APEX1 Expression as a Potential Diagnostic Biomarker of Clear Cell Renal Cell Carcinoma and Hepatobiliary Carcinomas
Abstract
:1. Introduction
2. Materials and Methods
2.1. Patients and Samples
2.2. Frozen Serum APEX1 Measurement by Enzyme-Linked Immunosorbent Assay (ELISA)
2.3. Tissue APEX1 Measurement by Immunohistochemical Staining Analysis
2.4. APEX1 mRNA Measurement by Reverse Transcriptase Digital Droplet PCR (RT-ddPCR)
2.5. Statistics
3. Results
3.1. Association between APEX1 Expression and Clinicopathological Characteristics
3.2. Cytoplasmic APEX1 May Predict Poor Prognosis with Relapse in HCC and Intrahepatic CC Cases
3.3. APEX1 mRNA Expression Levels Were Higher in HCC Tissues than in Non-Neoplastic Liver Tissues
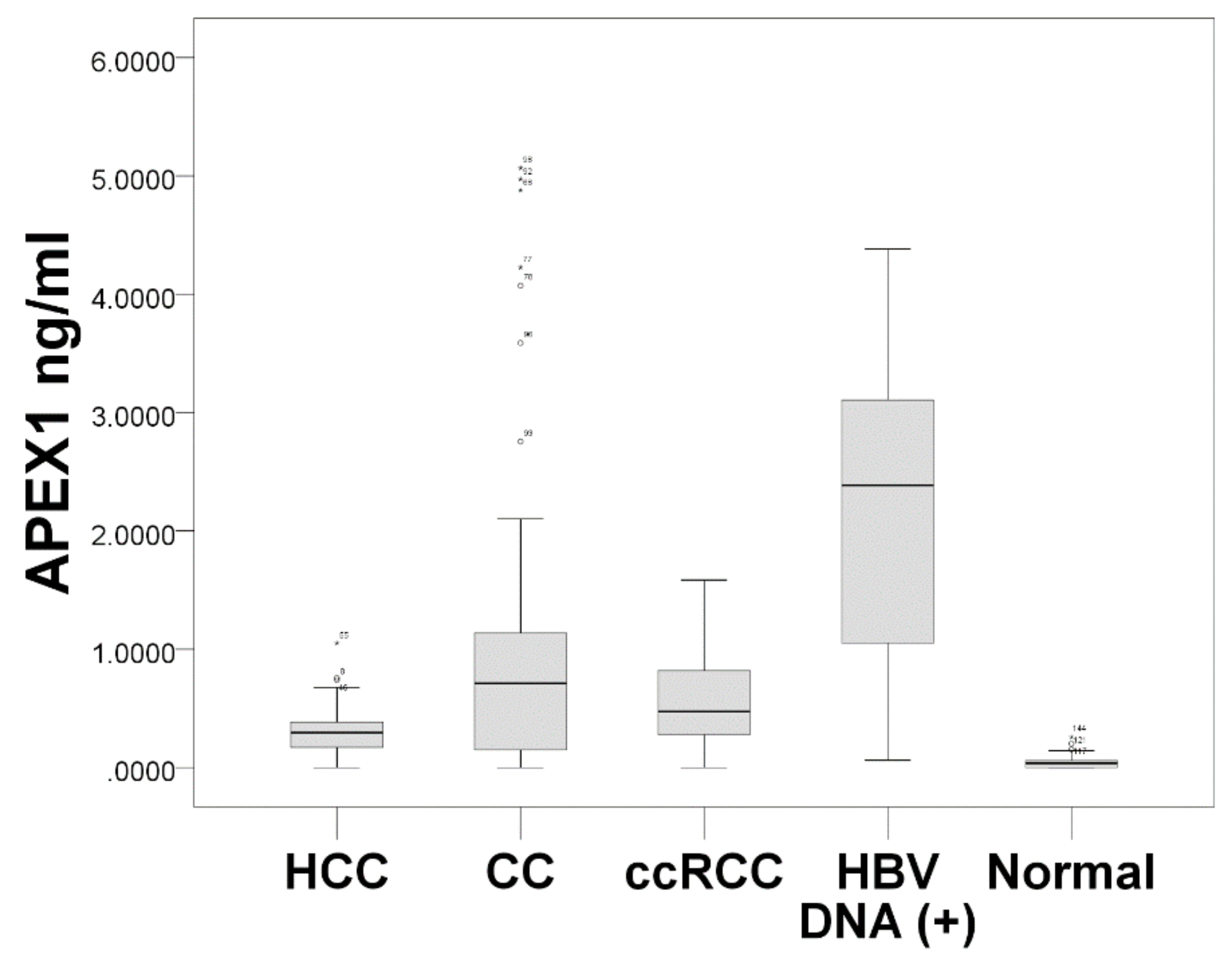
3.4. Serum APEX1 Expression Significantly Increased in Patients with HCC, CC, and ccRCC
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Tell, G.; Damante, G.; Caldwell, D.; Kelley, M.R. The intracellular localization of APE1/Ref-1: More than a passive phenomenon? Antioxid. Redox Signal. 2005, 7, 367–384. [Google Scholar] [CrossRef] [PubMed]
- O’Hara, A.M.; Bhattacharyya, A.; Mifflin, R.C.; Smith, M.F.; Ryan, K.A.; Scott, K.G.; Naganuma, M.; Casola, A.; Izumi, T.; Mitra, S.; et al. Interleukin-8 induction by Helicobacter pylori in gastric epithelial cells is dependent on apurinic/apyrimidinic endonuclease-1/redox factor-1. J. Immunol. 2006, 177, 7990–7999. [Google Scholar] [CrossRef]
- Lee, H.M.; Yuk, J.M.; Shin, D.M.; Yang, C.S.; Kim, K.K.; Choi, D.K.; Liang, Z.L.; Kim, J.M.; Jeon, B.H.; Kim, C.D.; et al. Apurinic/apyrimidinic endonuclease 1 is a key modulator of keratinocyte inflammatory responses. J. Immunol. 2009, 183, 6839–6848. [Google Scholar] [CrossRef] [PubMed]
- Cheng, T.L.; Chen, P.S.; Li, R.H.; Yuan, S.S.; Su, I.J.; Hung, J.H. Induction of apurinic endonuclease 1 overexpression by endoplasmic reticulum stress in hepatoma cells. Int. J. Mol. Sci. 2014, 15, 12442–12457. [Google Scholar] [CrossRef] [PubMed]
- Yuan, C.L.; He, F.; Ye, J.Z.; Wu, H.N.; Zhang, J.Y.; Liu, Z.H.; Li, Y.Q.; Luo, X.L.; Lin, Y.; Liang, R. APE1 overexpression is associated with poor survival in patients with solid tumors: A meta-analysis. Oncotarget 2017, 8, 59720–59728. [Google Scholar] [CrossRef]
- Pascut, D.; Sukowati, C.H.C.; Antoniali, G.; Mangiapane, G.; Burra, S.; Mascaretti, L.G.; Buonocore, M.R.; Croce, L.S.; Tiribelli, C.; Tell, G. Serum AP-endonuclease 1 (sAPE1) as novel biomarker for hepatocellular carcinoma. Oncotarget 2019, 10, 383–394. [Google Scholar] [CrossRef] [PubMed]
