Visfatin Connection: Present and Future in Osteoarthritis and Osteoporosis
Abstract
:1. Introduction
2. Essentials: OA, OP, and Visfatin
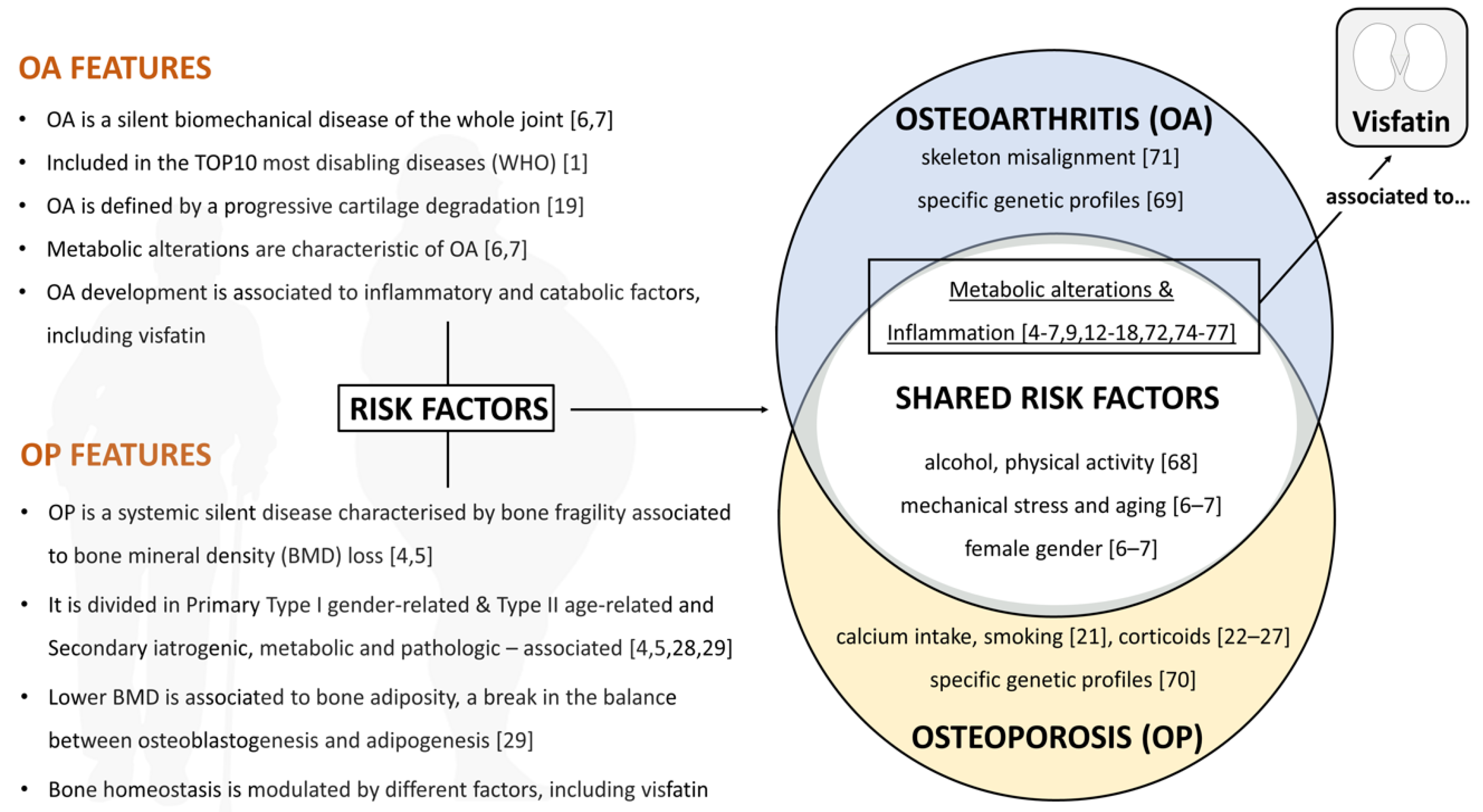
2.1. Osteoarthritis (OA)
2.2. Osteoporosis (OP)
2.3. Visfatin (NAMPT/PBEF)
3. Visfatin in OA & OP Shared Risk Factors
3.1. The Metabolic Component
3.2. The Inflammatory Component
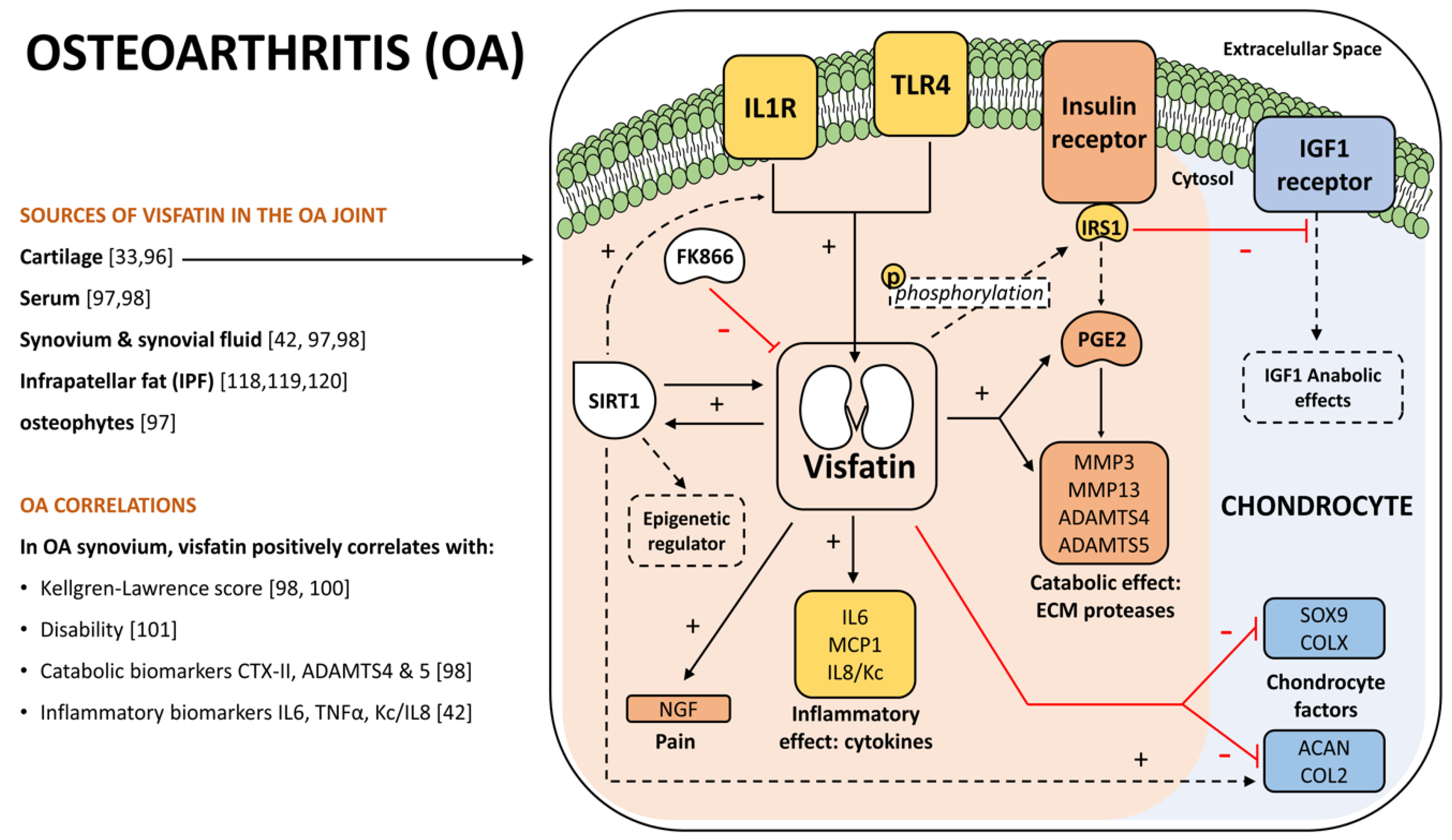
4. Visfatin Role in Osteoarthritis
4.1. OA and Visfatin Connection
4.2. OA Catabolism and Inflammation
4.3. OA Epigenetics and Circadian Rhythm
4.4. Other OA Joint Tissues
5. Visfatin’s Role in Osteoporosis
5.1. The OP and Visfatin Connection
5.2. Bone Catabolism and Inflammation
5.3. Bone Anabolism
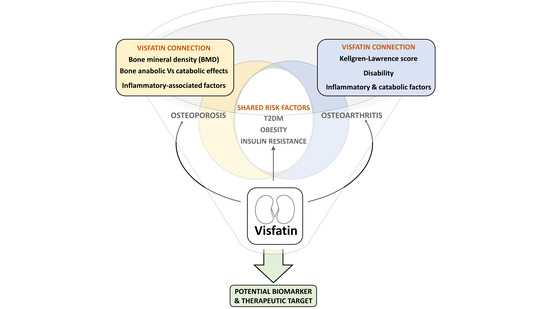
6. Visfatin as a Potential Biomarker and Therapeutic Target
7. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- World Health Organization Disease incidence, prevalence and disability. Glob. Burd. Dis. 2004, 3, 28–37.
