Catestatin in Acutely Decompensated Heart Failure Patients: Insights from the CATSTAT-HF Study
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Population
2.2. Data Measurement
2.3. Definitions
2.4. Echocardiography
2.5. Laboratory Analyses
2.6. Statistical Analysis
3. Results
3.1. Patients’ Baseline Characteristics
3.2. ADHF Patients with a History of MI and Those Without
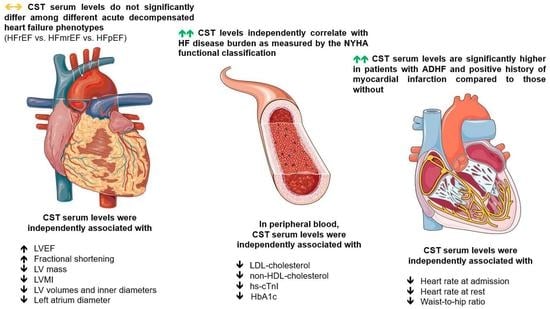
3.3. CST Serum Levels Stratified by the History of MI and Across Different LVEF Phenotypes
3.4. Associations of Serum CST Levels with Clinical and Laboratory Parameters
3.5. Associations of Serum CST Levels with Echocardiographic Parameters
4. Discussion
5. Conclusions
Author Contributions
Acknowledgments
Conflicts of Interest
References
- Kurmani, S.; Squire, I. Acute heart failure: Definition, classification and epidemiology. Curr. Heart Fail. Rep. 2017, 14, 385–392. [Google Scholar] [CrossRef] [PubMed]
- Roger, V.L. Epidemiology of heart failure. Circ. Res. 2013, 113, 646–659. [Google Scholar] [CrossRef] [PubMed]
- Savarese, G.; Lund, L.H. Global public health burden of heart failure. Card. Fail. Rev. 2017, 3, 7–11. [Google Scholar] [CrossRef]
- Ibrahim, N.E.; Januzzi, J.L. Established and emerging roles of biomarkers in heart failure. Circ. Res. 2018, 123, 614–629. [Google Scholar] [CrossRef] [PubMed]
- Viquerat, C.E.; Daly, P.; Swedberg, K.; Evers, C.; Curran, D.; Parmley, W.W.; Chatterjee, K. Endogenous catecholamine levels in chronic heart failure. Relation to the severity of hemodynamic abnormalities. Am. J. Med. 1985, 78, 455–460. [Google Scholar] [CrossRef]
- Deng, M.C.; Brisse, B.; Erren, M.; Khurana, C.; Breithardt, G.; Scheld, H.H. Ischemic versus idiopathic cardiomyopathy: Differing neurohumoral profiles despite comparable peak oxygen uptake. Int. J. Cardiol. 1997, 61, 261–268. [Google Scholar] [CrossRef]
- Notarius, C.F.; Spaak, J.; Morris, B.L.; Floras, J.S. Comparison of muscle sympathetic activity in ischemic and nonischemic heart failure. J. Card. Fail. 2007, 13, 470–475. [Google Scholar] [CrossRef]
- Fung, M.M.; Salem, R.M.; Mehtani, P.; Thomas, B.; Lu, C.F.; Perez, B.; Rao, F.; Stridsberg, M.; Ziegler, M.G.; Mahata, S.K.; et al. Direct vasoactive effects of the chromogranin A (CHGA) peptide catestatin in humans in vivo. Clin. Exp. Hypertens. 2010, 32, 278–287. [Google Scholar] [CrossRef]
- Mahata, S.K.; O’Connor, D.T.; Mahata, M.; Yoo, S.H.; Taupenot, L.; Wu, H.; Gill, B.M.; Parmer, R.J. Novel autocrine feedback control of catecholamine release. A discrete chromogranin a fragment is a noncompetitive nicotinic cholinergic antagonist. J. Clin. Invest. 1997, 100, 1623–1633. [Google Scholar] [CrossRef]
- Mahata, S.K.; Kiranmayi, M.; Mahapatra, N.R. Catestatin: A Master regulator of cardiovascular functions. Curr. Med. Chem. 2018, 25, 1352–1374. [Google Scholar] [CrossRef]
- Borovac, J.A.; Dogas, Z.; Supe-Domic, D.; Galic, T.; Bozic, J. Catestatin serum levels are increased in male patients with obstructive sleep apnea. Sleep Breath 2019, 23, 473–481. [Google Scholar] [CrossRef] [PubMed]
