An Unusual and Fatal Cause of Miliary Nodules on Chest Radiography
Abstract
:1. Introduction
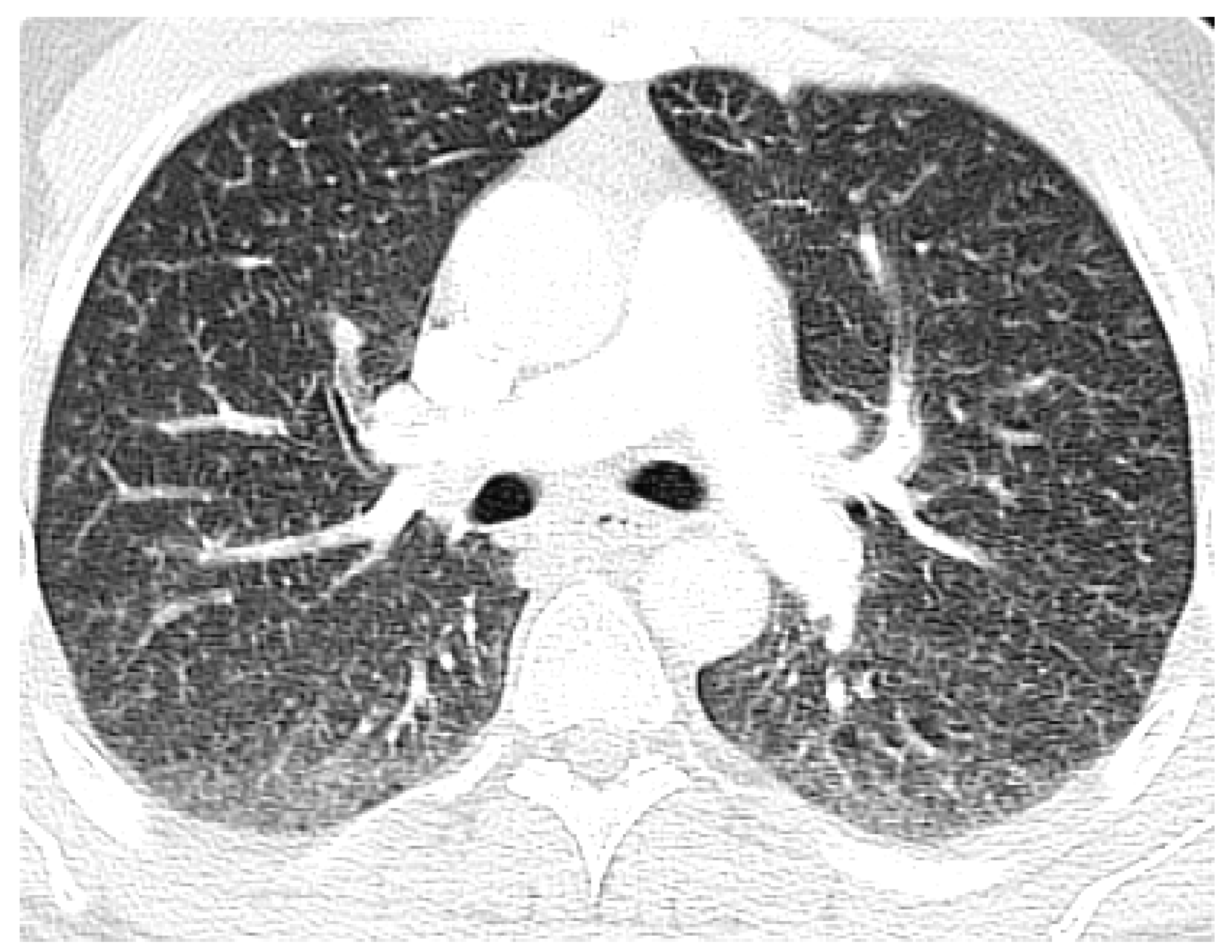
2. Case Report
3. Discussion
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Diaz-Ruiz, M.J.; Gallardo, X.; Castaner, E.; Mata, J.M.; Catala, J.; Ferreres, J.C. Cellulose granulomatosis of the lungs. Eur. Radiol. 1999, 9, 1203–1204. [Google Scholar] [CrossRef] [PubMed]
- Tomashefski, J.F.; Felo, J.A. The pulmonary pathology of illicit drug and substance abuse. Curr. Diagn. Pathol. 2004, 10, 413–426. [Google Scholar] [CrossRef]
- Ward, S.; Heyneman, L.E.; Reittner, P.; Kazerooni, E.A.; Godwin, J.D.; Muller, N.L. Talcosis associated with IV abuse of oral medications: CT findings. AJR Am. J. Roentgenol. 2000, 174, 789–793. [Google Scholar] [CrossRef] [PubMed]
- Fields, T.A.; McCall, S.J.; Reams, B.D.; Roggli, V.L.; Palmer, S.M.; Howell, D.N. Pulmonary embolization of microcrystalline cellulose in a lung transplant recipient. J. Heart Lung Transplant. 2005, 24, 624–627. [Google Scholar] [CrossRef] [PubMed]
- Ganesan, S.; Felo, J.; Saldana, M.; Kalasinsky, V.F.; Lewin-Smith, M.R.; Tomashefski, J.F., Jr. Embolized crospovidone (poly[N-vinyl-2-pyrrolidone]) in the lungs of intravenous drug users. Mod. Pathol. 2003, 16, 286–292. [Google Scholar] [CrossRef] [PubMed]
- Kringsholm, B.; Christoffersen, P. The nature and the occurrence of birefringent material in different organs in fatal drug addiction. Forensic Sci. Int. 1987, 34, 53–62. [Google Scholar] [CrossRef]
- Lamb, D.; Roberts, G. Starch and talc emboli in drug addicts’ lungs. J. Clin. Pathol. 1972, 25, 876–881. [Google Scholar] [CrossRef] [PubMed]
- Pare, J.P.; Cote, G.; Fraser, R.S. Long term follow-up of drug abusers with intravenous talcosis. Am. Rev. Respir. Dis. 1989, 139, 233–241. [Google Scholar] [CrossRef] [PubMed]
- Fraser, R. Embolic and thrombotic diseases of the lungs. In Diagnosis of Diseases of the Chest; WB Saunders: Philadelphia, PA, USA, 1990. [Google Scholar]