- National Collaborating Centre for Chronic Conditions (In Great Britain). Rheumatoid Arthritis: National Clinical Guideline for Management and Treatment in Adults; Royal College of Physicians of London: London, UK, 2009; ISBN 9780323054751. [Google Scholar]
- Barbour, K.E.; Helmick, C.G.; Boring, M.; Brady, T.J. Vital Signs: Prevalence of Doctor-Diagnosed Arthritis and Arthritis-Attributable Activity Limitation—United States, 2013–2015. MMWR. Morb. Mortal. Wkly. Rep. 2017, 66, 246–253. [Google Scholar] [CrossRef] [PubMed]
- Ginaldi, L.; Di Benedetto, M.; De Martinis, M.; Delmas, P.; Yun, A.; Lee, P.; Arron, J.; Choi, Y.; Lorenzo, J.; Mitra, D.; et al. Osteoporosis, inflammation and ageing. Immun. Ageing 2005, 2, 14. [Google Scholar] [CrossRef] [PubMed]
- Dobbs, M.B.; Buckwalter, J.; Saltzman, C. Osteoporosis: The increasing role of the orthopaedist. Iowa Orthop. J. 1999, 19, 43–52. [Google Scholar] [PubMed]
- Gómez, R.; Villalvilla, A.; Largo, R.; Gualillo, O.; Herrero-Beaumont, G. TLR4 signalling in osteoarthritis-finding targets for candidate DMOADs. Nat. Rev. Rheumatol. 2014, 11, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Sohn, D.; Sokolove, J.; Sharpe, O.; Erhart, J.C.; Chandra, P.E.; Lahey, L.J.; Lindstrom, T.M.; Hwang, I.; Boyer, K.A.; Andriacchi, T.P.; et al. Plasma proteins present in osteoarthritic synovial fluid can stimulate cytokine production via Toll-like receptor 4. Arthritis Res. Ther. 2012, 14, R7. [Google Scholar] [CrossRef] [PubMed]
- Herrero-Beaumont, G.; Roman-Blas, J.A.; Castañeda, S.; Jimenez, S.A. Primary Osteoarthritis No Longer Primary: Three Subsets with Distinct Etiological, Clinical, and Therapeutic Characteristics. Semin. Arthritis Rheum. 2009, 39, 71–80. [Google Scholar] [CrossRef]
- Karbaschian, Z.; Hosseinzadeh-Attar, M.J.; Giahi, L.; Golpaie, A.; Masoudkabir, F.; Talebpour, M.; Kosari, F.; Karbaschian, N.; Hoseini, M.; Mazaherioun, M. Portal and systemic levels of visfatin in morbidly obese subjects undergoing bariatric surgery. Endocrine 2013, 44, 114–118. [Google Scholar] [CrossRef]
- Auguet, T.; Terra, X.; Porras, J.A.; Orellana-Gavaldà, J.M.; Martinez, S.; Aguilar, C.; Lucas, A.; Pellitero, S.; Hernández, M.; Del Castillo, D.; et al. Plasma visfatin levels and gene expression in morbidly obese women with associated fatty liver disease. Clin. Biochem. 2013, 46, 202–208. [Google Scholar] [CrossRef]
- Lago, F.; Dieguez, C.; Gómez-Reino, J.; Gualillo, O. Adipokines as emerging mediators of immune response and inflammation. Nat. Clin. Pract. Rheumatol. 2007, 3, 716–724. [Google Scholar] [CrossRef]
- Fukuhara, A.; Matsuda, M.; Nishizawa, M.; Segawa, K.; Tanaka, M.; Kishimoto, K.; Matsuki, Y.; Murakami, M.; Ichisaka, T.; Murakami, H.; et al. Visfatin: A Protein Secreted by Visceral Fat That Mimics the Effects of Insulin. Science 2005, 307, 426–430. [Google Scholar] [CrossRef] [PubMed]
- Terra, X.; Auguet, T.; Quesada, I.; Aguilar, C.; Luna, A.M.; Hernández, M.; Sabench, F.; Porras, J.A.; Martínez, S.; Lucas, A.; et al. Increased levels and adipose tissue expression of visfatin in morbidly obese women: The relationship with pro-inflammatory cytokines. Clin. Endocrinol. 2012, 77, 691–698. [Google Scholar] [CrossRef] [PubMed]
- Aggeloussi, S.; Theodorou, A.A.; Paschalis, V.; Nikolaidis, M.G.; Fatouros, I.G.; Owolabi, E.O.; Kouretas, D.; Koutedakis, Y.; Jamurtas, A.Z. Adipocytokine Levels in Children: Effects of Fatness and Training. Pediatr. Exerc. Sci. 2012, 24, 461–471. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, R.Z.; Ma, X.Y.; Hu, X.F.; Kang, S.X.; Chen, S.K.; Cianflone, K.; Lu, H.L. Elevated visfatin levels in obese children are related to proinflammatory factors. J. Pediatr. Endocrinol. Metab. 2013, 26, 111–118. [Google Scholar] [CrossRef] [PubMed]
- Haider, D.G.; Pleiner, J.; Francesconi, M.; Wiesinger, G.F.; Müller, M.; Wolzt, M. Exercise training lowers plasma visfatin concentrations in patients with type 1 diabetes. J. Clin. Endocrinol. Metab. 2006, 91, 4702–4704. [Google Scholar] [CrossRef] [PubMed]
- Uslu, S.; Kebapçi, N.; Kara, M.; Bal, C. Relationship between adipocytokines and cardiovascular risk factors in patients with type 2 diabetes mellitus. Exp. Ther. Med. 2012, 4, 113–120. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, M.P.; Chung, F.M.; Chang, D.M.; Tsai, J.C.R.; Huang, H.F.; Shin, S.J.; Lee, Y.J. Elevated plasma level of visfatin/pre-B cell colony-enhancing factor in patients with type 2 diabetes mellitus. J. Clin. Endocrinol. Metab. 2006, 91, 295–299. [Google Scholar] [CrossRef] [PubMed]
- Litwic, A.; Registrar, S.; Edwards, M.; Clinical, M. Europe PMC Funders Group Epidemiology and Burden of Osteoarthritis. Br. Med. Bull. 2013, 44, 185–199. [Google Scholar] [CrossRef] [PubMed]
- Rousseau, J.C.; Garnero, P. Biological markers in osteoarthritis. Bone 2012, 51, 265–277. [Google Scholar] [CrossRef] [PubMed]
- Al-Bashaireh, A.M.; Haddad, L.G.; Weaver, M.; Chengguo, X.; Kelly, D.L.; Yoon, S. The Effect of Tobacco Smoking on Bone Mass: An Overview of Pathophysiologic Mechanisms. J. Osteoporos. 2018, 2018, 1206235. [Google Scholar] [CrossRef] [PubMed]
- Raterman, H.G.; Bultink, I.E.M.; Lems, W.F. Current Treatments and New Developments in the Management of Glucocorticoid-induced Osteoporosis. Drugs 2019, 79, 1065–1087. [Google Scholar] [CrossRef] [PubMed]
- Khosla, S.; Lufkin, E.G.; Hodgson, S.F.; Fitzpatrick, L.A.; Melton, L.J. Epidemiology and clinical features of osteoporosis in young individuals. Bone 1994, 15, 551–555. [Google Scholar] [CrossRef]
- Holland, E.G.; Taylor, A.T. Glucocorticoids in clinical practice. J. Fam. Pract. 1991, 32, 512–519. [Google Scholar] [PubMed]
- Gudbjornsson, B.; Juliusson, U.I.; Gudjonsson, F. V Prevalence of long term steroid treatment and the frequency of decision making to prevent steroid induced osteoporosis in daily clinical practice. Ann. Rheum. Dis. 2002, 61, 32–36. [Google Scholar] [CrossRef] [PubMed]
