Results of Treatment with Modern Fractionated Radiotherapy, Contemporary Stereotactic Radiosurgery, and Transsphenoidal Surgery in Nonfunctioning Pituitary Macroadenoma
Abstract
:1. Introduction
2. Patients and Methods
3. Results
4. Discussion
5. Conclusions
6. Novelty & Effect Statements
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| FRT | fractionated radiotherapy |
| ICD-9-CM | International Classification of Diseases, Ninth Revision, Clinical Modification |
| HR | hazard ratio |
| aHR | adjusted hazard ratio |
| CI | confidence interval |
| CCI | Charlson comorbidity index |
| LR | local recurrence |
| OS | overall survival |
| ASA | American Society of Anesthesiologists |
| Gy | gray |
References
- Arafah, B.M.; Nasrallah, M.P. Pituitary tumors: Pathophysiology, clinical manifestations and management. Endocr. Relat. Cancer 2001, 8, 287–305. [Google Scholar] [CrossRef]
- Freda, P.U.; Wardlaw, S.L. Clinical review 110: Diagnosis and treatment of pituitary tumors. J. Clin. Endocrinol. Metab. 1999, 84, 3859–3866. [Google Scholar] [CrossRef]
- Schaller, B. Gender-related differences in non-functioning pituitary adenomas. Neuro Endocrinol. Lett. 2003, 24, 425–430. [Google Scholar] [PubMed]
- Ciric, I.; Ragin, A.; Baumgartner, C.; Pierce, D. Complications of transsphenoidal surgery: Results of a national survey, review of the literature, and personal experience. Neurosurgery 1997, 40, 225–236. [Google Scholar] [CrossRef]
- Dekkers, O.M.; Pereira, A.M.; Romijn, J.A. Treatment and follow-up of clinically nonfunctioning pituitary macroadenomas. J. Clin. Endocrinol. Metab. 2008, 93, 3717–3726. [Google Scholar] [CrossRef]
- Barker, F.G., 2nd; Klibanski, A.; Swearingen, B. Transsphenoidal surgery for pituitary tumors in the United States, 1996-2000: Mortality, morbidity, and the effects of hospital and surgeon volume. J. Clin. Endocrinol. Metab. 2003, 88, 4709–4719. [Google Scholar] [CrossRef] [PubMed]
- Pollock, B.E.; Cochran, J.; Natt, N.; Brown, P.D.; Erickson, D.; Link, M.J.; Garces, Y.I.; Foote, R.L.; Stafford, S.L.; Schomberg, P.J. Gamma knife radiosurgery for patients with nonfunctioning pituitary adenomas: Results from a 15-year experience. Int. J. Radiat. Oncol. Biol. Phys. 2008, 70, 1325–1329. [Google Scholar] [CrossRef]
