Circulating Soluble Urokinase-Type Plasminogen Activator Receptor Levels Reflect Renal Function in Newly Diagnosed Patients with Multiple Myeloma Treated with Bortezomib-Based Induction
Abstract
:1. Introduction
2. Patients and Methods
3. Results
3.1. Patient Characteristics
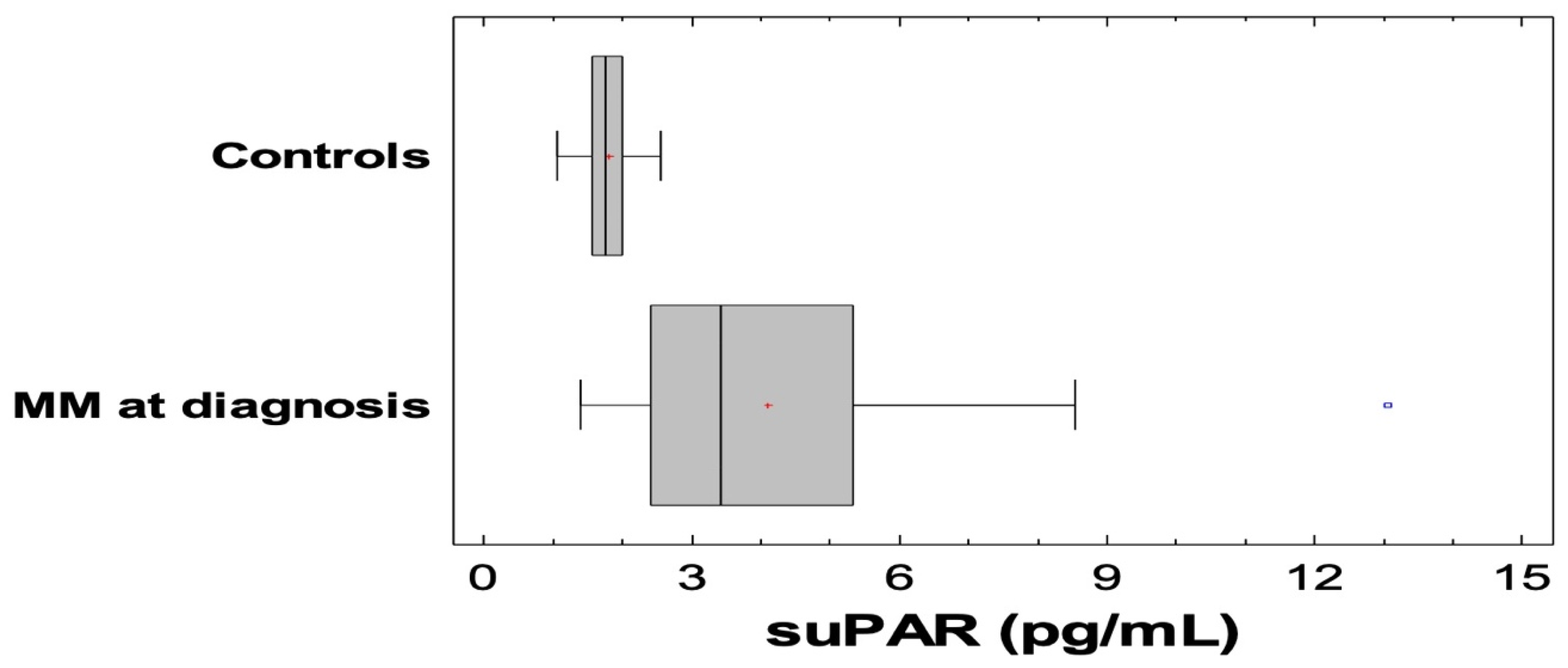
3.2. SuPAR and Other Biomarkers in MM and Controls
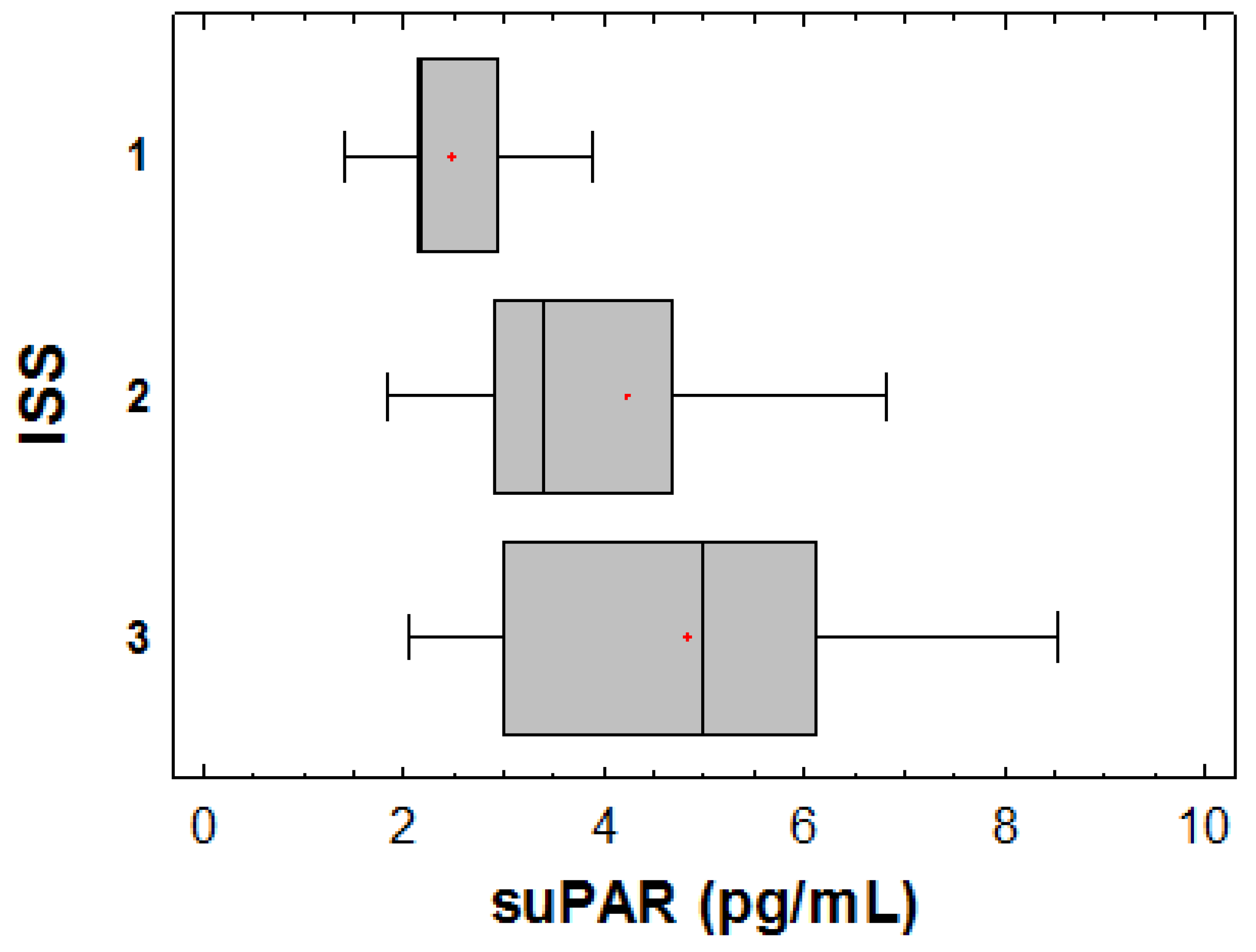
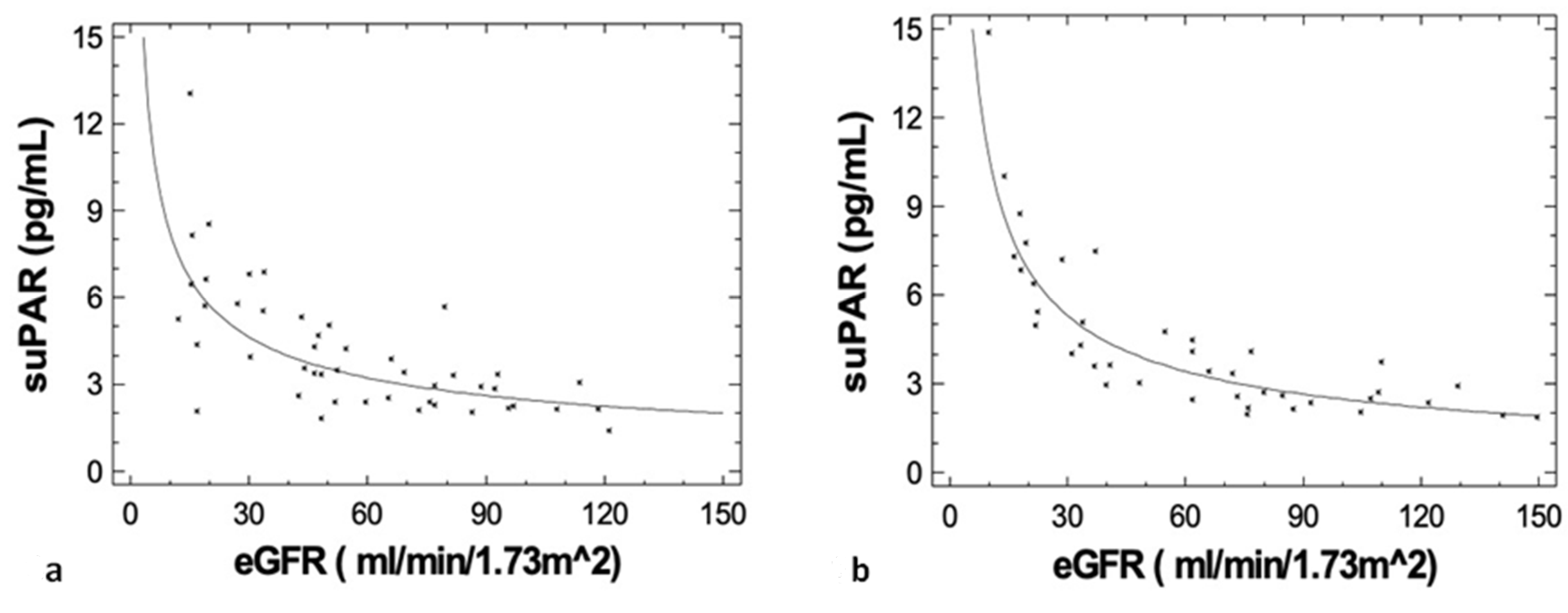
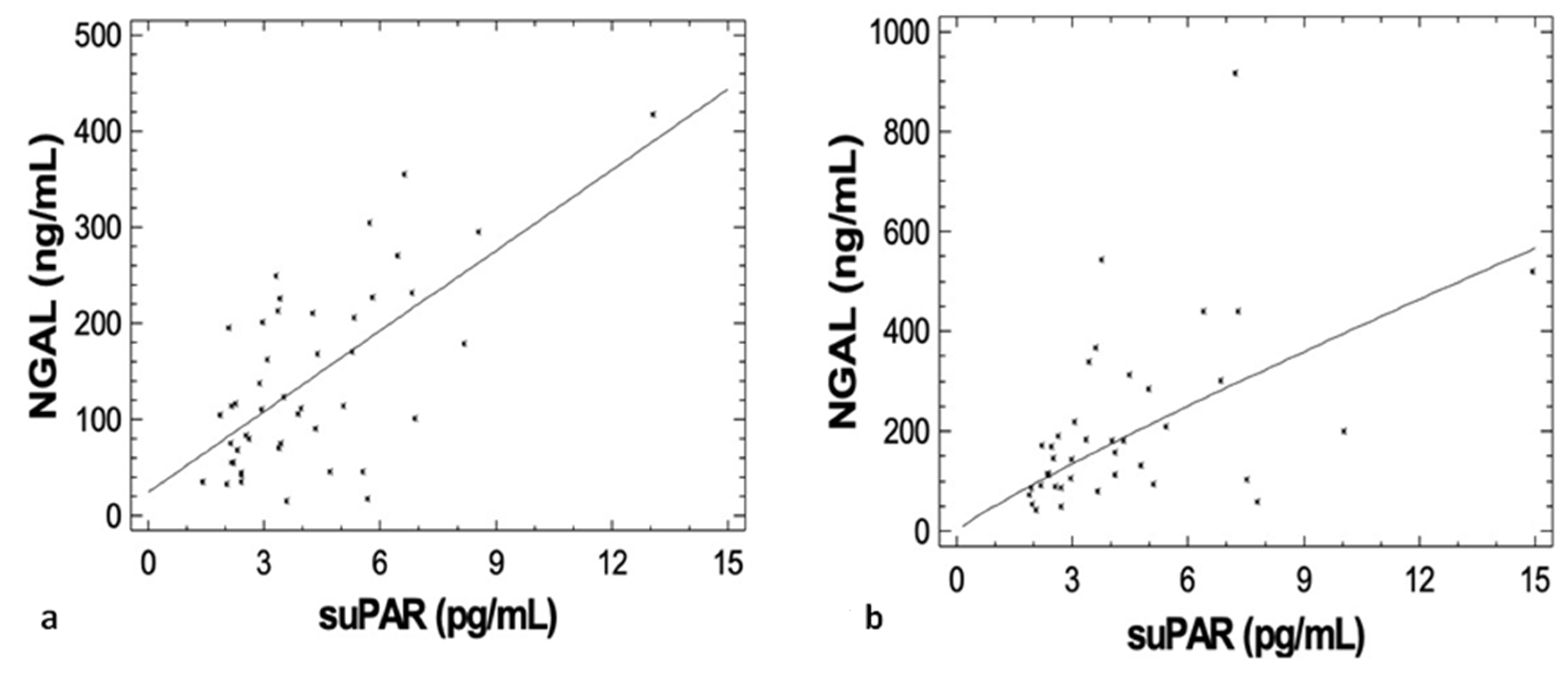
3.3. Correlations of suPAR with Disease Characteristics, Renal Function during Upfront Treatment, Cardio-Renal and Inflammatory Biomarkers
4. Discussion
Author Contributions
Funding
Conflicts of Interest
References
- Kumar, S.K.; Rajkumar, V.; Kyle, R.A.; van Duin, M.; Sonneveld, P.; Mateos, M.V.; Gay, F.; Anderson, K.C. Multiple myeloma. Nat. Rev. Dis. Primers 2017, 3, 17046. [Google Scholar] [CrossRef] [PubMed]
- Terpos, E.; Ntanasis-Stathopoulos, I.; Gavriatopoulou, M.; Dimopoulos, M.A. Pathogenesis of bone disease in multiple myeloma: From bench to bedside. Blood Cancer J. 2018, 8, 7. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hutchison, C.A.; Batuman, V.; Behrens, J.; Bridoux, F.; Sirac, C.; Dispenzieri, A.; Herrera, G.A.; Lachmann, H.; Sanders, P.W.; International, K.; et al. The pathogenesis and diagnosis of acute kidney injury in multiple myeloma. Nat. Rev. Nephrol. 2011, 8, 43–51. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tsakiris, D.J.; Stel, V.S.; Finne, P.; Fraser, E.; Heaf, J.; de Meester, J.; Schmaldienst, S.; Dekker, F.; Verrina, E.; Jager, K.J. Incidence and outcome of patients starting renal replacement therapy for end-stage renal disease due to multiple myeloma or light-chain deposit disease: An ERA-EDTA Registry study. Nephrol. Dial. Transplant. 2010, 25, 1200–1206. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Haynes, R.J.; Read, S.; Collins, G.P.; Darby, S.C.; Winearls, C.G. Presentation and survival of patients with severe acute kidney injury and multiple myeloma: A 20-year experience from a single centre. Nephrol. Dial. Transplant. 