- Karch, S.B. Synthetic Stimulants. In Karch’s Pathology of Drug Abuse, 3rd ed.; Karch, S.B., Ed.; CRC Press: Boca Raton, FL, USA, 2002; pp. 238–239. [Google Scholar]
- Huestis, M.A. Marijuana. In Principles of Forensic Toxicology; Levine, B., Ed.; American Association for Clinical Chemistry: Washington, DC, USA, 1999; pp. 246–247. [Google Scholar]
- Barsky, S.H.; Roth, M.D.; Kleerup, E.C.; Simmons, M.; Tashkin, D.P. Histopathological and molecular alterations in bronchial epithelium in habitual smokers of marijuana, cocaine and tobacco. J. Natl. Cancer Inst. 1998, 90, 1198–1205. [Google Scholar] [CrossRef] [PubMed]
- Gong, H., Jr.; Fligiel, S.; Tashkin, D.P.; Barbers, R.G. Tracheobronchial changes in habitual, heavy smokers of marijuana with and without tobacco. Am. Rev. Respir. Dis. 1987, 136, 142–149. [Google Scholar] [CrossRef] [PubMed]
- Marchiori, E.; Lourenco, S.; Gasparetto, T.D.; Zanetti, G.; Mano, C.M.; Nobre, L.F. Pulmonary talcosis: Imaging findings. Lung 2010, 188, 165–171. [Google Scholar] [CrossRef] [PubMed]
- Bendeck, S.E.; Leung, A.N.; Berry, G.J.; Daniel, D.; Ruoss, S.J. Cellulose granulomatosis presenting as centrilobular nodules: CT and histologic findings. AJR Am. J. Roentgenol. 2001, 177, 1151–1153. [Google Scholar] [CrossRef] [PubMed]
- Chute, D.J.; Rawley, J.; Cox, J.; Bready, R.J.; Reiber, K. Angiocentric systemic granulomatosis. Am. J. Forensic Med. Pathol. 2010, 31, 146–150. [Google Scholar] [CrossRef] [PubMed]
- Griffith, C.C.; Raval, J.S.; Nichols, L. Intravascular talcosis due to intravenous drug use is an underrecognized cause of pulmonary hypertension. Pulm. Med. 2012. [Google Scholar] [CrossRef] [PubMed]
- Giuliano, V.; Velez-Rivera, C.; Carlone, D. Cellulose granulomatosis of the lungs: CT findings. AJR Am. J. Roentgenol. 1994, 163, 220–221. [Google Scholar] [CrossRef] [PubMed]