- Kanis, J.A.; Johansson, H.; Oden, A.; Johnell, O.; de Laet, C.; Melton, L.J.; Tenenhouse, A.; Reeve, J.; Silman, A.J.; Pols, H.A.; et al. A Meta-Analysis of Prior Corticosteroid Use and Fracture Risk. J. Bone Miner. Res. 2004, 19, 893–899. [Google Scholar] [CrossRef] [PubMed]
- van Staa, T.P.; Leufkens, H.G.M.; Cooper, C. The Epidemiology of Corticosteroid-Induced Osteoporosis: A Meta-analysis. Osteoporos. Int. 2002, 13, 777–787. [Google Scholar] [CrossRef] [PubMed]
- Rizzoli, R. Bone: Towards a better management of glucocorticoid-induced osteoporosis? Nat. Rev. Rheumatol. 2017, 13, 635–636. [Google Scholar] [CrossRef] [PubMed]
- Cao, J.J. Effects of obesity on bone metabolism. J. Orthop. Surg. Res. 2011, 6, 30. [Google Scholar] [CrossRef]
- Abella, V.; Scotece, M.; Conde, J.; López, V.; Lazzaro, V.; Pino, J.; Gómez-Reino, J.J.; Gualillo, O. Adipokines, metabolic syndrome and rheumatic diseases. J. Immunol. Res. 2014, 2014, 343746. [Google Scholar] [CrossRef]
- Samal, B.; Sun, Y.; Stearns, G.; Xie, C.; Suggs, S.; McNiece, I. Cloning and characterization of the cDNA encoding a novel human pre-B-cell colony-enhancing factor. Mol. Cell. Biol. 1994, 14, 1431–1437. [Google Scholar] [CrossRef]
- Ognjanovic, S.; Bao, S.; Yamamoto, S.Y.; Garibay-Tupas, J.; Samal, B.; Bryant-Greenwood, G.D. Genomic organization of the gene coding for human pre-B-cell colony enhancing factor and expression in human fetal membranes. J. Mol. Endocrinol. 2001, 26, 107–117. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gosset, M.; Berenbaum, F.; Salvat, C.; Sautet, A.; Pigenet, A.; Tahiri, K.; Jacques, C. Crucial role of visfatin/pre-B cell colony-enhancing factor in matrix degradation and prostaglandin E2 synthesis in chondrocytes: Possible influence on osteoarthritis. Arthritis Rheum. 2008, 58, 1399–1409. [Google Scholar] [CrossRef] [PubMed]
- Luk, T.; Malam, Z.; Marshall, J.C. Pre-B cell colony-enhancing factor (PBEF)/visfatin: A novel mediator of innate immunity. J. Leukoc. Biol. 2008, 83, 804–816. [Google Scholar] [CrossRef] [PubMed]
- Moschen, A.R.; Kaser, A.; Enrich, B.; Mosheimer, B.; Theurl, M.; Niederegger, H.; Tilg, H. Visfatin, an adipocytokine with proinflammatory and immunomodulating properties. J. Immunol. 2007, 178, 1748–1758. [Google Scholar] [CrossRef] [PubMed]
- Revollo, J.R.; Grimm, A.A.; Imai, S. The regulation of nicotinamide adenine dinucleotide biosynthesis by Nampt/PBEF/visfatin in mammals. Curr. Opin. Gastroenterol. 2007, 23, 164–170. [Google Scholar] [CrossRef] [PubMed]
- Ye, S.Q.; Zhang, L.Q.; Adyshev, D.; Usatyuk, P.V.; Garcia, A.N.; Lavoie, T.L.; Verin, A.D.; Natarajan, V.; Garcia, J.G.N. Pre-B-cell-colony-enhancing factor is critically involved in thrombin-induced lung endothelial cell barrier dysregulation. Microvasc. Res. 2005, 70, 142–151. [Google Scholar] [CrossRef] [PubMed]
- Kitani, T.; Okuno, S.; Fujisawa, H. Growth phase-dependent changes in the subcellular localization of pre-B-cell colony-enhancing factor. FEBS Lett. 2003, 544, 74–78. [Google Scholar] [CrossRef]
- McGlothlin, J.R.; Gao, L.; Lavoie, T.; Simon, B.A.; Easley, R.B.; Ma, S.-F.; Rumala, B.B.; Garcia, J.G.N.; Ye, S.Q. Molecular cloning and characterization of canine pre-B-cell colony-enhancing factor. Biochem. Genet. 2005, 43, 127–141. [Google Scholar] [CrossRef] [PubMed]
- Khan, J.A.; Tao, X.; Tong, L. Molecular basis for the inhibition of human NMPRTase, a novel target for anticancer agents. Nat. Struct. Mol. Biol. 2006, 13, 582–588. [Google Scholar] [CrossRef]
- Wang, T.; Zhang, X.; Bheda, P.; Revollo, J.R.; Imai, S.; Wolberger, C. Structure of Nampt/PBEF/visfatin, a mammalian NAD+ biosynthetic enzyme. Nat. Struct. Mol. Biol. 2006, 13, 661–662. [Google Scholar] [CrossRef]
- Laiguillon, M.-C.; Houard, X.; Bougault, C.; Gosset, M.; Nourissat, G.; Sautet, A.; Jacques, C.; Berenbaum, F.; Sellam, J. Expression and function of visfatin (Nampt), an adipokine-enzyme involved in inflammatory pathways of osteoarthritis. Arthritis Res. Ther. 2014, 16, R38. [Google Scholar] [CrossRef] [PubMed]
- Körner, A.; Garten, A.; Blü, M.; Tauscher, R.; Rgen Kratzsch, J.; Kiess, W. Molecular Characteristics of Serum Visfatin and Differential Detection by Immunoassays. J. Clin. Endocrinol. Metab. 2007, 92, 4783–4791. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Garten, A.; Petzold, S.; Barnikol-Oettler, A.; Körner, A.; Thasler, W.E.; Kratzsch, J.; Kiess, W.; Gebhardt, R. Nicotinamide phosphoribosyltransferase (NAMPT/PBEF/visfatin) is constitutively released from human hepatocytes. Biochem. Biophys. Res. Commun. 2010, 391, 376–381. [Google Scholar] [CrossRef] [PubMed]
- Ognjanovic, S.; Bryant-Greenwood, G.D. Pre-B-cell colony-enhancing factor, a novel cytokine of human fetal membranes. Am. J. Obstet. Gynecol. 2002, 187, 1051–1058. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, M.; Nozaki, M.; Fukuhara, A.; Segawa, K.; Aoki, N.; Matsuda, M.; Komuro, R.; Shimomura, I. Visfatin is released from 3T3-L1 adipocytes via a non-classical pathway. Biochem. Biophys. Res. Commun. 2007, 359, 194–201. [Google Scholar] [CrossRef] [PubMed]
- Romacho, T.; Villalobos, L.A.; Cercas, E.; Carraro, R.; Sánchez-Ferrer, C.F.; Peiró, C. Visfatin as a Novel Mediator Released by Inflamed Human Endothelial Cells. PLoS ONE 2013, 8, 1–7. [Google Scholar] [CrossRef]
- Svoboda, P.; Krizova, E.; Sestakova, S.; Vapenkova, K.; Knejzlik, Z.; Rimpelova, S.; Rayova, D.; Volfova, N.; Krizova, I.; Rumlova, M.; et al. Nuclear transport of nicotinamide phosphoribosyltransferase is cell cycle–dependent in mammalian cells, and its inhibition slows cell growth. J. Biol. Chem. 2019, 294, 8676–8689. [Google Scholar] [CrossRef] [PubMed]
- Revollo, J.R.; Grimm, A.A.; Imai, S.I. The NAD biosynthesis pathway mediated by nicotinamide phosphoribosyltransferase regulates Sir2 activity in mammalian cells. J. Biol. Chem. 2004, 279, 50754–50763. [Google Scholar] [CrossRef]
- Nikiforov, A.; Dölle, C.; Niere, M.; Ziegler, M. Pathways and subcellular compartmentation of NAD biosynthesis in human cells: From entry of extracellular precursors to mitochondrial NAD generation. J. Biol. Chem. 2011, 286, 21767–21778. [Google Scholar] [CrossRef]
