multiple myeloma cells to form an essential survival loop. Eur. J. Haematol. 2009, 83, 119–129. [Google Scholar] [CrossRef]
- Mackay, F.; Schneider, P.; Rennert, P.; Browning, J. BAFF AND APRIL: A tutorial on B cell survival. Annu. Rev. Immunol. 2003, 21, 231–264. [Google Scholar] [CrossRef] [PubMed]
- Moreaux, J.; Legouffe, E.; Jourdan, E.; Quittet, P.; Rème, T.; Lugagne, C.; Moine, P.; Rossi, J.-F.; Klein, B.; Tarte, K. BAFF and APRIL protect myeloma cells from apoptosis induced by interleukin 6 deprivation and dexamethasone. Blood 2004, 103, 3148–3157. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jiang, P.; Yueguo, W.; Huiming, H.; Hongxiang, Y.; Mei, W.; Ju, S. B-Lymphocyte stimulator: A new biomarker for multiple myeloma. Eur. J. Haematol. 2009, 82, 267–276. [Google Scholar] [CrossRef] [PubMed]
- Fragioudaki, M.; Tsirakis, G.; Pappa, C.A.; Aristeidou, I.; Tsioutis, C.; Alegakis, A.; Kyriakou, D.S.; Stathopoulos, E.; Alexandrakis, M.G. Serum BAFF levels are related to angiogenesis and prognosis in patients with multiple myeloma. Leuk. Res. 2012, 36, 1004–1008. [Google Scholar] [CrossRef]
- Moreaux, J.; Cremer, F.W.; Reme, T.; Raab, M.; Mahtouk, K.; Kaukel, P.; Pantesco, V.; De Vos, J.; Jourdan, E.; Jauch, A. The level of TACI gene expression in myeloma cells is associated with a signature of microenvironment dependence versus a plasmablastic signature. Blood 2005, 106, 1021–1030. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- O’Connor, B.P.; Raman, V.S.; Erickson, L.D.; Cook, W.J.; Weaver, L.K.; Ahonen, C.; Lin, L.-L.; Mantchev, G.T.; Bram, R.J.; Noelle, R.J. BCMA is essential for the survival of long-lived bone marrow plasma cells. J. Exp. Med. 2004, 199, 91–98. [Google Scholar] [CrossRef]
- Tai, Y.T.; Mayes, P.A.; Acharya, C.; Zhong, M.Y.; Cea, M.; Cagnetta, A.; Craigen, J.; Yates, J.; Gliddon, L.; Fieles, W. Novel anti–B-cell maturation antigen antibody-drug conjugate (GSK2857916) selectively induces killing of multiple myeloma. Blood 2014, 123, 3128–3138. [Google Scholar] [CrossRef] [PubMed]
- Chiu, A.; Xu, W.; He, B.; Dillon, S.R.; Gross, J.A.; Sievers, E.; Qiao, X.; Santini, P.; Hyjek, E.; Lee, J.-w. Hodgkin lymphoma cells express TACI and BCMA receptors and generate survival and proliferation signals in response to BAFF and APRIL. Blood 2007, 109, 729–739. [Google Scholar] [CrossRef]
- Carpenter, R.O.; Evbuomwan, M.O.; Pittaluga, S.; Rose, J.J.; Raffeld, M.; Yang, S.; Gress, R.E.; Hakim, F.T.; Kochenderfer, J.N. B-cell maturation antigen is a promising target for adoptive T-cell therapy of multiple myeloma. Clin. Cancer Res. 2013, 19, 2048–2060. [Google Scholar] [CrossRef] [Green Version]
- Laurent, S.A.; Hoffmann, F.S.; Kuhn, P.-H.; Cheng, Q.; Chu, Y.; Schmidt-Supprian, M.; Hauck, S.M.; Schuh, E.; Krumbholz, M.; Rübsamen, H. γ-Secretase directly sheds the survival receptor BCMA from plasma cells. Nat. Commun. 2015, 6, 1–12. [Google Scholar] [CrossRef]