2010, 25, 419–426. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dimopoulos, M.A.; Delimpasi, S.; Katodritou, E.; Vassou, A.; Kyrtsonis, M.C.; Repousis, P.; Kartasis, Z.; Parcharidou, A.; Michael, M.; Michalis, E.; et al. Significant improvement in the survival of patients with multiple myeloma presenting with severe renal impairment after the introduction of novel agents. Ann. Oncol. 2014, 25, 195–200. [Google Scholar] [CrossRef] [PubMed]
- Hutchison, C.A.; Blade, J.; Cockwell, P.; Cook, M.; Drayson, M.; Fermand, J.P.; Kastritis, E.; Kyle, R.; Leung, N.; Pasquali, S.; et al. Novel approaches for reducing free light chains in patients with myeloma kidney. Nat. Rev. Nephrol. 2012, 8, 234–243. [Google Scholar] [CrossRef]
- Hayek, S.S.; Sever, S.; Ko, Y.A.; Trachtman, H.; Awad, M.; Wadhwani, S.; Altintas, M.M.; Wei, C.; Hotton, A.L.; French, A.L.; et al. Soluble Urokinase Receptor and Chronic Kidney Disease. N. Engl. J. Med. 2015, 373, 1916–1925. [Google Scholar] [CrossRef]
- Trimarchi, H. Primary focal and segmental glomerulosclerosis and soluble factor urokinase-type plasminogen activator receptor. World J. Nephrol. 2013, 2, 103–110. [Google Scholar] [CrossRef]
- Huai, Q.; Mazar, A.P.; Kuo, A.; Parry, G.C.; Shaw, D.E.; Callahan, J.; Li, Y.; Yuan, C.; Bian, C.; Chen, L.; et al. Structure of human urokinase plasminogen activator in complex with its receptor. Science 2006, 311, 656–659. [Google Scholar] [CrossRef]
- Hayek, S.S.; Leaf, D.E.; Samman Tahhan, A.; Raad, M.; Sharma, S.; Waikar, S.S.; Sever, S.; Camacho, A.; Wang, X.; Dande, R.R.; et al. Soluble Urokinase Receptor and Acute Kidney Injury. N. Engl. J. Med. 2020, 382, 416–426. [Google Scholar] [CrossRef] [PubMed]
- Hayek, S.S.; Koh, K.H.; Grams, M.E.; Wei, C.; Ko, Y.A.; Li, J.; Samelko, B.; Lee, H.; Dande, R.R.; Lee, H.W.; et al. A tripartite complex of suPAR, APOL1 risk variants and alphavbeta3 integrin on podocytes mediates chronic kidney disease. Nat. Med. 2017, 23, 945–953. [Google Scholar] [CrossRef] [PubMed]
