The Evolving Role of Natural Compounds in the Medical Treatment of Uterine Fibroids
Abstract
:1. Introduction
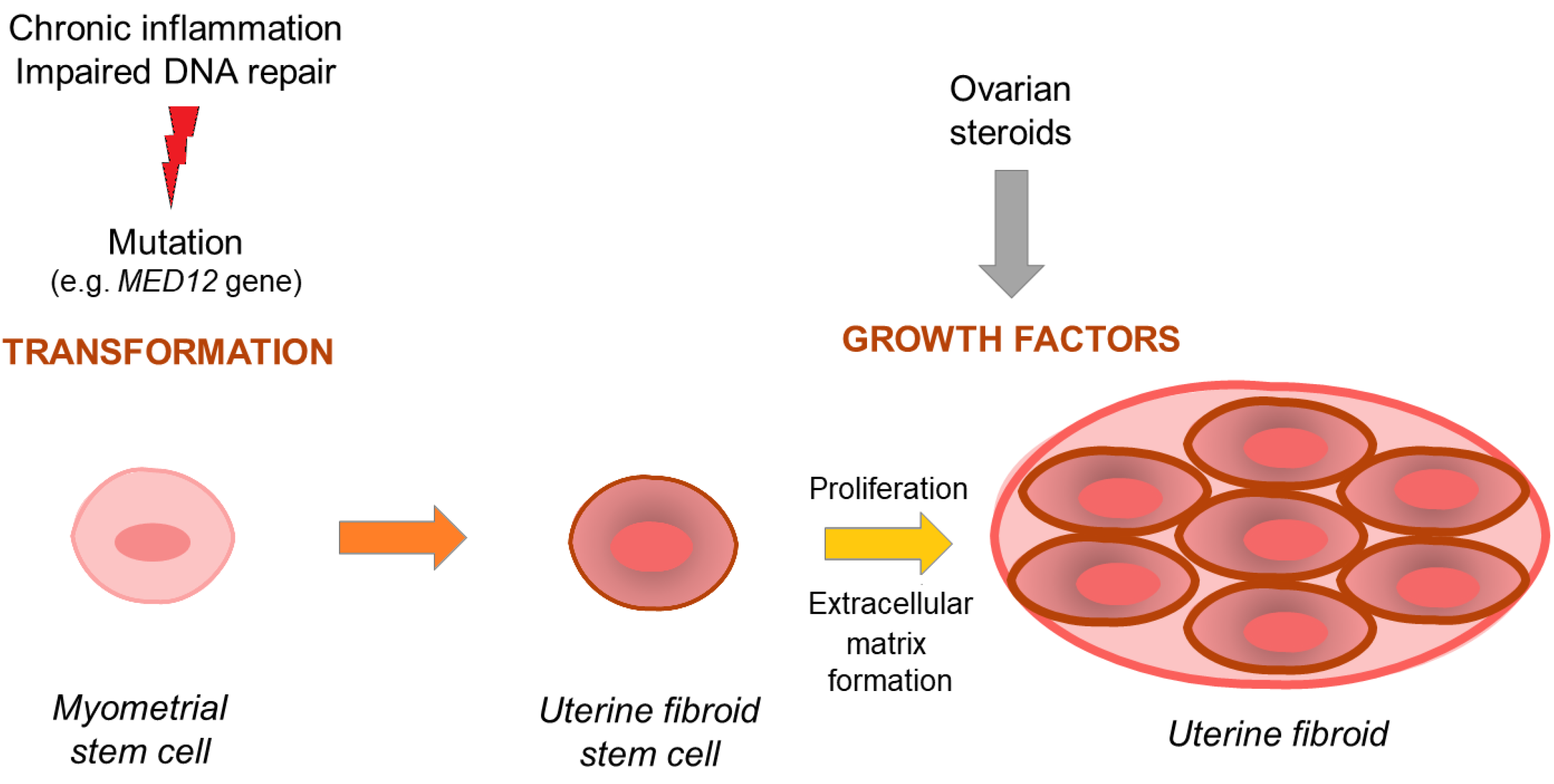
1.1. Uterine Fibroid Pathogenesis—Overview
1.2. Uterine Fibroid Treatment Challenges
2. Materials and Methods
3. Discussion
3.1. Natural Compounds
3.1.1. Vitamin D
3.1.2. Epigallocatechin Gallate (EGCG)
3.1.3. Berberine
3.1.4. Curcumin
3.1.5. Resveratrol
3.1.6. Fucoidans
3.1.7. Indole-3-Carbinol
3.1.8. Isoliquiritigenin
3.1.9. Quercetin
3.1.10. Sulforaphane
3.1.11. Anthocyanins
3.1.12. Omega-3 Fatty Acids
3.1.13. Methyl Jasmonate
3.1.14. Lycopene
3.1.15. Collagenase Clostridium histolyticum
3.2. Bioavailability of Natural Compounds
3.3. Clinical Application of Natural Compounds in UFs Management
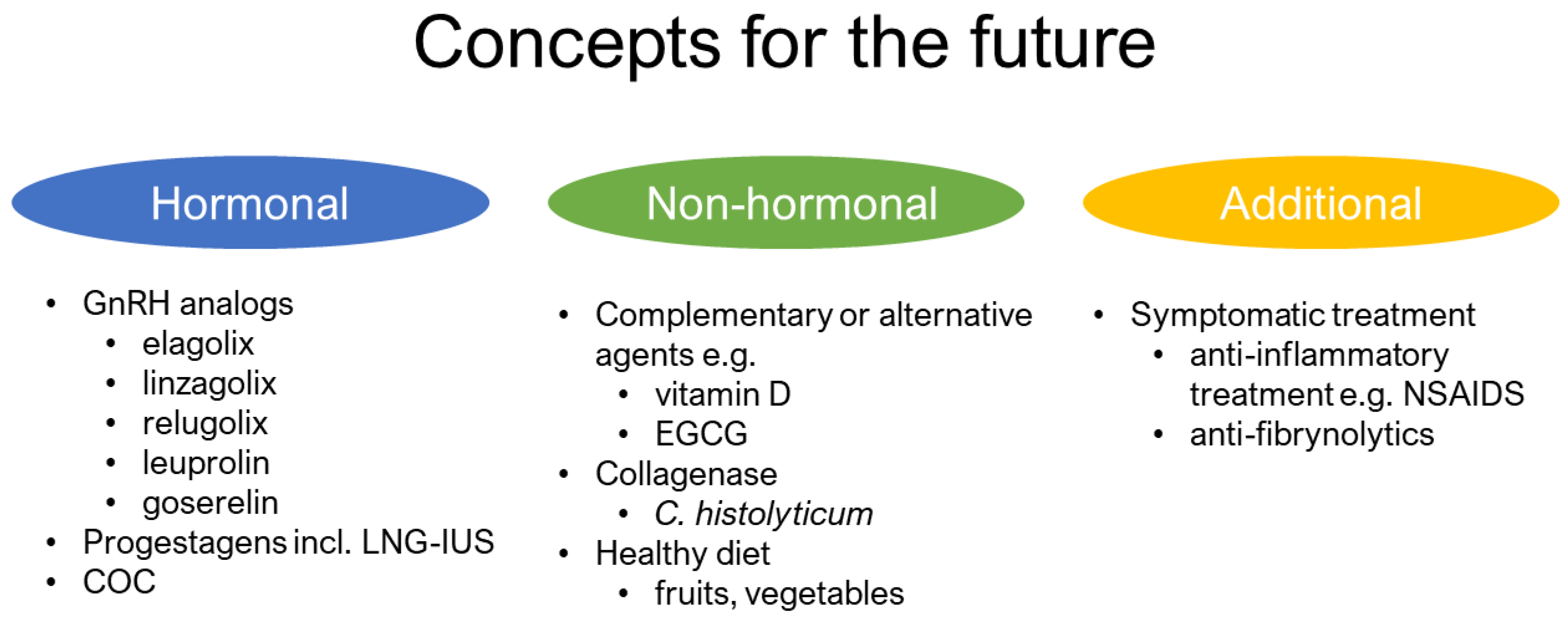
3.4. Future Direction
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Stewart, E.A.; Cookson, C.L.; Gandolfo, R.A.; Schulze-Rath, R. Epidemiology of uterine fibroids: A systematic review. BJOG 2017, 124, 1501–1512. [Google Scholar] [CrossRef] [PubMed]
- Wise, L.A.; Laughlin-Tommaso, S.K. Epidemiology of uterine fibroids: From menarche to menopause. Clin. Obstet. Gynecol. 2016, 59, 2–24. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stewart, E.A.; Laughlin-Tommaso, S.K.; Catherino, W.H.; Lalitkumar, S.; Gupta, D.; Vollenhoven, B. Uterine fibroids. Nat. Rev. Dis. Primers 2016, 2, 16043. [Google Scholar] [CrossRef] [PubMed]
- Murji, A.; Bedaiwy, M.; Singh, S.S.; Bougie, O.; Committee, C.R.S. Influence of ethnicity on clinical presentation and quality of life in women with uterine fibroids: Results from a prospective observational registry. J. Obstet. Gynaecol. Can. 2019. [Google Scholar] [CrossRef]
- Herve, F.; Katty, A.; Isabelle, Q.; Celine, S. Impact of uterine fibroids on quality of life: A national cross-sectional survey. Eur. J. Obstet. Gynecol. Reprod. Biol. 2018, 229, 32–37. [Google Scholar] [CrossRef]
- Merrill, R.M. Hysterectomy surveillance in the United States, 1997 through 2005. Med. Sci Monit 2008, 14, CR24–CR31. [Google Scholar]
- Al-Hendy, A.; Myers, E.R.; Stewart, E. Uterine fibroids: Burden and unmet medical need. Semin. Reprod. Med. 2017, 35, 473–480. [Google Scholar] [CrossRef] [Green Version]
- Harrington, A.; Bonine, N.G.; Banks, E.; Shih, V.; Stafkey-Mailey, D.; Fuldeore, R.M.; Yue, B.; Ye, J.M.; Ta, J.T.; Gillard, P. Direct costs incurred among women undergoing surgical procedures to treat uterine fibroids. J. Manag. Care Spec. Pharm. 2020, 26, S2–S10. [Google Scholar] [CrossRef]
- Cardozo, E.R.; Clark, A.D.; Banks, N.K.; Henne, M.B.; Stegmann, B.J.; Segars, J.H. The estimated annual cost of uterine leiomyomata in the United States. Am. J. Obstet. Gynecol. 2012, 206, e211–e219. [Google Scholar] [CrossRef] [Green Version]
- Soliman, A.M.; Yang, H.; Du, E.X.; Kelkar, S.S.; Winkel, C. The direct and indirect costs of uterine fibroid tumors: A systematic review of the literature between 2000 and 2013. Am. J. Obstet. Gynecol. 2015, 213, 141–160. [Google Scholar] [CrossRef]
- Ciebiera, M.; Lukaszuk, K.; Meczekalski, B.; Ciebiera, M.; Wojtyla, C.; Slabuszewska-Jozwiak, A.; Jakiel, G. Alternative oral agents in prophylaxis and therapy of uterine fibroids-an up-to-date review. Int J. Mol. Sci. 2017, 18. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mahase, E. Uterine fibroid drug is recalled after case of liver failure requiring transplant prompts EU review. BMJ 2020, 368, m1112. [Google Scholar] [CrossRef] [PubMed]
- Ulin, M.; Ali, M.; Chaudhry, Z.T.; Al-Hendy, A.; Yang, Q. Uterine fibroids in menopause and perimenopause. Menopause 2020, 27, 238–242. [Google Scholar] [CrossRef] [PubMed]
- Yang, Q.; Diamond, M.P.; Al-Hendy, A. Early life adverse environmental exposures increase the risk of uterine fibroid development: Role of epigenetic regulation. Front. Pharmacol. 2016, 7, 40. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Protic, O.; Toti, P.; Islam, M.S.; Occhini, R.; Giannubilo, S.R.; Catherino, W.H.; Cinti, S.; Petraglia, F.; Ciavattini, A.; Castellucci, M.; et al. Possible involvement of inflammatory/reparative processes in the development of uterine fibroids. Cell Tissue Res. 2016, 364, 415–427. [Google Scholar] [CrossRef] [PubMed]
- Elhusseini, H.; Elkafas, H.; Abdelaziz, M.; Halder, S.; Atabiekov, I.; Eziba, N.; Ismail, N.; El Andaloussi, A.; Al-Hendy, A. Diet-induced vitamin D deficiency triggers inflammation and DNA damage profile in murine myometrium. Int. J. Womens Health 2018, 10, 503–514. [Google Scholar] [CrossRef] [Green Version]
- Makinen, N.; Mehine, M.; Tolvanen, J.; Kaasinen, E.; Li, Y.; Lehtonen, H.J.; Gentile, M.; Yan, J.; Enge, M.; Taipale, M.; et al. MED12, the mediator complex subunit 12 gene, is mutated at high frequency in uterine leiomyomas. Science 2011, 334, 252–255. [Google Scholar] [CrossRef]
- Bulun, S.E. Uterine fibroids. N Engl. J. Med. 2013, 369, 1344–1355. [Google Scholar] [CrossRef] [Green Version]
- Halder, S.K.; Laknaur, A.; Miller, J.; Layman, L.C.; Diamond, M.; Al-Hendy, A. Novel MED12 gene somatic mutations in women from the southern united states with symptomatic uterine fibroids. Mol. Genet. Genomics 2015, 290, 505–511. [Google Scholar] [CrossRef]
- McWilliams, M.M.; Chennathukuzhi, V.M. Recent advances in uterine fibroid etiology. Semin. Reprod. Med. 2017, 35, 181–189. [Google Scholar] [CrossRef] [Green Version]
- Islam, M.S.; Ciavattini, A.; Petraglia, F.; Castellucci, M.; Ciarmela, P. Extracellular matrix in uterine leiomyoma pathogenesis: A potential target for future therapeutics. Hum. Reprod. Update 2018, 24, 59–85. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ciebiera, M.; Wlodarczyk, M.; Wrzosek, M.; Meczekalski, B.; Nowicka, G.; Lukaszuk, K.; Ciebiera, M.; Slabuszewska-Jozwiak, A.; Jakiel, G. Role of transforming growth factor beta in uterine fibroid biology. Int. J. Mol. Sci. 2017, 18. [Google Scholar] [CrossRef] [Green Version]
- Sozen, I.; Arici, A. Interactions of cytokines, growth factors, and the extracellular matrix in the cellular biology of uterine leiomyomata. Fertil. Steril. 2002, 78, 1–12. [Google Scholar] [CrossRef]
- Ishikawa, H.; Ishi, K.; Serna, V.A.; Kakazu, R.; Bulun, S.E.; Kurita, T. Progesterone is essential for maintenance and growth of uterine leiomyoma. Endocrinology 2010, 151, 2433–2442. [Google Scholar] [CrossRef] [Green Version]
- Borahay, M.A.; Al-Hendy, A.; Kilic, G.S.; Boehning, D. Signaling pathways in leiomyoma: Understanding pathobiology and implications for therapy. Mol. Med. 2015, 21, 242–256. [Google Scholar] [CrossRef]
- Ciarmela, P.; Islam, M.S.; Reis, F.M.; Gray, P.C.; Bloise, E.; Petraglia, F.; Vale, W.; Castellucci, M. Growth factors and myometrium: Biological effects in uterine fibroid and possible clinical implications. Hum. Reprod. Update 2011, 17, 772–790. [Google Scholar] [CrossRef] [Green Version]
- Curtis, S.W.; Washburn, T.; Sewall, C.; Di Augustine, R.; Lindzey, J.; Couse, J.F.; Korach, K.S. Physiological coupling of growth factor and steroid receptor signaling pathways: Estrogen receptor knockout mice lack estrogen-like response to epidermal growth factor. Proc. Natl Acad Sci USA 1996, 93, 12626–12630. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nilsson, S.; Makela, S.; Treuter, E.; Tujague, M.; Thomsen, J.; Andersson, G.; Enmark, E.; Pettersson, K.; Warner, M.; Gustafsson, J.A. Mechanisms of estrogen action. Physiol. Rev. 2001, 81, 1535–1565. [Google Scholar] [CrossRef] [PubMed]
- Andersen, J.; DyReyes, V.M.; Barbieri, R.L.; Coachman, D.M.; Miksicek, R.J. Leiomyoma primary cultures have elevated transcriptional response to estrogen compared with autologous myometrial cultures. J. Soc. Gynecol. Investig. 1995, 2, 542–551. [Google Scholar] [CrossRef]
- Wei, J.; Chiriboga, L.; Mizuguchi, M.; Yee, H.; Mittal, K. Expression profile of tuberin and some potential tumorigenic factors in 60 patients with uterine leiomyomata. Mod. Pathol. 2005, 18, 179–188. [Google Scholar] [CrossRef] [Green Version]
