Is there A Role for Alpha-Linolenic Acid in the Fetal Programming of Health?
Abstract
:1. Introduction
2. Dietary ω3 Fatty Acids and Health
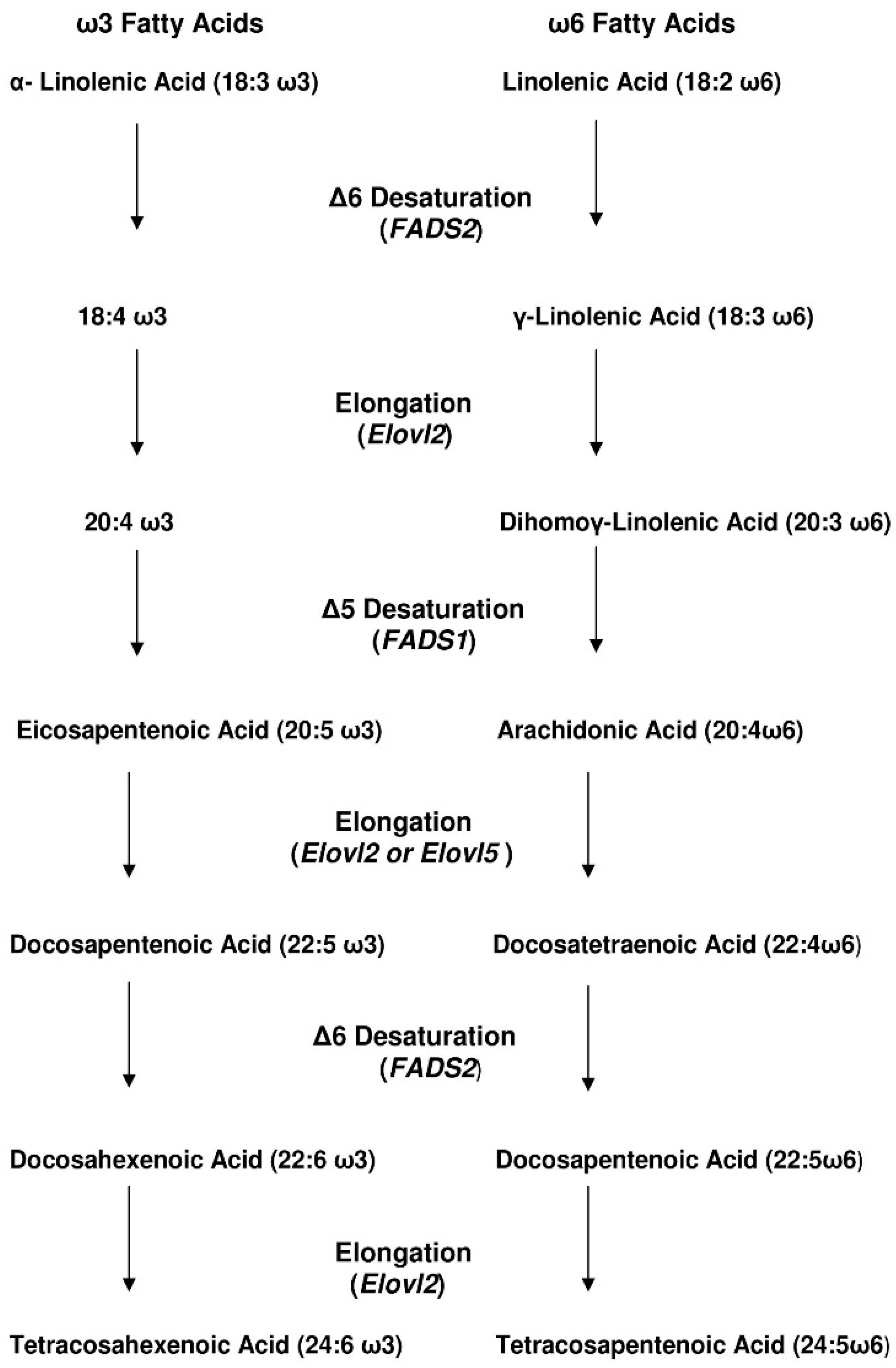
3. How Efficient Is ALA Conversion to EPA and DHA in Humans?
4. Adults
5. Fatty Acids Quality and Early Life
6. Perinatal Manipulation of ALA
7. Regulatory Mechanisms
8. Epigenetics
9. Final Thoughts and Recommendations
Acknowledgments
Conflicts of Interest
Abbreviations
| ALA | ω3 alpha linolenic acid |
| FA | fatty acid |
| CVD | cardiovascular risk disease |
| DHA | docosahexenoic acid |
| DPA | docosapentenoic acid |
| EFA | essential fatty acid |
| EPA | eicosapentenoic acid |
| Fads2 | gene of Δ6 desaturase enzyme |
| IR | insulin resistance |
| LA | linoleic acid |
| LDL | low density lipoproteins |
| Ppargc1a/Ppargc1a | peroxisome proliferative activated receptor gamma coactivator1a enzyle and gene, respectively |
| PUFA | polyunsaturated fatty acid |
| SCD1/Scd1 | stearoyl-CoA desaturase 1 enzyme and gene, respectively |
| SFA | saturated fatty acid |
References
- Burr, G.O.; Burr, M.M. The nature and role of the fatty acids essential in nutrition. J. Biol. Chem. 1930, 86, 587–621. [Google Scholar] [CrossRef]
- Sinopolus, A. Genetic variants in the metabolism of omega-6 and omega-3 fatty acids: Their role in the determination of nutritional requirements and chronic disease risk. Exp. Biol. Med. 2010, 235, 785–795. [Google Scholar]
- Merino, D.M.; Ma, D.W.L.; Mutch, D.M. Genetic variation in lipid desaturases and its impact on the development of human disease. Lipids Health Dis. 2010, 9, 63. [Google Scholar] [CrossRef] [PubMed]
- Innis, S.M. Essential fatty acid transfer and fetal development. Placenta 2005, 26, 570–575. [Google Scholar] [CrossRef] [PubMed]
- Hornstra, G. Essential fatty acids in mothers and their neonates. Am. J. Clin. Nutr. 2000, 71, 1262S–1269S. [Google Scholar] [PubMed]
- Barker, D.J.P. Fetal origins of coronary heart disease. BJM 1995, 311, 171–174. [Google Scholar] [CrossRef]
- Mennitti, L.V.; Oliveira, J.L.; Morais, C.A.; Estadella, D.; Oyama, L.M.; Nascimento, C.M.D.; Pisani, L.P. Type of fatty acids in maternal diets during pregnancy and/or lactation and metabolic consequences of the offspring. J. Nutr. Biochem. 2015, 26, 99–111. [Google Scholar] [CrossRef] [PubMed]
- Kabaran, S.; TanjuBesler, H.T. Do fatty acids affect fetal programming? J. Health Popul. Nutr. 2015, 33, 14. [Google Scholar] [CrossRef] [PubMed]
- Alfaradhi, M.; Ozanne, S.E. Developmental programming in response to maternal overnutrition. Front. Genet. 2011, 27, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Valenzuela, R.; Bascuñán, K.A.; Chamorro, R.; Barrera, C.; Sandoval, J.; Puigrredon, C.; Parraguez, G.; Orellana, P.; Gonzalez, V.; Valenzuela, A. Modification of Docosahexaenoic Acid Composition of Milk from Nursing Women Who Received Alpha Linolenic Acid from Chia Oil during Gestation and Nursing. Nutrients 2015, 7, 6405–6424. [Google Scholar] [CrossRef] [PubMed]
