Pulmonary Rehabilitation Reduces Subjective Fatigue in COPD: A Responder Analysis
Abstract
:1. Introduction
2. Methods
2.1. Study Design and Participants
2.2. Pulmonary Rehabilitation
2.3. Assessments and Questionnaires
2.3.1. Demographical Features
2.3.2. Clinical Features
2.3.3. Health Status and Clinical Features Obtained via NCSI
2.3.4. Collection of Post-PR Data
2.4. Statistical Analysis
3. Results
3.1. Patient Characteristics
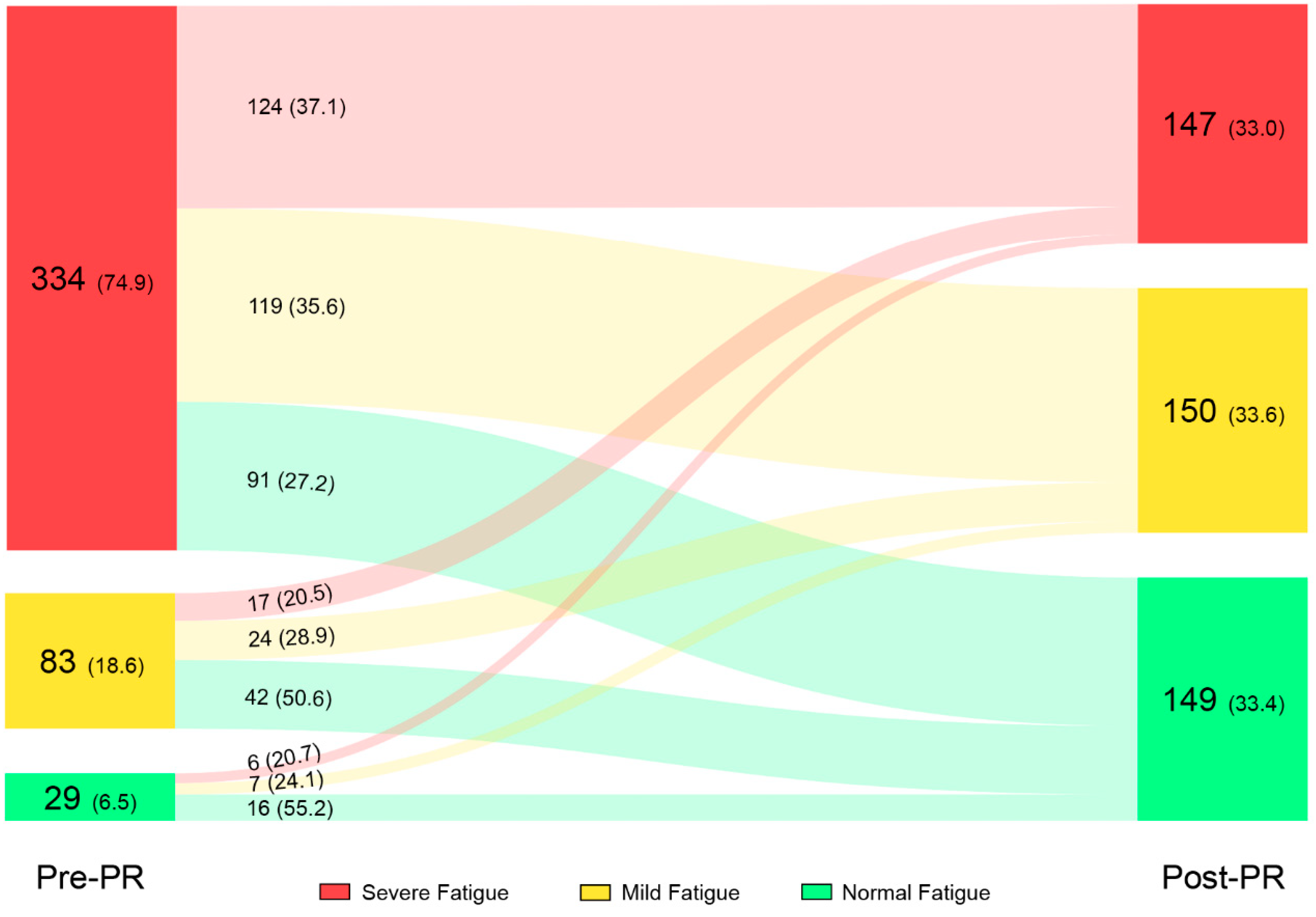
3.2. Effects of PR on Subjective Fatigue
3.3. Effects of PR on Other Outcome Measures