- Al-Hendy, A.; Diamond, M.P.; El-Sohemy, A.; Halder, S.K. 1,25-dihydroxyvitamin D3 regulates expression of sex steroid receptors in human uterine fibroid cells. J. Clin. Endocrinol. Metab. 2015, 100, E572–E582. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hassan, M.H.; Salama, S.A.; Arafa, H.M.; Hamada, F.M.; Al-Hendy, A. Adenovirus-mediated delivery of a dominant-negative estrogen receptor gene in uterine leiomyoma cells abrogates estrogen- and progesterone-regulated gene expression. J. Clin. Endocrinol. Metab. 2007, 92, 3949–3957. [Google Scholar] [CrossRef] [Green Version]
- Hassan, M.H.; Salama, S.A.; Zhang, D.; Arafa, H.M.; Hamada, F.M.; Fouad, H.; Walker, C.C.; Al-Hendy, A. Gene therapy targeting leiomyoma: Adenovirus-mediated delivery of dominant-negative estrogen receptor gene shrinks uterine tumors in Eker rat model. Fertil. Steril. 2010, 93, 239–250. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, J.J.; Kurita, T.; Bulun, S.E. Progesterone action in endometrial cancer, endometriosis, uterine fibroids, and breast cancer. Endocr. Rev. 2013, 34, 130–162. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stewart, E.A.; Friedman, A.J.; Peck, K.; Nowak, R.A. Relative overexpression of collagen type I and collagen type III messenger ribonucleic acids by uterine leiomyomas during the proliferative phase of the menstrual cycle. J. Clin. Endocrinol. Metab. 1994, 79, 900–906. [Google Scholar] [CrossRef]
- Leivonen, S.K.; Lazaridis, K.; Decock, J.; Chantry, A.; Edwards, D.R.; Kahari, V.M. Tgf-beta-elicited induction of tissue inhibitor of metalloproteinases (TIMP)-3 expression in fibroblasts involves complex interplay between smad3, p38alpha, and erk1/2. PLoS ONE 2013, 8, e57474. [Google Scholar] [CrossRef] [Green Version]
- Arici, A.; Sozen, I. Transforming growth factor-beta3 is expressed at high levels in leiomyoma where it stimulates fibronectin expression and cell proliferation. Fertil. Steril. 2000, 73, 1006–1011. [Google Scholar] [CrossRef]
- Islam, M.S.; Catherino, W.H.; Protic, O.; Janjusevic, M.; Gray, P.C.; Giannubilo, S.R.; Ciavattini, A.; Lamanna, P.; Tranquilli, A.L.; Petraglia, F.; et al. Role of activin-A and myostatin and their signaling pathway in human myometrial and leiomyoma cell function. J. Clin. Endocrinol Metab. 2014, 99, E775–E785. [Google Scholar] [CrossRef] [Green Version]
- Protic, O.; Islam, M.S.; Greco, S.; Giannubilo, S.R.; Lamanna, P.; Petraglia, F.; Ciavattini, A.; Castellucci, M.; Hinz, B.; Ciarmela, P. Activin A in inflammation, tissue repair, and fibrosis: Possible role as inflammatory and fibrotic mediator of uterine fibroid development and growth. Semin. Reprod. Med. 2017, 35, 499–509. [Google Scholar] [CrossRef]
- Tanwar, P.S.; Lee, H.J.; Zhang, L.; Zukerberg, L.R.; Taketo, M.M.; Rueda, B.R.; Teixeira, J.M. Constitutive activation of beta-catenin in uterine stroma and smooth muscle leads to the development of mesenchymal tumors in mice. Biol. Reprod. 2009, 81, 545–552. [Google Scholar] [CrossRef] [Green Version]
- Ko, Y.A.; Jamaluddin, M.F.B.; Adebayo, M.; Bajwa, P.; Scott, R.J.; Dharmarajan, A.M.; Nahar, P.; Tanwar, P.S. Extracellular matrix (ECM) activates beta-catenin signaling in uterine fibroids. Reproduction 2018, 155, 61–71. [Google Scholar] [CrossRef] [PubMed]
- Ono, M.; Yin, P.; Navarro, A.; Moravek, M.B.; Coon, J.S.T.; Druschitz, S.A.; Serna, V.A.; Qiang, W.; Brooks, D.C.; Malpani, S.S.; et al. Paracrine activation of Wnt/beta-catenin pathway in uterine leiomyoma stem cells promotes tumor growth. Proc. Natl Acad Sci USA 2013, 110, 17053–17058. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ono, M.; Yin, P.; Navarro, A.; Moravek, M.B.; Coon, V.J.; Druschitz, S.A.; Gottardi, C.J.; Bulun, S.E. Inhibition of canonical Wnt signaling attenuates human leiomyoma cell growth. Fertil. Steril. 2014, 101, 1441–1449. [Google Scholar] [CrossRef] [PubMed]
- Al-Hendy, A.; Laknaur, A.; Diamond, M.P.; Ismail, N.; Boyer, T.G.; Halder, S.K. Silencing MED12 gene reduces proliferation of human leiomyoma cells mediated via Wnt/beta-catenin signaling pathway. Endocrinology 2017, 158, 592–603. [Google Scholar] [CrossRef] [PubMed]
- El Andaloussi, A.; Al-Hendy, A.; Ismail, N.; Boyer, T.G.; Halder, S.K. Introduction of somatic mutation in MED12 induces Wnt4/beta-catenin and disrupts autophagy in human uterine myometrial cell. Reprod. Sci. 2020, 27, 823–832. [Google Scholar] [CrossRef] [PubMed]
- Ali, M.; Shahin, S.M.; Sabri, N.A.; Al-Hendy, A.; Yang, Q. Activation of beta-catenin signaling and its crosstalk with estrogen and histone deacetylases in human uterine fibroids. J. Clin. Endocrinol. Metab. 2020, 105. [Google Scholar] [CrossRef]
- Lewis, T.D.; Malik, M.; Britten, J.; Parikh, T.; Cox, J.; Catherino, W.H. Ulipristal acetate decreases active tgf-beta3 and its canonical signaling in uterine leiomyoma via two novel mechanisms. Fertil. Steril. 2019, 111, 806–815e801. [Google Scholar] [CrossRef]
- Vilos, G.A.; Allaire, C.; Laberge, P.Y.; Leyland, N.; Special, C. The management of uterine leiomyomas. J. Obstet. Gynaecol. Can. 2015, 37, 157–178. [Google Scholar] [CrossRef] [Green Version]
- Ali, M.; Chaudhry, Z.T.; Al-Hendy, A. Successes and failures of uterine leiomyoma drug discovery. Expert Opin. Drug Discov. 2018, 13, 169–177. [Google Scholar] [CrossRef]
- Schlaff, W.D.; Ackerman, R.T.; Al-Hendy, A.; Archer, D.F.; Barnhart, K.T.; Bradley, L.D.; Carr, B.R.; Feinberg, E.C.; Hurtado, S.M.; Kim, J.; et al. Elagolix for heavy menstrual bleeding in women with uterine fibroids. N Engl. J. Med. 2020, 382, 328–340. [Google Scholar] [CrossRef]
- Steinauer, J.; Pritts, E.A.; Jackson, R.; Jacoby, A.F. Systematic review of mifepristone for the treatment of uterine leiomyomata. Obstet. Gynecol. 2004, 103, 1331–1336. [Google Scholar] [CrossRef] [PubMed]
- Ali, M.; Al-Hendy, A. Selective progesterone receptor modulators for fertility preservation in women with symptomatic uterine fibroids. Biol. Reprod. 2017, 97, 337–352. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Donnez, J.; Dolmans, M.M. Uterine fibroid management: From the present to the future. Hum. Reprod. Update 2016, 22, 665–686. [Google Scholar] [CrossRef] [PubMed]
- Murji, A.; Whitaker, L.; Chow, T.L.; Sobel, M.L. Selective progesterone receptor modulators (sprms) for uterine fibroids. Cochrane Database Syst. Rev. 2017, 4, CD010770. [Google Scholar] [CrossRef] [PubMed]
- Spitz, I.M. Clinical utility of progesterone receptor modulators and their effect on the endometrium. Curr. Opin. Obstet. Gynecol. 2009, 21, 318–324. [Google Scholar] [CrossRef] [PubMed]
- Meunier, L.; Meszaros, M.; Pageaux, G.P.; Delay, J.M.; Herrero, A.; Pinzani, V.; Dominique, H.B. Acute liver failure requiring transplantation caused by ulipristal acetate. Clin. Res. Hepatol. Gastroenterol. 2020. [Google Scholar] [CrossRef] [PubMed]
- Donnez, J.; Arriagada, P.; Marciniak, M.; Larrey, D. Liver safety parameters of ulipristal acetate for the treatment of uterine fibroids: A comprehensive review of the clinical development program. Expert Opin. Drug Saf. 2018, 17, 1225–1232. [Google Scholar] [CrossRef]
- Ciebiera, M.; Vitale, S.G.; Ferrero, S.; Vilos, G.A.; Barra, F.; Caruso, S.; Lagana, A.S.; Sierant, A.; Cianci, A.; Jakiel, G. Vilaprisan, a new selective progesterone receptor modulator in uterine fibroid pharmacotherapy-will it really be a breakthrough? Curr. Pharm. Des. 2020, 26, 300–309. [Google Scholar] [CrossRef]
- Holick, M.F. Vitamin D: A millenium perspective. J. Cell Biochem. 2003, 88, 296–307. [Google Scholar] [CrossRef]
- Wetmore, J.B.; Kimber, C.; Mahnken, J.D.; Stubbs, J.R. Cholecalciferol v. Ergocalciferol for 25-hydroxyvitamin D (25(OH)D) repletion in chronic kidney disease: A randomised clinical trial. Br. J. Nutr. 2016, 116, 2074–2081. [Google Scholar] [CrossRef] [Green Version]