- Sanchez, E.; Li, M.; Kitto, A.; Li, J.; Wang, C.S.; Kirk, D.T.; Yellin, O.; Nichols, C.M.; Dreyer, M.P.; Ahles, C.P. Serum B-cell maturation antigen is elevated in multiple myeloma and correlates with disease status and survival. Br. J. Haematol. 2012, 158, 727–738. [Google Scholar] [CrossRef] [PubMed]
- Ghermezi, M.; Li, M.; Vardanyan, S.; Harutyunyan, N.M.; Gottlieb, J.; Berenson, A.; Spektor, T.M.; Andreu-Vieyra, C.; Petraki, S.; Sanchez, E. Serum B-cell maturation antigen: A novel biomarker to predict outcomes for multiple myeloma patients. Haematologica 2017, 102, 785–795. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sanchez, E.; Gillespie, A.; Tang, G.; Ferros, M.; Harutyunyan, N.M.; Vardanyan, S.; Gottlieb, J.; Li, M.; Wang, C.S.; Chen, H. Soluble B-cell maturation antigen mediates tumor-induced immune deficiency in multiple myeloma. Clin. Cancer Res. 2016, 22, 3383–3397. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ludwig, H.; Milosavljevic, D.; Berlanga, O.; Zojer, N.; Hübl, W.; Fritz, V.; Harding, S. Suppression of the noninvolved pair of the myeloma isotype correlates with poor survival in newly diagnosed and relapsed/refractory patients with myeloma. Am. J. Hematol. 2016, 91, 295–301. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bellucci, R.; Alyea, E.P.; Chiaretti, S.; Wu, C.J.; Zorn, E.; Weller, E.; Wu, B.; Canning, C.; Schlossman, R.; Munshi, N.C. Graft-versus-tumor response in patients with multiple myeloma is associated with antibody response to BCMA, a plasma-cell membrane receptor. Blood 2005, 105, 3945–3950. [Google Scholar] [CrossRef]
- Paíno, T.; Paiva, B.; Sayagués, J.M.; Mota, I.; Carvalheiro, T.; Corchete, L.; Aires-Mejia, I.; Perez, J.; Sanchez, M.; Barcena, P. Phenotypic identification of subclones in multiple myeloma with different chemoresistant, cytogenetic and clonogenic potential. Leukemia 2015, 29, 1186–1194. [Google Scholar] [CrossRef] [Green Version]
- Brudno, J.N.; Maric, I.; Hartman, S.D.; Rose, J.J.; Wang, M.; Lam, N.; Stetler-Stevenson, M.; Salem, D.; Yuan, C.; Pavletic, S. T cells genetically modified to express an anti–B-cell maturation antigen chimeric antigen receptor cause remissions of poor-prognosis relapsed multiple myeloma. J. Clin. Oncol. 2018, 36, 2267. [Google Scholar] [CrossRef]
- Pont, M.J.; Hill, T.; Cole, G.O.; Abbott, J.J.; Kelliher, J.; Salter, A.I.; Hudecek, M.; Comstock, M.L.; Rajan, A.; Patel, B.K. γ-Secretase inhibition increases efficacy of BCMA-specific chimeric antigen receptor T cells in multiple myeloma. Blood J. Am. Soc. Hematol. 2019, 134, 1585–1597. [Google Scholar] [CrossRef]
- Gagelmann, N.; Riecken, K.; Wolschke, C.; Berger, C.; Ayuk, F.A.; Fehse, B.; Kröger, N. Development of CAR-T cell therapies for multiple myeloma. Leukemia 2020, 130, 1–16. [Google Scholar] [CrossRef]