- Gaede, A.H.; Pilowsky, P.M. Catestatin, a chromogranin A-derived peptide, is sympathoinhibitory and attenuates sympathetic barosensitivity and the chemoreflex in rat CVLM. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2012, 302, R365–R372. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, X.; Xu, S.; Liang, Y.; Zhu, D.; Mi, L.; Wang, G.; Gao, W. Dramatic changes in catestatin are associated with hemodynamics in acute myocardial infarction. Biomarkers 2011, 16, 372–377. [Google Scholar] [CrossRef] [PubMed]
- Peng, F.; Chu, S.; Ding, W.; Liu, L.; Zhao, J.; Cui, X.; Li, R.; Wang, J. The predictive value of plasma catestatin for all-cause and cardiac deaths in chronic heart failure patients. Peptides 2016, 86, 112–117. [Google Scholar] [CrossRef] [PubMed]
- Ottesen, A.H.; Carlson, C.R.; Louch, W.E.; Dahl, M.B.; Sandbu, R.A.; Johansen, R.F.; Jarstadmarken, H.; Bjoras, M.; Hoiseth, A.D.; Brynildsen, J.; et al. Glycosylated chromogranin A in heart failure: Implications for processing and cardiomyocyte calcium homeostasis. Circ. Heart Fail. 2017, 10, e003675. [Google Scholar] [CrossRef] [PubMed]
- Meng, L.; Wang, J.; Ding, W.H.; Han, P.; Yang, Y.; Qi, L.T.; Zhang, B.W. Plasma catestatin level in patients with acute myocardial infarction and its correlation with ventricular remodelling. Postgrad. Med. J. 2013, 89, 193–196. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Ding, W.; Li, R.; Ye, X.; Zhao, J.; Jiang, J.; Meng, L.; Wang, J.; Chu, S.; Han, X.; et al. Plasma levels and diagnostic value of catestatin in patients with heart failure. Peptides 2013, 46, 20–25. [Google Scholar] [CrossRef] [PubMed]
- Zhu, D.; Wang, F.; Yu, H.; Mi, L.; Gao, W. Catestatin is useful in detecting patients with stage B heart failure. Biomarkers 2011, 16, 691–697. [Google Scholar] [CrossRef]
- McKee, P.A.; Castelli, W.P.; McNamara, P.M.; Kannel, W.B. The natural history of congestive heart failure: The Framingham study. N. Engl. J. Med. 1971, 285, 1441–1446. [Google Scholar] [CrossRef]
- Ponikowski, P.; Voors, A.A.; Anker, S.D.; Bueno, H.; Cleland, J.G.; Coats, A.J.; Falk, V.; Gonzalez-Juanatey, J.R.; Harjola, V.P.; Jankowska, E.A.; et al. 2016 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure: The task force for the diagnosis and treatment of acute and chronic heart failure of the European society of cardiology (ESC). Developed with the special contribution of the heart failure association (HFA) of the ESC. Eur. J. Heart Fail. 2016, 18, 891–975. [Google Scholar]
- Januzzi, J.L.; van Kimmenade, R.; Lainchbury, J.; Bayes-Genis, A.; Ordonez-Llanos, J.; Santalo-Bel, M.; Pinto, Y.M.; Richards, M. NT-proBNP testing for diagnosis and short-term prognosis in acute destabilized heart failure: An international pooled analysis of 1256 patients: The international collaborative of NT-proBNP study. Eur. Heart J. 2006, 27, 330–337. [Google Scholar] [CrossRef]