- Ciebiera, M.; Wlodarczyk, M.; Ciebiera, M.; Zareba, K.; Lukaszuk, K.; Jakiel, G. Vitamin D and uterine fibroids-review of the literature and novel concepts. Int. J. Mol. Sci. 2018, 19. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pludowski, P.; Holick, M.F.; Grant, W.B.; Konstantynowicz, J.; Mascarenhas, M.R.; Haq, A.; Povoroznyuk, V.; Balatska, N.; Barbosa, A.P.; Karonova, T.; et al. Vitamin D supplementation guidelines. J. Steroid Biochem. Mol. Biol. 2018, 175, 125–135. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Brakta, S.; Diamond, J.S.; Al-Hendy, A.; Diamond, M.P.; Halder, S.K. Role of vitamin D in uterine fibroid biology. Fertil. Steril. 2015, 104, 698–706. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhao, G.; Ford, E.S.; Tsai, J.; Li, C.; Croft, J.B. Factors associated with vitamin D deficiency and inadequacy among women of childbearing age in the United States. ISRN Obstet. Gynecol. 2012, 2012, 691486. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zadshir, A.; Tareen, N.; Pan, D.; Norris, K.; Martins, D. The prevalence of hypovitaminosis D among US adults: Data from the NHANES III. Ethn. Dis. 2005, 15, S5. [Google Scholar] [PubMed]
- Nesby-O’Dell, S.; Scanlon, K.S.; Cogswell, M.E.; Gillespie, C.; Hollis, B.W.; Looker, A.C.; Allen, C.; Doughertly, C.; Gunter, E.W.; Bowman, B.A. Hypovitaminosis D prevalence and determinants among african american and white women of reproductive age: Third national health and nutrition examination survey, 1988-1994. Am. J. Clin. Nutr. 2002, 76, 187–192. [Google Scholar] [CrossRef] [Green Version]
- Sabry, M.; Halder, S.K.; Allah, A.S.; Roshdy, E.; Rajaratnam, V.; Al-Hendy, A. Serum vitamin D3 level inversely correlates with uterine fibroid volume in different ethnic groups: A cross-sectional observational study. Int. J. Womens Health 2013, 5, 93–100. [Google Scholar] [CrossRef] [Green Version]
- Ciebiera, M.; Wlodarczyk, M.; Slabuszewska-Jozwiak, A.; Nowicka, G.; Jakiel, G. Influence of vitamin D and transforming growth factor beta3 serum concentrations, obesity, and family history on the risk for uterine fibroids. Fertil. Steril. 2016, 106, 1787–1792. [Google Scholar] [CrossRef] [Green Version]
- Baird, D.D.; Hill, M.C.; Schectman, J.M.; Hollis, B.W. Vitamin D and the risk of uterine fibroids. Epidemiology 2013, 24, 447–453. [Google Scholar] [CrossRef] [Green Version]
- Trump, D.L.; Aragon-Ching, J.B. Vitamin d in prostate cancer. Asian J. Androl. 2018, 20, 244–252. [Google Scholar] [CrossRef]
- Mahendra, A.; Karishma; Choudhury, B.K.; Sharma, T.; Bansal, N.; Bansal, R.; Gupta, S. Vitamin d and gastrointestinal cancer. J. Lab. Physicians 2018, 10, 1–5. [Google Scholar] [CrossRef] [PubMed]
- Welsh, J. Vitamin D and breast cancer: Past and present. J. Steroid Biochem. Mol. Biol. 2018, 177, 15–20. [Google Scholar] [CrossRef] [PubMed]
- Sharan, C.; Halder, S.K.; Thota, C.; Jaleel, T.; Nair, S.; Al-Hendy, A. Vitamin D inhibits proliferation of human uterine leiomyoma cells via catechol-O-methyltransferase. Fertil. Steril. 2011, 95, 247–253. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Halder, S.K.; Sharan, C.; Al-Hendy, A. 1,25-dihydroxyvitamin D3 treatment shrinks uterine leiomyoma tumors in the eker rat model. Biol. Reprod. 2012, 86, 116. [Google Scholar] [CrossRef] [PubMed]
- Halder, S.K.; Sharan, C.; Al-Hendy, O.; Al-Hendy, A. Paricalcitol, a vitamin D receptor activator, inhibits tumor formation in a murine model of uterine fibroids. Reprod. Sci. 2014, 21, 1108–1119. [Google Scholar] [CrossRef] [PubMed]
- Halder, S.K.; Goodwin, J.S.; Al-Hendy, A. 1,25-dihydroxyvitamin D3 reduces tgf-beta3-induced fibrosis-related gene expression in human uterine leiomyoma cells. J. Clin. Endocrinol. Metab. 2011, 96, E754–E762. [Google Scholar] [CrossRef]
- Halder, S.K.; Osteen, K.G.; Al-Hendy, A. Vitamin D3 inhibits expression and activities of matrix metalloproteinase-2 and -9 in human uterine fibroid cells. Hum. Reprod. 2013, 28, 2407–2416. [Google Scholar] [CrossRef] [Green Version]
- Halder, S.K.; Osteen, K.G.; Al-Hendy, A. 1,25-dihydroxyvitamin D3 reduces extracellular matrix-associated protein expression in human uterine fibroid cells. Biol. Reprod. 2013, 89, 150. [Google Scholar] [CrossRef]
- Corachan, A.; Ferrero, H.; Escrig, J.; Monleon, J.; Faus, A.; Cervello, I.; Pellicer, A. Long-term vitamin d treatment decreases human uterine leiomyoma size in a xenograft animal model. Fertil. Steril. 2020, 113, 205–216e204. [Google Scholar] [CrossRef]
- Ciavattini, A.; Delli Carpini, G.; Serri, M.; Vignini, A.; Sabbatinelli, J.; Tozzi, A.; Aggiusti, A.; Clemente, N. Hypovitaminosis D and “small burden” uterine fibroids: Opportunity for a vitamin D supplementation. Medicine (Baltimore) 2016, 95, e5698. [Google Scholar] [CrossRef]
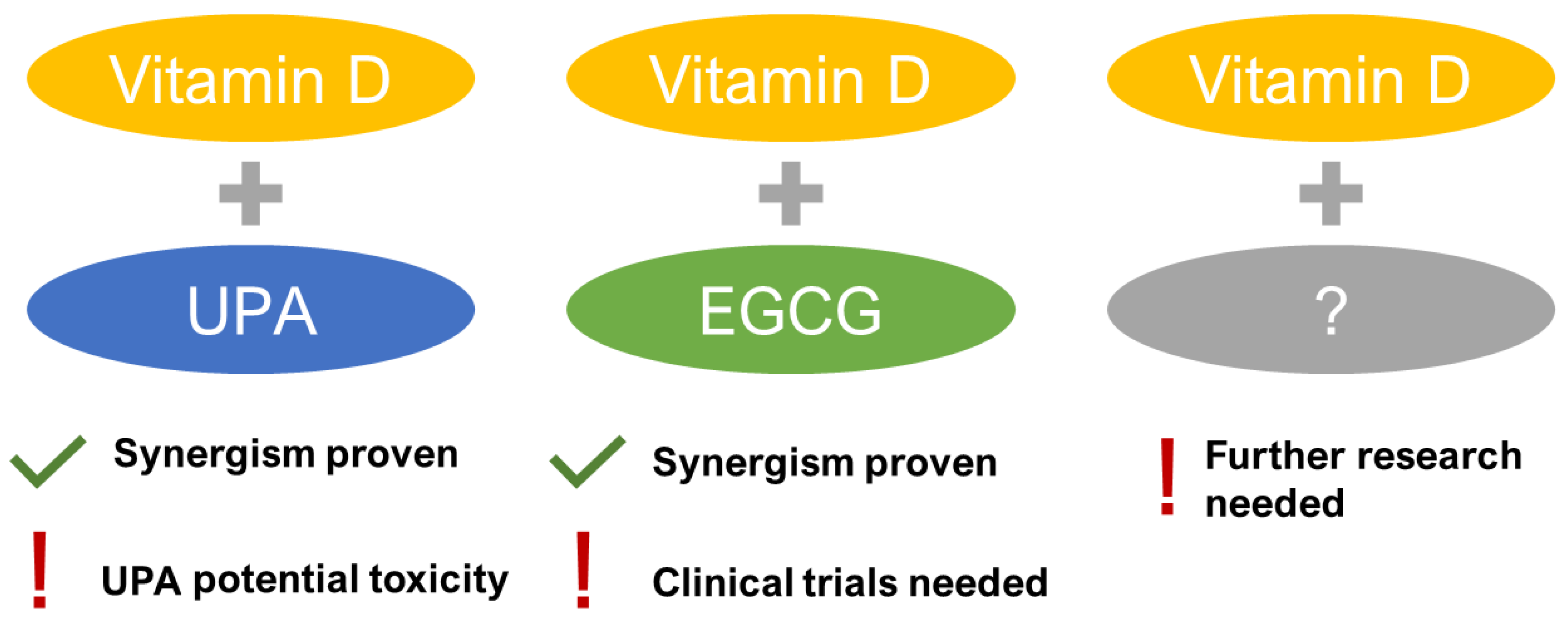
- Porcaro, G.; Santamaria, A.; Giordano, D.; Angelozzi, P. Vitamin D plus epigallocatechin gallate: A novel promising approach for uterine myomas. Eur. Rev. Med. Pharmacol. Sci. 2020, 24, 3344–3351. [Google Scholar] [CrossRef] [PubMed]
- Ali, M.; Shahin, S.M.; Sabri, N.A.; Al-Hendy, A.; Yang, Q. 1,25 dihydroxyvitamin D3 enhances the antifibroid effects of ulipristal acetate in human uterine fibroids. Reprod. Sci. 2019, 26, 812–828. [Google Scholar] [CrossRef] [PubMed]
- Ciebiera, M.; Meczekalski, B.; Lukaszuk, K.; Jakiel, G. Potential synergism between ulipristal acetate and vitamin D3 in uterine fibroid pharmacotherapy - 2 case studies. Gynecol. Endocrinol. 2019, 35, 473–477. [Google Scholar] [CrossRef] [PubMed]
- Arjeh, S.; Darsareh, F.; Asl, Z.A.; Kutenaei, M.A. Effect of oral consumption of vitamin d on uterine fibroids: A randomized clinical trial. Complement. Ther. Clin. Pract. 2020, 39, 101159. [Google Scholar] [CrossRef] [PubMed]
- Prasanth, M.I.; Sivamaruthi, B.S.; Chaiyasut, C.; Tencomnao, T. A review of the role of green tea (Camellia sinensis) in antiphotoaging, stress resistance, neuroprotection, and autophagy. Nutrients 2019, 11. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, D.; Al-Hendy, M.; Richard-Davis, G.; Montgomery-Rice, V.; Sharan, C.; Rajaratnam, V.; Khurana, A.; Al-Hendy, A. Green tea extract inhibits proliferation of uterine leiomyoma cells in vitro and in nude mice. Am. J. Obstet. Gynecol. 2010, 202, 289.e1–289.e9. [Google Scholar] [CrossRef] [Green Version]
- Graham, H.N. Green tea composition, consumption, and polyphenol chemistry. Prev. Med. 1992, 21, 334–350. [Google Scholar] [CrossRef]
- Lin, J.K.; Liang, Y.C.; Lin-Shiau, S.Y. Cancer chemoprevention by tea polyphenols through mitotic signal transduction blockade. Biochem. Pharmacol. 1999, 58, 911–915. [Google Scholar] [CrossRef]
- Chow, H.H.; Cai, Y.; Hakim, I.A.; Crowell, J.A.; Shahi, F.; Brooks, C.A.; Dorr, R.T.; Hara, Y.; Alberts, D.S. Pharmacokinetics and safety of green tea polyphenols after multiple-dose administration of epigallocatechin gallate and polyphenon e in healthy individuals. Clin. Cancer Res. 2003, 9, 3312–3319. [Google Scholar]
- Thangapazham, R.L.; Singh, A.K.; Sharma, A.; Warren, J.; Gaddipati, J.P.; Maheshwari, R.K. Green tea polyphenols and its constituent epigallocatechin gallate inhibits proliferation of human breast cancer cells in vitro and in vivo. Cancer Lett. 2007, 245, 232–241. [Google Scholar] [CrossRef]
- Tang, Y.; Zhao, D.Y.; Elliott, S.; Zhao, W.; Curiel, T.J.; Beckman, B.S.; Burow, M.E. Epigallocatechin-3 gallate induces growth inhibition and apoptosis in human breast cancer cells through survivin suppression. Int. J. Oncol. 2007, 31, 705–711. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liang, Y.C.; Lin-Shiau, S.Y.; Chen, C.F.; Lin, J.K. Inhibition of cyclin-dependent kinases 2 and 4 activities as well as induction of cdk inhibitors p21 and p27 during growth arrest of human breast carcinoma cells by (-)-epigallocatechin-3-gallate. J. Cell Biochem. 1999, 75, 1–12. [Google Scholar] [CrossRef]
- Chuang, T.Y.; Min, J.; Wu, H.L.; McCrary, C.; Layman, L.C.; Diamond, M.P.; Azziz, R.; Al-Hendy, A.; Chen, Y.H. Berberine inhibits uterine leiomyoma cell proliferation via downregulation of cyclooxygenase 2 and pituitary tumor-transforming gene 1. Reprod. Sci. 2017, 24, 1005–1013. [Google Scholar] [CrossRef]
- Gupta, S.; Ahmad, N.; Nieminen, A.L.; Mukhtar, H. Growth inhibition, cell-cycle dysregulation, and induction of apoptosis by green tea constituent (-)-epigallocatechin-3-gallate in androgen-sensitive and androgen-insensitive human prostate carcinoma cells. Toxicol. Appl. Pharmacol. 2000, 164, 82–90. [Google Scholar] [CrossRef] [PubMed]