- Imai, S. The NAD World 2.0: The importance of the inter-tissue communication mediated by NAMPT/NAD+/SIRT1 in mammalian aging and longevity control. NPJ Syst. Biol. Appl. 2016, 2, 16018. [Google Scholar] [CrossRef]
- Imai, S. The NAD World: A new systemic regulatory network for metabolism and aging--Sirt1, systemic NAD biosynthesis, and their importance. Cell Biochem. Biophys. 2009, 53, 65–74. [Google Scholar] [CrossRef] [PubMed]
- Rongvaux, A.; She, R.J.; Mulks, M.H.; Gigot, D.; Urbain, J.; Leo, O.; Andris, F. Pre-B-cell colony-enhancing factor, whose expression is up-regulated in activated lymphocytes, is a nicotinamide phosphoribosyltransferase, a cytosolic enzyme involved in NAF biosynthesis. Eur. J. Immunol. 2002, 32, 3225–3234. [Google Scholar] [CrossRef]
- Martin, P.R.; Martin, P.R.; Shea, R.J.; Shea, R.J.; Mulks, M.H.; Mulks, M.H. Identi cation of a Plasmid-Encoded Gene from. Society 2001, 183, 1168–1174. [Google Scholar]
- Houtkooper, R.H.; Cantó, C.; Wanders, R.J.; Auwerx, J. The secret life of NAD+: An old metabolite controlling new metabolic signaling pathways. Endocr. Rev. 2010, 31, 194–223. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.; Yang, T.; Baur, J.A.; Perez, E.; Matsui, T.; Carmona, J.J.; Lamming, D.W.; Souza-Pinto, N.C.; Bohr, V.A.; Rosenzweig, A.; et al. Nutrient-sensitive mitochondrial NAD+ levels dictate cell survival. Cell 2007, 130, 1095–1107. [Google Scholar] [CrossRef] [PubMed]
- Harlan, B.A.; Pehar, M.; Sharma, D.R.; Beeson, G.; Beeson, C.C.; Vargas, M.R. Enhancing NAD+ salvage pathway reverts the toxicity of primary astrocytes expressing amyotrophic lateral sclerosis-linked mutant SOD1. J. Biol. Chem. 2016, 291, 10836–10846. [Google Scholar] [CrossRef] [PubMed]
- Pittelli, M.; Formentini, L.; Faraco, G.; Lapucci, A.; Rapizzi, E.; Cialdai, F.; Romano, G.; Moneti, G.; Moroni, F.; Chiarugi, A. Inhibition of nicotinamide phosphoribosyltransferase: Cellular bioenergetics reveals a mitochondrial insensitive NAD pool. J. Biol. Chem. 2010, 285, 34106–34114. [Google Scholar] [CrossRef]
- Nakagawa, T.; Lomb, D.J.; Haigis, M.C.; Guarente, L. SIRT5 Deacetylates carbamoyl phosphate synthetase 1 and regulates the urea cycle. Cell 2009, 137, 560–570. [Google Scholar] [CrossRef]
- Kim, S.R.; Bae, Y.H.; Bae, S.K.; Choi, K.S.; Yoon, K.H.; Koo, T.H.; Jang, H.O.; Yun, I.; Kim, K.W.; Kwon, Y.G.; et al. Visfatin enhances ICAM-1 and VCAM-1 expression through ROS-dependent NF-kappaB activation in endothelial cells. Biochim. Biophys. Acta 2008, 1783, 886–895. [Google Scholar] [CrossRef]
- Koltai, E.; Bori, Z.; Chabert, C.; Dubouchaud, H.; Naito, H.; Machida, S.; Davies, K.J.; Murlasits, Z.; Fry, A.C.; Boldogh, I.; et al. SIRT1 may play a crucial role in overload induced hypertrophy of skeletal muscle. J. Physiol. 2017. [Google Scholar] [CrossRef]
- Li, Y.; Zhang, Y.; Dorweiler, B.; Cui, D.; Wang, T.; Woo, C.W.; Brunkan, C.S.; Wolberger, C.; Imai, S.I.; Tabas, I. Extracellular nampt promotes macrophage survival via a nonenzymatic interleukin-6/STAT3 signaling mechanism. J. Biol. Chem. 2008, 283, 34833–34843. [Google Scholar] [CrossRef]
- Wang, W.; Hu, Y.; Wang, X.; Wang, Q.; Deng, H. ROS-Mediated 15-Hydroxyprostaglandin Dehydrogenase Degradation via Cysteine Oxidation Promotes NAD+-Mediated Epithelial-Mesenchymal Transition. Cell Chem. Biol. 2018, 25, e4. [Google Scholar] [CrossRef]
- Yu, A.; Dang, W. Regulation of stem cell aging by SIRT1–Linking metabolic signaling to epigenetic modifications. Mol. Cell. Endocrinol. 2017, 455, 75–82. [Google Scholar] [CrossRef]
- Aksoy, P.; White, T.A.; Thompson, M.; Chini, E.N. Regulation of intracellular levels of NAD: A novel role for CD38. Biochem. Biophys. Res. Commun. 2006, 345, 1386–1392. [Google Scholar] [CrossRef]
- Bai, P. Biology of Poly(ADP-Ribose) Polymerases: The Factotums of Cell Maintenance. Mol. Cell 2015, 58, 947–958. [Google Scholar] [CrossRef] [Green Version]
- Imai, S.; Guarente, L. NAD+ and sirtuins in aging and disease. Trends Cell Biol. 2014, 24, 464–471. [Google Scholar] [CrossRef]
- Borer, K.T. Physical activity in the prevention and amelioration of osteoporosis in women: Interaction of mechanical, hormonal and dietary factors. Sports Med. 2005, 35, 779–830. [Google Scholar] [CrossRef]
- Tachmazidou, I.; Hatzikotoulas, K.; Southam, L.; Esparza-Gordillo, J.; Haberland, V.; Zheng, J.; Johnson, T.; Koprulu, M.; Zengini, E.; Steinberg, J.; et al. Identification of new therapeutic targets for osteoarthritis through genome-wide analyses of UK Biobank data. Nat. Genet. 2019, 51, 230–236. [Google Scholar] [CrossRef]
- Yang, C.; Ren, J.; Li, B.; Jin, C.; Ma, C.; Cheng, C.; Sun, Y.; Shi, X. Identification of gene biomarkers in patients with postmenopausal osteoporosis. Mol. Med. Rep. 2019, 19, 1065–1073. [Google Scholar] [CrossRef]
- Sharma, L.; Song, J.; Dunlop, D.; Felson, D.; Lewis, C.E.; Segal, N.; Torner, J.; Cooke, T.D.V.; Hietpas, J.; Lynch, J.; et al. Varus and Valgus Alignment and Incident and Progressive Knee Osteoarthritis. Ann. Rheum. Dis. 2010, 69, 1940. [Google Scholar] [CrossRef]
- Erem, S.; Atfi, A.; Razzaque, M.S. Anabolic effects of vitamin D and magnesium in aging bone. J. Steroid Biochem. Mol. Biol. 2019, 193, 105400. [Google Scholar] [CrossRef]
- Novak, S.; Divkovic, D.; Drenjancevic, I.; Cosic, A.; Selthofer-Relatic, K. Visfatin serum level and expression in subcutaneous and visceral adipose tissue in prepubertal boys. Pediatr. Obes. 2016, 11, 411–417. [Google Scholar] [CrossRef]
- Ouchi, N.; Parker, J.L.; Lugus, J.J.; Walsh, K. Adipokines in inflammation and metabolic disease. Nat. Rev. Immunol. 2011, 11, 85–97. [Google Scholar] [CrossRef]
- Kowalska, I.; Karczewska-Kupczewska, M.; Adamska, A.; Nikolajuk, A.; Otziomek, E.; Straczkowski, M. Serum visfatin is differentially regulated by insulin and free Fatty acids in healthy men. J. Clin. Endocrinol. Metab. 2013, 98, E293–E297. [Google Scholar] [CrossRef]