- Mahmood, N.; Mihalcioiu, C.; Rabbani, S.A. Multifaceted Role of the Urokinase-Type Plasminogen Activator (uPA) and Its Receptor (uPAR): Diagnostic, Prognostic, and Therapeutic Applications. Front. Oncol. 2018, 12, 24. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dimopoulos, M.A.; Sonneveld, P.; Leung, N.; Merlini, G.; Ludwig, H.; Kastritis, E.; Goldschmidt, H.; Joshua, D.; Orlowski, R.Z.; Powles, R.; et al. International Myeloma Working Group Recommendations for the Diagnosis and Management of Myeloma-Related Renal Impairment. J. Clin. Oncol. 2016, 34, 1544–1557. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kumar, S.; Paiva, B.; Anderson, K.C.; Durie, B.; Landgren, O.; Moreau, P.; Munshi, N.; Lonial, S.; Blade, J.; Mateos, M.V.; et al. International Myeloma Working Group consensus criteria for response and minimal residual disease assessment in multiple myeloma. Lancet Oncol. 2016, 17, e328–e346. [Google Scholar] [CrossRef]
- Inker, L.A.; Schmid, C.H.; Tighiouart, H.; Eckfeldt, J.H.; Feldman, H.I.; Greene, T.; Kusek, J.W.; Manzi, J.; Van Lente, F.; Zhang, Y.L.; et al. Estimating glomerular filtration rate from serum creatinine and cystatin C. N. Engl. J. Med. 2012, 367, 20–29. [Google Scholar] [CrossRef] [Green Version]
- Musolino, C.; Allegra, A.; Innao, V.; Allegra, A.G.; Pioggia, G.; Gangemi, S. Inflammatory and Anti-Inflammatory Equilibrium, Proliferative and Antiproliferative Balance: The Role of Cytokines in Multiple Myeloma. Mediat. Inflamm. 2017, 2017, 1852517. [Google Scholar] [CrossRef] [Green Version]
- Ni, W.; Han, Y.; Zhao, J.; Cui, J.; Wang, K.; Wang, R.; Liu, Y. Serum soluble urokinase-type plasminogen activator receptor as a biological marker of bacterial infection in adults: A systematic review and meta-analysis. Sci. Rep. 2016, 6, 39481. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Q.; Li, L.; Chen, H.; Zhang, G.; Zhu, S.; Kong, R.; Chen, H.; Wang, G.; Sun, B. Soluble urokinase plasminogen activator receptor associates with higher risk, advanced disease severity as well as inflammation, and might serve as a prognostic biomarker of severe acute pancreatitis. J. Clin. Lab. Anal. 2020, 34, e23097. [Google Scholar] [CrossRef]