3.4. Relationship between Change in Fatigue and Change in Other Outcomes
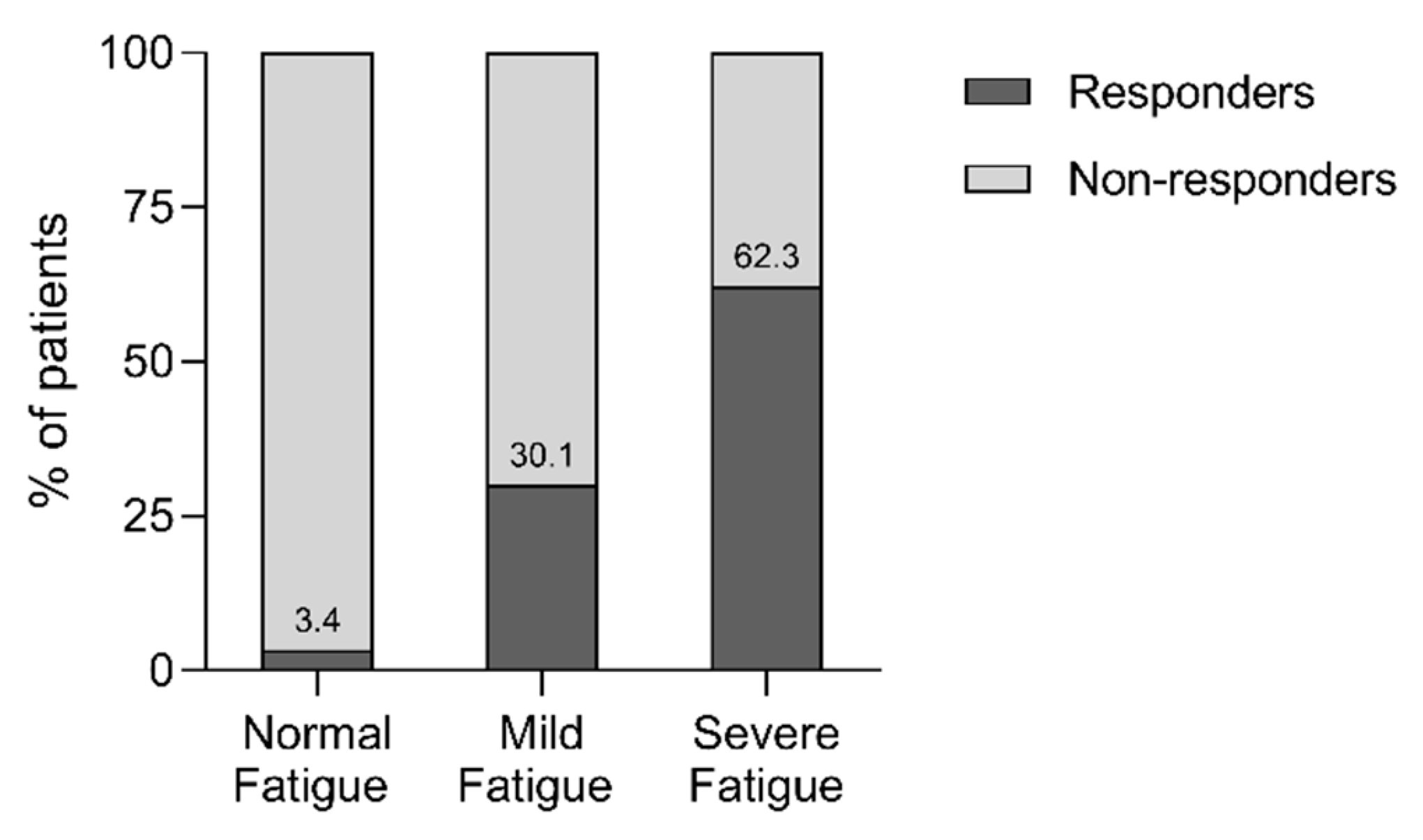
3.5. Responders versus Non-Responders on Fatigue
3.6. Responder Analysis
4. Discussion
Strengths, Limitations, and Clinical Implications
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Global Initiative for Chronic Obstructive Lung Disease. Global Strategy for the Diagnosis, Management, and Prevention of Chronic Obstructive Pulmonary Disease (2018 Report). Available online: https://goldcopd.org/wp-content/uploads/2017/11/GOLD-2018-v6.0-FINAL-revised-20-Nov_WMS.pdf (accessed on 7 November 2018).