- Swanson, D.; Block, R.; Mousa, S.A. Omega-3 fatty acids EPA and DHA: Health benefits throughout life. Adv. Nutr. 2012, 3, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Larque, E.; Gil-Sanchez, A.; Prieto-Sanchez, M.T.; Koletzko, B. Omega 3 fatty acids, gestation and pregnancy outcomes. Br. J. Nutr. 2012, 107, S77–S84. [Google Scholar] [CrossRef] [PubMed]
- Fonseca, B.M.; Correia-da-Silva, G.; Teixeira, N.A. The rat as an animal model for fetoplacental development: A reappraisal of the post-implantation period. Reprod. Biol. 2012, 12, 97–118. [Google Scholar] [CrossRef]
- Brennan, R.O.; Mulligan, W.C. Nutrigenetics: New Concepts for Relieving Hypoglycemia; M Evans & Co.: New York, NY, USA, 1975; p. 258. [Google Scholar]
- Mutch, D.M.; Wahli, W.; Williamson, G. Nutrigenomics and nutrigenetics: The emerging faces of Nutrition. FASEB. 2005, 19, 1602–1616. [Google Scholar] [CrossRef] [PubMed]
- Dayton, S.; Pearce, M.L.; Goldman, H. Controlled trial of a diet high in unsaturated fat for prevention of atherosclerotic complications. Lancet 1968, 16, 1060–1062. [Google Scholar] [CrossRef]
- Lorente-Cebrián, S.; Costa, A.G.; Navas-Carretero, S.; Zabala, M.; Martínez, J.A.; Moreno-Aliaga, M.J. Role of omega-3 fatty acids in obesity, metabolic syndrome, and cardiovascular diseases: A review of the evidence. Physiol. Biochem. 2013, 69, 633–651. [Google Scholar] [CrossRef] [PubMed]
- Gebauer, S.K.; Psota, T.L.; Harris, W.S.; Kris-Etherton, P.M. n-3 Fatty acid dietary recommendations and food sources to achieve essentiality and cardiovascular benefits. Am. J. Clin. Nutr. 2006, 83, S1526–S1535. [Google Scholar]
- Poudyal, H.; Panchal, S.K.; Diwan, V.; Brown, L. Omega-3 fatty acids and metabolic syndrome: Effects and emerging mechanisms of action. Prog. Lipid Res. 2011, 50, 372–387. [Google Scholar] [CrossRef] [PubMed]
- Abeywardena, M.W.; Patten, G.S. Role of ω3 Long chain Polyunsaturated Fatty Acids in Reducing Cardio-Metabolic Risk Factors. Endocr. Metab. Immune Disord. Drug Targets 2011, 11, 232–246. [Google Scholar] [CrossRef] [PubMed]
- Larsson, S.C.; Orsini, N.; Wolk, A. Long-chain omega-3 polyunsaturated fatty acids and risk of stroke: A meta-analysis. Eur. J. Epidemiol. 2012, 27, 895–901. [Google Scholar] [CrossRef] [PubMed]
- Kris-Etherton, P.M.; Harris, W.S.; Appel, L.J. Fish Consumption, Fish Oil, Omega-3 Fatty Acids, and Cardiovascular Disease. Circulation 2002, 106, 2747–2757. [Google Scholar] [CrossRef] [PubMed]
- Coste, T.C.; Gerbi, A.; Vague, P.; Pieroni, G.; Raccah, D. Neuroprotective Effect of Docosahexaenoic Acid-Enriched Phospholipids in Experimental Diabetic Neuropathy. Diabetes 2003, 52, 2578–2585. [Google Scholar] [CrossRef] [PubMed]