- Mingione, V.; Yen, C.P.; Vance, M.L.; Steiner, M.; Sheehan, J.; Laws, E.R.; Steiner, L. Gamma surgery in the treatment of nonsecretory pituitary macroadenoma. J. Neurosurg. 2006, 104, 876–883. [Google Scholar] [CrossRef] [PubMed]
- Gupta, S.; Laviraj, M.A.; Kunhiparambath, H.; Sharma, D.; Rajendran, M.; Julka, P.K.; Rath, G.K. Comparative dosimetric study of three-dimensional conformal (3DCRT), intensity modulated radiotherapy (IMRT), and volumetric modulated arc therapy (VMAT) for treatment in pituitary adenomas. J. Clin. Oncol. 2017. [Google Scholar] [CrossRef]
- Mackley, H.B.; Reddy, C.A.; Lee, S.Y.; Harnisch, G.A.; Mayberg, M.R.; Hamrahian, A.H.; Suh, J.H. Intensity-modulated radiotherapy for pituitary adenomas: The preliminary report of the Cleveland Clinic experience. Int. J. Radiat. Oncol. Biol. Phys. 2007, 67, 232–239. [Google Scholar] [CrossRef]
- Tsang, R.W.; Brierley, J.D.; Panzarella, T.; Gospodarowicz, M.K.; Sutcliffe, S.B.; Simpson, W.J. Radiation therapy for pituitary adenoma: Treatment outcome and prognostic factors. Int. J. Radiat. Oncol. Biol. Phys. 1994, 30, 557–565. [Google Scholar] [CrossRef]
- Sheehan, J.P.; Niranjan, A.; Sheehan, J.M.; Jane, J.A., Jr.; Laws, E.R.; Kondziolka, D.; Flickinger, J.; Landolt, A.M.; Loeffler, J.S.; Lunsford, L.D. Stereotactic radiosurgery for pituitary adenomas: An intermediate review of its safety, efficacy, and role in the neurosurgical treatment armamentarium. J. Neurosurg. 2005, 102, 678–691. [Google Scholar] [CrossRef]
- Meeks, S.L.; Pukala, J.; Ramakrishna, N.; Willoughby, T.R.; Bova, F.J. Radiosurgery technology development and use. J. Radiosurg. SBRT 2011, 1, 21–29. [Google Scholar]
- Chiang, C.J.; You, S.L.; Chen, C.J.; Yang, Y.W.; Lo, W.C.; Lai, M.S. Quality assessment and improvement of nationwide cancer registration system in Taiwan: A review. Jpn. J. Clin. Oncol. 2015, 45, 291–296. [Google Scholar] [CrossRef]
- Wen, C.P.; Tsai, S.P.; Chung, W.S. A 10-year experience with universal health insurance in Taiwan: Measuring changes in health and health disparity. Ann. Intern. Med. 2008, 148, 258–267. [Google Scholar] [CrossRef]