- Kessler, R.; Partridge, M.R.; Miravitlles, M.; Cazzola, M.; Vogelmeier, C.; Leynaud, D.; Ostinelli, J. Symptom variability in patients with severe COPD: A pan-European cross-sectional study. Eur. Respir. J. 2011, 37, 264–272. [Google Scholar] [CrossRef] [PubMed]
- Goërtz, Y.M.J.; Spruit, M.A.; Van’t Hul, A.J.; Vercoulen, J.H.; Van Herck, M.; Nakken, N.; Djamin, R.S.; Burtin, C.; Thong, M.S.Y.; Coors, A.; et al. Fatigue is highly prevalent in patients with COPD and correlates poorly with the degree of airflow limitation. Ther. Adv. Respir. Dis 2019, in press. [Google Scholar]
- Small, S.; Lamb, M. Fatigue in chronic illness: The experience of individuals with chronic obstructive pulmonary disease and with asthma. J. Adv. Nurs. 1999, 30, 469–478. [Google Scholar] [CrossRef] [PubMed]
- Kentson, M.; Tödt, K.; Skargren, E.; Jakobsson, P.; Ernerudh, J.; Unosson, M.; Theander, K. Factors associated with experience of fatigue, and functional limitations due to fatigue in patients with stable COPD. Ther. Adv. Respir. Dis. 2016, 10, 410–424. [Google Scholar] [CrossRef] [PubMed]
- Todt, K.; Skargren, E.; Jakobsson, P.; Theander, K.; Unosson, M. Factors associated with low physical activity in patients with chronic obstructive pulmonary disease: A cross-sectional study. Scand. J. Caring Sci. 2015, 29, 697–707. [Google Scholar] [CrossRef]
- Kapella, M.C.; Larson, J.L.; Patel, M.K.; Covey, M.K.; Berry, J.K. Subjective fatigue, influencing variables, and consequences in chronic obstructive pulmonary disease. Nurs. Res. 2006, 55, 10–17. [Google Scholar] [CrossRef] [PubMed]
- Kouijzer, M.; Brusse-Keizer, M.; Bode, C. COPD-related fatigue: Impact on daily life and treatment opportunities from the patient’s perspective. Respir. Med. 2018, 141, 47–51. [Google Scholar] [CrossRef] [PubMed]
- Stridsman, C.; Skär, L.; Hedman, L.; Rönmark, E.; Lindberg, A. Fatigue Affects Health Status and Predicts Mortality Among Subjects with COPD: Report from the Population-Based OLIN COPD Study. J. Chronic Obstr. Pulm. Dis. 2015, 12, 199–206. [Google Scholar] [CrossRef]
- Baghai-Ravary, R.; Quint, J.K.; Goldring, J.J.; Hurst, J.R.; Donaldson, G.C.; Wedzicha, J.A. Determinants and impact of fatigue in patients with chronic obstructive pulmonary disease. Respir. Med. 2009, 103, 216–223. [Google Scholar] [CrossRef] [Green Version]
- Paddison, J.S.; Effing, T.W.; Quinn, S.; Frith, P.A. Fatigue in COPD: Association with functional status and hospitalisations. Eur. Respir. J. 2013, 41, 565–570. [Google Scholar] [CrossRef]
- Postma, D.S.; Wijkstra, P.J.; Hiemstra, P.S.; Melgert, B.N.; Braunstahl, G.J.; Hylkema, M.N.; Sterk, P.J. The Dutch National Program for Respiratory Research. Lancet Respir. Med. 2016, 4, 356–357. [Google Scholar] [CrossRef]
- Peters, J.B.; Heijdra, Y.F.; Daudey, L.; Boer, L.M.; Molema, J.; Dekhuijzen, P.R.; Schermer, T.R.; Vercoulen, J.H. Course of normal and abnormal fatigue in patients with chronic obstructive pulmonary disease, and its relationship with domains of health status. Patient Educ. Couns. 2011, 85, 281–285. [Google Scholar] [CrossRef]
- Spruit, M.A.; Wouters, E.F.M. Organizational aspects of pulmonary rehabilitation in chronic respiratory diseases. Respirology 2019, 24, 838–843. [Google Scholar] [CrossRef] [PubMed]
- Spruit, M.A.; Singh, S.J.; Garvey, C.; ZuWallack, R.; Nici, L.; Rochester, C.; Hill, K.; Holland, A.E.; Lareau, S.C.; Man, W.D.C.; et al. An official American Thoracic Society/European Respiratory Society statement: Key concepts and advances in pulmonary rehabilitation. Am. J. Respir. Crit. Care Med. 2013, 188, e13–e64. [Google Scholar] [CrossRef] [PubMed]