- Barceló-Coblijn, G.; Murphy, E.J. Alpha-linolenic acid and its conversion to longer chain n-3 fatty acids: Benefits for human health and a role in maintaining tissue n-3 fatty acid levels. Prog. Lipid Res. 2009, 48, 355–374. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez-Leyva, D.; Bassett, C.M.C.; McCullough, R.; Pierce, P. The cardiovascular effects of flaxseed and its omega-3 fatty acid, alpha-linolenic acid. Can. J. Cardiol. 2010, 26, 489–496. [Google Scholar] [CrossRef]
- Baxheinrich, A.; Stratmann, B.; Lee-Barkey, Y.H.; Tschoepe, D.; Wahrburg, U. Effects of an energy-restricted diet rich in plant-derived a-linolenic acid on systemic inflammation and endothelial function in overweight-to-obese patients with metabolic syndrome traits. Br. J. Nutr. 2014, 112, 1315–1322. [Google Scholar]
- Egert, S.; Baxheinrich, A.; Lee-Barkey, Y.H.; Tschoepe, D.; Wahrburg, U.; Stratmann, B. Effects of a rapeseed oil-enriched hypoenergetic diet with a high content of α-linolenic acid on body weight and cardiovascular risk profile in patients with the metabolic syndrome. Br. J. Nutr. 2012, 108, 682–691. [Google Scholar]
- Poudyal, H.; Panchal, S.K.; Ward, L.C.; Brown, L. Effects of ALA, EPA and DHA in high-carbohydrate, high-fat diet-induced metabolic syndrome in rats. J. Nutr. Biochem. 2013, 24, 1041–1052. [Google Scholar] [CrossRef] [PubMed]
- Commor, W.E.; Bendich, A. Highly unsaturated fatty acids in nutrition and disease prevention. Am. J. Clin. Nutr. 2000, 71 (Suppl. 1), 169S–398S. [Google Scholar]
- Hu, F.B.; Stampfer, M.J.; Manson, J.A.E.; Rimm, E.B.; Wolk, A.; Colditz, G.A.; Hennekens, C.H.; Willett, W.C. Dietary intake of a-linolenic acid and risk of fatal ischemic heart disease among women. Am. J. Clin. Nutr. 1999, 69, 890–897. [Google Scholar] [PubMed]
- Truong, H.; di Bello, J.R.; Ruiz-Narvaez, E.; Kraft, P.; Campos, H.; Baylin, A. Does genetic variation in the Δ6-desaturase promoter modify the association between α-linolenic acid and the prevalence of metabolic syndrome? Am. J. Clin. Nutr. 2009, 89, 920–925. [Google Scholar] [CrossRef] [PubMed]
- Fleming, J.A.; Kris-Etherton, P.M. The evidence for α-linolenic acid and cardiovascular disease benefits: Comparisons with eicosapentaenoic acid and docosahexaenoic acid. Adv. Nutr. 2014, 5, 863S–876S. [Google Scholar] [CrossRef] [PubMed]
- Decsi, T.; Koletzko, B. n-3 Fatty acids and pregnancy outcomes. Curr. Opin. Clin. Nutr. Metab. Care 2005, 8, 161–166. [Google Scholar] [CrossRef] [PubMed]
- Brenna, J.T. Efficiency of conversion of α -linolenic acid to long chain n-3 fatty acids in man. Curr. Opin. Clin. Nutr. Metab. Care 2002, 5, 127–132. [Google Scholar] [CrossRef]
- Burdge, G.C. Metabolism of α-linolenic acid in humans. Prostaglandins Leukot. Essent. Fat. Acids 2006, 75, 161–168. [Google Scholar] [CrossRef] [PubMed]