- Shao, Y.J.; Chan, T.S.; Tsai, K.; Wu, S.Y. Association between proton pump inhibitors and the risk of hepatocellular carcinoma. Aliment. Pharmacol. Ther. 2018, 48, 460–468. [Google Scholar] [CrossRef]
- Wu, S.Y.; Chou, H.Y.; Yuh, C.H.; Mekuria, S.L.; Kao, Y.C.; Tsai, H.C. Radiation-Sensitive Dendrimer-Based Drug Delivery System. Adv. Sci. (Weinh) 2018, 5, 1700339. [Google Scholar] [CrossRef]
- Hsieh, M.C.; Chang, W.W.; Yu, H.H.; Lu, C.Y.; Chang, C.L.; Chow, J.M.; Chen, S.U.; Cheng, Y.; Wu, S.Y. Adjuvant radiotherapy and chemotherapy improve survival in patients with pancreatic adenocarcinoma receiving surgery: Adjuvant chemotherapy alone is insufficient in the era of intensity modulation radiation therapy. Cancer Med. 2018, 7, 2328–2338. [Google Scholar] [CrossRef]
- Yen, Y.C.; Hsu, H.L.; Chang, J.H.; Lin, W.C.; Chang, Y.C.; Chang, C.L.; Chow, J.M.; Yuan, K.S.; Wu, A.T.H.; Wu, S.Y. Efficacy of thoracic radiotherapy in patients with stage IIIB-IV epidermal growth factor receptor-mutant lung adenocarcinomas who received and responded to tyrosine kinase inhibitor treatment. Radiother. Oncol. 2018, 129, 52–60. [Google Scholar] [CrossRef]
- Lin, Y.K.; Hsieh, M.C.; Wang, W.W.; Lin, Y.C.; Chang, W.W.; Chang, C.L.; Cheng, Y.F.; Wu, S.Y. Outcomes of adjuvant treatments for resectable intrahepatic cholangiocarcinoma: Chemotherapy alone, sequential chemoradiotherapy, or concurrent chemoradiotherapy. Radiother. Oncol. 2018, 128, 575–583. [Google Scholar] [CrossRef]
- Chen, T.M.; Lin, K.C.; Yuan, K.S.; Chang, C.L.; Chow, J.M.; Wu, S.Y. Treatment of advanced nasopharyngeal cancer using low- or high-dose concurrent chemoradiotherapy with intensity-modulated radiotherapy: A propensity score-matched, nationwide, population-based cohort study. Radiother. Oncol. 2018, 129, 23–29. [Google Scholar] [CrossRef]
- Chang, C.L.; Tsai, H.C.; Lin, W.C.; Chang, J.H.; Hsu, H.L.; Chow, J.M.; Yuan, K.S.; Wu, A.T.H.; Wu, S.Y. Dose escalation intensity-modulated radiotherapy-based concurrent chemoradiotherapy is effective for advanced-stage thoracic esophageal squamous cell carcinoma. Radiother. Oncol. 2017, 125, 73–79. [Google Scholar] [CrossRef]
- Charlson, M.; Szatrowski, T.P.; Peterson, J.; Gold, J. Validation of a combined comorbidity index. J. Clin. Epidemiol. 1994, 47, 1245–1251. [Google Scholar] [CrossRef]