- Chang, Y.H.; Chang, D.M.; Lin, K.C.; Shin, S.J.; Lee, Y.J. Visfatin in overweight/obesity, type 2 diabetes mellitus, insulin resistance, metabolic syndrome and cardiovascular diseases: A meta-analysis and systemic review. Diabetes. Metab. Res. Rev. 2011, 27, 515–527. [Google Scholar] [CrossRef]
- Veronese, N.; Cooper, C.; Reginster, J.Y.; Hochberg, M.; Branco, J.; Bruyère, O.; Chapurlat, R.; Al-Daghri, N.; Dennison, E.; Herrero-Beaumont, G.; et al. Type 2 diabetes mellitus and osteoarthritis. Semin. Arthritis Rheum. 2019, 49, 9–19. [Google Scholar] [CrossRef]
- Catalán, V.; Gómez-Ambrosi, J.; Rodríguez, A.; Ramírez, B.; Silva, C.; Rotellar, F.; Cienfuegos, J.A.; Salvador, J.; Frühbeck, G. Association of increased Visfatin/PBEF/NAMPT circulating concentrations and gene expression levels in peripheral blood cells with lipid metabolism and fatty liver in human morbid obesity. Nutr. Metab. Cardiovasc. Dis. 2010, 21, 245–253. [Google Scholar] [CrossRef]
- Friebe, D.; Neef, M.; Kratzsch, J.; Erbs, S.; Dittrich, K.; Garten, A.; Petzold-Quinque, S.; Blüher, S.; Reinehr, T.; Stumvoll, M.; et al. Leucocytes are a major source of circulating nicotinamide phosphoribosyltransferase (NAMPT)/pre-B cell colony (PBEF)/visfatin linking obesity and inflammation in humans. Diabetologia 2011, 54, 1200–1211. [Google Scholar] [CrossRef] [Green Version]
- Curat, C.A.; Wegner, V.; Sengenès, C.; Miranville, A.; Tonus, C.; Busse, R.; Bouloumié, A. Macrophages in human visceral adipose tissue: Increased accumulation in obesity and a source of resistin and visfatin. Diabetologia 2006, 49, 744–747. [Google Scholar] [CrossRef]
- Taşçilar, M.E.; Cekmez, F.; Meral, C.; Pirgon, O.; Tanju, I.A.; Canpolat, F.E.; Abaci, A.; Tapan, S.; Eker, I. Evaluation of adipocytokines in obese children with insulin resistance. Turk. J. Pediatr. 2011, 53, 269–273. [Google Scholar]
- Ha, C.H.; Swearingin, B.; Jeon, Y.K. Relationship of visfatin level to pancreatic endocrine hormone level, HOMA-IR index, and HOMA β-cell index in overweight women who performed hydraulic resistance exercise. J. Phys. Ther. Sci. 2015, 27, 2965–2969. [Google Scholar] [CrossRef]
- Zahorska-Markiewicz, B.; Olszanecka-Glinianowicz, M.; Janowska, J.; Kocełak, P.; Semik-Grabarczyk, E.; Holecki, M.; Dąbrowski, P.; Skorupa, A. Serum concentration of visfatin in obese women. Metabolism 2007, 56, 1131–1134. [Google Scholar] [CrossRef]
- Kolsgaard, M.L.; Wangensteen, T.; Brunborg, C.; Joner, G.; Holven, K.B.; Halvorsen, B.; Aukrust, P.; Tonstad, S. Elevated visfatin levels in overweight and obese children and adolescents with metabolic syndrome. Scand. J. Clin. Lab. Investig. 2009, 69, 858–864. [Google Scholar] [CrossRef]
- Lundberg, I.E.; Tj, A.; Bottai, M.; Werth, V.P.; Pilkington, C.; de Visser, M.; Alfredsson, L.; Amato, A.A.; Barohn, R.J.; Liang, M.H.; et al. 2017 European League Against Rheumatism/American College of Rheumatology Classification Criteria for Adult and Juvenile Idiopathic Inflammatory Myopathies and Their Major Subgroups. ARTHRITIS Rheumatol. 2017, 69, 2271–2282. [Google Scholar] [CrossRef]
- Dogru, T.; Sonmez, A.; Tasci, I.; Bozoglu, E.; Yilmaz, M.I.; Genc, H.; Erdem, G.; Gok, M.; Bingol, N.; Kilic, S.; et al. Plasma visfatin levels in patients with newly diagnosed and untreated type 2 diabetes mellitus and impaired glucose tolerance. Diabetes Res. Clin. Pract. 2007, 76, 24–29. [Google Scholar] [CrossRef] [PubMed]
- Liang, H.; Wang, H.; Luo, L.; Fan, S.; Zhou, L.; Liu, Z.; Yao, S.; Zhang, X.; Zhong, K.; Zhao, H.; et al. Toll-like receptor 4 promotes high glucose-induced catabolic and inflammatory responses in chondrocytes in an NF-κB-dependent manner. Life Sci. 2019, 228, 258–265. [Google Scholar] [CrossRef] [PubMed]
- Camp, S.M.; Ceco, E.; Evenoski, C.L.; Danilov, S.M.; Zhou, T.; Chiang, E.T.; Moreno-Vinasco, L.; Mapes, B.; Zhao, J.; Gursoy, G.; et al. Unique Toll-Like Receptor 4 Activation by NAMPT/PBEF Induces NFκB Signaling and Inflammatory Lung Injury. Sci. Rep. 2015, 5, 13135. [Google Scholar] [CrossRef]
- Oita, R.C.; Camp, S.M.; Ma, W.; Ceco, E.; Harbeck, M.; Singleton, P.; Messana, J.; Sun, X.; Wang, T.; Garcia, J.G.N. Novel Mechanism for Nicotinamide Phosphoribosyltransferase Inhibition of TNF-α-Mediated Apoptosis in Human Lung Endothelial Cells. Am. J. Respir. Cell Mol. Biol. 2018. [Google Scholar] [CrossRef] [PubMed]
- Alonso-Pérez, A.; Franco-Trepat, E.; Guillán-Fresco, M.; Jorge-Mora, A.; López, V.; Pino, J.; Gualillo, O.; Gómez, R. Role of Toll-Like Receptor 4 on Osteoblast Metabolism and Function. Front. Physiol. 2018, 9, 504. [Google Scholar] [CrossRef]
- Herrero-Beaumont, G.; Pérez-Baos, S.; Sánchez-Pernaute, O.; Roman-Blas, J.A.; Lamuedra, A.; Largo, R. Targeting chronic innate inflammatory pathways, the main road to prevention of osteoarthritis progression. Biochem. Pharmacol. 2019, 165, 24–32. [Google Scholar] [CrossRef]
- Srivastava, R.K.; Dar, H.Y.; Mishra, P.K. Immunoporosis: Immunology of Osteoporosis-Role of T Cells. Front. Immunol. 2018, 9, 657. [Google Scholar] [CrossRef] [PubMed]
- Kalaitzoglou, E.; Barboza, E.; Lopes, P.; Fu, Y.; Herron, J.C.; Flaming, J.M.; Donovan, E.L.; Hu, Y.; Filiberti, A.; Griffin, T.M.; et al. TLR4 Promotes and DAP12 Limits Obesity-Induced Osteoarthritis in Aged Female Mice. JBMR Plus 2018, 3, e10079. [Google Scholar] [CrossRef] [PubMed]
- Fukuhara, A.; Matsuda, M.; Nishizawa, M.; Segawa, K.; Tanaka, M.; Kishimoto, K.; Matsuki, Y.; Murakami, M.; Ichisaka, T.; Murakami, H.; et al. Retraction. Science 2007, 318, 565. [Google Scholar] [CrossRef] [PubMed]
- Rho, Y.H.; Solus, J.; Sokka, T.; Oeser, A.; Chung, C.P.; Gebretsadik, T.; Shintani, A.; Pincus, T.; Stein, C.M. Adipocytokines are associated with radiographic joint damage in rheumatoid arthritis. Arthritis Rheum. 2009, 60, 1906–1914. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hong, E.H.; Yun, H.S.; Kim, J.; Um, H.D.; Lee, K.H.; Kang, C.M.; Lee, S.J.; Chun, J.S.; Hwang, S.G. Nicotinamide phosphoribosyltransferase is essential for interleukin-1beta-mediated dedifferentiation of articular chondrocytes via SIRT1 and extracellular signal-regulated kinase (ERK) complex signaling. J. Biol. Chem. 2011, 286, 28619–28631. [Google Scholar] [CrossRef] [PubMed]