- Shin, J.H.; Choi, S.; Lee, Y.R.; Park, M.S.; Na, Y.G.; Irani, K.; Lee, S.D.; Park, J.B.; Kim, J.M.; Lim, J.S.; et al. APE1/Ref-1 as a Serological Biomarker for the Detection of Bladder Cancer. Cancer Res. Treat. 2015, 47, 823–833. [Google Scholar] [CrossRef] [Green Version]
- Zhang, S.; He, L.; Dai, N.; Guan, W.; Shan, J.; Yang, X.; Zhong, Z.; Qing, Y.; Jin, F.; Chen, C.; et al. Serum APE1 as a predictive marker for platinum-based chemotherapy of non-small cell lung cancer patients. Oncotarget 2016, 7, 77482–77494. [Google Scholar] [CrossRef] [Green Version]
- Amin, M.; Gress, D.; Vega, M.; Laura, R.; Edge, S.; Greene, F.; Byrd, D.R.; Brookland, R.K.; Washington, M.K. AJCC Cancer Staging Manual, 8th ed.; Springer: Chicago, IL, USA, 2017; pp. 739–748. [Google Scholar]
- Lee, Y.M.; Kim, J.M.; Lee, H.J.; Seong, I.O.; Kim, K.H. Immunohistochemical expression of CD44, matrix metalloproteinase2 and matrix metalloproteinase9 in renal cell carcinomas. Urol. Oncol. 2019. [Google Scholar] [CrossRef]
- Lee, Y.R.; Park, M.S.; Joo, H.K.; Kim, K.M.; Kim, J.; Jeon, B.H.; Choi, S. Therapeutic positioning of secretory acetylated APE1/Ref-1 requirement for suppression of tumor growth in triple-negative breast cancer in vivo. Sci. Rep. 2018, 8, 8701. [Google Scholar] [CrossRef]
- Allred, D.C.; Harvey, J.M.; Berardo, M.; Clark, G.M. Prognostic and predictive factors in breast cancer by immunohistochemical analysis. Mod. Pathol. 1998, 11, 155–168. [Google Scholar]
- Eun, H.S.; Cho, S.Y.; Lee, B.S.; Kim, S.; Song, I.S.; Chun, K.; Oh, C.H.; Yeo, M.K.; Kim, S.H.; Kim, K.H. Cytochrome P450 4A11 expression in tumor cells: A favorable prognostic factor for hepatocellular carcinoma patients. J. Gastroenterol. Hepatol. 2019, 34, 224–233. [Google Scholar] [CrossRef]
- Lee, Y.R.; Kim, K.M.; Jeon, B.H.; Choi, S. Extracellularly secreted APE1/Ref-1 triggers apoptosis in triple-negative breast cancer cells via RAGE binding, which is mediated through acetylation. Oncotarget 2015, 6, 23383–23398. [Google Scholar] [CrossRef] [Green Version]
- Duguid, J.R.; Eble, J.N.; Wilson, T.M.; Kelley, M.R. Differential cellular and subcellular expression of the human multifunctional apurinic/apyrimidinic endonuclease (APE/ref-1) DNA repair enzyme. Cancer Res. 1995, 55, 6097–6102. [Google Scholar]
- Kakolyris, S.; Kaklamanis, L.; Giatromanolaki, A.; Koukourakis, M.; Hickson, I.D.; Barzilay, G.; Turley, H.; Leek, R.D.; Kanavaros, P.; Georgoulias, V.; et al. Expression and subcellular localization of human AP endonuclease 1 (HAP1/Ref-1) protein: A basis for its role in human disease. Histopathology 1998, 33, 561–569. [Google Scholar] [CrossRef]