- Jung, Y.D.; Kim, M.S.; Shin, B.A.; Chay, K.O.; Ahn, B.W.; Liu, W.; Bucana, C.D.; Gallick, G.E.; Ellis, L.M. EGCG, a major component of green tea, inhibits tumour growth by inhibiting VEGF induction in human colon carcinoma cells. Br. J. Cancer 2001, 84, 844–850. [Google Scholar] [CrossRef] [Green Version]
- Demeule, M.; Brossard, M.; Page, M.; Gingras, D.; Beliveau, R. Matrix metalloproteinase inhibition by green tea catechins. Biochim Biophys Acta 2000, 1478, 51–60. [Google Scholar] [CrossRef]
- Ozercan, I.H.; Sahin, N.; Akdemir, F.; Onderci, M.; Seren, S.; Sahin, K.; Kucuk, O. Chemoprevention of fibroid tumors by [-]-epigallocatechin-3-gallate in quail. Nutr. Res. 2008, 28, 92–97. [Google Scholar] [CrossRef]
- Zhang, D.; Al-Hendy, M.; Richard-Davis, G.; Montgomery-Rice, V.; Rajaratnam, V.; Al-Hendy, A. Antiproliferative and proapoptotic effects of epigallocatechin gallate on human leiomyoma cells. Fertil. Steril. 2010, 94, 1887–1893. [Google Scholar] [CrossRef] [Green Version]
- Ahmad, N.; Gupta, S.; Mukhtar, H. Green tea polyphenol epigallocatechin-3-gallate differentially modulates nuclear factor kappa beta in cancer cells versus normal cells. Arch. Biochem. Biophys. 2000, 376, 338–346. [Google Scholar] [CrossRef]
- Beck, S.E.; Jung, B.H.; Fiorino, A.; Gomez, J.; Rosario, E.D.; Cabrera, B.L.; Huang, S.C.; Chow, J.Y.; Carethers, J.M. Bone morphogenetic protein signaling and growth suppression in colon cancer. Am. J. Physiol. Gastrointest. Liver Physiol. 2006, 291, G135–G145. [Google Scholar] [CrossRef]
- Horvath, L.G.; Henshall, S.M.; Kench, J.G.; Turner, J.J.; Golovsky, D.; Brenner, P.C.; O’Neill, G.F.; Kooner, R.; Stricker, P.D.; Grygiel, J.J.; et al. Loss of BMP2, Smad8, and Smad4 expression in prostate cancer progression. Prostate 2004, 59, 234–242. [Google Scholar] [CrossRef]
- Al-Hendy, A.; Salama, S.A. Catechol-O-methyltransferase polymorphism is associated with increased uterine leiomyoma risk in different ethnic groups. J. Soc. Gynecol. Investig. 2006, 13, 136–144. [Google Scholar] [CrossRef]
- Salama, S.A.; Ho, S.L.; Wang, H.Q.; Tenhunen, J.; Tilgmann, C.; Al-Hendy, A. Hormonal regulation of catechol-o-methyl transferase activity in women with uterine leiomyomas. Fertil. Steril. 2006, 86, 259–262. [Google Scholar] [CrossRef]
- Othman, E.E.; Al-Hendy, A. Molecular genetics and racial disparities of uterine leiomyomas. Best Pract. Res. Clin. Obstet. Gynaecol. 2008, 22, 589–601. [Google Scholar] [CrossRef] [Green Version]
- Zhu, B.T.; Shim, J.Y.; Nagai, M.; Bai, H.W. Molecular modelling study of the mechanism of high-potency inhibition of human catechol-O-methyltransferase by (-)-epigallocatechin-3-o-gallate. Xenobiotica 2008, 38, 130–146. [Google Scholar] [CrossRef]
- Roshdy, E.; Rajaratnam, V.; Maitra, S.; Sabry, M.; Allah, A.S.; Al-Hendy, A. Treatment of symptomatic uterine fibroids with green tea extract: A pilot randomized controlled clinical study. Int. J. Womens Health 2013, 5, 477–486. [Google Scholar] [CrossRef] [Green Version]
- Ahmed, R.S.; Liu, G.; Renzetti, A.; Farshi, P.; Yang, H.; Soave, C.; Saed, G.; El-Ghoneimy, A.A.; El-Banna, H.A.; Foldes, R.; et al. Biological and mechanistic characterization of novel prodrugs of green tea polyphenol epigallocatechin gallate analogs in human leiomyoma cell lines. J. Cell Biochem. 2016, 117, 2357–2369. [Google Scholar] [CrossRef]
- Wang, N.; Tan, H.Y.; Li, L.; Yuen, M.F.; Feng, Y. Berberine and coptidis rhizoma as potential anticancer agents: Recent updates and future perspectives. J. Ethnopharmacol. 2015, 176, 35–48. [Google Scholar] [CrossRef]
- Lee, T.K.; Kim, D.I.; Song, Y.L.; Lee, Y.C.; Kim, H.M.; Kim, C.H. Differential inhibition of Scutellaria barbata D. Don (Lamiaceae) on HCG-promoted proliferation of cultured uterine leiomyomal and myometrial smooth muscle cells. Immunopharmacol. Immunotoxicol. 2004, 26, 329–342. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Xun, K.; Wang, Y.; Chen, X. A systematic review of the anticancer properties of berberine, a natural product from chinese herbs. Anticancer Drugs 2009, 20, 757–769. [Google Scholar] [CrossRef] [Green Version]
- Fu, L.; Chen, W.; Guo, W.; Wang, J.; Tian, Y.; Shi, D.; Zhang, X.; Qiu, H.; Xiao, X.; Kang, T.; et al. Berberine targets AP-2/hTERT, NF-kappaB/COX-2, HIF-1alpha/VEGF and cytochrome-C/caspase signaling to suppress human cancer cell growth. PLoS ONE 2013, 8, e69240. [Google Scholar] [CrossRef] [Green Version]
- Lee, T.K.; Kim, D.I.; Han, J.Y.; Kim, C.H. Inhibitory effects of Scutellaria barbata D. Don. and Euonymus Alatus Sieb. On aromatase activity of human leiomyomal cells. Immunopharmacol. Immunotoxicol. 2004, 26, 315–327. [Google Scholar] [CrossRef] [PubMed]
- Wu, H.L.; Chuang, T.Y.; Al-Hendy, A.; Diamond, M.P.; Azziz, R.; Chen, Y.H. Berberine inhibits the proliferation of human uterine leiomyoma cells. Fertil. Steril. 2015, 103, 1098–1106. [Google Scholar] [CrossRef] [PubMed]
- Tsai, S.J.; Lin, S.J.; Cheng, Y.M.; Chen, H.M.; Wing, L.Y. Expression and functional analysis of pituitary tumor transforming gene-1 in uterine leiomyomas. J. Clin. Endocrinol. Metab. 2005, 90, 3715–3723. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Park, S.B.; Jee, B.C.; Kim, S.H.; Cho, Y.J.; Han, M. Cyclooxygenase-2 inhibitor, celecoxib, inhibits leiomyoma cell proliferation through the nuclear factor kappaB pathway. Reprod. Sci. 2014, 21, 1187–1195. [Google Scholar] [CrossRef] [PubMed]
- Ke, X.; Dou, F.; Cheng, Z.; Dai, H.; Zhang, W.; Qu, X.; Ding, P.; Zuo, X. High expression of cyclooxygenase-2 in uterine fibroids and its correlation with cell proliferation. Eur. J. Obstet. Gynecol. Reprod. Biol. 2013, 168, 199–203. [Google Scholar] [CrossRef] [PubMed]
- Hewlings, S.J.; Kalman, D.S. Curcumin: A review of its' effects on human health. Foods 2017, 6. [Google Scholar] [CrossRef]
- Cheng, I.C.; Li, R.K.; Leung, G.P.; Li, S.L.; Kong, M.; Lao, L.X.; Zhang, Z.J.; Lin, W.L.; Ng, E.H.; Rong, J.H.; et al. Application of UPLC-MS/MS to simultaneously detect four bioactive compounds in the tumour-shrinking decoction (FM1523) for uterine fibroids treatment. Phytochem. Anal. 2019, 30, 447–455. [Google Scholar] [CrossRef]
- Bajracharya, P.; Lee, E.J.; Lee, D.M.; Shim, S.H.; Kim, K.J.; Lee, S.H.; Bae, J.J.; Chun, S.S.; Lee, T.K.; Kwon, S.H.; et al. Effect of different ingredients in traditional korean medicine for human uterine leiomyoma on normal myometrial and leiomyomal smooth muscle cell proliferation. Arch. Pharm. Res. 2009, 32, 1555–1563. [Google Scholar] [CrossRef]
- Chen, X.; Chen, X.; Shi, X.; Gao, Z.; Guo, Z. Curcumin attenuates endothelial cell fibrosis through inhibiting endothelial-interstitial transformation. Clin. Exp. Pharmacol. Physiol. 2020. [Google Scholar] [CrossRef]
- Malik, M.; Mendoza, M.; Payson, M.; Catherino, W.H. Curcumin, a nutritional supplement with antineoplastic activity, enhances leiomyoma cell apoptosis and decreases fibronectin expression. Fertil Steril 2009, 91, 2177–2184. [Google Scholar] [CrossRef] [PubMed]
- Tsuiji, K.; Takeda, T.; Li, B.; Wakabayashi, A.; Kondo, A.; Kimura, T.; Yaegashi, N. Inhibitory effect of curcumin on uterine leiomyoma cell proliferation. Gynecol. Endocrinol. 2011, 27, 512–517. [Google Scholar] [CrossRef] [PubMed]
- Yu, C.H.; Zhao, J.S.; Zhao, H.; Peng, T.; Shen, D.C.; Xu, Q.X.; Li, Y.; Webb, R.C.; Wang, M.H.; Shi, X.M.; et al. Transcriptional profiling of uterine leiomyoma rats treated by a traditional herb pair, Curcumae rhizoma and Sparganii rhizoma. Braz. J. Med. Biol. Res. 2019, 52, e8132. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Biswas, S.; Chen, S.; Liang, G.; Feng, B.; Cai, L.; Khan, Z.A.; Chakrabarti, S. Curcumin analogs reduce stress and inflammation indices in experimental models of diabetes. Front. Endocrinol. (Lausanne) 2019, 10, 887. [Google Scholar] [CrossRef] [PubMed]
- Feng, T.; Wei, Y.; Lee, R.J.; Zhao, L. Liposomal curcumin and its application in cancer. Int. J. Nanomedicine 2017, 12, 6027–6044. [Google Scholar] [CrossRef] [Green Version]