- Mohrhauer, H.; Holman, R.T. Effect of linolenic acid upon the metabolism of linoleic acid. J. Nutr. 1963, 81, 67–74. [Google Scholar] [PubMed]
- Brenner, R.R.; Peluffo, R.O. Effect of saturated and unsaturated fatty acids on the desatu-ration in vitro of palmitic, stearic, oleic, linoleic, and linolenic acids. J. Biol. Chem. 1966, 241, 5213–5219. [Google Scholar] [PubMed]
- Brenna, J.T.; Salem, N., Jr.; Sinclair, A.J.; Cunnane, S.C. Alpha-Linolenic acid supplementation and conversion to n-3 long-chain polyunsaturated fatty acids in humans. Prostaglandins Leukot Essent Fatty Acids. 2009, 80, 85–91. [Google Scholar] [CrossRef] [PubMed]
- Anderson, B.M.; Ma, D.W. Are all n-3 polyunsaturated fatty acids created equal? Lipids Health Dis. 2009, 10, 33. [Google Scholar] [CrossRef] [PubMed]
- Stoffel, W.; Holz, B.; Jenke, B.; Binczek, E.; Gunter, R.H.; Kiss, C.; Karakesisoglou, I.; Thevis, M.; Weber, A.A.; Arnhold, S.; et al. Delta6-desaturase (FADS2) deficiency unveils the role of omega3- and omega6-polyunsaturated fatty acids. EMBO J. 2008, 27, 2281–2292. [Google Scholar] [CrossRef] [PubMed]
- Burdge, G. Alpha-linolenic acid metabolism in men and women: Nutritional and biological implications. Curr. Opin. Clin. Nutr. Metab. Care 2004, 7, 137–144. [Google Scholar] [CrossRef] [PubMed]
- DeLany, J.P.; Windhauser, M.M.; Champagne, C.M.; Bray, G.A. Differential oxidation of individual dietary fatty acids in humans. Am. J. Clin. Nutr. 2000, 72, 905–911. [Google Scholar] [PubMed]
- Burdge, G.C.; Jones, A.E.; Wootton, S.A. Eicosapentaenoic and docosapentaenoic acids are the principal products of α-linolenic acid metabolism in young men. Br. J. Nutr. 2002, 88, 355–363. [Google Scholar] [CrossRef] [PubMed]
- Burdge, G.C.; Wootton, S.A. Conversion of α-linolenic acid to eicosapentaenoic, docosapentaenoic and docosahexaenoic acids in young women. Br. J. Nutr. 2002, 88, 411–420. [Google Scholar] [CrossRef] [PubMed]
- Burdge, G.C.; Calder, P.C. Conversion of alpha-linolenic acid to longer-chain polyunsaturated fatty acids in human adults. Reprod. Nutr. Dev. 2005, 45, 581–597. [Google Scholar] [CrossRef] [PubMed]
- Childs, C.; Kew, E.S.; Finnegan, Y.E.; Minihane, A.M.; Leigh-Firban, E.C.; Williams, C.M.; Calder, P.C. Increased dietary α-linolenic acid has sex-specific effects upon eicosapentaenoic acid status in humans: Re-examination of data from a randomized, placebo-controlled, parallel study. Nutr. J. 2014, 13, 113–117. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Molto-Puigmarti, C.; Plat, J.; Mensink, R.P.; Muller, A.; Jansen, E.; Zeegers, M.P.; Thijs, C. FADS1 FADS2 gene variants modify the association between fishintake and the docosahexaenoic acid proportions in human milk. Am. J. Clin. Nutr. 2010, 91, 1368–1376. [Google Scholar] [CrossRef] [PubMed]