- Choi, S.; Joo, H.K.; Jeon, B.H. Dynamic Regulation of APE1/Ref-1 as a Therapeutic Target Protein. Chonnam Med. J. 2016, 52, 75–80. [Google Scholar] [CrossRef] [Green Version]
- Li, M.; Zhong, Z.; Zhu, J.; Xiang, D.; Dai, N.; Cao, X.; Qing, Y.; Yang, Z.; Xie, J.; Li, Z.; et al. Identification and characterization of mitochondrial targeting sequence of human apurinic/apyrimidinic endonuclease 1. J. Biol. Chem. 2010, 285, 14871–14881. [Google Scholar] [CrossRef]
- Barchiesi, A.; Wasilewski, M.; Chacinska, A.; Tell, G.; Vascotto, C. Mitochondrial translocation of APE1 relies on the MIA pathway. Nucleic Acids Res. 2015, 43, 5451–5464. [Google Scholar] [CrossRef] [Green Version]
- Li, M.X.; Shan, J.L.; Wang, D.; He, Y.; Zhou, Q.; Xia, L.; Zeng, L.L.; Li, Z.P.; Wang, G.; Yang, Z.Z. Human apurinic/apyrimidinic endonuclease 1 translocalizes to mitochondria after photodynamic therapy and protects cells from apoptosis. Cancer Sci. 2012, 103, 882–888. [Google Scholar] [CrossRef]
- Lucarelli, G.; Loizzo, D.; Franzin, R.; Battaglia, S.; Ferro, M.; Cantiello, F.; Castellano, G.; Bettocchi, C.; Ditonno, P.; Battaglia, M. Metabolomic insights into pathophysiological mechanisms and biomarker discovery in clear cell renal cell carcinoma. Expert Rev. Mol. Diagn. 2019, 19, 397–407. [Google Scholar] [CrossRef]
- Lucarelli, G.; Rutigliano, M.; Sallustio, F.; Ribatti, D.; Giglio, A.; Lepore Signorile, M.; Grossi, V.; Sanese, P.; Napoli, A.; Maiorano, E.; et al. Integrated multi-omics characterization reveals a distinctive metabolic signature and the role of NDUFA4L2 in promoting angiogenesis, chemoresistance, and mitochondrial dysfunction in clear cell renal cell carcinoma. Aging (Albany N. Y.) 2018, 10, 3957–3985. [Google Scholar] [CrossRef]
- Lai, R.K.; Xu, I.M.; Chiu, D.K.; Tse, A.P.; Wei, L.L.; Law, C.T.; Lee, D.; Wong, C.M.; Wong, M.P.; Ng, I.O.; et al. NDUFA4L2 Fine-tunes Oxidative Stress in Hepatocellular Carcinoma. Clin. Cancer Res. 2016, 22, 3105–3117. [Google Scholar] [CrossRef] [Green Version]
- Kakolyris, S.; Kaklamanis, L.; Engels, K.; Turley, H.; Hickson, I.D.; Gatter, K.C.; Harris, A.L. Human apurinic endonuclease 1 expression in a colorectal adenoma-carcinoma sequence. Cancer Res. 1997, 57, 1794–1797. [Google Scholar]
- Al-Attar, A.; Gossage, L.; Fareed, K.R.; Shehata, M.; Mohammed, M.; Zaitoun, A.M.; Soomro, I.; Lobo, D.N.; Abbotts, R.; Chan, S.; et al. Human apurinic/apyrimidinic endonuclease (APE1) is a prognostic factor in ovarian, gastro-oesophageal and pancreatico-biliary cancers. Br. J. Cancer 2010, 102, 704–709. [Google Scholar] [CrossRef] [Green Version]