- Lambert, J.M.; Morris, C.Q. Antibody–drug conjugates (ADCs) for personalized treatment of solid tumors: A review. Adv. Ther. 2017, 34, 1015–1035. [Google Scholar] [CrossRef] [Green Version]
- Ryan, M.C.; Hering, M.; Peckham, D.; McDonagh, C.F.; Brown, L.; Kim, K.M.; Meyer, D.L.; Zabinski, R.F.; Grewal, I.S.; Carter, P.J. Antibody targeting of B-cell maturation antigen on malignant plasma cells. Mol. Cancer Ther. 2007, 6, 3009–3018. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kinneer, K.; Flynn, M.; Thomas, S.B.; Meekin, J.; Varkey, R.; Xiao, X.; Zhong, H.; Breen, S.; Hynes, P.G.; Fleming, R. Preclinical assessment of an antibody–PBD conjugate that targets BCMA on multiple myeloma and myeloma progenitor cells. Leukemia 2019, 33, 766–771. [Google Scholar] [CrossRef] [PubMed]
- Pahl, A.; Lutz, C.; Hechler, T. Amanitins and their development as a payload for antibody-drug conjugates. Drug Discov. Today Technol. 2018, 30, 85–89. [Google Scholar] [CrossRef] [PubMed]
- Singh, R.K.; Jones, R.J.; Hong, S.; Shirazi, F.; Wang, H.; Kuiatse, I.; Pahl, A.; Orlowski, R.Z. HDP101, a novel B-cell maturation antigen (BCMA)-targeted antibody conjugated to α-Amanitin, is active against myeloma with preferential efficacy against pre-clinical models of deletion 17p. Blood 2018, 132, 593. [Google Scholar] [CrossRef]
- Trudel, S.; Lendvai, N.; Popat, R.; Voorhees, P.M.; Reeves, B.; Libby, E.N.; Richardson, P.G.; Anderson, L.D., Jr.; Sutherland, H.J.; Yong, K. Targeting B-cell maturation antigen with GSK2857916 antibody–drug conjugate in relapsed or refractory multiple myeloma (BMA117159): A dose escalation and expansion phase 1 trial. Lancet Oncol. 2018, 19, 1641–1653. [Google Scholar] [CrossRef]
- Trudel, S.; Lendvai, N.; Popat, R.; Voorhees, P.M.; Reeves, B.; Libby, E.N.; Richardson, P.G.; Hoos, A.; Gupta, I.; Bragulat, V. Antibody–drug conjugate, GSK2857916, in relapsed/refractory multiple myeloma: An update on safety and efficacy from dose expansion phase I study. Blood Cancer J. 2019, 9, 1–10. [Google Scholar] [CrossRef] [Green Version]
- Lonial, S.; Lee, H.C.; Badros, A.; Trudel, S.; Nooka, A.K.; Chari, A.; Abdallah, A.-O.; Callander, N.; Lendvai, N.; Sborov, D. Belantamab mafodotin for relapsed or refractory multiple myeloma (DREAMM-2): A two-arm, randomised, open-label, phase 2 study. Lancet Oncol. 2020, 21, 207–221. [Google Scholar] [CrossRef]
- Lonial, S.; Lee, H.C.; Badros, A.; Trudel, S.; Nooka, A.K.; Chari, A.; Abdallah, A.-O.A.; Callander, N.S.; Sborov, D.W.; Suvannasankha, A. Pivotal DREAMM-2 Study: Single-Agent Belantamab Mafodotin (GSK2857916) in Patients With Relapsed/refractory Multiple Myeloma (RRMM) Refractory to Proteasome Inhibitors (PIs), Immunomodulatory Agents, and Refractory and/or Intolerant to Anti-CD38 Monoclonal Antibodies (mAbs); American Society of Clinical Oncology: Alexandria, VA, USA, 2020. [Google Scholar]