- Chen, J.H.; Yen, Y.C.; Yang, H.C.; Liu, S.H.; Yuan, S.P.; Wu, L.L.; Lee, F.P.; Lin, K.C.; Lai, M.T.; Wu, C.C.; et al. Curative-Intent Aggressive Treatment Improves Survival in Elderly Patients With Locally Advanced Head and Neck Squamous Cell Carcinoma and High Comorbidity Index. Medicine 2016, 95, e3268. [Google Scholar] [CrossRef]
- Fernandez, A.; Karavitaki, N.; Wass, J.A. Prevalence of pituitary adenomas: A community-based, cross-sectional study in Banbury (Oxfordshire, UK). Clin. Endocrinol. 2010, 72, 377–382. [Google Scholar] [CrossRef]
- Day, P.F.; Loto, M.G.; Glerean, M.; Picasso, M.F.; Lovazzano, S.; Giunta, D.H. Incidence and prevalence of clinically relevant pituitary adenomas: Retrospective cohort study in a Health Management Organization in Buenos Aires, Argentina. Arch. Endocrinol. Metab. 2016, 60, 554–561. [Google Scholar] [CrossRef]
- Onnestam, L.; Berinder, K.; Burman, P.; Dahlqvist, P.; Engstrom, B.E.; Wahlberg, J.; Nystrom, H.F. National incidence and prevalence of TSH-secreting pituitary adenomas in Sweden. J. Clin. Endocrinol. Metab. 2013, 98, 626–635. [Google Scholar] [CrossRef]
- Gruppetta, M.; Mercieca, C.; Vassallo, J. Prevalence and incidence of pituitary adenomas: A population based study in Malta. Pituitary 2013, 16, 545–553. [Google Scholar] [CrossRef]
- Karavitaki, N. Prevalence and incidence of pituitary adenomas. Ann. Endocrinol. 2012, 73, 79–80. [Google Scholar] [CrossRef]
- Ntali, G.; Wass, J.A. Epidemiology, clinical presentation and diagnosis of non-functioning pituitary adenomas. Pituitary 2018, 21, 111–118. [Google Scholar] [CrossRef]
- Penn, D.L.; Burke, W.T.; Laws, E.R. Management of non-functioning pituitary adenomas: Surgery. Pituitary 2018, 21, 145–153. [Google Scholar] [CrossRef]
- Losa, M.; Mortini, P.; Barzaghi, R.; Ribotto, P.; Terreni, M.R.; Marzoli, S.B.; Pieralli, S.; Giovanelli, M. Early results of surgery in patients with nonfunctioning pituitary adenoma and analysis of the risk of tumor recurrence. J. Neurosurg. 2008, 108, 525–532. [Google Scholar] [CrossRef]
- O’Sullivan, E.P.; Woods, C.; Glynn, N.; Behan, L.A.; Crowley, R.; O’Kelly, P.; Smith, D.; Thompson, C.J.; Agha, A. The natural history of surgically treated but radiotherapy-naive nonfunctioning pituitary adenomas. Clin. Endocrinol. 2009, 71, 709–714. [Google Scholar] [CrossRef]