- McCarthy, B.; Casey, D.; Devane, D.; Murphy, K.; Murphy, E.; Lacasse, Y. Pulmonary rehabilitation for chronic obstructive pulmonary disease. Cochrane Database Syst. Rev. 2015, 2, Cd003793. [Google Scholar]
- Houben-Wilke, S.; Janssen, D.J.; Franssen, F.M.; Vanfleteren, L.E.; Wouters, E.F.; Spruit, M.A. Contribution of individual COPD assessment test (CAT) items to CAT total score and effects of pulmonary rehabilitation on CAT scores. Health Qual. Life Outcomes 2018, 16, 205. [Google Scholar] [CrossRef] [PubMed]
- Vercoulen, J.H.; Swanink, C.M.; Fennis, J.F.; Galama, J.M.; van der Meer, J.W.; Bleijenberg, G. Dimensional assessment of chronic fatigue syndrome. J. Psychosom. Res. 1994, 38, 383–392. [Google Scholar] [CrossRef] [Green Version]
- Peters, J.B.; Boer, L.M.; Molema, J.; Heijdra, Y.F.; Prins, J.B.; Vercoulen, J.H. Integral Health Status-Based Cluster Analysis in Moderate-Severe COPD Patients Identifies Three Clinical Phenotypes: Relevant for Treatment As Usual and Pulmonary Rehabilitation. Int. J. Behav. Med. 2017, 24, 571–583. [Google Scholar] [CrossRef] [PubMed]
- Verhage, F. Intelligence and Age; Van Gorcum: Assen, The Netherlands, 1964. (In Dutch) [Google Scholar]
- Landbo, C.; Prescott, E.V.; Lange, P.; Vestbo, J.; Almdal, T.P. Prognostic value of nutritional status in chronic obstructive pulmonary disease. Am. J. Respir. Crit. Care Med. 1999, 160, 1856–1861. [Google Scholar] [CrossRef]
- World Health Organization. Physical status: The use and interpretation of anthropometry. Report of a WHO Expert Committee. In WHO Technical Report Series 854; World Health Organization: Geneva, Switzerland, 1995. [Google Scholar]
- Holland, A.E.; Spruit, M.A.; Troosters, T.; Puhan, M.A.; Pepin, V.; Saey, D.; McCormack, M.C.; Carlin, B.W.; Sciurba, F.C.; Pitta, F.; et al. An official European Respiratory Society/American Thoracic Society technical standard: Field walking tests in chronic respiratory disease. Eur. Respir. J. 2014, 44, 1428–1446. [Google Scholar] [CrossRef]
- Troosters, T.; Gosselink, R.; Decramer, M. Six minute walking distance in healthy elderly subjects. Eur. Respir. J. 1999, 14, 270–274. [Google Scholar] [CrossRef] [Green Version]
- Koolen, E.H.; van Hees, H.W.; van Lummel, R.C.; Dekhuijzen, R.; Djamin, R.S.; Spruit, M.A.; van’t Hul, A.J. “Can do” versus “do do”: A Novel Concept to Better Understand Physical Functioning in Patients with Chronic Obstructive Pulmonary Disease. J. Clin. Med. 2019, 8, 340. [Google Scholar] [CrossRef] [PubMed]
- Singh, S.J.; Puhan, M.A.; Andrianopoulos, V.; Hernandes, N.A.; Mitchell, K.E.; Hill, C.J.; Lee, A.L.; Camillo, C.A.; Troosters, T.; Spruit, M.A.; et al. An official systematic review of the European Respiratory Society/American Thoracic Society: Measurement properties of field walking tests in chronic respiratory disease. Eur. Respir. J. 2014, 44, 1447–1478. [Google Scholar] [CrossRef]
- Carrozzino, D.; Vassend, O.; Bjørndal, F.; Pignolo, C.; Olsen, L.R.; Bech, P. A clinimetric analysis of the Hopkins Symptom Checklist (SCL-90-R) in general population studies (Denmark, Norway, and Italy). Nord. J. Psychiatry 2016, 70, 374–379. [Google Scholar] [CrossRef]