- Eugen-Olsen, J.; Andersen, O.; Linneberg, A.; Ladelund, S.; Hansen, T.W.; Langkilde, A.; Petersen, J.; Pielak, T.; Moller, L.N.; Jeppesen, J.; et al. Circulating soluble urokinase plasminogen activator receptor predicts cancer, cardiovascular disease, diabetes and mortality in the general population. J. Intern. Med. 2010, 268, 296–308. [Google Scholar] [CrossRef]
- Rasmussen, L.J.; Ladelund, S.; Haupt, T.H.; Ellekilde, G.; Poulsen, J.H.; Iversen, K.; Eugen-Olsen, J.; Andersen, O. Soluble urokinase plasminogen activator receptor (suPAR) in acute care: A strong marker of disease presence and severity, readmission and mortality. A retrospective cohort study. Emerg. Med. J. EMJ 2016, 33, 769–775. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Petersen, J.E.V.; Kallemose, T.; Barton, K.D.; Caspi, A.; Rasmussen, L.J.H. Soluble urokinase plasminogen activator receptor (suPAR) as a prognostic marker of mortality in healthy, general and patient populations: Protocol for a systematic review and meta-analysis. BMJ Open 2020, 10, e036125. [Google Scholar] [CrossRef] [PubMed]
- Hjertner, O.; Qvigstad, G.; Hjorth-Hansen, H.; Seidel, C.; Woodliff, J.; Epstein, J.; Waage, A.; Sundan, A.; Borset, M. Expression of urokinase plasminogen activator and the urokinase plasminogen activator receptor in myeloma cells. Br. J. Haematol. 2000, 109, 815–822. [Google Scholar] [CrossRef] [PubMed]
- Asimakopoulos, F.; Hope, C.; Johnson, M.G.; Pagenkopf, A.; Gromek, K.; Nagel, B. Extracellular matrix and the myeloid-in-myeloma compartment: Balancing tolerogenic and immunogenic inflammation in the myeloma niche. J. Leukoc. Biol. 2017, 102, 265–275. [Google Scholar] [CrossRef]
- Hahm, E.; Wei, C.; Fernandez, I.; Li, J.; Tardi, N.J.; Tracy, M.; Wadhwani, S.; Cao, Y.; Peev, V.; Zloza, A.; et al. Bone marrow-derived immature myeloid cells are a main source of circulating suPAR contributing to proteinuric kidney disease. Nat. Med. 2017, 23, 100–106. [Google Scholar] [CrossRef] [Green Version]