- Dekkers, O.M.; van der Klaauw, A.A.; Pereira, A.M.; Biermasz, N.R.; Honkoop, P.J.; Roelfsema, F.; Smit, J.W.; Romijn, J.A. Quality of life is decreased after treatment for nonfunctioning pituitary macroadenoma. J. Clin. Endocrinol. Metab. 2006, 91, 3364–3369. [Google Scholar] [CrossRef]
- Churilla, T.M.; Chowdhury, I.H.; Handorf, E.; Collette, L.; Collette, S.; Dong, Y.; Alexander, B.M.; Kocher, M.; Soffietti, R.; Claus, E.B.; et al. Comparison of Local Control of Brain Metastases With Stereotactic Radiosurgery vs Surgical Resection: A Secondary Analysis of a Randomized Clinical Trial. JAMA Oncol. 2018. [Google Scholar] [CrossRef]
- Larson, D.A.; Flickinger, J.C.; Loeffler, J.S. The radiobiology of radiosurgery. Int. J. Radiat. Oncol. Biol. Phys. 1993, 25, 557–561. [Google Scholar] [CrossRef]
- Hall, E.J.; Brenner, D.J. The radiobiology of radiosurgery: Rationale for different treatment regimes for AVMs and malignancies. Int. J. Radiat. Oncol. Biol. Phys. 1993, 25, 381–385. [Google Scholar] [CrossRef]
- Tome, W.A. Universal survival curve and single fraction equivalent dose: Useful tools in understanding potency of ablative radiotherapy: In regard to Parks et al. (Int J Radiat Oncol Biol Phys 2008;70:847-852). Int. J. Radiat. Oncol. Biol. Phys. 2009, 73, 1286. [Google Scholar] [CrossRef]
- Park, C.; Papiez, L.; Zhang, S.; Story, M.; Timmerman, R.D. Universal survival curve and single fraction equivalent dose: Useful tools in understanding potency of ablative radiotherapy. Int. J. Radiat. Oncol. Biol. Phys. 2008, 70, 847–852. [Google Scholar] [CrossRef]
- Kajiwara, K.; Saito, K.; Yoshikawa, K.; Ideguchi, M.; Nomura, S.; Fujii, M.; Suzuki, M. Stereotactic radiosurgery/radiotherapy for pituitary adenomas: A review of recent literature. Neurol. Med. Chir. 2010, 50, 749–755. [Google Scholar] [CrossRef]
- Brada, M.; Rajan, B.; Traish, D.; Ashley, S.; Holmes-Sellors, P.J.; Nussey, S.; Uttley, D. The long-term efficacy of conservative surgery and radiotherapy in the control of pituitary adenomas. Clin. Endocrinol. 1993, 38, 571–578. [Google Scholar] [CrossRef]