- Di Maso, V.; Avellini, C.; Croce, L.S.; Rosso, N.; Quadrifoglio, F.; Cesaratto, L.; Codarin, E.; Bedogni, G.; Beltrami, C.A.; Tell, G.; et al. Subcellular localization of APE1/Ref-1 in human hepatocellular carcinoma: Possible prognostic significance. Mol. Med. 2007, 13, 89–96. [Google Scholar] [CrossRef]
- Wu, H.H.; Chu, Y.C.; Wang, L.; Tsai, L.H.; Lee, M.C.; Chen, C.Y.; Shieh, S.H.; Cheng, Y.W.; Lee, H. Cytoplasmic Ape1 expression elevated by p53 aberration may predict survival and relapse in resected non-small cell lung cancer. Ann. Surg. Oncol. 2013. [Google Scholar] [CrossRef]
- Choi, S.; Lee, Y.R.; Park, M.S.; Joo, H.K.; Cho, E.J.; Kim, H.S.; Kim, C.S.; Park, J.B.; Irani, K.; Jeon, B.H. Histone deacetylases inhibitor trichostatin A modulates the extracellular release of APE1/Ref-1. Biochem. Biophys. Res. Commun. 2013, 435, 403–407. [Google Scholar] [CrossRef]



| Case | No. Individuals |
|---|---|
| FFPE tissue specimen group | 269 |
| Hepatocellular carcinoma | 131 |
| HCC with HBV | 106 |
| HCC without HBV | 25 |
| Intrahepatic cholangiocarcinoma | 32 |
| Clear cell renal cell carcinoma | 106 |
| Frozen serum specimen group | 216 |
| Healthy control | 39 |
| Hepatitis B viral DNA (+) * | 32 |
| Hepatocellular carcinoma | 59 |
| Cholangiocarcinoma | 46 |
| Intrahepatic | 13 |
| hilar | 5 |
| distal | 28 |
| Clear cell renal cell carcinoma | 40 |
| Frozen tissue specimen group | 20 |
| Hepatocellular carcinoma | 20 |
| Characteristics | No. | Nuclear APEX1 | Cytoplasmic APEX1 | ||
|---|---|---|---|---|---|
| Median (IQR) | p * | Median (IQR) | p * | ||
| Age at operation | 0.778 | 0.778 | |||
| ≤65 | 54 | 7 (7–7) | 2 (0–4) | ||
| >65 | 52 | 7 (6.25–7) | 2 (0–3.75) | ||
| Sex | 0.885 | 0.383 | |||
| Male | 75 | 7 (7–7) | 2 (0–3) | ||
| Female | 31 | 7 (7–7) | 2 (0–5) | ||
| Histologic nuclear grade | 0.016 | <0.001 | |||
| I–II | 72 | 7 (7–7) | 0 (0–2) | ||
| III–IV | 34 | 7 (5.75–7) | 4.5 (2–6) | ||
| Pathologic T stage | 0.189 | 0.512 | |||
| I | 44 | 7 (7–7.75) | 2 (0–4.75) | ||
| II–IV | 62 | 7 (6.75–7) | 2 (0–3) | ||
| Characteristics | No. | Nuclear APEX1 | Cytoplasmic APEX1 | ||
|---|---|---|---|---|---|
| Median (IQR) | p | Median (IQR) | p | ||
| Age at operation | 0.018 * | 0.396 * | |||
| ≤65 | 109 | 7 (6–7.50) | 5 (3–6) | ||
| >65 | 22 | 8 (6.75–8) | 4 (2–6.25) | ||
| Sex | 0.121 * | 0.355 * | |||
| Male | 102 | 7 (6–8) | 5 (3–6) | ||
| Female | 29 | 7 (5.5–7) | 5 (4–6) | ||
| HBV infection | 0.421 * | 0.747 * | |||
| No | 25 | 7 (7–8) | 5 (3.5–6) | ||
| Yes | 106 | 7 (6–8) | 5 3–6) | ||
| Cirrhosis | 0.355 * | 0.534 * | |||