- Bene, M.C.; Castoldi, G.; Knapp, W.; Rigolin, G.M.; Escribano, L.; Lemez, P.; Ludwig, W.D.; Matutes, E.; Orfao, A.; Lanza, F.; et al. CD87 (urokinase-type plasminogen activator receptor), function and pathology in hematological disorders: A review. Leukemia 2004, 18, 394–400. [Google Scholar] [CrossRef]
- Rigolin, G.M.; Tieghi, A.; Ciccone, M.; Bragotti, L.Z.; Cavazzini, F.; Della Porta, M.; Castagnari, B.; Carroccia, R.; Guerra, G.; Cuneo, A.; et al. Soluble urokinase-type plasminogen activator receptor (suPAR) as an independent factor predicting worse prognosis and extra-bone marrow involvement in multiple myeloma patients. Br. J. Haematol. 2003, 120, 953–959. [Google Scholar] [CrossRef]
- Rubio-Jurado, B.; Tello-Gonzalez, A.; Bustamante-Chavez, L.; de la Pena, A.; Riebeling-Navarro, C.; Nava-Zavala, A.H. Circulating Levels of Urokinase-Type Plasminogen Activator Receptor and D-Dimer in Patients With Hematological Malignancies. Clin. Lymphoma Myeloma Leuk. 2015, 15, 621–626. [Google Scholar] [CrossRef]
- Zhuang, T.; Chelluboina, B.; Ponnala, S.; Velpula, K.; Rehman, A.; Chetty, C.; Zakharian, E.; Rao, J.; Veeravalli, K. Involvement of nitric oxide synthase in matrix metalloproteinase-9- and/or urokinase plasminogen activator receptor-mediated glioma cell migration. BMC Cancer 2013, 11, 590. [Google Scholar] [CrossRef] [Green Version]
- Shen, J.; Wang, Q.; Wang, J.; Su, G.H.; Wang, J.; Guo, S.H.; Liu, Y.A.; Wu, Z.; Liu, R.F.; Li, X.; et al. Analysis of soluble urokinase plasminogen activator receptor in multiple myeloma for predicting prognosis. Oncol. Lett. 2015, 10, 2403–2409. [Google Scholar] [CrossRef]
- Kastritis, E.; Papassotiriou, I.; Roussou, M.; Gavriatopoulou, M.; Mantzou, A.; Psimenou, E.; Marinaki, S.; Gakiopoulou, C.; Margeli, A.; Kanellias, N.; et al. Soluble Urokinase-Type Plasminogen Activator Receptor (suPAR) Is a Renal Biomarker with Potential Clinical Applications in Monoclonal Gammopathy of Renal Significance (MGRS). Blood 2019, 134 (Suppl. S1), 3126. [Google Scholar] [CrossRef]