- Derogatis, L.R.; Fitzpatrick, M.; Maruish, M.E. The Use of Psychological Testing for Treatment Planning and Outcomes Assessment, 3rd ed.; Instruments for Adults The SCL-90-R, the Brief Symptom Inventory (BSI), and the BSI-18; Lawrence Erlbaum Associates Publishers: Mahwah, NJ, USA, 2004; Volume 3. [Google Scholar]
- Arrindell, W.A.; Ettema, J.H.M. SCL-90: Handleiding Bij Een Multidimensionele Psychopathologie-Indicator; Swets & Zeitlinger: Lisse, The Netherlands, 1986. [Google Scholar]
- Kloens, G.J.; Barelds, D.P.H.; Luteijn, F.; Schaap, C.P.D.R. De waarde van enige vragenlijsten in de eerstelijn. Diagn. Wijzer 2002, 5, 130–148. [Google Scholar]
- Vercoulen, J.H.; Daudey, L.; Molema, J.; Vos, P.J.; Peters, J.B.; Top, M.; Folgering, H. An Integral assessment framework of health status in chronic obstructive pulmonary disease (COPD). Int. J. Behav. Med. 2008, 15, 263–279. [Google Scholar] [CrossRef]
- Peters, J.B.; Daudey, L.; Heijdra, Y.F.; Molema, J.; Dekhuijzen, P.R.; Vercoulen, J.H. Development of a battery of instruments for detailed measurement of health status in patients with COPD in routine care: The Nijmegen Clinical Screening Instrument. Qual. Life Res. 2009, 18, 901–912. [Google Scholar] [CrossRef] [PubMed]
- Maille, A.R.; Koning, C.J.; Zwinderman, A.H.; Willems, L.N.; Dijkman, J.H.; Kaptein, A.A. The development of the ‘Quality-of-life for Respiratory Illness Questionnaire (QOL-RIQ)’: A disease-specific quality-of-life questionnaire for patients with mild to moderate chronic non-specific lung disease. Respir. Med. 1997, 91, 297–309. [Google Scholar] [CrossRef]
- Van Stel, H.F.; Maillé, A.R.; Colland, V.T.; Everaerd, W. Interpretation of change and longitudinal validity of the quality of life for respiratory illness questionnaire (QoLRIQ) in inpatient pulmonary rehabilitation. Qual. Life Res. 2003, 12, 133–145. [Google Scholar] [CrossRef]
- Bergner, M.; Bobbitt, R.A.; Carter, W.B.; Gilson, B.S. The Sickness Impact Profile: Development and final revision of a health status measure. Med. Care 1981, 19, 787–805. [Google Scholar] [CrossRef]
- Beck, A.T.; Guth, D.; Steer, R.A.; Ball, R. Screening for major depression disorders in medical inpatients with the Beck Depression Inventory for Primary Care. Behav. Res. Ther. 1997, 35, 785–791. [Google Scholar] [CrossRef]
- Diener, E.; Emmons, R.A.; Larsen, R.J.; Griffin, S. The Satisfaction With Life Scale. J. Personal. Assess. 1985, 49, 71–75. [Google Scholar] [CrossRef] [PubMed]
- Beurskens, A.J.; Bültmann, U.; Kant, I.; Vercoulen, J.H.; Bleijenberg, G.; Swaen, G.M. Fatigue among working people: Validity of a questionnaire measure. Occup. Environ. Med. 2000, 57, 353–357. [Google Scholar] [CrossRef]
- Worm-Smeitink, M.; Gielissen, M.; Bloot, L.; Van Laarhoven, H.W.; Van Engelen, B.G.; Van Riel, P.; Bleijenberg, G.; Nikolaus, S.; Knoop, H. The assessment of fatigue: Psychometric qualities and norms for the Checklist individual strength. J. Psychosom. Res. 2017, 98, 40–46. [Google Scholar] [CrossRef] [PubMed]
- Van Herck, M.; Spruit, M.A.; Burtin, C.; Djamin, R.; Antons, J.; Goërtz, Y.M.J.; Ebadi, Z.; Janssen, D.J.A.; Vercoulen, J.H.; Peters, J.B.; et al. Fatigue is Highly Prevalent in Patients with Asthma and Contributes to the Burden of Disease. J. Clin. Med. 2018, 7, 471. [Google Scholar] [CrossRef] [PubMed]
- Vercoulen, J.H.M.M.; Alberts, M.; Bleijenberg, G. De Checklist Individual Strength (CIS). Gedragstherapie 1999, 32, 131–136. [Google Scholar]