| No | 33 | 7 (5.5–8) | 5 (3–6) | ||
| Yes | 98 | 7 (6–8) | 5 (3–6) | ||
| Tumor size (the greatest diameter) | 0.174 * | 0.096 * | |||
| ≤5.0 cm | 110 | 7 (6–8) | 5 (3.75–6) | ||
| >5.0 cm | 21 | 7 (6.5–8) | 4 (3–5.5) | ||
| Lymphovascular invasion | 0.559 * | 0.569 * | |||
| No | 31 | 7 (6–8) | 5 (3–6) | ||
| Yes | 100 | 7 (6–8) | 5 (3–6) | ||
| Histologic grade | 0.947 * | 0.449 * | |||
| 1–2 (well and moderate) | 110 | 7 (6–8) | 5 (3–6) | ||
| 3–4 (poorly and undiff.) | 21 | 7 (5–8) | 5 (3.5–7) | ||
| Pathologic stage | 0.870 ** | 0.663 ** | |||
| I | 45 | 7 (6–7.5) | 5 (4–6) | ||
| II | 78 | 7 (6–8) | 5 (3–6) | ||
| III | 5 | 7 (6.5–7.5) | 6 (3.5–6.5) | ||
| IV | 3 | 7 (NA) | 5 (NA) | ||
| Characteristics | No. | Nuclear APEX1 | Cytoplasmic APEX1 | ||
|---|---|---|---|---|---|
| Median (IQR) | p | Median (IQR) | p | ||
| Age at operation | 0.785 * | 0.696 * | |||
| ≤65 | 21 | 7 (3–7) | 6 (3–7) | ||
| >65 | 11 | 6 (6–7) | 6 (3–7) | ||
| Sex | 0.229 * | 0.458 * | |||
| Male | 23 | 7 (5–7) | 6 3–7) | ||
| Female | 9 | 6 (2.5–7) | 5 (2–6.5) | ||
| HBV infection | 0.018 * | 0.480 * | |||
| No | 24 | 6 (3–7) | 6 (3.25–7) | ||
| Yes | 8 | 7 (6.25–8) | 4 (3–6.5) | ||
| Cirrhosis | 0.092 * | 0.207 * | |||
| No | 25 | 7 (3–7) | 6 (3.5–7) | ||
| Yes | 7 | 4 (6–8) | 3 (3–5) | ||
| Histologic grade | 0.285 * | 0.308 * | |||
| 1–2 (well & moderate) | 26 | 7 (5.75–7) | 6 (3–7) | ||
| 3–4 (poorly & undiff.) | 6 | 3.5 (0–7) | 5 (0–6.25) | ||
| Pathologic stage | 0.355 ** | 0.099 ** | |||
| I | 3 | 7 (NA) | 6 (NA) | ||
| II | 27 | 7 (5–7) | 6 (3–7) | ||
| III | 2 | NA | NA | ||
| IV | 0 | NA | NA | ||
| Prognostic Factor | Overall Survival | Disease-Free Survival | ||
|---|---|---|---|---|
| HR (95% CI) | p * | HR (95% CI) | p * | |
| Cytoplasmic APEX1 | 1.106 (0.936–1.307) | 0.237 | 1.153 (1.036–1.284) | 0.009 |
| Age at operation | 0.161 | 0.908 | ||
| ≤65 | 1 (reference) | 1 (reference) | ||
| >65 | 0.401 (0.112–1.437) | 0.963 (0.510–1.819) | ||
| Sex | 0.374 | 0.826 | ||
| Male | 1 (reference) | 1 (reference) | ||
| Female | 0.629 (0.226–1.749) | 0.934 (0.509–1.714) | ||
| HBV infection | 0.640 | 0.564 | ||
| No | 1 (reference) | 1 (reference) | ||
| Yes | 1.259 (0.479–3.308) | 1.202 (0.643–2.246) | ||
| Cirrhosis | 0.757 | 0.092 | ||
| No | 1 (reference) | 1 (reference) | ||
| Yes | 0.881 (0.396–1.963) | 1.645 (0.923–2.931) | ||
| Tumor size (the greatest diameter) | 0.004 | 0.022 | ||
| ≤5.0 cm | 1 (reference) | 1 (reference) | ||
| >5.0 cm | 4.400 (1.606–12.054) | 2.219 (1.122–4.389) | ||
| Lymphovascular invasion | 0.121 | 0.419 | ||
| No | 1 (reference) | 1 (reference) | ||