- Gavriatopoulou, M.; Terpos, E.; Kastritis, E.; Dimopoulos, M.A. Current treatments for renal failure due to multiple myeloma. Expert Opin. Pharmacother. 2016, 17, 2165–2177. [Google Scholar] [CrossRef] [PubMed]
- Kastritis, E.; Kanellias, N.; Theodorakakou, F.; Psimenou, E.; Gakiopoulou, C.; Marinaki, S.; Roussou, M.; Gavriatopoulou, M.; Migkou, M.; Fotiou, D.; et al. Renal pathology in patients with monoclonal gammopathy or multiple myeloma: Monoclonal immunoglobulins are not always the cause. Leuk. Lymphoma 2020, 1–4. [Google Scholar] [CrossRef] [PubMed]
- Martinez-Sanchez, J.; Palomo, M.; Torramade-Moix, S.; Moreno-Castaño, A.; Rovira, M.; Gutiérrez-García, G.; Fernández-Avilés, F.; Escolar, G.; Penack, O.; Rosiñol, L.; et al. The induction strategies administered in the treatment of multiple myeloma exhibit a deleterious effect on the endothelium. Bone Marrow Transplant. 2020. [Google Scholar] [CrossRef] [PubMed]
- Piccin, A.; Sartori, M.; Bisogno, G.; Van Schilfgaarde, M.; Saggiorato, G.; Pierro, A.; Corvetta, D.; Marcheselli, L.; Andrea, M.; Gastl, G.; et al. New insights into sinusoidal obstruction syndrome. Intern. Med. J. 2017, 47, 1173–1183. [Google Scholar] [CrossRef] [PubMed]
- Dimopoulos, M.; Roussou, M.; Gavriatopoulou, M.; Psimenou, E.; Ziogas, D.; Eleutherakis-Papaiakovou, E.; Fotiou, D.; Migkou, M.; Kanellias, N.; Panagiotidis, I.; et al. Cardiac and renal complications of carfilzomib in patients with multiple myeloma. Blood Adv. 2017, 1, 449–454. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hutchison, C.A.; Cockwell, P.; Stringer, S.; Bradwell, A.; Cook, M.; Gertz, M.A.; Dispenzieri, A.; Winters, J.L.; Kumar, S.; Rajkumar, S.V.; et al. Early reduction of serum-free light chains associates with renal recovery in myeloma kidney. J. Am. Soc. Nephrol. JASN 2011, 22, 1129–1136. [Google Scholar] [CrossRef] [Green Version]