- Liu, P.; Li, H.; Cepeda, J.; Zhang, L.Q.; Cui, X.; Garcia, J.G.N.; Ye, S.Q. Critical role of PBEF expression in pulmonary cell inflammation and permeability. Cell Biol. Int. 2009, 33, 19–30. [Google Scholar] [CrossRef] [PubMed]
- Dahl, T.B.; Yndestad, A.; Skjelland, M.; Øie, E.; Dahl, A.; Michelsen, A.; Damås, J.K.; Tunheim, S.H.; Ueland, T.; Smith, C.; et al. Increased expression of visfatin in macrophages of human unstable carotid and coronary atherosclerosis: Possible role in inflammation and plaque destabilization. Circulation 2007, 115, 972–980. [Google Scholar] [CrossRef]
- Dvir-Ginzberg, M.; Gagarina, V.; Lee, E.J.; Hall, D.J. Regulation of cartilage-specific gene expression in human chondrocytes by SirT1 and nicotinamide phosphoribosyltransferase. J. Biol. Chem. 2008, 283, 36300–36310. [Google Scholar] [CrossRef]
- Chen, W.P.; Bao, J.P.; Feng, J.; Hu, P.F.; Shi, Z.L.; Wu, L.D. Increased serum concentrations of visfatin and its production by different joint tissues in patients with osteoarthritis. Clin. Chem. Lab. Med. 2010, 48, 1141–1145. [Google Scholar] [CrossRef]
- Duan, Y.; Hao, D.; Li, M.; Wu, Z.; Li, D.; Yang, X.; Qiu, G. Increased synovial fluid visfatin is positively linked to cartilage degradation biomarkers in osteoarthritis. Rheumatol. Int. 2012, 32, 985–990. [Google Scholar] [CrossRef]
- Yang, W.; Kang, X.; Liu, J.; Li, H.; Ma, Z.; Jin, X.; Qian, Z.; Xie, T.; Qin, N.; Feng, D.; et al. Clock gene Bmal1 modulates human cartilage gene expression by crosstalk with Sirt1. Endocrinology 2016, 157, 3096–3107. [Google Scholar] [CrossRef]
- Calvet, J.; Orellana, C.; Gratacós, J.; Berenguer-Llergo, A.; Caixàs, A.; Chillarón, J.J.; Pedro-Botet, J.; García-Manrique, M.; Navarro, N.; Larrosa, M. Synovial fluid adipokines are associated with clinical severity in knee osteoarthritis: A cross-sectional study in female patients with joint effusion. Arthritis Res. Ther. 2016, 18, 207. [Google Scholar] [CrossRef]
- Calvet, J.; Orellana, C.; Albiñana Giménez, N.; Berenguer-Llergo, A.; Caixàs, A.; García-Manrique, M.; Galisteo Lencastre, C.; Navarro, N.; Larrosa, M.; Gratacós, J. Differential involvement of synovial adipokines in pain and physical function in female patients with knee osteoarthritis. A cross-sectional study. Osteoarthr. Cartil. 2017, 26, 276–284. [Google Scholar] [CrossRef]
- Oh, H.; Kwak, J.S.; Yang, S.; Gong, M.K.; Kim, J.H.; Rhee, J.; Kim, S.K.; Kim, H.E.; Ryu, J.H.; Chun, J.S. Reciprocal regulation by hypoxia-inducible factor-2α and the NAMPT-NAD+-SIRT axis in articular chondrocytes is involved in osteoarthritis. Osteoarthr. Cartil. 2015, 23, 2288–2296. [Google Scholar] [CrossRef]
- Yammani, R.R.; Loeser, R.F. Extracellular nicotinamide phosphoribosyltransferase (NAMPT/visfatin) inhibits insulin-like growth factor-1 signaling and proteoglycan synthesis in human articular chondrocytes. Arthritis Res. Ther. 2012, 14, R23. [Google Scholar] [CrossRef]
- Davies, L.C.; Blain, E.J.; Gilbert, S.J.; Caterson, B.; Duance, V.C. The Potential of IGF-1 and TGFβ1 for Promoting “Adult” Articular Cartilage Repair: An In Vitro Study. Tissue Eng. Part A 2008, 14, 1251–1261. [Google Scholar] [CrossRef]
- Jacques, C.; Holzenberger, M.; Mladenovic, Z.; Salvat, C.; Pecchi, E.; Berenbaum, F.; Gosset, M. Proinflammatory actions of visfatin/nicotinamide phosphoribosyltransferase (Nampt) involve regulation of insulin signaling pathway and Nampt enzymatic activity. J. Biol. Chem. 2012, 287, 15100–15108. [Google Scholar] [CrossRef]
- McNulty, A.L.; Miller, M.R.; O’Connor, S.K.; Guilak, F. The effects of adipokines on cartilage and meniscus catabolism. Connect. Tissue Res. 2011, 52, 523–533. [Google Scholar] [CrossRef]
- Junker, S.; Frommer, K.W.; Krumbholz, G.; Tsiklauri, L.; Gerstberger, R.R.; Rehart, S.; Steinmeyer, J.J.; Rickert, M.; Wenisch, S.; Schett, G.; et al. Expression of adipokines in osteoarthritis osteophytes and their effect on osteoblasts. Matrix Biol. 2017, 62, 75–91. [Google Scholar] [CrossRef]
- Won, Y.; Shin, Y.; Chun, C.H.; Cho, Y.; Ha, C.W.; Kim, J.H.; Chun, J.S. Pleiotropic roles of metallothioneins as regulators of chondrocyte apoptosis and catabolic and anabolic pathways during osteoarthritis pathogenesis. Ann. Rheum. Dis. 2016, 75, 2045–2052. [Google Scholar] [CrossRef] [Green Version]
- Pecchi, E.; Priam, S.; Gosset, M.; Pigenet, A.; Sudre, L.; Laiguillon, M.-C.; Berenbaum, F.; Houard, X. Induction of nerve growth factor expression and release by mechanical and inflammatory stimuli in chondrocytes: Possible involvement in osteoarthritis pain. Arthritis Res. Ther. 2014, 16, R16. [Google Scholar] [CrossRef]
- Guo, J.Y.; Li, F.; Wen, Y.B.; Cui, H.X.; Guo, M.L.; Zhang, L.; Zhang, Y.F.; Guo, Y.J.; Guo, Y.X. Melatonin inhibits Sirt1-dependent NAMPT and NFAT5 signaling in chondrocytes to attenuate osteoarthritis. Oncotarget 2017, 8, 55967–55983. [Google Scholar] [CrossRef] [Green Version]
- Gossan, N.; Boot-Handford, R.P.; Meng, Q.J. Ageing and osteoarthritis: A circadian rhythm connection. Biogerontology 2015, 16, 209–219. [Google Scholar] [CrossRef]
- Matsushita, T.; Sasaki, H.; Takayama, K.; Ishida, K.; Matsumoto, T.; Kubo, S.; Matsuzaki, T.; Nishida, K.; Kurosaka, M.; Kuroda, R. The overexpression of SIRT1 inhibited osteoarthritic gene expression changes induced by interleukin-1β in human chondrocytes. J. Orthop. Res. 2013, 31, 531–537. [Google Scholar] [CrossRef]
- Haas, S.; Straub, R.H. Disruption of rhythms of molecular clocks in primary synovial fibroblasts of patients with osteoarthritis and rheumatoid arthritis, role of IL-1β/TNF. Arthritis Res. Ther. 2012, 14, R122. [Google Scholar] [CrossRef]