- Januzzi, J.L., Jr.; Camargo, C.A.; Anwaruddin, S.; Baggish, A.L.; Chen, A.A.; Krauser, D.G.; Tung, R.; Cameron, R.; Nagurney, J.T.; Chae, C.U.; et al. The N-terminal Pro-BNP investigation of dyspnea in the emergency department (PRIDE) study. Am. J. Cardiol. 2005, 95, 948–954. [Google Scholar] [CrossRef]
- Januzzi, J.L.; Chen-Tournoux, A.A.; Christenson, R.H.; Doros, G.; Hollander, J.E.; Levy, P.D.; Nagurney, J.T.; Nowak, R.M.; Pang, P.S.; Patel, D.; et al. N-terminal pro–B-type natriuretic peptide in the emergency department: The ICON-RELOADED study. J. Am. Coll. Cardiol. 2018, 71, 1191–1200. [Google Scholar] [CrossRef]
- Mosteller, R.D. Simplified calculation of body-surface area. N. Engl. J. Med. 1987, 317, 1098. [Google Scholar]
- Williams, B.; Mancia, G.; Spiering, W.; Agabiti Rosei, E.; Azizi, M.; Burnier, M.; Clement, D.L.; Coca, A.; de Simone, G.; Dominiczak, A.; et al. 2018 ESC/ESH Guidelines for the management of arterial hypertension. Eur. Heart J. 2018, 39, 3021–3104. [Google Scholar] [CrossRef]
- Levey, A.S.; Stevens, L.A.; Schmid, C.H.; Zhang, Y.L.; Castro, A.F., 3rd; Feldman, H.I.; Kusek, J.W.; Eggers, P.; Van Lente, F.; Greene, T.; et al. A new equation to estimate glomerular filtration rate. Ann. Intern. Med. 2009, 150, 604–612. [Google Scholar] [CrossRef]
- American Diabetes Association. (2) Classification and diagnosis of diabetes. Diabetes Care 2015, 38, S8–S16. [Google Scholar]
- Surawicz, B.; Childers, R.; Deal Barbara, J.; Gettes Leonard, S. AHA/ACCF/HRS Recommendations for the Standardization and interpretation of the electrocardiogram. Circulation 2009, 119, e235–e240. [Google Scholar] [CrossRef]
- Lang, R.M.; Badano, L.P.; Mor-Avi, V.; Afilalo, J.; Armstrong, A.; Ernande, L.; Flachskampf, F.A.; Foster, E.; Goldstein, S.A.; Kuznetsova, T.; et al. Recommendations for cardiac chamber quantification by echocardiography in adults: An update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. Eur. Heart J. Cardiovasc. Imaging 2015, 16, 233–270. [Google Scholar] [CrossRef]
- Folland, E.D.; Parisi, A.F.; Moynihan, P.F.; Jones, D.R.; Feldman, C.L.; Tow, D.E. Assessment of left ventricular ejection fraction and volumes by real-time, two-dimensional echocardiography. A comparison of cineangiographic and radionuclide techniques. Circulation 1979, 60, 760–766. [Google Scholar] [CrossRef]
- Remme, W.J. The sympathetic nervous system and ischaemic heart disease. Eur. Heart J. 1998, 19, F62–F71. [Google Scholar]
- Liu, L.; Ding, W.; Zhao, F.; Shi, L.; Pang, Y.; Tang, C. Plasma levels and potential roles of catestatin in patients with coronary heart disease. Scand. Cardiovasc. J. 2013, 47, 217–224. [Google Scholar] [CrossRef]
- Taupenot, L.; Harper, K.L.; O’Connor, D.T. The chromogranin-secretogranin family. N. Engl. J. Med. 2003, 348, 1134–1149. [Google Scholar] [CrossRef]
- Helle, K.B. The granin family of uniquely acidic proteins of the diffuse neuroendocrine system: Comparative and functional aspects. Biol. Rev. Camb. Philos. Soc. 2004, 79, 769–794. [Google Scholar] [CrossRef]
- Gayen, J.R.; Gu, Y.; O’Connor, D.T.; Mahata, S.K. Global disturbances in autonomic function yield cardiovascular instability and hypertension in the chromogranin a null mouse. Endocrinology 2009, 150, 5027–5035. [Google Scholar] [CrossRef]