- Basnayake, K.; Cheung, C.K.; Sheaff, M.; Fuggle, W.; Kamel, D.; Nakoinz, S.; Hutchison, C.A.; Cook, M.; Stoves, J.; Bradwell, A.R.; et al. Differential progression of renal scarring and determinants of late renal recovery in sustained dialysis dependent acute kidney injury secondary to myeloma kidney. J. Clin. Pathol. 2010, 63, 884–887. [Google Scholar] [CrossRef]
- Hutchison, C.A.; Bridoux, F. Renal impairment in multiple myeloma: Time is of the essence. J. Clin. Oncol. 2011, 29, e312–e313, author reply e314. [Google Scholar] [CrossRef]
- Kastritis, E.; Fotiou, D.; Theodorakakou, F.; Dialoupi, I.; Migkou, M.; Roussou, M.; Karatrasoglou, E.A.; Tselegkidi, M.I.; Ntalianis, A.; Kanellias, N.; et al. Timing and impact of a deep response in the outcome of patients with systemic light chain (AL) amyloidosis. Amyloid 2020, 1–9. [Google Scholar] [CrossRef]
- Papassotiriou, G.P.; Kastritis, E.; Gkotzamanidou, M.; Christoulas, D.; Eleutherakis-Papaiakovou, E.; Migkou, M.; Gavriatopoulou, M.; Roussou, M.; Margeli, A.; Papassotiriou, I.; et al. Neutrophil Gelatinase—Associated Lipocalin and Cystatin C Are Sensitive Markers of Renal Injury in Patients With Multiple Myeloma. Clin. Lymphoma Myeloma Leuk. 2016, 16, 29–35. [Google Scholar] [CrossRef] [PubMed]
- Dimopoulos, M.A.; Roussou, M.; Gavriatopoulou, M.; Zagouri, F.; Migkou, M.; Matsouka, C.; Barbarousi, D.; Christoulas, D.; Primenou, E.; Grapsa, I.; et al. Reversibility of renal impairment in patients with multiple myeloma treated with bortezomib-based regimens: Identification of predictive factors. Clin. Lymphoma Myeloma 2009, 9, 302–306. [Google Scholar] [CrossRef]
- Terpos, E.; Katodritou, E.; Tsiftsakis, E.; Kastritis, E.; Christoulas, D.; Pouli, A.; Michalis, E.; Verrou, E.; Anargyrou, K.; Tsionos, K.; et al. Cystatin-C is an independent prognostic factor for survival in multiple myeloma and is reduced by bortezomib administration. Haematologica 2009, 94, 372–379. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bargnoux, A.S.; Kuster, N.; Morena, M.; Baptista, G.; Chenine, L.; Badiou, S.; Leray, H.; Dupuy, A.M.; Cristol, J.P. How to interpret cardiac biomarkers in renal failure and elderly? Ann. Biol. Clin. 2016, 74, 413–419. [Google Scholar] [CrossRef] [PubMed]