- Gossan, N.; Zeef, L.; Hensman, J.; Hughes, A.; Bateman, J.F.; Rowley, L.; Little, C.B.; Piggins, H.D.; Rattray, M.; Boot-Handford, R.P.; et al. The circadian clock in murine chondrocytes regulates genes controlling key aspects of cartilage homeostasis. Arthritis Rheum. 2013, 65, 2334–2345. [Google Scholar] [CrossRef]
- Chang, H.C.; Guarente, L. SIRT1 and other sirtuins in metabolism. Trends Endocrinol. Metab. 2014, 25, 138–145. [Google Scholar] [CrossRef]
- Ramsey, K.M.; Yoshino, J.; Brace, C.S.; Abrassart, D.; Kobayashi, Y.; Marcheva, B.; Hong, H.; Chong, J.L.; Ethan, D.; Lee, C.; et al. Circadian Clock Feedback Cycle Through NAMPT-Mediated NAD+ Biosynthesis. Science 2009, 324, 651–654. [Google Scholar] [CrossRef]
- Nakahata, Y.; Sahar, S.; Astarita, G.; Kaluzova, M.; Sassone-Corsi, P. Circadian control of the NAD+ salvage pathway by CLOCK-SIRT1. Science 2009, 324, 654–657. [Google Scholar] [CrossRef]
- Gandhi, R.; Takahashi, M.; Virtanen, C.; Syed, K.; Davey, J.R.; Mahomed, N.N. Microarray analysis of the infrapatellar fat pad in knee osteoarthritis: Relationship with joint inflammation. J. Rheumatol. 2011, 38, 1966–1972. [Google Scholar] [CrossRef]
- Gandhi, R.; Takahashi, M.; Smith, H.; Rizek, R.; Mahomed, N.N. The synovial fluid adiponectin-leptin ratio predicts pain with knee osteoarthritis. Clin. Rheumatol. 2010, 29, 1223–1228. [Google Scholar] [CrossRef]
- Klein-Wieringa, I.R.; Kloppenburg, M.; Bastiaansen-Jenniskens, Y.M.; Yusuf, E.; Kwekkeboom, J.C.; El-Bannoudi, H.; Nelissen, R.G.H.H.; Zuurmond, A.; Stojanovic-Susulic, V.; Van Osch, G.J.V.M.; et al. The infrapatellar fat pad of patients with osteoarthritis has an inflammatory phenotype. Ann. Rheum. Dis. 2011, 70, 851–857. [Google Scholar] [CrossRef] [Green Version]
- Brentano, F.; Schorr, O.; Ospelt, C.; Stanczyk, J.; Gay, R.E.; Gay, S.; Kyburz, D. Pre-B cell colony-enhancing factor/visfatin, a new marker of inflammation in rheumatoid arthritis with proinflammatory and matrix-degrading activities. Arthritis Rheum. 2007, 56, 2829–2839. [Google Scholar] [CrossRef]
- Wu, M.H.; Tsai, C.H.; Huang, Y.L.; Fong, Y.C.; Tang, C.H. Visfatin Promotes IL-6 and TNF-α Production in Human Synovial Fibroblasts by Repressing miR-199a-5p through ERK, p38 and JNK Signaling Pathways. Int. J. Mol. Sci. 2018, 19, 190. [Google Scholar] [CrossRef]
- Nishimuta, J.F.; Levenston, M.E. Meniscus is more susceptible than cartilage to catabolic and anti-anabolic effects of adipokines. Osteoarthr. Cartil. 2015, 23, 1551–1562. [Google Scholar] [CrossRef] [Green Version]
- Han, R.L.; Li, Z.J.; Li, M.J.; Li, J.Q.; Lan, X.Y.; Sun, G.R.; Kang, X.T.; Chen, H. Novel 9-bp indel in visfatin gene and its associations with chicken growth. Br. Poult. Sci. 2011, 52, 52–57. [Google Scholar] [CrossRef]
- Ma, C.; Pi, C.; Yang, Y.; Lin, L.; Shi, Y.; Li, Y.; Li, Y.; He, X. Nampt Expression Decreases Age-Related Senescence in Rat Bone Marrow Mesenchymal Stem Cells by Targeting Sirt1. PLoS ONE 2017, 12, e0170930. [Google Scholar] [CrossRef]
- Biver, E.; Salliot, C.; Combescure, C.; Gossec, L.; Hardouin, P.; Legroux-Gerot, I.; Cortet, B. Influence of adipokines and ghrelin on bone mineral density and fracture risk: A systematic review and meta-analysis. J. Clin. Endocrinol. Metab. 2011, 96, 2703–2713. [Google Scholar] [CrossRef]
- Peng, X.D.; Xie, H.; Zhao, Q.; Wu, X.P.; Sun, Z.Q.; Liao, E.Y. Relationships between serum adiponectin, leptin, resistin, visfatin levels and bone mineral density, and bone biochemical markers in Chinese men. Clin. Chim. Acta 2008, 387, 31–35. [Google Scholar] [CrossRef]
- Zhang, H.; Xie, H.; Zhao, Q.; Xie, G.Q.; Wu, X.P.; Liao, E.Y.; Luo, X.H. Relationships between serum adiponectin, apelin, leptin, resistin, visfatin levels and bone mineral density, and bone biochemical markers in post-menopausal Chinese women. J. Endocrinol. Investig. 2010, 33, 707–711. [Google Scholar] [CrossRef]
- Van den Bergh, R.; Florence, E.; Vlieghe, E.; Boonefaes, T.; Grooten, J.; Houthuys, E.; Tran, H.T.T.; Gali, Y.; De Baetselier, P.; Vanham, G.; et al. Transcriptome analysis of monocyte-HIV interactions. Retrovirology 2010, 7, 53. [Google Scholar] [CrossRef]
- Gruodyte, R.; Jurimae, J.; Cicchella, A.; Stefanelli, C.; Passariello, C.; Jurimae, T. Adipocytokines and bone mineral density in adolescent female athletes. Acta Paediatr. 2010, 99, 1879–1884. [Google Scholar] [CrossRef]
- Tohidi, M.; Akbarzadeh, S.; Larijani, B.; Kalantarhormozi, M.; Ostovar, A.; Assadi, M.; Vahdat, K.; Farrokhnia, M.; Sanjdideh, Z.; Amirinejad, R.; et al. Omentin-1, visfatin and adiponectin levels in relation to bone mineral density in Iranian postmenopausal women. Bone 2012, 51, 876–881. [Google Scholar] [CrossRef] [Green Version]
- Śliwicka, E.; Nowak, A.; Zep, W.; Leszczyński, P.; Pilaczyńska-Szcześniak, Ł. Bone mass and bone metabolic indices in male master rowers. J. Bone Miner. Metab. 2015, 33, 540–546. [Google Scholar] [CrossRef]
- Kochetkova, E.A.; Ugai, L.G.; Maistrovskaia, Y.V.; Nevzorova, V.A. Adipokines: A Possible Contribution to Vascular and Bone Remodeling in Idiopathic Pulmonary Arterial Hypertension. Calcif. Tissue Int. 2017, 100, 325–331. [Google Scholar] [CrossRef]
- Sucunza, N.; Barahona, M.J.; Resmini, E.; Fernández-Real, J.M.; Ricart, W.; Farrerons, J.; Rodríguez Espinosa, J.; Marin, A.M.; Puig, T.; Webb, S.M. A link between bone mineral density and serum adiponectin and visfatin levels in acromegaly. J. Clin. Endocrinol. Metab. 2009, 94, 3889–3896. [Google Scholar] [CrossRef]
- Linossier, M.T.; Amirova, L.E.; Thomas, M.; Normand, M.; Bareille, M.P.; Gauquelin-Koch, G.; Beck, A.; Costes-Salon, M.C.; Bonneau, C.; Gharib, C.; et al. Effects of short-term dry immersion on bone remodeling markers, insulin and adipokines. PLoS ONE 2017, 12, e0182970. [Google Scholar] [CrossRef]
- Chen, Z.; Zhao, G.H.; Zhang, Y.K.; Shen, G.S.; Xu, Y.J.; Xu, N.W. Research on the correlation of diabetes mellitus complicated with osteoporosis with lipid metabolism, adipokines and inflammatory factors and its regression analysis. Eur. Rev. Med. Pharmacol. Sci. 2017, 21, 3900–3905. [Google Scholar]