- Xie, L.; Innis, S. Genetic variants of the FADS1 FADS2 gene cluster are associated with altered (n-6) and (n-3) essential fatty acids in plasma and erythrocyte phospholipids in women during pregnancy and in breast milk during lactation. J. Nutr. 2008, 138, 2222–2228. [Google Scholar] [CrossRef] [PubMed]
- Caspi, A.; Williams, B.; Kim-Cohen, J.; Craig, I.W.; Milne, B.J.; Poulton, R.; Schalkwyk, L.C.; Taylor, A.; Werts, H.; Moffitt, T.E. Moderation of breastfeeding effects on the IQ by genetic variation in fatty acid metabolism. Proc. Natl. Acad. Sci. USA 2007, 104, 18860–18865. [Google Scholar] [CrossRef] [PubMed]
- Burdge, G.; Calder, P.C. Dietary α-linolenic acid and health-related outcomes: A metabolic perspective. Nutr. Res. Rev. 2006, 19, 26–52. [Google Scholar] [CrossRef] [PubMed]
- Arterburn, L.M.; Hall, E.B.; Oken, H. Distribution, interconversion, and dose response of n-3 fatty acids in humans. Am. J. Clin. Nutr. 2006, 83, S1467–1476S. [Google Scholar]
- Niculescu, M.D.; Lupu, D.S.; Craciunescu, C.N. Perinatal manipulation of α-linolenic acid intake induces epigenetic changes in maternal and offspring livers. FASEB J. 2013, 27, 350–358. [Google Scholar] [CrossRef] [PubMed]
- Koren, G. Polyunsaturated fatty acids and fetal brain development Unfulfilled promises. Can. Fam. Phys. 2015, 61, 41–42. [Google Scholar]
- Church, M.W.; Jen, K.-L.C.; Anumba, J.I.; Jackson, D.A.; Adams, B.R.; Hotra, J.W. Excess Omega-3 Fatty Acid Consumption by Mothers during Pregnancy and Lactation Caused Shorter Life Span and Abnormal ABRs in Old Adult Offspring. Neurotoxicol. Teratol. 2010, 32, 171–181. [Google Scholar] [CrossRef] [PubMed]
- Uauy, R.; Mena, P.; Wegher, B.; Nieto, S.; Salem, N. Long chain polyunsaturated fatty acid formation in neonates: Effect of gestational age and intrauterine growth. Pediatr. Res. 2000, 47, 127. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez, A.; Sarda, P.; Nessmann, C.; Boulot, P.; Poisson, J.P.; Leger, C.L.; Descomps, B. Fatty acid desaturase activities and polyunsaturated fatty acid composition in human liver between the seventeenth and thirty-sixth gestational weeks. Am. J. Obstet. Gynecol. 1998, 179, 1063–1070. [Google Scholar] [CrossRef]
- Miles, E.A.; Calder, P.C. Omega-6 and omega-3 polyunsaturated fatty acids and allergic diseases in infancy and childhood. Curr. Pharm. Des. 2014, 20, 946–953. [Google Scholar] [CrossRef] [PubMed]
- Niculescu, M.D. Alpha-Linolenic Acid Alters Cell Cycle, Apoptosis, and DNA Methyltransferase Expression in Mouse Neural Stem Cells, but Not Global DNA Methylation. J. Hum. Nutr. Food Sci. 2014, 2, 1026. [Google Scholar]
- Hollander, K.S.; TempelBrami, C.; Konikoff, F.M.; Fainaru, M.; Leikin-Frenkel, A. Dietary enrichment with alpha-linolenic acid during pregnancy attenuates insulin resistance in adult offspring in mice. Arch. Physiol. Biochem. 2014, 120, 99–111. [Google Scholar] [CrossRef] [PubMed]