- Salehi, B.; Mishra, A.P.; Nigam, M.; Sener, B.; Kilic, M.; Sharifi-Rad, M.; Fokou, P.V.T.; Martins, N.; Sharifi-Rad, J. Resveratrol: A double-edged sword in health benefits. Biomedicines 2018, 6. [Google Scholar] [CrossRef] [Green Version]
- Bhullar, K.S.; Hubbard, B.P. Lifespan and healthspan extension by resveratrol. Biochim. Biophys. Acta 2015, 1852, 1209–1218. [Google Scholar] [CrossRef] [Green Version]
- Li, L.; Qiu, R.L.; Lin, Y.; Cai, Y.; Bian, Y.; Fan, Y.; Gao, X.J. Resveratrol suppresses human cervical carcinoma cell proliferation and elevates apoptosis via the mitochondrial and p53 signaling pathways. Oncol. Lett. 2018, 15, 9845–9851. [Google Scholar] [CrossRef] [Green Version]
- Zhou, Z.X.; Mou, S.F.; Chen, X.Q.; Gong, L.L.; Ge, W.S. Anti-inflammatory activity of resveratrol prevents inflammation by inhibiting NFkappaB in animal models of acute pharyngitis. Mol. Med. Rep. 2018, 17, 1269–1274. [Google Scholar] [CrossRef] [Green Version]
- Kim, D.I.; Lee, T.K.; Lim, I.S.; Kim, H.; Lee, Y.C.; Kim, C.H. Regulation of IGF-1 production and proliferation of human leiomyomal smooth muscle cells by Scutellaria barbata D. Don in vitro: Isolation of flavonoids of apigenin and luteolin as acting compounds. Toxicol. Appl. Pharmacol. 2005, 205, 213–224. [Google Scholar] [CrossRef]
- Wu, C.H.; Shieh, T.M.; Wei, L.H.; Cheng, T.F.; Chen, H.Y.; Huang, T.C.; Wang, K.L.; Hsia, S.M. Resveratrol inhibits proliferation of myometrial and leiomyoma cells and decreases extracellular matrix-associated protein expression. J. Funct. Foods 2016, 23, 241–252. [Google Scholar] [CrossRef]
- Ho, Y.; Sh Yang, Y.C.; Chin, Y.T.; Chou, S.Y.; Chen, Y.R.; Shih, Y.J.; Whang-Peng, J.; Changou, C.A.; Liu, H.L.; Lin, S.J.; et al. Resveratrol inhibits human leiomyoma cell proliferation via crosstalk between integrin alphavbeta3 and IGF-1R. Food Chem. Toxicol. 2018, 120, 346–355. [Google Scholar] [CrossRef]
- Chen, H.Y.; Lin, P.H.; Shih, Y.H.; Wang, K.L.; Hong, Y.H.; Shieh, T.M.; Huang, T.C.; Hsia, S.M. Natural antioxidant resveratrol suppresses uterine fibroid cell growth and extracellular matrix formation in vitro and in vivo. Antioxidants 2019, 8. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Collins, K.G.; Fitzgerald, G.F.; Stanton, C.; Ross, R.P. Looking beyond the terrestrial: The potential of seaweed derived bioactives to treat non-communicable diseases. Mar. Drugs 2016, 14. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Charboneau, A.J.; Delaney, J.P.; Beilman, G. Fucoidans inhibit the formation of post-operative abdominal adhesions in a rat model. PLoS ONE 2018, 13, e0207797. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Wu, N.; Chen, Y.; Tan, J.; Wang, J.; Geng, L.; Qin, Y.; Zhang, Q. Degradation of different molecular weight fucoidans and their inhibition of tgf-beta1 induced epithelial-mesenchymal transition in mouse renal tubular epithelial cells. Int. J. Biol. Macromol. 2020, 151, 545–553. [Google Scholar] [CrossRef]
- Wang, L.; Zhang, P.; Li, X.; Zhang, Y.; Zhan, Q.; Wang, C. Low-molecular-weight fucoidan attenuates bleomycin-induced pulmonary fibrosis: Possible role in inhibiting tgf-beta1-induced epithelial-mesenchymal transition through ERK pathway. Am. J. Transl. Res. 2019, 11, 2590–2602. [Google Scholar]
- Wu, S.Y.; Chen, Y.T.; Tsai, G.Y.; Hsu, F.Y.; Hwang, P.A. Protective effect of low-molecular-weight fucoidan on radiation-induced fibrosis through tgf-beta1/Smad pathway-mediated inhibition of collagen i accumulation. Mar. Drugs 2020, 18. [Google Scholar] [CrossRef] [Green Version]
- Chen, H.Y.; Huang, T.C.; Lin, L.C.; Shieh, T.M.; Wu, C.H.; Wang, K.L.; Hong, Y.H.; Hsia, S.M. Fucoidan inhibits the proliferation of leiomyoma cells and decreases extracellular matrix-associated protein expression. Cell Physiol. Biochem. 2018, 49, 1970–1986. [Google Scholar] [CrossRef]
- Licznerska, B.; Baer-Dubowska, W. Indole-3-carbinol and its role in chronic diseases. Adv. Exp. Med. Biol. 2016, 928, 131–154. [Google Scholar] [CrossRef]
- Ahmad, A.; Biersack, B.; Li, Y.; Kong, D.; Bao, B.; Schobert, R.; Padhye, S.B.; Sarkar, F.H. Targeted regulation of PI3K/Akt/mTOR/NF-kappaB signaling by indole compounds and their derivatives: Mechanistic details and biological implications for cancer therapy. Anticancer Agents Med. Chem. 2013, 13, 1002–1013. [Google Scholar] [CrossRef] [PubMed]
- Li, B.; Cong, M.; Zhu, Y.; Xiong, Y.; Jin, W.; Wan, Y.; Zhou, Y.; Ao, Y.; Wang, H. Indole-3-carbinol induces apoptosis of hepatic stellate cells through K63 de-ubiquitination of RIP1 in rats. Cell Physiol. Biochem. 2017, 41, 1481–1490. [Google Scholar] [CrossRef] [PubMed]
- Greco, S.; Islam, M.S.; Zannotti, A.; Delli Carpini, G.; Giannubilo, S.R.; Ciavattini, A.; Petraglia, F.; Ciarmela, P. Quercetin and indole-3-carbinol inhibit extracellular matrix expression in human primary uterine leiomyoma cells. Reprod. Biomed. Online 2020. [Google Scholar] [CrossRef] [PubMed]
- Cho, S.; Kim, S.; Jin, Z.; Yang, H.; Han, D.; Baek, N.I.; Jo, J.; Cho, C.W.; Park, J.H.; Shimizu, M.; et al. Isoliquiritigenin, a chalcone compound, is a positive allosteric modulator of GABAA receptors and shows hypnotic effects. Biochem. Biophys. Res. Commun. 2011, 413, 637–642. [Google Scholar] [CrossRef]
- Park, S.J.; Song, H.Y.; Youn, H.S. Suppression of the TRIF-dependent signaling pathway of toll-like receptors by isoliquiritigenin in RAW264.7 macrophages. Mol. Cells 2009, 28, 365–368. [Google Scholar] [CrossRef]
- Feldman, M.; Santos, J.; Grenier, D. Comparative evaluation of two structurally related flavonoids, isoliquiritigenin and liquiritigenin, for their oral infection therapeutic potential. J. Nat. Prod. 2011, 74, 1862–1867. [Google Scholar] [CrossRef]
- Watanabe, Y.; Nagai, Y.; Honda, H.; Okamoto, N.; Yamamoto, S.; Hamashima, T.; Ishii, Y.; Tanaka, M.; Suganami, T.; Sasahara, M.; et al. Isoliquiritigenin attenuates adipose tissue inflammation in vitro and adipose tissue fibrosis through inhibition of innate immune responses in mice. Sci. Rep. 2016, 6, 23097. [Google Scholar] [CrossRef] [Green Version]
- Xiang, S.; Chen, H.; Luo, X.; An, B.; Wu, W.; Cao, S.; Ruan, S.; Wang, Z.; Weng, L.; Zhu, H.; et al. Isoliquiritigenin suppresses human melanoma growth by targeting miR-301b/LRIG1 signaling. J. Exp. Clin. Cancer Res. 2018, 37, 184. [Google Scholar] [CrossRef]
- Li, J.; Kang, S.W.; Kim, J.L.; Sung, H.Y.; Kwun, I.S.; Kang, Y.H. Isoliquiritigenin entails blockade of tgf-beta1-Smad signaling for retarding high glucose-induced mesangial matrix accumulation. J. Agric. Food Chem. 2010, 58, 3205–3212. [Google Scholar] [CrossRef]
- Kim, D.C.; Ramachandran, S.; Baek, S.H.; Kwon, S.H.; Kwon, K.Y.; Cha, S.D.; Bae, I.; Cho, C.H. Induction of growth inhibition and apoptosis in human uterine leiomyoma cells by isoliquiritigenin. Reprod. Sci. 2008, 15, 552–558. [Google Scholar] [CrossRef]
- Lin, P.H.; Kung, H.L.; Chen, H.Y.; Huang, K.C.; Hsia, S.M. Isoliquiritigenin suppresses E2-induced uterine leiomyoma growth through the modulation of cell death program and the repression of ecm accumulation. Cancers 2019, 11. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Formica, J.V.; Regelson, W. Review of the biology of quercetin and related bioflavonoids. Food Chem. Toxicol. 1995, 33, 1061–1080. [Google Scholar] [CrossRef]
- Andres, S.; Pevny, S.; Ziegenhagen, R.; Bakhiya, N.; Schafer, B.; Hirsch-Ernst, K.I.; Lampen, A. Safety aspects of the use of quercetin as a dietary supplement. Mol. Nutr. Food Res. 2018, 62. [Google Scholar] [CrossRef]
- Murakami, A.; Ashida, H.; Terao, J. Multitargeted cancer prevention by quercetin. Cancer Lett. 2008, 269, 315–325. [Google Scholar] [CrossRef] [PubMed]
- Van der Woude, H.; Ter Veld, M.G.; Jacobs, N.; van der Saag, P.T.; Murk, A.J.; Rietjens, I.M. The stimulation of cell proliferation by quercetin is mediated by the estrogen receptor. Mol. Nutr. Food Res. 2005, 49, 763–771. [Google Scholar] [CrossRef]
- Ganbold, M.; Shimamoto, Y.; Ferdousi, F.; Tominaga, K.; Isoda, H. Antifibrotic effect of methylated quercetin derivatives on tgfbeta-induced hepatic stellate cells. Biochem. Biophys. Rep. 2019, 20, 100678. [Google Scholar] [CrossRef]