- Terzoudis, S.; Malliaraki, N.; Damilakis, J.; Dimitriadou, D.A.; Zavos, C.; Koutroubakis, I.E. Chemerin, visfatin, and vaspin serum levels in relation to bone mineral density in patients with inflammatory bowel disease. Eur. J. Gastroenterol. Hepatol. 2016, 28, 814–819. [Google Scholar] [CrossRef]
- Park, K.H.; Kim, D.K.; Huh, Y.H.; Lee, G.; Lee, S.H.; Hong, Y.; Kim, S.H.; Kook, M.S.; Koh, J.T.; Chun, J.S.; et al. NAMPT enzyme activity regulates catabolic gene expression in gingival fibroblasts during periodontitis. Exp. Mol. Med. 2017, 49, e368. [Google Scholar] [CrossRef]
- Evans, L.; Williams, A.S.; Hayes, A.J.; Jones, S.A.; Nowell, M. Suppression of leukocyte infiltration and cartilage degradation by selective inhibition of pre-B cell colony-enhancing factor/visfatin/nicotinamide phosphoribosyltransferase: Apo866-mediated therapy in human fibroblasts and murine collagen-induced arthrit. Arthritis Rheum. 2011, 63, 1866–1877. [Google Scholar] [CrossRef]
- Briana, D.D.; Boutsikou, M.; Boutsikou, T.; Malamitsi-Puchner, A. Associations of novel adipocytokines with bone biomarkers in intra uterine growth-restricted fetuses/neonates at term. J. Matern. Fetal. Neonatal Med. 2014, 27, 984–988. [Google Scholar] [CrossRef]
- Iacobellis, G.; Iorio, M.; Napoli, N.; Cotesta, D.; Zinnamosca, L.; Marinelli, C.; Petramala, L.; Minisola, S.; D’Erasmo, E.; Letizia, C. Relation of adiponectin, visfatin and bone mineral density in patients with metabolic syndrome. J. Endocrinol. Investig. 2011, 34, e12–e15. [Google Scholar] [CrossRef]
- Siviero-Miachon, A.A.; Spinola-Castro, A.M.; de Martino Lee, M.L.; Calixto, A.R.; Geloneze, B.; Lazaretti-Castro, M.; Guerra-Junior, G. Visfatin is a positive predictor of bone mineral density in young survivors of acute lymphocytic leukemia. J. Bone Miner. Metab. 2017, 35, 73–82. [Google Scholar] [CrossRef]
- Briana, D.D.; Boutsikou, M.; Boutsikou, T.; Malamitsi-Puchner, A. Relationships between maternal novel adipocytokines and bone biomarkers in complicated by gestational hypertensive disorders and normal pregnancies. J. Matern. Fetal. Neonatal Med. 2013, 26, 1219–1222. [Google Scholar] [CrossRef]
- He, X.; He, J.; Shi, Y.; Pi, C.; Yang, Y.; Sun, Y.; Ma, C.; Lin, L.; Zhang, L.; Li, Y.Y.; et al. Nicotinamide phosphoribosyltransferase (Nampt) may serve as the marker for osteoblast differentiation of bone marrow-derived mesenchymal stem cells. Exp. Cell Res. 2017, 352, 45–52. [Google Scholar] [CrossRef]
- Ling, M.; Huang, P.; Islam, S.; Heruth, D.P.; Li, X.; Zhang, L.Q.; Li, D.-Y.; Hu, Z.; Ye, S.Q. Epigenetic regulation of Runx2 transcription and osteoblast differentiation by nicotinamide phosphoribosyltransferase. Cell Biosci. 2017, 7, 27. [Google Scholar] [CrossRef]
- Riddle, R.C.; Clemens, T.L. Insulin, osteoblasts, and energy metabolism: Why bone counts calories. J. Clin. Investig. 2014, 124, 1465–1467. [Google Scholar] [CrossRef]
- Xie, H.; Tang, S.Y.; Luo, X.H.; Huang, J.; Cui, R.R.; Yuan, L.Q.; Zhou, H.D.; Wu, X.P.; Liao, E.Y. Insulin-Like Effects of Visfatin on Human Osteoblasts. Calcif. Tissue Int. 2007, 80, 201–210. [Google Scholar] [CrossRef]
- Li, Y.Y.; He, X.; Li, Y.Y.; He, J.; Anderstam, B.; Andersson, G.; Lindgren, U. Nicotinamide phosphoribosyltransferase (Nampt) affects the lineage fate determination of mesenchymal stem cells: A possible cause for reduced osteogenesis and increased adipogenesis in older individuals. J. Bone Miner. Res. 2011, 26, 2656–2664. [Google Scholar] [CrossRef]
- Li, Y.Y.; He, J.; He, X.; Li, Y.Y.; Lindgren, U. Nampt expression increases during osteogenic differentiation of multi- and omnipotent progenitors. Biochem. Biophys. Res. Commun. 2013, 434, 117–123. [Google Scholar] [CrossRef]
- Zainabadi, K. The variable role of SIRT1 in the maintenance and differentiation of mesenchymal stem cells. Regen. Med. 2018, 13, 343–356. [Google Scholar] [CrossRef]
- Moschen, A.R.; Geiger, S.; Gerner, R.; Tilg, H. Pre-B cell colony enhancing factor/NAMPT/visfatin and its role in inflammation-related bone disease. Mutat. Res. Fundam. Mol. Mech. Mutagen. 2010, 690, 95–101. [Google Scholar] [CrossRef]
- Baek, J.M.; Ahn, S.J.; Cheon, Y.H.; Lee, M.S.; Oh, J.; Kim, J.Y. Nicotinamide phosphoribosyltransferase inhibits receptor activator of nuclear factor-κB ligand-induced osteoclast differentiation in vitro. Mol. Med. Rep. 2017, 15, 784–792. [Google Scholar] [CrossRef]
- Kacso, A.C.; Bondor, C.I.; Coman, A.L.; Potra, A.R.; Georgescu, C.E. Determinants of visfatin in type 2 diabetes patients with diabetic kidney disease: Relationship to inflammation, adiposity and undercarboxylated osteocalcin. Scand. J. Clin. Lab. Investig. 2016, 76, 217–225. [Google Scholar] [CrossRef]
- Silva, B.C.; Bilezikian, J.P. Parathyroid hormone: Anabolic and catabolic actions on the skeleton. Curr. Opin. Pharmacol. 2015, 22, 41–50. [Google Scholar] [CrossRef]
- Nishikawa, A.; Ishida, T.; Taketsuna, M.; Yoshiki, F.; Enomoto, H. Safety and effectiveness of daily teriparatide in a prospective observational study in patients with osteoporosis at high risk of fracture in Japan: Final report. Clin. Interv. Aging 2016, 11, 913–925. [Google Scholar] [CrossRef]


© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Franco-Trepat, E.; Guillán-Fresco, M.; Alonso-Pérez, A.; Jorge-Mora, A.; Francisco, V.; Gualillo, O.; Gómez, R. Visfatin Connection: Present and Future in Osteoarthritis and Osteoporosis. J. Clin. Med. 2019, 8, 1178. https://doi.org/10.3390/jcm8081178
Franco-Trepat E, Guillán-Fresco M, Alonso-Pérez A, Jorge-Mora A, Francisco V, Gualillo O, Gómez R. Visfatin Connection: Present and Future in Osteoarthritis and Osteoporosis. Journal of Clinical Medicine. 2019; 8(8):1178. https://doi.org/10.3390/jcm8081178
Chicago/Turabian StyleFranco-Trepat, Eloi, María Guillán-Fresco, Ana Alonso-Pérez, Alberto Jorge-Mora, Vera Francisco, Oreste Gualillo, and Rodolfo Gómez. 2019. "Visfatin Connection: Present and Future in Osteoarthritis and Osteoporosis" Journal of Clinical Medicine 8, no. 8: 1178. https://doi.org/10.3390/jcm8081178