- Pascual-Figal, D.A.; Manzano-Fernandez, S.; Boronat, M.; Casas, T.; Garrido, I.P.; Bonaque, J.C.; Pastor-Perez, F.; Valdes, M.; Januzzi, J.L. Soluble ST2, high-sensitivity troponin T-and N-terminal pro-B-type natriuretic peptide: Complementary role for risk stratification in acutely decompensated heart failure. Eur. J. Heart Fail. 2011, 13, 718–725. [Google Scholar] [CrossRef]
- Aimo, A.; Januzzi, J.L., Jr.; Vergaro, G.; Ripoli, A.; Latini, R.; Masson, S.; Magnoli, M.; Anand, I.S.; Cohn, J.N.; Tavazzi, L.; et al. High-sensitivity troponin T, NT-proBNP and glomerular filtration rate: A multimarker strategy for risk stratification in chronic heart failure. Int. J. Cardiol. 2019, 277, 166–172. [Google Scholar] [CrossRef] [Green Version]
- Ahmed, A. A propensity matched study of New York Heart Association class and natural history end points in heart failure. Am. J. Cardiol. 2007, 99, 549–553. [Google Scholar] [CrossRef]
- Ahmed, A.; Aronow, W.S.; Fleg, J.L. Higher New York Heart Association classes and increased mortality and hospitalization in patients with heart failure and preserved left ventricular function. Am. Heart J. 2006, 151, 444–450. [Google Scholar] [CrossRef] [Green Version]
- Muntwyler, J.; Abetel, G.; Gruner, C.; Follath, F. One-year mortality among unselected outpatients with heart failure. Eur. Heart J. 2002, 23, 1861–1866. [Google Scholar] [CrossRef] [Green Version]
- Wang, D.; Liu, T.; Shi, S.; Li, R.; Shan, Y.; Huang, Y.; Hu, D.; Huang, C. Chronic administration of catestatin improves autonomic function and exerts cardioprotective effects in myocardial infarction rats. J. Cardiovasc. Pharmacol. Ther. 2016, 21, 526–535. [Google Scholar] [CrossRef]
- Zhu, D.; Xie, H.; Wang, X.; Liang, Y.; Yu, H.; Gao, W. Correlation of plasma catestatin level and the prognosis of patients with acute myocardial infarction. PLoS ONE 2015, 10, e0122993. [Google Scholar] [CrossRef]
- Meng, L.; Ye, X.J.; Ding, W.H.; Yang, Y.; Di, B.B.; Liu, L.; Huo, Y. Plasma catecholamine release-inhibitory peptide catestatin in patients with essential hypertension. J. Cardiovasc. Med. 2011, 12, 643–647. [Google Scholar] [CrossRef]
- Mahapatra, N.R.; O’Connor, D.T.; Vaingankar, S.M.; Hikim, A.P.; Mahata, M.; Ray, S.; Staite, E.; Wu, H.; Gu, Y.; Dalton, N.; et al. Hypertension from targeted ablation of chromogranin A can be rescued by the human ortholog. J. Clin. Invest. 2005, 115, 1942–1952. [Google Scholar] [CrossRef] [Green Version]
- Ying, W.; Mahata, S.; Bandyopadhyay, G.K.; Zhou, Z.; Wollam, J.; Vu, J.; Mayoral, R.; Chi, N.W.; Webster, N.J.G.; Corti, A.; et al. Catestatin inhibits obesity-induced macrophage infiltration and inflammation in the liver and suppresses hepatic glucose production, leading to improved insulin sensitivity. Diabetes 2018, 67, 841–848. [Google Scholar] [CrossRef]
- Gallo, M.P.; Femmino, S.; Antoniotti, S.; Querio, G.; Alloatti, G.; Levi, R. Catestatin induces glucose uptake and GLUT4 trafficking in adult rat cardiomyocytes. Biomed. Res. Int. 2018, 2018, 2086109. [Google Scholar] [CrossRef]