- Steer, R.A.; Cavalieri, T.A.; Leonard, D.M.; Beck, A.T. Use of the Beck Depression Inventory for Primary Care to screen for major depression disorders. Gen. Hosp. Psychiatry 1999, 21, 106–111. [Google Scholar] [CrossRef]
- Cohen, J. Statistical Power Analysis for the Behavioral Sciences; Routledge Academic: New York, NY, USA, 1988. [Google Scholar]
- Wong, C.J.; Goodridge, D.; Marciniuk, D.D.; Rennie, D. Fatigue in patients with COPD participating in a pulmonary rehabilitation program. Int J Chronic Obstr. Pulm. Dis. 2010, 5, 319–326. [Google Scholar] [CrossRef]
- Chen, Y.W.; Camp, P.G.; Coxson, H.O.; Road, J.D.; Guenette, J.A.; Hunt, M.A.; Reid, W.D. A Comparison of Pain, Fatigue, Dyspnea and their Impact on Quality of Life in Pulmonary Rehabilitation Participants with Chronic Obstructive Pulmonary Disease. J. Chronic Obstr. Pulm. Dis. 2018, 15, 65–72. [Google Scholar] [CrossRef] [PubMed]
- Luk, E.K.; Khan, F.; Irving, L. Maintaining Gains Following Pulmonary Rehabilitation. Lung 2015, 193, 709–715. [Google Scholar] [CrossRef] [PubMed]
- Lewko, A.; Bidgood, P.L.; Jewell, A.; Garrod, R. Evaluation of multidimensional COPD-related subjective fatigue following a pulmonary rehabilitation programme. Respir. Med. 2014, 108, 95–102. [Google Scholar] [CrossRef] [PubMed]
- Williams, J.E.; Singh, S.J.; Sewell, L.; Morgan, M.D.L. Health status measurement: Sensitivity of the self-reported Chronic Respiratory Questionnaire (CRQ-SR) in pulmonary rehabilitation. Thorax 2003, 58, 515–518. [Google Scholar] [CrossRef]
- Singh, V.; Khandelwal, D.C.; Khandelwal, R.; Abusaria, S. Pulmonary rehabilitation in patients with chronic obstructive pulmonary disease. Indian J. Chest Dis. Allied Sci. 2003, 45, 13–17. [Google Scholar] [PubMed]
- Sundararajan, L.; Balami, J.; Packham, S. Effectiveness of outpatient pulmonary rehabilitation in elderly patients with chronic obstructive pulmonary disease. J. Cardiopulm. Rehabil. Prev. 2010, 30, 121–125. [Google Scholar] [CrossRef] [PubMed]
- Mitchell, K.E.; Johnson-Warrington, V.; Apps, L.D.; Bankart, J.; Sewell, L.; Williams, J.E.; Singh, S.J. A self-management programme for COPD: A randomised controlled trial. Eur. Respir. J. 2014, 44, 1538–1547. [Google Scholar] [CrossRef] [PubMed]
- Guell, R.; Resqueti, V.; Sangenis, M.; Morante, F.; Martorell, B.; Casan, P.; Guyatt, G.H. Impact of pulmonary rehabilitation on psychosocial morbidity in patients with severe COPD. Chest 2006, 129, 899–904. [Google Scholar] [CrossRef]
- Rochester, C.L. Patient assessment and selection for pulmonary rehabilitation. Respirology 2019, 24, 844–853. [Google Scholar] [CrossRef]
- Stoilkova, A.; Janssen, D.J.; Franssen, F.M.; Spruit, M.A.; Wouters, E.F. Coping styles in patients with COPD before and after pulmonary rehabilitation. Respir. Med. 2013, 107, 825–833. [Google Scholar] [CrossRef] [Green Version]
- Pirraglia, P.A.; Casserly, B.; Velasco, R.; Borgia, M.L.; Nici, L. Association of change in depression and anxiety symptoms with functional outcomes in pulmonary rehabilitation patients. J. Psychosom. Res. 2011, 71, 45–49. [Google Scholar] [CrossRef]
- Spruit, M.A.; Vercoulen, J.H.; Sprangers, M.A.; Wouters, E.F. Fatigue in COPD: An important yet ignored symptom. Lancet Respir. Med. 2017, 5, 542–544. [Google Scholar] [CrossRef]
- Lee, J.; Nguyen, H.Q.; Jarrett, M.E.; Mitchell, P.H.; Pike, K.C.; Fan, V.S. Effect of symptoms on physical performance in COPD. Heart Lung 2018, 47, 149–156. [Google Scholar] [CrossRef] [PubMed]