| Yes | 0.415 (0.137–1.262) | 1.382 (0.631–3.028) | ||
| Histologic grade | 0.004 | 0.019 | ||
| 1–2 (well and moderate) | 1 (reference) | 1 (reference) | ||
| 3–4 (poorly and undiff.) | 3.326 (1.455–7.599) | 1.970 (1.116–3.479) | ||
| Pathologic stage | 0.401 | 0.234 | ||
| I | 1 (reference) | 1 (reference) | ||
| II–IV | 1.525 (0.570–4.081) | 1.464 (0.782–2.743) | ||
| Prognostic Factor | Overall Survival | Disease-Free Survival | ||
|---|---|---|---|---|
| HR (95% CI) | p * | HR (95% CI) | p * | |
| Cytoplasmic APEX1 | 1.563 (0.886–2.757) | 0.123 | 1.407 (1.081–1.831) | 0.011 |
| Age at operation | 0.122 | 0.343 | ||
| ≤65 | 1 (reference) | 1 (reference) | ||
| >65 | 0.181 (0.021–1.578) | 0.596 (0.205–1.736) | ||
| Sex | 0.525 | 0.802 | ||
| Male | 1 (reference) | 1 (reference) | ||
| Female | 0.585 (0.112–3.049) | 1.150 (0.385–3.438) | ||
| HBV infection | 0.517 | 0.182 | ||
| No | 1 (reference) | 1 (reference) | ||
| Yes | 0.469 (0.048–4.627) | 0.224 (0.025–2.017) | ||
| Cirrhosis | 0.403 | 0.133 | ||
| No | 1 (reference) | 1 (reference) | ||
| Yes | 3.598 (0.179–72.492) | 5.979 (0.580–61.672) | ||
| Histologic grade | 0.485 | 0.831 | ||
| 1–2 (well and moderate) | 1 (reference) | 1 (reference) | ||
| 3–4 (poorly and undiff.) | 0.459 (0.052–4.073) | 1.145 (0.331–3.966) | ||
| Pathologic stage | 0.787 | 0.718 | ||
| I | 1 (reference) | 1 (reference) | ||
| II–IV | 0.734 (0.078–6.943) | 1.356 (0.260–7.089) | ||
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, J.-M.; Yeo, M.-K.; Lim, J.S.; Song, I.-S.; Chun, K.; Kim, K.-H. APEX1 Expression as a Potential Diagnostic Biomarker of Clear Cell Renal Cell Carcinoma and Hepatobiliary Carcinomas. J. Clin. Med. 2019, 8, 1151. https://doi.org/10.3390/jcm8081151
Kim J-M, Yeo M-K, Lim JS, Song I-S, Chun K, Kim K-H. APEX1 Expression as a Potential Diagnostic Biomarker of Clear Cell Renal Cell Carcinoma and Hepatobiliary Carcinomas. Journal of Clinical Medicine. 2019; 8(8):1151. https://doi.org/10.3390/jcm8081151
Chicago/Turabian StyleKim, Ji-Myung, Min-Kyung Yeo, Jae Sung Lim, In-Sang Song, Kwangsik Chun, and Kyung-Hee Kim. 2019. "APEX1 Expression as a Potential Diagnostic Biomarker of Clear Cell Renal Cell Carcinoma and Hepatobiliary Carcinomas" Journal of Clinical Medicine 8, no. 8: 1151. https://doi.org/10.3390/jcm8081151
APA StyleKim, J.-M., Yeo, M.-K., Lim, J. S., Song, I.-S., Chun, K., & Kim, K.-H. (2019). APEX1 Expression as a Potential Diagnostic Biomarker of Clear Cell Renal Cell Carcinoma and Hepatobiliary Carcinomas. Journal of Clinical Medicine, 8(8), 1151. https://doi.org/10.3390/jcm8081151