- Simunovic, M.; Supe-Domic, D.; Karin, Z.; Degoricija, M.; Paradzik, M.; Bozic, J.; Unic, I.; Skrabic, V. Serum catestatin concentrations are decreased in obese children and adolescents. Pediatr. Diabetes 2019. Epub ahead of print. [Google Scholar] [CrossRef]
- Bandyopadhyay, G.K.; Vu, C.U.; Gentile, S.; Lee, H.; Biswas, N.; Chi, N.W.; O’Connor, D.T.; Mahata, S.K. Catestatin (chromogranin A (352–372)) and novel effects on mobilization of fat from adipose tissue through regulation of adrenergic and leptin signaling. J. Biol. Chem. 2012, 287, 23141–23151. [Google Scholar] [CrossRef]
- Bandyopadhyay, G.K.; Mahata, S.K. Chromogranin A regulation of obesity and peripheral insulin sensitivity. Front. Endocrinol. 2017, 8, 20. [Google Scholar] [CrossRef]
- Durakoglugil, M.E.; Ayaz, T.; Kocaman, S.A.; Kirbas, A.; Durakoglugil, T.; Erdogan, T.; Cetin, M.; Sahin, O.Z.; Cicek, Y. The relationship of plasma catestatin concentrations with metabolic and vascular parameters in untreated hypertensive patients: Influence on high-density lipoprotein cholesterol. Anatol. J. Cardiol. 2015, 15, 577–585. [Google Scholar] [CrossRef]
- Kojima, M.; Ozawa, N.; Mori, Y.; Takahashi, Y.; Watanabe-Kominato, K.; Shirai, R.; Watanabe, R.; Sato, K.; Matsuyama, T.A.; Ishibashi-Ueda, H.; et al. Catestatin prevents macrophage-driven atherosclerosis but not arterial injury-induced neointimal hyperplasia. Thromb. Haemost. 2018, 118, 182–194. [Google Scholar] [CrossRef]
- Chen, Y.; Wang, X.; Yang, C.; Su, X.; Yang, W.; Dai, Y.; Han, H.; Jiang, J.; Lu, L.; Wang, H.; et al. Decreased circulating catestatin levels are associated with coronary artery disease: The emerging anti-inflammatory role. Atherosclerosis 2019, 281, 78–88. [Google Scholar] [CrossRef]
- Wettersten, N.; Maisel, A. Role of cardiac troponin levels in acute heart failure. Card. Fail. Rev. 2015, 1, 102–106. [Google Scholar] [CrossRef]
- Januzzi, J.L., Jr.; Filippatos, G.; Nieminen, M.; Gheorghiade, M. Troponin elevation in patients with heart failure: On behalf of the third Universal definition of myocardial infarction global task force: Heart failure section. Eur. Heart J. 2012, 33, 2265–2271. [Google Scholar] [CrossRef]
- Angelone, T.; Quintieri, A.M.; Brar, B.K.; Limchaiyawat, P.T.; Tota, B.; Mahata, S.K.; Cerra, M.C. The antihypertensive chromogranin a peptide catestatin acts as a novel endocrine/paracrine modulator of cardiac inotropism and lusitropism. Endocrinology 2008, 149, 4780–4793. [Google Scholar] [CrossRef]
- Imbrogno, S.; Garofalo, F.; Cerra, M.C.; Mahata, S.K.; Tota, B. The catecholamine release-inhibitory peptide catestatin (chromogranin A344–363) modulates myocardial function in fish. J. Exp. Biol. 2010, 213, 3636–3643. [Google Scholar] [CrossRef]
- Mazza, R.; Gattuso, A.; Mannarino, C.; Brar, B.K.; Barbieri, S.F.; Tota, B.; Mahata, S.K. Catestatin (chromogranin A344–364) is a novel cardiosuppressive agent: Inhibition of isoproterenol and endothelin signaling in the frog heart. Am. J. Physiol. Heart Circ. Physiol. 2008, 295, H113–122. [Google Scholar] [CrossRef]