- Baltzan, M.A.; Scott, A.S.; Wolkove, N.; Bailes, S.; Bernard, S.; Bourbeau, J.; Canadian COPD Pulmonary Rehabilitation Research Group. Fatigue in COPD: Prevalence and effect on outcomes in pulmonary rehabilitation. Chronic Respir. Dis. 2011, 8, 119–128. [Google Scholar]
- Yohannes, A.M.; Alexopoulos, G.S. Depression and anxiety in patients with COPD. Eur. Respir. Rev. 2014, 23, 345–349. [Google Scholar] [CrossRef] [PubMed] [Green Version]
| Domain | Sub-Domain | Definition | Instruments/Measurement | Items |
|---|---|---|---|---|
| Symptoms | Subjective dyspnoea | The patient’s overall burden of pulmonary symptoms | PARS-D global dyspnea activity [31] PARS-D global dyspnea burden [31] | 2 |
| Dyspnoea emotions | The level of frustration and anxiety a person experiences when dyspnoeic | DEQ frustration [31] DEQ anxiety [31] | 6 | |
| Fatigue | The level of experienced fatigue | CIS subjective fatigue [18] | 8 | |
| Functional impairment | Subjective impairment | The experienced degree of impairment in general | QoLRiQ general activities [33,34] | 4 |
| Behavioural impairment | The extent to which a person cannot perform specific and concrete activities as a result of having the disease | SIP home management [35] SIP ambulation [35] | 22 | |
| Quality of life | General QoL | Mood and the satisfaction of a person with his/her life as a whole | BDI for primary care [36] Satisfaction with life scale [37] | 12 |
| HRQoL | Satisfaction related to physical functioning and the future | Satisfaction physiological functioning [31] Satisfaction future [31] | 2 | |
| Satisfaction relations | Satisfaction with the (absent) relationships with spouse and others | Satisfaction spouse [31] Satisfaction social [31] | 2 |
| Demographical Features | |
|---|---|
| Age, y | 60.5 ± 8.8 |
| Male, n (%) | 238 (53.4) |
| Education level, low/middle/high, n a | 229/151/60 |
| Tobacco use, b non-/ex-/smoker, n | 23/376/47 |
| COPD diagnosis > 10 years, n (%) c | 145 (32.5) |
| ≥1 self-reported comorbidity, n (%) b | 336 (76.2) |
| Clinical Features | |
| BMI, kg/m2 | 25.9 ± 5.5 |
| BMI classification, Uw/No/Ow/Ob, n | 69/157/140/80 |
| FEV1, L | 1.2 ± 0.5 |
| FEV1, % predicted | 42.5 ± 17.7 |
| GOLD grade, I/II/III/IV, n | 17/99/210/120 |
| 6MWD, m d | 383.2 ± 105.8 |
| 6MWD, % predicted d | 58.2 ± 15.4 |
| <70% predicted, n (%) | 290 (78.4) |
| Anxiety (SCL-90-A, 10–50), p b | 17.6 ± 7.2 |
| Anxiety score ≥ 23, n (%) | 95 (21.6) |
| Health Status (NCSI) | |
| Fatigue (CIS-Fatigue, 8–56), p | 41.9 ± 9.3 |
| Fatigue severity, normal/mild/severe, n | 29/83/334 |
| Dyspnoea (Dyspnoea VAS, 0–10), p | 5.8 ± 1.9 |
| HRQoL (2-10) *, p | 5.8 ± 1.7 |
| Depression (BDI-PC, 0–21) *, p | 3.4 ± 3.0 |
| Depression score ≥ 4, n (%) | 172 (38.6) |
| Responders 1 (n = 233) | Non-Responders 2 (n = 184) | p-Value | |
|---|---|---|---|
| Demographical Features | |||
| Age, y | 59.5 ± 8.8 | 61.8 ± 8.6 | 0.011 |
| Male, n (%) | 115 (49.4) | 106 (57.6) | 0.094 |
| Tobacco use a non-/ex-/smoker, n | 7/194/29 | 10/156/17 | 0.291 |
| COPD diagnosis > 10 years, n (%) b | 82 (38.7) | 54 (32.7) | 0.233 |
| ≥1 self-reported comorbidity, n (%) a | 178 (77.4) | 142 (77.6) | 0.961 |
| Clinical Features | |||
| BMI, kg/m2 | 26.1 ± 5.6 | 25.9 ± 5.4 | 0.879 |
| FFMi, kg/m2 c | 16.4 ± 2.2 | 16.7 ± 2.4 | 0.220 |
| FEV1, L | 1.3 ± 0.6 | 1.2 ± 0.5 | 0.217 |
| FEV1, % predicted | 44.0 ± 18.0 | 41.4 ± 17.2 | 0.102 |
| GOLD grade I/II/III/IV, n | 10/58/106/59 | 6/36/91/51 | 0.543 |
| 6MWD, m d | 391.4 ± 105.0 | 369.0 ± 104.8 | 0.051 |