- Xiao, Y.; Zhou, L.; Zhang, T.; Qin, C.; Wei, P.; Luo, L.; Luo, L.; Huang, G.; Chen, A.; Liu, G. Anti-fibrosis activity of quercetin attenuates rabbit tracheal stenosis via the tgf-beta/Akt/mTOR signaling pathway. Life Sci. 2020, 250, 117552. [Google Scholar] [CrossRef]
- Cavalcante, M.B.; Saccon, T.D.; Nunes, A.D.C.; Kirkland, J.L.; Tchkonia, T.; Schneider, A.; Masternak, M.M. Dasatinib plus quercetin prevents uterine age-related dysfunction and fibrosis in mice. Aging (Albany NY) 2020, 12, 2711–2722. [Google Scholar] [CrossRef]
- Borahay, M.A.; Asoglu, M.R.; Mas, A.; Adam, S.; Kilic, G.S.; Al-Hendy, A. Estrogen receptors and signaling in fibroids: Role in pathobiology and therapeutic implications. Reprod. Sci. 2017, 24, 1235–1244. [Google Scholar] [CrossRef]
- Houghton, C.A.; Fassett, R.G.; Coombes, J.S. Sulforaphane: Translational research from laboratory bench to clinic. Nutr. Rev. 2013, 71, 709–726. [Google Scholar] [CrossRef]
- Fix, C.; Carver-Molina, A.; Chakrabarti, M.; Azhar, M.; Carver, W. Effects of the isothiocyanate sulforaphane on tgf-beta1-induced rat cardiac fibroblast activation and extracellular matrix interactions. J. Cell Physiol. 2019, 234, 13931–13941. [Google Scholar] [CrossRef]
- Milito, A.; Brancaccio, M.; D’Argenio, G.; Castellano, I. Natural sulfur-containing compounds: An alternative therapeutic strategy against liver fibrosis. Cells 2019, 8. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Oh, C.J.; Kim, J.Y.; Min, A.K.; Park, K.G.; Harris, R.A.; Kim, H.J.; Lee, I.K. Sulforaphane attenuates hepatic fibrosis via NF-E2-related factor 2-mediated inhibition of transforming growth factor-beta/Smad signaling. Free Radic. Biol. Med. 2012, 52, 671–682. [Google Scholar] [CrossRef] [PubMed]
- Kyung, S.Y.; Kim, D.Y.; Yoon, J.Y.; Son, E.S.; Kim, Y.J.; Park, J.W.; Jeong, S.H. Sulforaphane attenuates pulmonary fibrosis by inhibiting the epithelial-mesenchymal transition. BMC Pharmacol. Toxicol. 2018, 19, 13. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sun, C.; Li, S.; Li, D. Sulforaphane mitigates muscle fibrosis in mdx mice via Nrf2-mediated inhibition of TGF-beta/smad signaling. J. Appl. Physiol. 2016, 120, 377–390. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Islam, M.S.; Afrin., S.; Brennan, J.; Segars, J. The active phytochemical of cruciferous vegetables, sulforaphane, reduces proliferation and inflammation of uterine fibroid cells. In Meeting: The Basic Science of Uterine Fibroids; NC, 27701. 28/FEB/2020; National Institute of Environmental Health Sciences: Research Triangle Park, CA, USA.
- Lila, M.A.; Burton-Freeman, B.; Grace, M.; Kalt, W. Unraveling anthocyanin bioavailability for human health. Annu. Rev. Food Sci. Technol. 2016, 7, 375–393. [Google Scholar] [CrossRef] [PubMed]
- Islam, M.S.; Giampieri, F.; Janjusevic, M.; Gasparrini, M.; Forbes-Hernandez, T.Y.; Mazzoni, L.; Greco, S.; Giannubilo, S.R.; Ciavattini, A.; Mezzetti, B.; et al. An anthocyanin rich strawberry extract induces apoptosis and ROS while decreases glycolysis and fibrosis in human uterine leiomyoma cells. Oncotarget 2017, 8, 23575–23587. [Google Scholar] [CrossRef] [Green Version]
- Giampieri, F.; Islam, M.S.; Greco, S.; Gasparrini, M.; Forbes Hernandez, T.Y.; Delli Carpini, G.; Giannubilo, S.R.; Ciavattini, A.; Mezzetti, B.; Mazzoni, L.; et al. Romina: A powerful strawberry with in vitro efficacy against uterine leiomyoma cells. J. Cell Physiol. 2019, 234, 7622–7633. [Google Scholar] [CrossRef]
- Giampieri, F.; Tulipani, S.; Alvarez-Suarez, J.M.; Quiles, J.L.; Mezzetti, B.; Battino, M. The strawberry: Composition, nutritional quality, and impact on human health. Nutrition 2012, 28, 9–19. [Google Scholar] [CrossRef]
- Wang, S.Y.; Feng, R.; Lu, Y.; Bowman, L.; Ding, M. Inhibitory effect on activator protein-1, nuclear factor-kappaB, and cell transformation by extracts of strawberries (Fragaria x ananassa duch.). J. Agric. Food Chem. 2005, 53, 4187–4193. [Google Scholar] [CrossRef]
- Sokola-Wysoczanska, E.; Wysoczanski, T.; Wagner, J.; Czyz, K.; Bodkowski, R.; Lochynski, S.; Patkowska-Sokola, B. Polyunsaturated fatty acids and their potential therapeutic role in cardiovascular system disorders-a review. Nutrients 2018, 10. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ibarguren, M.; Lopez, D.J.; Escriba, P.V. The effect of natural and synthetic fatty acids on membrane structure, microdomain organization, cellular functions and human health. Biochim. Biophys. Acta 2014, 1838, 1518–1528. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rizos, E.C.; Ntzani, E.E.; Bika, E.; Kostapanos, M.S.; Elisaf, M.S. Association between omega-3 fatty acid supplementation and risk of major cardiovascular disease events: A systematic review and meta-analysis. JAMA 2012, 308, 1024–1033. [Google Scholar] [CrossRef] [PubMed]
- Calder, P.C.; Grimble, R.F. Polyunsaturated fatty acids, inflammation and immunity. Eur. J. Clin. Nutr. 2002, 56 (Suppl. 3), S14–S19. [Google Scholar] [CrossRef] [Green Version]
- Li, K.; Huang, T.; Zheng, J.; Wu, K.; Li, D. Effect of marine-derived n-3 polyunsaturated fatty acids on c-reactive protein, interleukin 6 and tumor necrosis factor alpha: A meta-analysis. PLoS ONE 2014, 9, e88103. [Google Scholar] [CrossRef] [Green Version]
- Islam, M.S.; Castellucci, C.; Fiorini, R.; Greco, S.; Gagliardi, R.; Zannotti, A.; Giannubilo, S.R.; Ciavattini, A.; Frega, N.G.; Pacetti, D.; et al. Omega-3 fatty acids modulate the lipid profile, membrane architecture, and gene expression of leiomyoma cells. J. Cell Physiol. 2018, 233, 7143–7156. [Google Scholar] [CrossRef]
- Baldwin, I.T. Jasmonate-induced responses are costly but benefit plants under attack in native populations. Proc. Natl. Acad. Sci. USA 1998, 95, 8113–8118. [Google Scholar] [CrossRef] [Green Version]
- Wasternack, C. Jasmonates: An update on biosynthesis, signal transduction and action in plant stress response, growth and development. Ann. Bot. 2007, 100, 681–697. [Google Scholar] [CrossRef] [Green Version]
- Gunjegaonkar, S.M.; Shanmugarajan, T.S. Molecular mechanism of plant stress hormone methyl jasmonate for its anti-inflammatory activity. Plant. Signal. Behav. 2019, 14, e1642038. [Google Scholar] [CrossRef]
- Cesari, I.M.; Carvalho, E.; Figueiredo Rodrigues, M.; Mendonca Bdos, S.; Amoedo, N.D.; Rumjanek, F.D. Methyl jasmonate: Putative mechanisms of action on cancer cells cycle, metabolism, and apoptosis. Int. J. Cell Biol. 2014, 2014, 572097. [Google Scholar] [CrossRef]
- Sa-Nakanishi, A.B.; Soni-Neto, J.; Moreira, L.S.; Goncalves, G.A.; Silva, F.M.S.; Bracht, L.; Bersani-Amado, C.A.; Peralta, R.M.; Bracht, A.; Comar, J.F. Anti-inflammatory and antioxidant actions of methyl jasmonate are associated with metabolic modifications in the liver of arthritic rats. Oxid. Med. Cell Longev. 2018, 2018, 2056250. [Google Scholar] [CrossRef] [PubMed]
- Umukoro, S.; Alabi, A.O.; Eduviere, A.T.; Ajayi, A.M.; Oluwole, O.G. Anti-inflammatory and membrane stabilizing properties of methyl jasmonate in rats. Chin. J. Nat. Med. 2017, 15, 202–209. [Google Scholar] [CrossRef]
- Pereira-Marostica, H.V.; Castro, L.S.; Goncalves, G.A.; Silva, F.M.S.; Bracht, L.; Bersani-Amado, C.A.; Peralta, R.M.; Comar, J.F.; Bracht, A.; Sa-Nakanishi, A.B. Methyl jasmonate reduces inflammation and oxidative stress in the brain of arthritic rats. Antioxidants 2019, 8. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ribera-Fonseca, A.; Jimenez, D.; Leal, P.; Riquelme, I.; Roa, J.C.; Alberdi, M.; Peek, R.M.; Reyes-Diaz, M. The anti-proliferative and anti-invasive effect of leaf extracts of blueberry plants treated with methyl jasmonate on human gastric cancer in vitro is related to their antioxidant properties. Antioxidants 2020, 9. [Google Scholar] [CrossRef] [Green Version]
- Ali, M.; Al-Hendy, A.; Yang, Q. Natural compound methyl jasmonate shows promising anti-fibroid effects via inhibition of EZH2 mediated Wnt/β-catenin signaling pathway activation in human uterine fibroids. In Meeting: The Basic Science of Uterine Fibroids; NC, 27701. 28/FEB/2020; National Institute of Environmental Health Sciences: Research Triangle Park, CA, USA.