- Lonial, S.; Lee, H.C.; Badros, A.; Trudel, S.; Nooka, A.K.; Chari, A.; Abdallah, A.-O.; Callander, N.; Lendvai, N.; Sborov, D.; et al. DREAMM-2: Single-agent belantamab mafodotin in patients with relapsed/refractory multiple myeloma (RRMM)–outcomes by prior therapies. Hema Sphere 2020, 4, 8519. [Google Scholar]
- Nooka, A.K.; Mateos, M.V.; Nizar Bahlis, N.; Katja Weisel, K.; Oriol, A.; Alonso, A.; Suvannasankha, A.; Holkova, B.; Luptakova, K.; Fecteau, D.; et al. DREAMM-4: Evaluating safety and clinical activity of belantamab mafodotin in combination with pembrolizumab in patients with relapsed/ refractory multiple myeloma (RRMM). Hema Sphere 2020, 4, 433–434. [Google Scholar]
- Hipp, S.; Tai, Y.; Blanset, D.; Deegen, P.; Wahl, J.; Thomas, O.; Rattel, B.; Adam, P.; Anderson, K.; Friedrich, M. A novel BCMA/CD3 bispecific T-cell engager for the treatment of multiple myeloma induces selective lysis in vitro and in vivo. Leukemia 2017, 31, 1743–1751. [Google Scholar] [CrossRef]
- Topp, M.S.; Duell, J.; Zugmaier, G.; Attal, M.; Moreau, P.; Langer, C.; Krönke, J.; Facon, T.; Salnikov, A.V.; Lesley, R. Anti–B-cell maturation antigen BiTE molecule AMG 420 induces responses in multiple myeloma. J. Clin. Oncol. 2020, 38, 775–783. [Google Scholar] [CrossRef] [PubMed]
- Zhou, X.; Einsele, H.; Danhof, S. Bispecific Antibodies: A New Era of Treatment for Multiple Myeloma. J. Clin. Med. 2020, 9, 2166. [Google Scholar] [CrossRef] [PubMed]
- Seckinger, A.; Delgado, J.A.; Moser, S.; Moreno, L.; Neuber, B.; Grab, A.; Lipp, S.; Merino, J.; Prosper, F.; Emde, M. Target expression, generation, preclinical activity, and pharmacokinetics of the BCMA-T cell bispecific antibody EM801 for multiple myeloma treatment. Cancer Cell 2017, 31, 396–410. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Costa, L.J.; Wong, S.W.; Bermúdez, A.; de la Rubia, J.; Mateos, M.-V.; Ocio, E.M.; Rodríguez-Otero, P.; San-Miguel, J.; Li, S.; Sarmiento, R. First Clinical Study of the B-Cell Maturation Antigen (BCMA) 2+ 1 T Cell Engager (TCE) CC-93269 in Patients (Pts) with Relapsed/Refractory Multiple Myeloma (RRMM): Interim Results of a Phase 1 Multicenter Trial; American Society of Hematology: Washington, DC, USA, 2019. [Google Scholar]
- Cho, S.F.; Lin, L.; Xing, L.; Wen, K.; Yu, T.; Hsieh, P.A.; Li, Y.; Munshi, N.C.; Wahl, J.; Matthes, K. AMG 701 Potently Induces Anti-Multiple Myeloma (MM) Functions of T Cells and IMiDs Further Enhance Its Efficacy to Prevent MM Relapse in Vivo; American Society of Hematology: Washington, DC, USA, 2019. [Google Scholar]
- Usmani, S.Z.; Mateos, M.-V.; Nahi, H.; Krishnan, A.Y.; van de Donk, N.W.; San Miguel, J.; Oriol, A.; Rosiñol, L.; Chari, A.; Adams, H. Phase I Study of Teclistamab, a Humanized B-cell Maturation Antigen (BCMA) x CD3 Bispecific antibody, in Relapsed/Refractory Multiple Myeloma (R/R MM); American Society of Clinical Oncology: Washington, DC, USA, 2020. [Google Scholar]