- Dev, N.B.; Gayen, J.R.; O’Connor, D.T.; Mahata, S.K. Chromogranin a and the autonomic system: Decomposition of heart rate variability and rescue by its catestatin fragment. Endocrinology 2010, 151, 2760–2768. [Google Scholar] [CrossRef]




| VARIABLE | Total Cohort N = 90 | ADHF without MI N = 50 | ADHF with MI N = 40 | p-Value * |
|---|---|---|---|---|
| Demographics and clinical profile | ||||
| Age, years | 70.3 ± 10.2 | 69.8 ± 10.8 | 70.9 ± 9.6 | 0.610 |
| Female sex | 47 (52.2) | 33 (66.0) | 14 (35.0) | 0.003 |
| Body mass index, kg/m2 | 30.2 ± 4.2 | 30.8 ± 4.4 | 29.6 ± 3.9 | 0.182 |
| Body surface area, m2 | 2.02 ± 0.18 | 2.02 ± 0.19 | 2.03 ± 0.17 | 0.792 |
| Waist-to-hip ratio | 0.98 ± 0.08 | 0.97 ± 0.09 | 0.99 ± 0.06 | 0.095 |
| Systolic blood pressure, mmHg | 137 ± 28 | 134 ± 23 | 140 ± 32 | 0.285 |
| Diastolic blood pressure, mmHg | 80 ± 13 | 79 ± 12 | 82 ± 14 | 0.279 |
| Heart rate at admission, beats/min | 95 ± 31 | 94 ± 28 | 96 ± 35 | 0.726 |
| Heart rate at rest, beats/min | 88 ± 26 | 90 ± 28 | 84 ± 22 | 0.300 |
| HF-related hospitalization event in a previous year | 36 (40.0) | 15 (30.0) | 21 (52.5) | 0.030 |
| Pacemaker/ICD/CRT device | 13 (14.4) | 3 (6.0) | 10 (25.0) | 0.011 |
| Left bundle branch block | 35 (38.9) | 19 (38.0) | 16 (40.0) | 0.847 |
| NYHA functional class | 3.0 ± 0.62 | 2.9 ± 0.53 | 3.2 ± 0.69 | 0.031 |
| CKD stage | 2.6 ± 0.9 | 2.3 ± 1.0 | 2.9 ± 0.8 | 0.004 |
| LVEF ≤35%, biplane Simpson | 37 (41.1) | 16 (32.0) | 20 (50.0) | 0.083 |
| SaO2 <90% at admission | 34 (37.8) | 18 (36.0) | 10 (40.0) | 0.777 |
| Comorbidities and concomitant clinical conditions | ||||
| Diabetes mellitus | 37 (41.1) | 17 (34.0) | 20 (50.0) | 0.125 |
| Anemia | 36 (40.0) | 12 (24.0) | 14 (35.0) | 0.253 |
| Obesity | 38 (42.2) | 25 (55.6) | 13 (35.1) | 0.065 |
| Hyperuricemia | 75 (83.3) | 40 (83.3) | 35 (87.5) | 0.193 |
| Dyslipidemia | 60 (66.6) | 33 (66.6) | 27 (67.5) | 0.743 |
| Chronic obstructive pulmonary disease | 21 (23.3) | 14 (28.0) | 7 (17.5) | 0.242 |
| Chronic kidney disease | 46 (51.1) | 21 (42.0) | 25 (62.5) | 0.053 |
| Arterial hypertension | 84 (93.3) | 45 (90.0) | 39 (97.5) | 0.156 |
| Atrial fibrillation | 50 (55.6) | 28 (56.0) | 22 (55.0) | 0.924 |
| Peripheral artery disease | 19 (21.1) | 9 (18.0) | 10 (25.0) | 0.419 |
| Smoker, present or former | 34 (37.8) | 14 (28.0) | 20 (50.0) | 0.032 |
| History of stroke or transient ischemic attack | 7 (7.8) | 4 (8.0) | 3 (7.5) | 0.930 |
| Pharmacotherapy | ||||
| ACE inhibitor or ARB | 70 (77.8) | 41 (82.0) | 29 (72.5) | 0.245 |
| Sacubitril-valsartan | 24 (26.7) | 11 (22.0) | 13 (32.5) | 0.154 |
| β-blocker | 81 (90.0) | 43 (86.0) | 38 (95.0) | 0.235 |
| Ca2+ channel blocker | 13 (14.4) | 9 (18.0) | 4 (10.0) | 0.305 |
| MRA | 42 (46.7) | 24 (48.0) | 18 (45.0) | 0.953 |
| Diuretics | 82 (91.1) | 45 (90.0) | 37 (92.5) | 0.274 |
| Digoxin | 18 (20.0) | 9 (18.0) | 9 (22.5) | 0.554 |