| 6MWD, % predicted d | 59.4 ± 14.9 | 56.4 ±16.1 | 0.072 |
| <70 % predicted, n (%) | 151 (76.7) | 122 (81.9) | 0.238 |
| Quadriceps muscle strength, Nm e | 294.5 ± 102.9 | 284.9 ± 98.4 | 0.515 |
| Anxiety (SCL-90-A, 10–50), p f | 17.7 ± 7.0 | 18.1 ± 7.5 | 0.620 |
| Anxiety score ≥ 23, n (%) | 48 (20.9) | 44 (24.3) | 0.406 |
| NCSI—Symptoms | |||
| Subjective dyspnoea, p # | 13.1 ± 3.8 | 13.3 ± 3.7 | 0.868 |
| Dyspnoea (Dyspnoea VAS, 0–10) p # | 4.3 ± 1.9 | 5.4 ± 1.9 | 0.519 |
| Dyspnoea emotions | 13.0 ± 3.9 | 13.0 ± 4.1 | 0.926 |
| Fatigue (CIS-Fatigue, 8–56), p | 45.4 ± 7.3 | 40.5 ± 7.8 | <0.001 |
| Mild fatigue, n (%) | 25 (10.7) | 58 (31.5) ⱡ | <0.001 |
| Severe fatigue, n (%) | 208 (89.3) | 126 (68.5) ⱡ | |
| NCSI—Quality of Life | |||
| General QoL, p | 28.0 ± 14.8 | 26.8 ± 14.3 | 0.615 |
| HRQoL (2–10), p * | 6.1 ± 1.6 | 5.7 ± 1.7 | 0.004 |
| Depression (BDI-PC, 0–21), p * | 3.6 ± 3.1 | 3.3 ± 2.9 | 0.384 |
| Depression score ≥ 4, n (%) | 93 (39.3) | 74 (40.2) | 0.950 |
| Satisfaction with relations, p | 4.0 ± 1.8 | 3.8 ±1.9 | 0.056 |
| NCSI—Functional Impairment | |||
| Subjective impairment, p | 16.5 ± 5.2 | 16.9 ± 5.1 | 0.555 |
| Behaviour impairment, p | 27.1 ± 13.7 | 28.1 ± 14.3 | 0.517 |
| Responders 1 (n = 233) | Non-Responders 2 (n = 184) | p-Value | |
|---|---|---|---|
| Clinical Features | |||
| ΔBMI, kg/m2 a | −0.0 ± 1.4 | −0.1 ± 1.3 | 0.914 |
| ΔFFMi, kg/m2 b | 0.3 ± 1.0 | −0.1 ± 1.3 | 0.012 |
| ΔFEV1, L c | 0.1 ± 0.3 | 0.0 ± 0.3 | 0.572 |
| Δ6MWD, m d | 70.7 ± 74.4 | 38.3 ± 70.3 | 0.001 |
| ΔQuadriceps muscle strength, Nm e | 25.5 ± 62.0 | 26.5 ± 68.7 | 0.901 |
| ΔAnxiety (SCL-90-A, 10–50), p f | −4.1 ± 5.5 | −1.8 ± 6.0 | <0.001 |
| NCSI—Symptoms | |||
| ΔSubjective dyspnoea, p # g | −4.2 ± 4.2 | −2.1 ± 4.3 | <0.001 |
| ΔDyspnoea (Dyspnoea VAS, 0–10), p # g | −1.5 ± 2.1 | −0.6 ± 2.0 | <0.001 |
| ΔDyspnoea emotions, p h | −2.6 ± 3.9 | −1.2 ± 3.7 | <0.001 |
| ΔFatigue (CIS-Fatigue, 8–56) | −19.2 ± 7.2 | −1.7 ± 6.4 | <0.001 |
| NCSI—Quality of Life | |||
| ΔGeneral QoL, p i | −10.3 ± 12.3 | −5.4 ± 12.0 | <0.001 |
| ΔHRQoL, p * i | −2.5 ± 1.9 | −1.1 ± 1.8 | <0.001 |
| ΔDepression (BDI-PC, 0–21), p * | −2.1 ± 2.6 | −1.0 ± 2.6 | <0.001 |
| ΔSatisfaction with relationship, p | −0.9 ± 1.9 | −0.2 ± 2.3 | 0.003 |
| NCSI—Functional Impairment | |||
| ΔSubjective impairment, p | −4.8 ± 5.6 | −1.9 ± 5.0 | <0.001 |
| ΔBehaviour impairment, p | −2.9 ± 13.6 | −0.6 ± 16.0 | 0.106 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Van Herck, M.; Antons, J.; Vercoulen, J.H.; Goërtz, Y.M.J.; Ebadi, Z.; Burtin, C.; Janssen, D.J.A.; Thong, M.S.Y.; Otker, J.; Coors, A.; et al. Pulmonary Rehabilitation Reduces Subjective Fatigue in COPD: A Responder Analysis. J. Clin. Med. 2019, 8, 1264. https://doi.org/10.3390/jcm8081264
Van Herck M, Antons J, Vercoulen JH, Goërtz YMJ, Ebadi Z, Burtin C, Janssen DJA, Thong MSY, Otker J, Coors A, et al. Pulmonary Rehabilitation Reduces Subjective Fatigue in COPD: A Responder Analysis. Journal of Clinical Medicine. 2019; 8(8):1264. https://doi.org/10.3390/jcm8081264
Chicago/Turabian StyleVan Herck, Maarten, Jeanine Antons, Jan H. Vercoulen, Yvonne M. J. Goërtz, Zjala Ebadi, Chris Burtin, Daisy J. A. Janssen, Melissa S. Y. Thong, Jacqueline Otker, Arnold Coors, and et al. 2019. "Pulmonary Rehabilitation Reduces Subjective Fatigue in COPD: A Responder Analysis" Journal of Clinical Medicine 8, no. 8: 1264. https://doi.org/10.3390/jcm8081264