- Chang, E.F.; Zada, G.; Kim, S.; Lamborn, K.R.; Quinones-Hinojosa, A.; Tyrrell, J.B.; Wilson, C.B.; Kunwar, S. Long-term recurrence and mortality after surgery and adjuvant radiotherapy for nonfunctional pituitary adenomas. J. Neurosurg. 2008, 108, 736–745. [Google Scholar] [CrossRef] [PubMed]
- Sheehan, J.P.; Pouratian, N.; Steiner, L.; Laws, E.R.; Vance, M.L. Gamma Knife surgery for pituitary adenomas: Factors related to radiological and endocrine outcomes. J. Neurosurg. 2011, 114, 303–309. [Google Scholar] [CrossRef] [PubMed]
- Liscak, R.; Vladyka, V.; Marek, J.; Simonova, G.; Vymazal, J. Gamma knife radiosurgery for endocrine-inactive pituitary adenomas. Acta Neurochir. (Wien) 2007, 149, 999–1006, discussion 1006. [Google Scholar] [CrossRef]

| Fractionated Radiotherapy | Stereotactic Radiosurgery | Transsphenoidal Surgery | p-Value | |
|---|---|---|---|---|
| N, % | N, % | N, % | ||
| Sex | 133 | 53 | 362 | 0.877 |
| Male | 79 (59.4) | 32 (60.4) | 208 (57.5) | |
| Female | 54 (40.6) | 21 (39.6) | 154 (42.5) | |
| Age | 0.024 | |||
| 1–17 | 22 (16.5) | 9 (17.0) | 96 (26.5) | |
| 18–29 | 11 (8.3) | 7 (13.2) | 48 (13.3) | |
| 30–39 | 26 (19.5) | 12 (22.6) | 46 (12.7) | |
| 40–49 | 24 (18.0) | 8 (15.1) | 67 (18.5) | |
| 50–59 | 17 (12.8) | 6 (11.3) | 50 (13.8) | |
| 60–69 | 19 (14.3) | 3 (5.7) | 35 (9.7) | |
| ≥70 | 14 (10.5) | 8 (15.1) | 20 (5.5) | |
| Urbanization level | 0.111 | |||
| 1 (most urbanized) | 30 (22.6) | 19 (35.8) | 84 (23.2) | |
| 2 | 17 (12.8) | 6 (11.3) | 69 (19.1) | |
| 3 | 6 (4.5) | 3 (5.7) | 32 (8.8) | |
| 4 | 13 (9.8) | 6 (11.3) | 27 (7.5) | |
| 5 (least urbanized) | 67 (50.4) | 19 (35.8) | 150 (41.4) | |
| Monthly income | 0.583 | |||
| ≤NTD15,840 | 23 (17.3) | 10 (18.9) | 84 (23.2) | |
| NTD15,841–25,000 | 59 (44.4) | 26 (49.1) | 153 (42.3) | |
| ≥NTD25,001 | 51 (38.3) | 17 (32.1) | 125 (34.5) | |
| CCI | 0.029 | |||
| 0 | 93 (69.9) | 38 (71.7) | 286 (79.0) | |
| 1–2 | 34 (25.6) | 9 (17.0) | 51 (14.1) | |
| 3+ | 6 (4.5) | 6 (11.3) | 25 (6.9) | |
| ASA Scores | 0.868 | |||
| ASA = 1 | 84 (63.2) | 32 (60.4) | 232 (64.1) | |
| ASA > 1 | 49 (36.8) | 21 (39.6) | 130 (35.9) | |
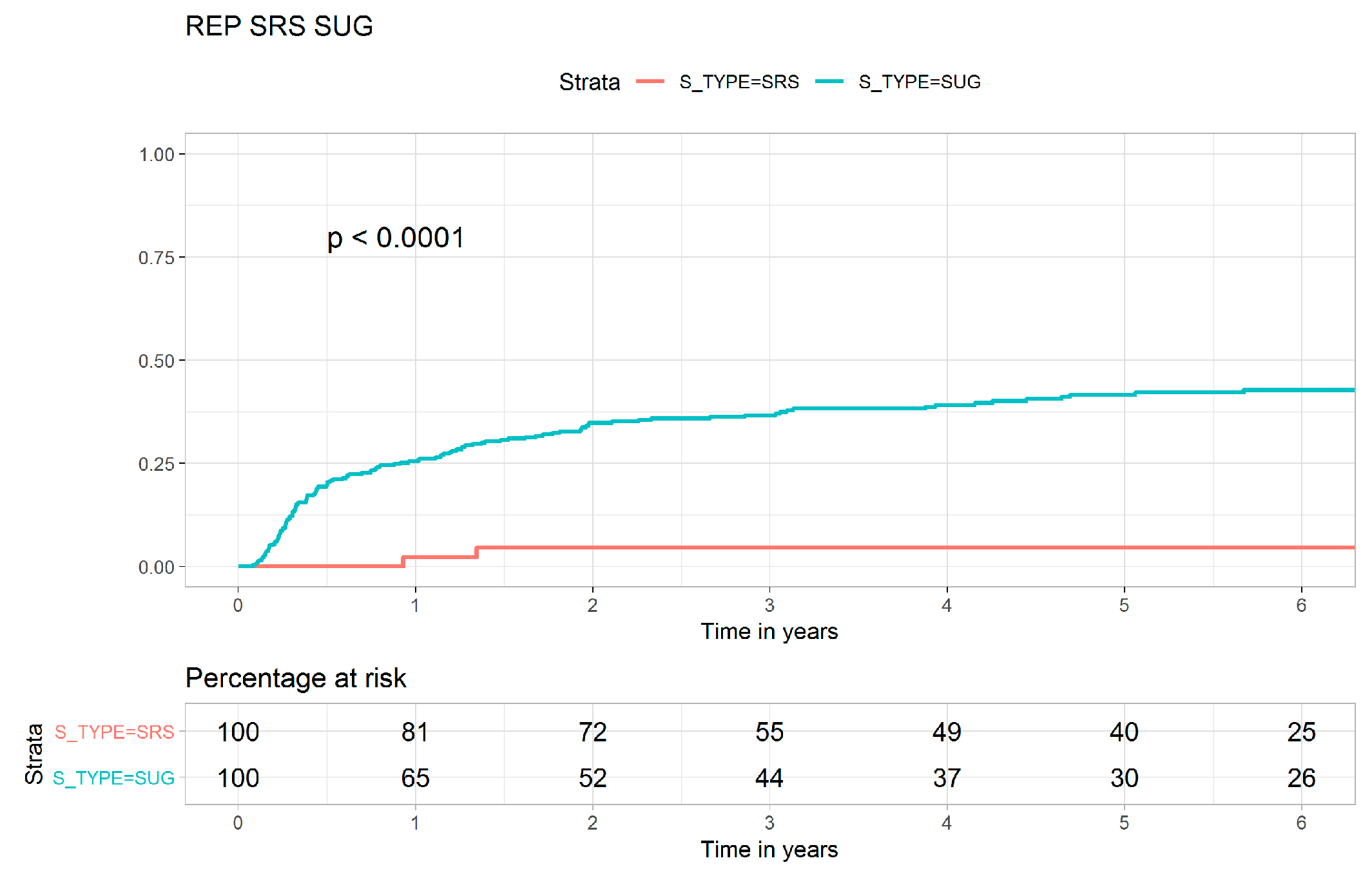
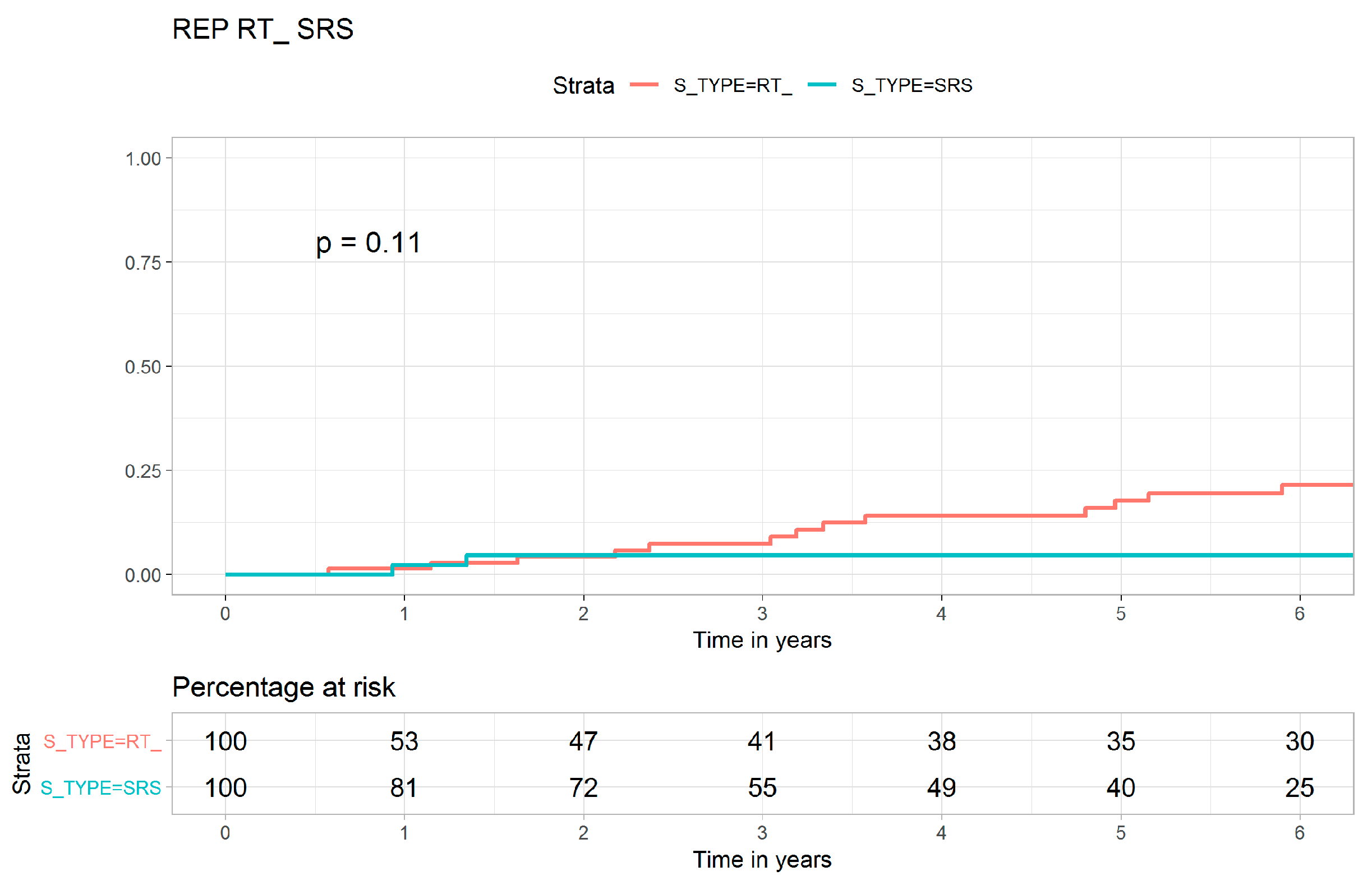
| Local recurrence | 19 (14.3) | 3 (5.7) | 135 (37.3) | <0.001 |