- Shomonov-Wagner, L.; Raz, A.; Leikin-Frenkel, A. Alpha linolenic acid in maternal diet halts the lipid disarray due to saturated fatty acids in the liver of mice offspring at weaning. Lipids Health Dis. 2015, 14, 14. [Google Scholar] [CrossRef] [PubMed]
- Leikin-Frenkel, A.; Shomonov-Wagner, L.; Juknat, A.; Pasmanik-Chor, M. Maternal Diet Enriched with alpha-Linolenic or Saturated Fatty Acids Differentially Regulates Gene Expression in the Liver of Mouse Offspring. J. Nutrigenet. Nutrigenom. 2015, 8, 185–194. [Google Scholar]
- Clarke, S.D. Polyunsaturated Fatty Acid Regulation of Gene Transcription: A Molecular Mechanism to Improve the Metabolic Syndrome. J. Nutr. 2001, 131, 1129–1132. [Google Scholar] [CrossRef]
- Deckelbaum, R.J.; Worgall, T.S.; Seo, T. n-3 Fatty acids and gene expression. J. Clin. Nutr. 2006, 83 (Suppl. 6), 1520S–1525S. [Google Scholar]
- Ahmed, A.A.; Balogun, K.A.; Bykova, N.V.; Cheema, S.K. Novel regulatory roles of omega-3 fatty acids in metabolic pathways: A proteomics approach. Nutr. Metab. 2014, 11, 6. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ong, T.P.; Ozanne, S.E. Developmental programming of type 2 diabetes: Early nutrition and epigenetic mechanisms. Curr. Opin. Clin. Nutr. Metab. Care 2015, 18, 354–360. [Google Scholar] [CrossRef] [PubMed]
- Waterland, R.A.; Rached, M.-T. Developmental establishment of epigenotype: A role for dietary fatty acids? Scand. J. Food Nutr. 2006, 50 (Suppl. 2), 21–26. [Google Scholar] [CrossRef]
- Wang, J.; Wu, Z.; Li, D.; Li, N.; Dindot, S.V.; Satterfield, M.C.; Bazer, F.W.; Wu, G. Nutrition Epigenetics and Metabolic Syndrome. Antioxid. Redox Signal. 2012, 17, 282–301. [Google Scholar] [CrossRef] [PubMed]
- Vickers, M.H. Early Life Nutrition, Epigenetics and Programming of Later Life Disease. Nutrients 2004, 6, 2165–2178. [Google Scholar] [CrossRef] [PubMed]
- Burdge, G.C.; Lillycrop, K.A. Fatty acids and epigenetics. Curr. Opin. Clin. Nutr. Metab. Care 2014, 17, 156–161. [Google Scholar] [CrossRef] [PubMed]
© 2016 by the author; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons by Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Leikin-Frenkel, A.I. Is there A Role for Alpha-Linolenic Acid in the Fetal Programming of Health? J. Clin. Med. 2016, 5, 40. https://doi.org/10.3390/jcm5040040
Leikin-Frenkel AI. Is there A Role for Alpha-Linolenic Acid in the Fetal Programming of Health? Journal of Clinical Medicine. 2016; 5(4):40. https://doi.org/10.3390/jcm5040040
Chicago/Turabian StyleLeikin-Frenkel, Alicia I. 2016. "Is there A Role for Alpha-Linolenic Acid in the Fetal Programming of Health?" Journal of Clinical Medicine 5, no. 4: 40. https://doi.org/10.3390/jcm5040040