- Vire, E.; Brenner, C.; Deplus, R.; Blanchon, L.; Fraga, M.; Didelot, C.; Morey, L.; Van Eynde, A.; Bernard, D.; Vanderwinden, J.M.; et al. The polycomb group protein ezh2 directly controls DNA methylation. Nature 2006, 439, 871–874. [Google Scholar] [CrossRef]
- Wise, L.A.; Radin, R.G.; Palmer, J.R.; Kumanyika, S.K.; Boggs, D.A.; Rosenberg, L. Intake of fruit, vegetables, and carotenoids in relation to risk of uterine leiomyomata. Am. J. Clin. Nutr. 2011, 94, 1620–1631. [Google Scholar] [CrossRef]
- Valko, M.; Leibfritz, D.; Moncol, J.; Cronin, M.T.; Mazur, M.; Telser, J. Free radicals and antioxidants in normal physiological functions and human disease. Int. J. Biochem. Cell Biol. 2007, 39, 44–84. [Google Scholar] [CrossRef]
- Valko, M.; Rhodes, C.J.; Moncol, J.; Izakovic, M.; Mazur, M. Free radicals, metals and antioxidants in oxidative stress-induced cancer. Chem. Biol. Interact. 2006, 160, 1–40. [Google Scholar] [CrossRef]
- Gajowik, A.; Dobrzynska, M.M. The evaluation of protective effect of lycopene against genotoxic influence of X-irradiation in human blood lymphocytes. Radiat. Environ. Biophys. 2017, 56, 413–422. [Google Scholar] [CrossRef] [Green Version]
- Gajowik, A.; Dobrzynska, M.M. Lycopene - antioxidant with radioprotective and anticancer properties. A review. Roczniki Państwowego Zakładu Higieny 2014, 65, 263–271. [Google Scholar]
- Srinivasan, M.; Devipriya, N.; Kalpana, K.B.; Menon, V.P. Lycopene: An antioxidant and radioprotector against gamma-radiation-induced cellular damages in cultured human lymphocytes. Toxicology 2009, 262, 43–49. [Google Scholar] [CrossRef] [PubMed]
- Torbergsen, A.C.; Collins, A.R. Recovery of human lymphocytes from oxidative DNA damage; the apparent enhancement of DNA repair by carotenoids is probably simply an antioxidant effect. Eur. J. Nutr. 2000, 39, 80–85. [Google Scholar] [CrossRef] [PubMed]
- Sun, H.; Valdivia, L.A.; Subbotin, V.; Aitouche, A.; Fung, J.J.; Starzl, T.E.; Rao, A.S. Improved surgical technique for the establishment of a murine model of aortic transplantation. Microsurgery 1998, 18, 368–371. [Google Scholar] [CrossRef] [Green Version]
- Aydemir, G.; Kasiri, Y.; Birta, E.; Beke, G.; Garcia, A.L.; Bartok, E.M.; Ruhl, R. Lycopene-derived bioactive retinoic acid receptors/retinoid-X receptors-activating metabolites may be relevant for lycopene's anti-cancer potential. Mol. Nutr. Food Res. 2013, 57, 739–747. [Google Scholar] [CrossRef] [PubMed]
- Sharoni, Y.; Linnewiel-Hermoni, K.; Zango, G.; Khanin, M.; Salman, H.; Veprik, A.; Danilenko, M.; Levy, J. The role of lycopene and its derivatives in the regulation of transcription systems: Implications for cancer prevention. Am. J. Clin. Nutr. 2012, 96, 1173S–1178S. [Google Scholar] [CrossRef]
- Tanaka, T.; Shnimizu, M.; Moriwaki, H. Cancer chemoprevention by carotenoids. Molecules 2012, 17, 3202–3242. [Google Scholar] [CrossRef]
- Cho, K.S.; Shin, M.; Kim, S.; Lee, S.B. Recent advances in studies on the therapeutic potential of dietary carotenoids in neurodegenerative diseases. Oxid Med. Cell Longev. 2018, 2018, 4120458. [Google Scholar] [CrossRef]
- Terry, K.L.; Missmer, S.A.; Hankinson, S.E.; Willett, W.C.; De Vivo, I. Lycopene and other carotenoid intake in relation to risk of uterine leiomyomata. Am. J. Obstet. Gynecol. 2008, 198, 37.e1–37.e8. [Google Scholar] [CrossRef] [Green Version]
- Heber, D.; Lu, Q.Y. Overview of mechanisms of action of lycopene. Exp. Biol. Med. (Maywood) 2002, 227, 920–923. [Google Scholar] [CrossRef]
- Rao, A.V.; Rao, L.G. Carotenoids and human health. Pharmacol. Res. 2007, 55, 207–216. [Google Scholar] [CrossRef]
- Sahin, K.; Ozercan, R.; Onderci, M.; Sahin, N.; Gursu, M.F.; Khachik, F.; Sarkar, F.H.; Munkarah, A.; Ali-Fehmi, R.; Kmak, D.; et al. Lycopene supplementation prevents the development of spontaneous smooth muscle tumors of the oviduct in Japanese Quail. Nutr. Cancer 2004, 50, 181–189. [Google Scholar] [CrossRef] [PubMed]
- Sahin, K.; Ozercan, R.; Onderci, M.; Sahin, N.; Khachik, F.; Seren, S.; Kucuk, O. Dietary tomato powder supplementation in the prevention of leiomyoma of the oviduct in the japanese quail. Nutr. Cancer 2007, 59, 70–75. [Google Scholar] [CrossRef] [PubMed]
- Beecher, G.R. Nutrient content of tomatoes and tomato products. Proc. Soc. Exp. Biol. Med. 1998, 218, 98–100. [Google Scholar] [CrossRef] [PubMed]
- Abushita, A.A.; Daood, H.G.; Biacs, P.A. Change in carotenoids and antioxidant vitamins in tomato as a function of varietal and technological factors. J. Agric. Food Chem. 2000, 48, 2075–2081. [Google Scholar] [CrossRef]
- Hwang, E.S.; Bowen, P.E. Can the consumption of tomatoes or lycopene reduce cancer risk? Integr. Cancer Ther. 2002, 1, 121–132. [Google Scholar] [CrossRef] [Green Version]
- Bicsak, T.A.; Harper, E. Purification of nonspecific protease-free collagenase from clostridium histolyticum. Anal. Biochem. 1985, 145, 286–291. [Google Scholar] [CrossRef]
- Brunengraber, L.N.; Jayes, F.L.; Leppert, P.C. Injectable Clostridium histolyticum collagenase as a potential treatment for uterine fibroids. Reprod. Sci. 2014, 21, 1452–1459. [Google Scholar] [CrossRef] [Green Version]
- Jayes, F.L.; Liu, B.; Moutos, F.T.; Kuchibhatla, M.; Guilak, F.; Leppert, P.C. Loss of stiffness in collagen-rich uterine fibroids after digestion with purified collagenase clostridium histolyticum. Am. J. Obstet. Gynecol. 2016, 215, 596.e591–596.e598. [Google Scholar] [CrossRef]
- Singh, B.; Sims, H.; Truehart, I.; Simpson, K.; Wang, K.; Patzkowsky, K.; Wegman, T.; Soma, J.M.; Dixon, R.; Jayes, F.; et al. Results of a phase 1 clinical trial to assess safety and tolerability of injectable collagenase in women with symptomatic uterine fibroids. In Meeting: The Basic Science of Uterine Fibroids; NC, 27701. 28/FEB/2020; National Institute of Environmental Health Sciences: Research Triangle Park, CA, USA.