| Aspirin | 35 (38.9) | 13 (26.0) | 22 (55.0) | 0.005 |
| Warfarin | 23 (25.6) | 14 (28.0) | 9 (22.5) | 0.617 |
| NOAC | 22 (24.4) | 13 (26.0) | 9 (22.5) | 0.821 |
| Statin | 33 (36.7) | 14 (28.0) | 19 (47.5) | 0.035 |
| Variable | Total Cohort N = 90 | ADHF without Prior MI N = 50 | ADHF with Prior MI N = 40 | p-Value * |
|---|---|---|---|---|
| Laboratory values | ||||
| Sodium, mmol/L | 138.9 ± 3.7 | 139.0 ± 4.1 | 138.7 ± 3.2 | 0.725 |
| Potassium, mmol/L | 4.2 ± 0.4 | 4.1 ± 0.4 | 4.2 ± 0.5 | 0.252 |
| Creatinine, µmol/L | 117.6 ± 59.5 | 99.9 ± 42.0 | 139.8 ± 70.4 | 0.001 |
| BUN, mmol/L | 4.9 ± 2.6 | 4.5 ± 2.5 | 5.4 ± 2.8 | 0.132 |
| eGFR, mL/min/1.73 m2 | 57.3 ± 24.9 | 63.8 ± 25.7 | 49.2 ± 21.7 | 0.005 |
| Uric acid, µmol/L | 535 ± 165 | 511 ± 179 | 565 ± 143 | 0.130 |
| Albumin, g/L | 38.7 ± 4.1 | 38.6 ± 4.1 | 38.8 ± 4.2 | 0.849 |
| Total proteins, g/L | 68.2 ± 7.2 | 67.5 ± 7.8 | 69.0 ± 6.3 | 0.314 |
| Hemoglobin, g/L | 133.4 ± 19.2 | 134.1 ± 18.7 | 132.6 ± 19.9 | 0.706 |
| WBC count, ×109/L | 8.08 ± 2.59 | 7.73 ± 2.49 | 8.51 ± 2.66 | 0.156 |
| Erythrocytes, ×1012/L | 4.48 ± 0.69 | 4.46 ± 0.69 | 4.51 ± 0.71 | 0.775 |
| Thrombocytes, ×109/L | 214 ± 60 | 217 ± 61 | 209 ± 58 | 0.522 |
| Lymphocytes, ×109/L | 1.50 ± 1.35 | 1.49 ± 0.70 | 1.50 ± 0.68 | 0.970 |
| Neutrophils, ×109/L | 5.64 ± 2.18 | 5.41 ± 2.11 | 5.92 ± 2.24 | 0.278 |
| Fasting glucose, mmol/L | 8.2 ± 3.0 | 7.7 ± 2.6 | 8.8 ± 3.4 | 0.091 |
| HbA1c, % | 6.61 ± 1.26 | 6.33 ± 0.94 | 6.97 ± 1.50 | 0.017 |
| NT-proBNP, pg/mL | 3586 (1361–7787) | 2286 (1110–5976) | 5277 (3079–12004) | 0.008 |
| hs-cTnI, ng/L | 22.9 (11.6–49.0) | 16.0 (10.0–27.3) | 35.8 (19.3–84.2) | 0.001 |
| CRP, mg/L | 8.4 (4.9–20.5) | 7.9 (4.7–17.7) | 11.5 (6.6–30.6) | 0.110 |
| D-dimer, mg/L | 1.62 ± 1.35 | 1.55 ± 1.33 | 1.72 ± 1.40 | 0.718 |
| Triglycerides, mmol/L | 1.56 ± 0.64 | 1.51 ± 0.64 | 1.61 ± 0.65 | 0.471 |
| Total cholesterol, mmol/L | 4.4 ± 1.3 | 4.7 ± 1.3 | 4.1 ± 1.3 | 0.030 |
| HDL cholesterol, mmol/L | 1.0 (0.8–1.2) | 1.0 (0.9–1.2) | 0.9 (0.8–1.1) | 0.023 |
| LDL cholesterol, mmol/L | 2.7 ± 1.1 | 2.9 ± 1.1 | 2.4 ± 1.1 | 0.029 |
| Variable | Total Cohort N = 90 | ADHF without Prior MI N = 50 | ADHF with Prior MI N = 40 | p-Value * |
|---|---|---|---|---|
| LVEF, biplane Simpson, % | 43.4 ± 16.4 | 46.3 ± 15.8 | 39.9 ± 16.6 | 0.066 |
| LV mass, g | 254.4 ± 95.7 | 250.2 ± 93.9 | 258.5 ± 97.5 | 0.686 |
| LVMI, g/m2 | 119.0 ± 47.3 | 117.3 ± 46.5 | 120.7 ± 48.1 | 0.744 |
| LV EDd, mm | 57.9 ± 9.4 | 56.9 ± 8.5 | 58.9 ± 10.4 | 0.322 |
| LV ESd, mm | 42.6 ± 12.1 | 40.9 ± 11.3 | 44.6 ± 12.9 | 0.152 |
| IVSd, mm | 11 (10–13) | 11 (10–13.5) | 11 (10–12) | 0.405 |
| LV PWd, mm | 10.9 ± 2.0 | 11.0 ± 1.9 | 10.9 ± 2.1 | 0.734 |
| FS, % | 27.3 ± 11.5 | 28.4 ± 11.4 | 25.9 ± 11.6 | 0.326 |
| LV EDV, mL/m2 ** | 85.2 ± 32.3 | 80.4 ± 26.4 | 91.1 ± 38.0 | 0.142 |
| LV ESV, mL/m2 ** | 45.0 ± 29.7 | 40.0 ± 25.4 | 51.3 ± 33.6 | 0.096 |
| LA diameter, mm | 49.9 ± 8.9 | 49.5 ± 9.7 | 50.3 ± 7.7 | 0.685 |
| Aortic root diameter, mm | 33.8 ± 5.1 | 33.3 ± 5.3 | 34.5 ± 4.9 | 0.295 |