| TRIAL NAME | NCT Number | PHASE | Number of Patients (Treatment Arm) | Previous Lines of Treatment (Median) | Previous Drugs | Combinatio n with Other Drugs | Efficacy | Aes (Grade 3–4) | Reference |
|---|---|---|---|---|---|---|---|---|---|
| BMA117159 | NCT02064387 | I | 35 (part 2) | 1–≥10 | IMIDs PIs Alkilators | / | ORR 60% Median PFS 12 Months | Corneal Events (9%), Thrombocytopenia (26%), Anaemia (14%) | Trudel S, Blood Cancer Journal (2019) 9:37 |
| DREAMM-2 | NCT03525678 | II | 196 | 3–21 (7) | IMIDs PIs Anti-CD38 | / | ORR: 31% (2.5 mg/kg), 34% (3.4 mg/kg)Median PFS: 2.8 (2.5 mg/kg), 3.9 Months (3.4 mg/kg) | Kerathopathy (27% and 21%) Thrombocytopenia (20% and 33%) Anemia (20% And 25%) | Lonial S, Lancet Oncol 2020; 21:207–221 |
| DREAMM-4 | NCT03848845 | I/II | 13 (part 1) | 3–13 (7.5)–2.5 mg/kg cohort 3–8 (5)–3.4 mg/kg cohort | IMIDs PIs Anti-CD38 | Pembrolizumab | ORR: 67% (2.5 mg/kg Cohort), 14% (3.4 mg/kg Cohort) | Kerathopathy (33% and 57%) | Nooka AJ, EHA2020, Abs EP955 |
| DRUG NAME | TRIAL NAME | NCT Number | PHASE | Setting | Combination with Other Drugs | Comparator Arm |
|---|---|---|---|---|---|---|
| GSK2857916 | BMA117159 | NCT02064387 | I | RRMM | / | / |
| DREAMM-2 | NCT03525678 | II | RRMM | / | / | |
| DREAMM-3 | NCT04162210 | III | RRMM | / | Pomalidomide/Dexamethasone | |
| DREAMM-4 | NCT03848845 | I/II | RRMM | Pembrolizumab | / | |
| DREAMM-5 | NCT04126200 | I/II | RRMM | GSK3174998 GSK3359609 Nirogacestat | / | |
| DREAMM-6 | NCT03544281 | I/II | RRMM | Lenalidomide/Dexamethasone Bortezomib/Dexamethasone | / | |
| DREAMM-7 | NCT04246047 | III | RRMM | Bortezomib/Dexamethasone | Daratumumab/Bortezomib/Dexamethasone | |
| DREAMM-8 | NCT04484623 | III | RRMM | Pomalidomide/Dexamethasone | Pomalidomide/Bortezomib/dexamethasone | |
| MCRN 007 | NCT03715478 | I/II | RRMM | Pomalidomide/Dexamethasone | / | |
| 209664 | NCT04091126 | I | NDMM | Bortezomib/Lenalidomide/Dexamethasone | / | |
| MEDI2228 | D7900C00001 | NCT03489525 | I | RRMM | / | / |
| CC99712 | CC-99712-MM-001 | NCT04036461 | I | RRMM | / | / |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dalla Palma, B.; Marchica, V.; Catarozzo, M.T.; Giuliani, N.; Accardi, F. Monoclonal and Bispecific Anti-BCMA Antibodies in Multiple Myeloma. J. Clin. Med. 2020, 9, 3022. https://doi.org/10.3390/jcm9093022
Dalla Palma B, Marchica V, Catarozzo MT, Giuliani N, Accardi F. Monoclonal and Bispecific Anti-BCMA Antibodies in Multiple Myeloma. Journal of Clinical Medicine. 2020; 9(9):3022. https://doi.org/10.3390/jcm9093022
Chicago/Turabian StyleDalla Palma, Benedetta, Valentina Marchica, Maria Teresa Catarozzo, Nicola Giuliani, and Fabrizio Accardi. 2020. "Monoclonal and Bispecific Anti-BCMA Antibodies in Multiple Myeloma" Journal of Clinical Medicine 9, no. 9: 3022. https://doi.org/10.3390/jcm9093022