- Rein, M.J.; Renouf, M.; Cruz-Hernandez, C.; Actis-Goretta, L.; Thakkar, S.K.; da Silva Pinto, M. Bioavailability of bioactive food compounds: A challenging journey to bioefficacy. Br. J. Clin. Pharmacol. 2013, 75, 588–602. [Google Scholar] [CrossRef] [Green Version]
- Borel, P.; Caillaud, D.; Cano, N.J. Vitamin D bioavailability: State of the art. Crit. Rev. Food Sci. Nutr. 2015, 55, 1193–1205. [Google Scholar] [CrossRef]
- Dube, A.; Nicolazzo, J.A.; Larson, I. Chitosan nanoparticles enhance the intestinal absorption of the green tea catechins (+)-catechin and (-)-epigallocatechin gallate. Eur. J. Pharm. Sci. 2010, 41, 219–225. [Google Scholar] [CrossRef]
- Granja, A.; Neves, A.R.; Sousa, C.T.; Pinheiro, M.; Reis, S. EGCG intestinal absorption and oral bioavailability enhancement using folic acid-functionalized nanostructured lipid carriers. Heliyon 2019, 5, e02020. [Google Scholar] [CrossRef] [Green Version]
- Kunnumakkara, A.B.; Harsha, C.; Banik, K.; Vikkurthi, R.; Sailo, B.L.; Bordoloi, D.; Gupta, S.C.; Aggarwal, B.B. Is curcumin bioavailability a problem in humans: Lessons from clinical trials. Expert Opin. Drug Metab. Toxicol. 2019, 15, 705–733. [Google Scholar] [CrossRef] [PubMed]
- Jamwal, R. Bioavailable curcumin formulations: A review of pharmacokinetic studies in healthy volunteers. J. Integr. Med. 2018, 16, 367–374. [Google Scholar] [CrossRef] [PubMed]
- Chimento, A.; De Amicis, F.; Sirianni, R.; Sinicropi, M.S.; Puoci, F.; Casaburi, I.; Saturnino, C.; Pezzi, V. Progress to improve oral bioavailability and beneficial effects of resveratrol. Int. J. Mol. Sci. 2019, 20. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Baenas, N.; Suarez-Martinez, C.; Garcia-Viguera, C.; Moreno, D.A. Bioavailability and new biomarkers of cruciferous sprouts consumption. Food Res. Int. 2017, 100, 497–503. [Google Scholar] [CrossRef] [PubMed]
- Boots, A.W.; Haenen, G.R.; Bast, A. Health effects of quercetin: From antioxidant to nutraceutical. Eur. J. Pharmacol. 2008, 585, 325–337. [Google Scholar] [CrossRef]
- Petyaev, I.M. Lycopene deficiency in ageing and cardiovascular disease. Oxid. Med. Cell. Longev. 2016, 2016, 3218605. [Google Scholar] [CrossRef] [Green Version]
- Sies, H.; Stahl, W. Lycopene: Antioxidant and biological effects and its bioavailability in the human. Proc. Soc. Exp. Biol. Med. 1998, 218, 121–124. [Google Scholar] [CrossRef]
- Newman, D.J.; Cragg, G.M. Natural products as sources of new drugs over the 30 years from 1981 to 2010. J. Nat. Prod. 2012, 75, 311–335. [Google Scholar] [CrossRef] [Green Version]
- Islam, M.S.; Akhtar, M.M.; Ciavattini, A.; Giannubilo, S.R.; Protic, O.; Janjusevic, M.; Procopio, A.D.; Segars, J.H.; Castellucci, M.; Ciarmela, P. Use of dietary phytochemicals to target inflammation, fibrosis, proliferation, and angiogenesis in uterine tissues: Promising options for prevention and treatment of uterine fibroids? Mol. Nutr. Food Res. 2014, 58, 1667–1684. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Clarke, T.C.; Black, L.I.; Stussman, B.J.; Barnes, P.M.; Nahin, R.L. Trends in the Use of Complementary Health Approaches Among Adults: United States, 2002-2012. In National Health Statistics Reports; No. 79; National Center for Health Statistics: Hyattsville, MD, USA, 2015. [Google Scholar]
- Lozinski, T.; Filipowska, J.; Gurynowicz, G.; Zgliczynska, M.; Kluz, T.; Jedra, R.; Skowyra, A.; Ciebiera, M. The effect of high-intensity focused ultrasound guided by magnetic resonance therapy on obstetrical outcomes in patients with uterine fibroids - experiences from the main polish center and a review of current data. Int. J. Hyperthermia 2019, 36, 582–590. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Marsh, E.E.; Ekpo, G.E.; Cardozo, E.R.; Brocks, M.; Dune, T.; Cohen, L.S. Racial differences in fibroid prevalence and ultrasound findings in asymptomatic young women (18-30 years old): A pilot study. Fertil. Steril. 2013, 99, 1951–1957. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- American College of Obstetricians and Gynecologists (ACOG). Vitamin D: Screening and supplementation during pregnancy. Committee Opinion No. 495. Obstet. Gynecol. 2011, 118, 197–198. [Google Scholar]
- Rusinska, A.; Pludowski, P.; Walczak, M.; Borszewska-Kornacka, M.K.; Bossowski, A.; Chlebna-Sokol, D.; Czech-Kowalska, J.; Dobrzanska, A.; Franek, E.; Helwich, E.; et al. Vitamin D supplementation guidelines for general population and groups at risk of vitamin D deficiency in poland-recommendations of the Polish Society of Pediatric Endocrinology and Diabetes and the expert panel with participation of national specialist consultants and representatives of scientific societies-2018 update. Front. Endocrinol. (Lausanne) 2018, 9, 246. [Google Scholar] [CrossRef]
- American College of Obstetricians and Gynecologists (ACOG). Moderate caffeine consumption during pregnancy. Committee Opinion No. 462. Obstet. Gynecol. 2010, 116, 467–468. [Google Scholar]
- Tenore, G.C.; Daglia, M.; Ciampaglia, R.; Novellino, E. Exploring the nutraceutical potential of polyphenols from black, green and white tea infusions - an overview. Curr. Pharm. Biotechnol. 2015, 16, 265–271. [Google Scholar] [CrossRef] [Green Version]
- Zhong, J.; Xu, C.; Reece, E.A.; Yang, P. The green tea polyphenol EGCG alleviates maternal diabetes-induced neural tube defects by inhibiting DNA hypermethylation. Am. J. Obstet. Gynecol. 2016, 215, 368.e1–368.e10. [Google Scholar] [CrossRef] [Green Version]
- Wu, X.K.; Wang, Y.Y.; Liu, J.P.; Liang, R.N.; Xue, H.Y.; Ma, H.X.; Shao, X.G.; Ng, E.H.; Reproductive Developmental Network in Chinese, M. Randomized controlled trial of letrozole, berberine, or a combination for infertility in the polycystic ovary syndrome. Fertil. Steril. 2016, 106, 757–765. [Google Scholar] [CrossRef] [Green Version]
- Spinozzi, S.; Colliva, C.; Camborata, C.; Roberti, M.; Ianni, C.; Neri, F.; Calvarese, C.; Lisotti, A.; Mazzella, G.; Roda, A. Berberine and its metabolites: Relationship between physicochemical properties and plasma levels after administration to human subjects. J. Nat. Prod. 2014, 77, 766–772. [Google Scholar] [CrossRef]
- National Institutes of Health (NIH). National Center for Complementary and Integrative Health. Goldenseal; 2018. Available online: http://nccih.nih.gov/health/goldenseal (accessed on 11 May 2020).
- Li, L.; Li, C.; Pan, P.; Chen, X.; Wu, X.; Ng, E.H.; Yang, D. A single arm pilot study of effects of berberine on the menstrual pattern, ovulation rate, hormonal and metabolic profiles in anovulatory chinese women with polycystic ovary syndrome. PLoS ONE 2015, 10, e0144072. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chiaffarino, F.; Parazzini, F.; La Vecchia, C.; Chatenoud, L.; Di Cintio, E.; Marsico, S. Diet and uterine myomas. Obstet. Gynecol. 1999, 94, 395–398. [Google Scholar] [CrossRef] [PubMed]
- Chen, N.N.; Han, M.; Yang, H.; Yang, G.Y.; Wang, Y.Y.; Wu, X.K.; Liu, J.P. Chinese herbal medicine guizhi fuling formula for treatment of uterine fibroids: A systematic review of randomised clinical trials. BMC Complement. Altern. Med. 2014, 14, 2. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chwalisz, K.; Taylor, H. Current and emerging medical treatments for uterine fibroids. Semin. Reprod. Med. 2017, 35, 510–522. [Google Scholar] [CrossRef] [PubMed]
- Kasinski, A.L.; Du, Y.; Thomas, S.L.; Zhao, J.; Sun, S.Y.; Khuri, F.R.; Wang, C.Y.; Shoji, M.; Sun, A.; Snyder, J.P.; et al. Inhibition of IkappaB kinase-nuclear factor-kappab signaling pathway by 3,5-bis(2-flurobenzylidene)piperidin-4-one (EF24), a novel monoketone analog of curcumin. Mol. Pharmacol. 2008, 74, 654–661. [Google Scholar] [CrossRef]

| Compound | Molecular Target |
|---|---|
| Vitamin D | MMPs inhibition Catechol-O-methyltransferase suppression TGF-β induced ECM production inhibition Wnt/β-catenin pathway inhibition Steroid receptor expression decrease Anti-inflammatory effect Apoptosis induction/proliferation inhibition |
| EGCG | MMPs inhibition Catechol-O-methyltransferase suppression Anti-inflammatory effect Apoptosis induction/proliferation inhibition BMP2 expression upregulation |
| Berberine | Cyclooxygenase 2 inhibition Anti-inflammatory effect PTTG1 inhibition Apoptosis induction |
| Curcumin | PPARγ activation TGF-β induced ECM production inhibition Apoptosis induction Anti-inflammatory effect |
| Resveratrol | MMPs inhibition ECM production inhibition Apoptosis induction |
| Fucoidan | Epithelial–mesenchymal transition inhibition ECM production inhibition Wnt/β-catenin pathway inhibition |
| Indolo-3-carbinol | ECM production inhibition Anti-inflammatory effect Apoptosis induction |
| Isoliquiritigenin | MMPs inhibition ECM production inhibition Anti-inflammatory effect Apoptosis induction |
| Quercetin | Effect on steroid receptors TGF-β induced ECM production inhibition Anti-inflammatory effect |
| Sulforaphane | Effect on TGF-β pathway Anti-inflammatory effect |
| Anthocyanins | ECM production inhibition Anti-inflammatory effect |
| Omega-3 fatty acids | Anti-inflammatory effect Lipid profile modulation |
| Methyl jasmonate | Enhancer of zeste homolog 2 inhibition ECM production inhibition Wnt/β-catenin pathway inhibition Apoptosis induction |
| Lycopene | Immunomodulation Apoptosis induction |
| Collagenase C. histolyticum | ECM degradation |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ciebiera, M.; Ali, M.; Prince, L.; Jackson-Bey, T.; Atabiekov, I.; Zgliczyński, S.; Al-Hendy, A. The Evolving Role of Natural Compounds in the Medical Treatment of Uterine Fibroids. J. Clin. Med. 2020, 9, 1479. https://doi.org/10.3390/jcm9051479
Ciebiera M, Ali M, Prince L, Jackson-Bey T, Atabiekov I, Zgliczyński S, Al-Hendy A. The Evolving Role of Natural Compounds in the Medical Treatment of Uterine Fibroids. Journal of Clinical Medicine. 2020; 9(5):1479. https://doi.org/10.3390/jcm9051479
Chicago/Turabian StyleCiebiera, Michał, Mohamed Ali, Lillian Prince, Tia Jackson-Bey, Ihor Atabiekov, Stanisław Zgliczyński, and Ayman Al-Hendy. 2020. "The Evolving Role of Natural Compounds in the Medical Treatment of Uterine Fibroids" Journal of Clinical Medicine 9, no. 5: 1479. https://doi.org/10.3390/jcm9051479