| LA/Ao ratio | 1.49 ± 0.28 | 1.49 ± 0.31 | 1.48 ± 0.24 | 0.757 |
| Variable | Univariate β | p-Value * | B | SE | β | t | p-Value ** |
|---|---|---|---|---|---|---|---|
| NYHA class | 0.533 | <0.001 | 0.361 | 0.082 | 0.491 | 4.257 | <0.001 |
| WHR | −0.335 | 0.012 | −1.360 | 0.615 | −0.237 | −2.231 | 0.026 |
| Heart rate at admission, bpm | −0.164 | 0.125 | −0.933 | 0.418 | −0.201 | −1.960 | 0.036 |
| Heart rate at rest, bpm | −0.189 | 0.098 | −0.951 | 0.451 | −0.242 | −2.177 | 0.030 |
| Total cholesterol, mmol/L | −0.108 | 0.268 | |||||
| Triglycerides, mmol/L | −0.201 | 0.101 | |||||
| HDL-c, mmol/L | −0.082 | 0.508 | |||||
| LDL-c, mmol/L | −0.272 | 0.019 | −0.089 | 0.040 | −0.231 | −2.123 | 0.029 |
| Non-HDL cholesterol, mmol/L | −0.281 | 0.014 | −0.094 | 0.045 | −0.237 | −2.298 | 0.026 |
| NT-proBNP, pg/mL | −0.193 | 0.074 | |||||
| hs-cTnI, ng/L | −0.260 | 0.015 | −0.080 | 0.111 | −0.221 | −0.799 | 0.030 |
| CRP, mg/L | 0.147 | 0.490 | |||||
| D-dimer, mg/L | −0.094 | 0.770 | |||||
| Glucose, fasting, mmol/L | −0.165 | 0.159 | |||||
| HbA1c, % | −0.264 | 0.023 | −0.085 | 0.039 | −0.235 | −1.248 | 0.027 |
| Variable | Univariate β | p-Value * | B | SE | β | t | p-Value ** |
|---|---|---|---|---|---|---|---|
| LVEF, % | 0.323 | 0.010 | 0.700 | 0.299 | 0.271 | 2.350 | 0.022 |
| LV mass, g | −0.312 | 0.019 | −0.001 | 0.001 | −0.249 | −2.185 | 0.031 |
| LVMI, g/m2 | −0.301 | 0.022 | −0.002 | 0.001 | −0.237 | −2.488 | 0.015 |
| LV EDd, mm | −0.463 | <0.001 | −0.020 | 0.005 | −0.341 | −3.203 | 0.001 |
| LV ESd, mm | −0.411 | <0.001 | −0.013 | 0.004 | −0.311 | −2.762 | 0.005 |
| IVSd, mm | 0.181 | 0.139 | |||||
| LV PWd, mm | −0.165 | 0.180 | |||||
| LV EDV, indexed to BSA, mL/m2 | −0.375 | <0.001 | −0.002 | 0.001 | −0.324 | −3.211 | 0.002 |
| LV ESV, indexed to BSA, mL/m2 | −0.349 | <0.001 | −0.003 | 0.001 | −0.328 | −3.157 | 0.002 |
| LA diameter, mm | −0.297 | 0.010 | −0.012 | 0.005 | −0.262 | −2.415 | 0.019 |
| Aortic root diameter, mm | −0.070 | 0.574 | |||||
| LA/Ao ratio | −0.032 | 0.795 | |||||
| LAEDV, mL | −0.171 | 0.251 | |||||
| LAVI, mL/m2 | −0.233 | 0.101 | |||||
| Fractional shortening, % | 0.292 | 0.021 | 0.011 | 0.003 | 0.255 | 2.198 | 0.029 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Borovac, J.A.; Glavas, D.; Susilovic Grabovac, Z.; Supe Domic, D.; D’Amario, D.; Bozic, J. Catestatin in Acutely Decompensated Heart Failure Patients: Insights from the CATSTAT-HF Study. J. Clin. Med. 2019, 8, 1132. https://doi.org/10.3390/jcm8081132
Borovac JA, Glavas D, Susilovic Grabovac Z, Supe Domic D, D’Amario D, Bozic J. Catestatin in Acutely Decompensated Heart Failure Patients: Insights from the CATSTAT-HF Study. Journal of Clinical Medicine. 2019; 8(8):1132. https://doi.org/10.3390/jcm8081132
Chicago/Turabian StyleBorovac, Josip A., Duska Glavas, Zora Susilovic Grabovac, Daniela Supe Domic, Domenico D’Amario, and Josko Bozic. 2019. "Catestatin in Acutely Decompensated Heart Failure Patients: Insights from the CATSTAT-HF Study" Journal of Clinical Medicine 8, no. 8: 1132. https://doi.org/10.3390/jcm8081132