| Radiation dose (median, Gy) | 50.4 | 18 | 0 | <0.001 |
| Death | 0.126 | |||
| No | 94 (70.7) | 45 (84.9) | 273 (75.4) | |
| Yes | 39 (29.3) | 8 (15.1) | 89 (24.6) |
| Crude HR (95% CI) | Adjusted HR * (95% CI) | p-Value | |
|---|---|---|---|
| Therapeutic modality (REF: Fractionated radiotherapy) | |||
| Stereotactic radiosurgery | 0.28 (0.10–0.95) | 0.27 (0.10–0.91) | 0.0345 |
| Transsphenoidal surgery | 2.12 (1.31–2.42) | 1.95 (1.25–2.37) | 0.0044 |
| Sex (REF: male) | |||
| Female | 0.935 (0.73–1.23) | 0.990 (0.77–1.12) | 0.7397 |
| Age (REF: 1–17) | |||
| 18–29 | 0.667 (0.4–1.12) | 0.576 (0.34–1.98) | 0.5120 |
| 30–39 | 0.602 (0.37–0.99) | 0.731 (0.44–1.22) | 0.1043 |
| 40–49 | 0.851 (0.55–1.32) | 0.844 (0.53–1.33) | 0.2326 |
| 50–59 | 0.751 (0.44–1.3) | 0.737 (0.42–1.31) | 0.4665 |
| 60~69 | 0.46 (0.23–0.93) | 0.473 (0.22–1.03) | 0.2937 |
| ≥70 | 0.276 (0.1–0.76) | 0.344 (0.12–1.02) | 0.0601 |
| CCI (REF: CCI = 0) | |||
| 1–2 | 1.09 (0.69–1.72) | 1.388 (0.85–2.28) | 0.1939 |
| 3+ | 0.82 (0.36–1.86) | 1.436 (0.59–3.48) | 0.4229 |
| Income (REF: ≤NTD15,840/month) | |||
| NTD15,841–25,000 | 0.893 (0.6–1.34) | 0.897 (0.59–1.37) | 0.6141 |
| ≥NTD25,001 | 0.847 (0.56–1.29) | 0.885 (0.57–1.38) | 0.5878 |
| Residential area (REF: 1 (most urbanized)) | |||
| 2 | 1.031 (0.63–1.68) | 0.889 (0.54–1.47) | 0.6483 |
| 3 | 0.919 (0.47–1.79) | 0.753 (0.38–1.49) | 0.4147 |
| 4 | 1.567 (0.85–2.89) | 1.639 (0.87–3.01) | 0.1294 |
| 5 (least urbanized) | 1.185 (0.8–1.76) | 1.097 (0.73–1.66) | 0.6605 |
| ASA Scores (REF: ASA = 1) | |||
| >1 | 0.723 (0.51–1.03) | 0.790 (0.51–1.22) | 0.2897 |
| Crude HR (95% CI) | Adjusted HR * (95% CI) | p-Value | |
|---|---|---|---|
| Therapeutic modality (REF: Fractionated radiotherapy) | |||
| Stereotactic radiosurgery | 0.551 (0.26–1.18) | 0.36 (0.15–0.85) | 0.0190 |
| Transsphenoidal surgery | 0.921 (0.63–1.34) | 1.03 (0.68–1.56) | 0.8948 |
| Sex (REF: male) | |||
| Female | 0.937 (0.94–1.47) | 0.903 (0.79–1.26) | 0.6452 |
| Age (REF: 1–17) | |||
| 18–29 | 1.65 (0.8–3.43) | 0.96 (0.75–2.29) | 0.2324 |
| 30–39 | 1.09 (0.51–2.34) | 0.98 (0.45–2.15) | 0.9664 |
| 40–49 | 2.77 (1.47–5.21) | 2.03 (1.16–4.31) | 0.0166 |