- Terpos, E.; Christoulas, D.; Kastritis, E.; Katodritou, E.; Pouli, A.; Michalis, E.; Papassotiriou, I.; Dimopoulos, M.A.; Greek Myeloma Study, G. The Chronic Kidney Disease Epidemiology Collaboration cystatin C (CKD-EPI-CysC) equation has an independent prognostic value for overall survival in newly diagnosed patients with symptomatic multiple myeloma; is it time to change from MDRD to CKD-EPI-CysC equations? Eur. J. Haematol. 2013, 91, 347–355. [Google Scholar] [CrossRef] [PubMed]
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Terpos, E.; Ntanasis-Stathopoulos, I.; Papassotiriou, G.-P.; Kastritis, E.; Margeli, A.; Kanellias, N.; Eleutherakis-Papaiakovou, E.; Migkou, M.; Fotiou, D.; Roussou, M.; et al. Circulating Soluble Urokinase-Type Plasminogen Activator Receptor Levels Reflect Renal Function in Newly Diagnosed Patients with Multiple Myeloma Treated with Bortezomib-Based Induction. J. Clin. Med. 2020, 9, 3201. https://doi.org/10.3390/jcm9103201
Terpos E, Ntanasis-Stathopoulos I, Papassotiriou G-P, Kastritis E, Margeli A, Kanellias N, Eleutherakis-Papaiakovou E, Migkou M, Fotiou D, Roussou M, et al. Circulating Soluble Urokinase-Type Plasminogen Activator Receptor Levels Reflect Renal Function in Newly Diagnosed Patients with Multiple Myeloma Treated with Bortezomib-Based Induction. Journal of Clinical Medicine. 2020; 9(10):3201. https://doi.org/10.3390/jcm9103201
Chicago/Turabian StyleTerpos, Evangelos, Ioannis Ntanasis-Stathopoulos, Gerasimos-Petros Papassotiriou, Efstathios Kastritis, Alexandra Margeli, Nikolaos Kanellias, Evangelos Eleutherakis-Papaiakovou, Magdalini Migkou, Despina Fotiou, Maria Roussou, and et al. 2020. "Circulating Soluble Urokinase-Type Plasminogen Activator Receptor Levels Reflect Renal Function in Newly Diagnosed Patients with Multiple Myeloma Treated with Bortezomib-Based Induction" Journal of Clinical Medicine 9, no. 10: 3201. https://doi.org/10.3390/jcm9103201