- Padley, S.P.G.; Adler, B.D.; Staples, C.A.; Miller, R.R.; Muller, N.L. Pulmonary talcosis: CT findings in three cases. Radiology 1993, 186, 125–127. [Google Scholar] [CrossRef] [PubMed]
- Poletti, V.; Chilosi, M.; Olivieri, D. Diagnostic invasive procedures in diffuse infiltrative lung diseases. Respiration 2004, 71, 107–119. [Google Scholar] [CrossRef] [PubMed]
- Halkos, M.E.; Gal, A.A.; Kerendi, F.; Miller, D.L.; Miller, J.I., Jr. Role of thoracic surgeons in the diagnosis of idiopathic interstitial lung disease. Ann. Thorac. Surg. 2005, 79, 2172–2179. [Google Scholar] [CrossRef] [PubMed]
- Paré, J.A.; Fraser, R.G.; Hogg, J.C.; Howlett, J.G.; Murphy, S.B. Pulmonary ‘mainline’ granulomatosis: Talcosis of intravenous methadone abuse. Medicine (Baltimore) 1979, 58, 229–239. [Google Scholar] [CrossRef] [PubMed]
- Chau, C.H.; Yew, W.W.; Lee, J. Inhaled budesonide in the treatment of talc-induced pulmonary granulomatosis. Respiration 2003, 70, 439. [Google Scholar] [CrossRef] [PubMed]
- Hashimoto, M.; Kobayashi, K.; Yamagata, N.; Katsura, T.; Sugihara, S.; Iwabuchi, H.; Kasama, T.; Kasahara, K.; Takahashi, T.; Takeshita, K.; et al. Suppression of pulmonary granulomatous inflammation by immunomodulating agents. Agents Actions 1992, 37, 99–106. [Google Scholar] [CrossRef] [PubMed]

© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cheema, A.; Chaughtai, S.; Mazahir, U.; Roy, M.; Hossain, M.A. An Unusual and Fatal Cause of Miliary Nodules on Chest Radiography. J. Clin. Med. 2018, 7, 164. https://doi.org/10.3390/jcm7070164
Cheema A, Chaughtai S, Mazahir U, Roy M, Hossain MA. An Unusual and Fatal Cause of Miliary Nodules on Chest Radiography. Journal of Clinical Medicine. 2018; 7(7):164. https://doi.org/10.3390/jcm7070164
Chicago/Turabian StyleCheema, Anmol, Saira Chaughtai, Usman Mazahir, Manimala Roy, and Mohammad A. Hossain. 2018. "An Unusual and Fatal Cause of Miliary Nodules on Chest Radiography" Journal of Clinical Medicine 7, no. 7: 164. https://doi.org/10.3390/jcm7070164