| 50–59 | 3.35 (1.70–6.16) | 2.12 (1.52–6.56) | 0.0020 |
| 60–69 | 4.34 (2.27–8.28) | 2.77 (0.83–3.77) | 0.0399 |
| ≥70 | 6.25 (3.29–8.88) | 2.99 (1.41–5.38) | 0.0044 |
| CCI (REF: CCI = 0) | |||
| 1–2 | 3.21 (2.15–4.77) | 2.08 (1.33–3.26) | 0.0014 |
| 3+ | 6.57 (4.02–8.73) | 4.56 (2.51–7.28) | <.0001 |
| Income (REF: ≤NTD15,840/month) | |||
| NTD15,841–25,000 | 0.849 (0.56–1.28) | 0.953 (0.61–1.48) | 0.8307 |
| ≥NTD25,001 | 0.649 (0.41–1.02) | 0.690 (0.42–1.12) | 0.1362 |
| Regions of residence (REF: 1 (most urbanized)) | |||
| 2 | 1.313 (0.75–2.31) | 1.417 (0.79–2.54) | 0.2401 |
| 3 | 1.804 (0.94–3.47) | 1.521 (0.76–3.04) | 0.2356 |
| 4 | 3.885 (2.18–6.92) | 1.787 (0.47–5.3) | 0.3018 |
| 5 (least urbanized) | 1.388 (0.86–2.24) | 1.08 (0.65–1.8) | 0.7674 |
| ASA Scores (REF: ASA = 1) | |||
| >1 | 3.319 (2.34–4.7) | 1.603 (0.99–2.6) | 0.0552 |
| Fractionated Radiotherapy | Stereotactic Radiosurgery | Transsphenoidal Surgery | p-Value | |
|---|---|---|---|---|
| N, % | N, % | N, % | ||
| Secondary primary brain or head and neck cancers | 26 (19.55) | 7 (13.21) | 64 (17.68) | 0.593 |
| Hypopituitarism | 14 (10.53) | 5 (9.43) | 37 (10.22) | 0.976 |
| Visual field deficit | 47 (35.34) | 12 (22.64) | 95 (26.24) | 0.089 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hsiao, P.-K.; Chang, C.-L.; Yuan, K.S.-P.; Wu, A.T.H.; Wu, S.-Y. Results of Treatment with Modern Fractionated Radiotherapy, Contemporary Stereotactic Radiosurgery, and Transsphenoidal Surgery in Nonfunctioning Pituitary Macroadenoma. J. Clin. Med. 2019, 8, 518. https://doi.org/10.3390/jcm8040518
Hsiao P-K, Chang C-L, Yuan KS-P, Wu ATH, Wu S-Y. Results of Treatment with Modern Fractionated Radiotherapy, Contemporary Stereotactic Radiosurgery, and Transsphenoidal Surgery in Nonfunctioning Pituitary Macroadenoma. Journal of Clinical Medicine. 2019; 8(4):518. https://doi.org/10.3390/jcm8040518
Chicago/Turabian StyleHsiao, Ping-Kun, Chia-Lun Chang, Kevin Sheng-Po Yuan, Alexander T.H. Wu, and Szu-Yuan Wu. 2019. "Results of Treatment with Modern Fractionated Radiotherapy, Contemporary Stereotactic Radiosurgery, and Transsphenoidal Surgery in Nonfunctioning Pituitary Macroadenoma" Journal of Clinical Medicine 8, no. 4: 518. https://doi.org/10.3390/jcm8040518






