Safety and Effectiveness of Volumetric Modulated Arc Therapy-Based Stereotactic Radiosurgery for Posterior Fossa Brain Metastases: A Single-Centre Experience
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Study Cohort
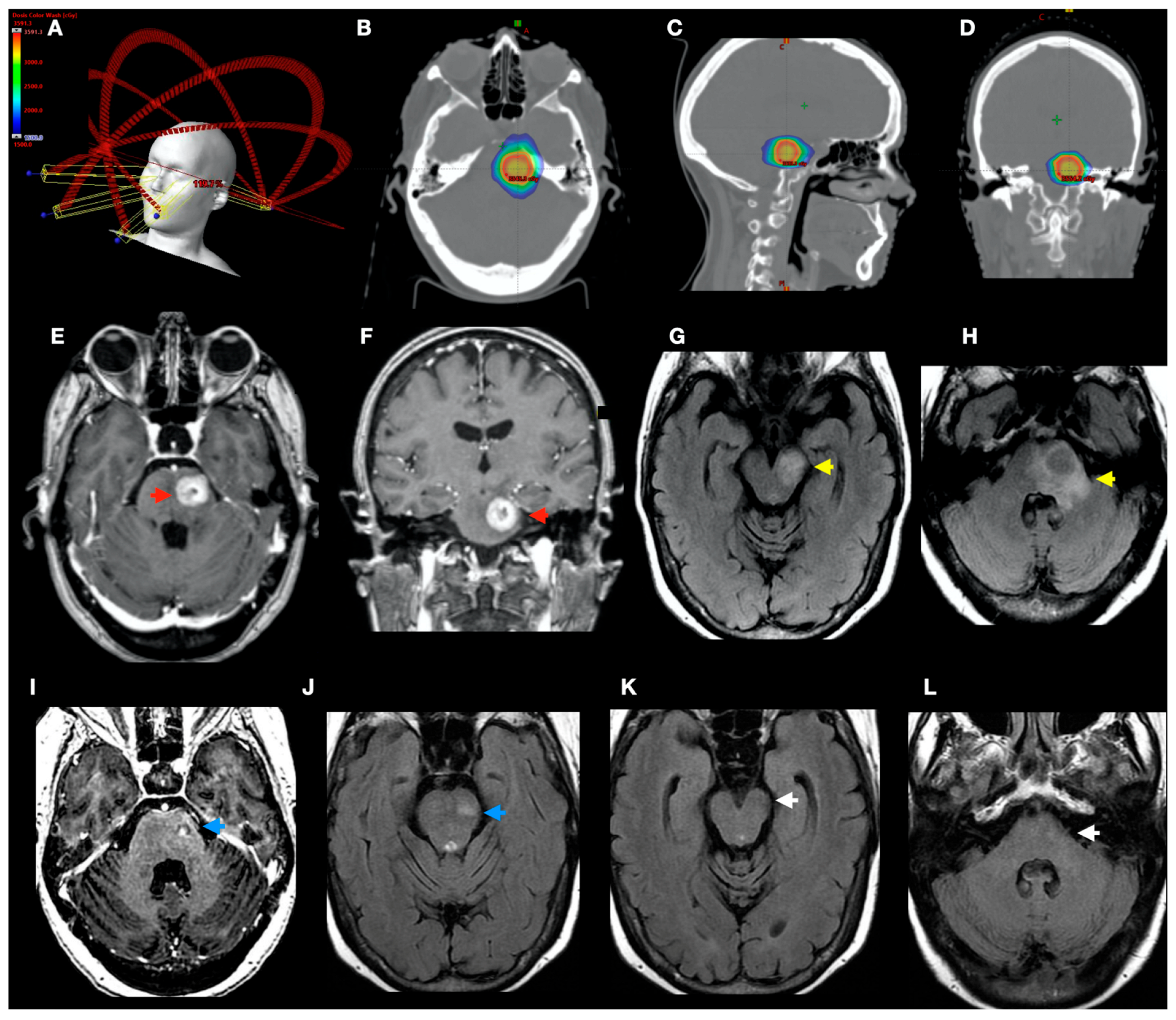
2.3. Treatment Technique
2.4. Statistical Analysis
3. Results
3.1. Cohort Characteristics
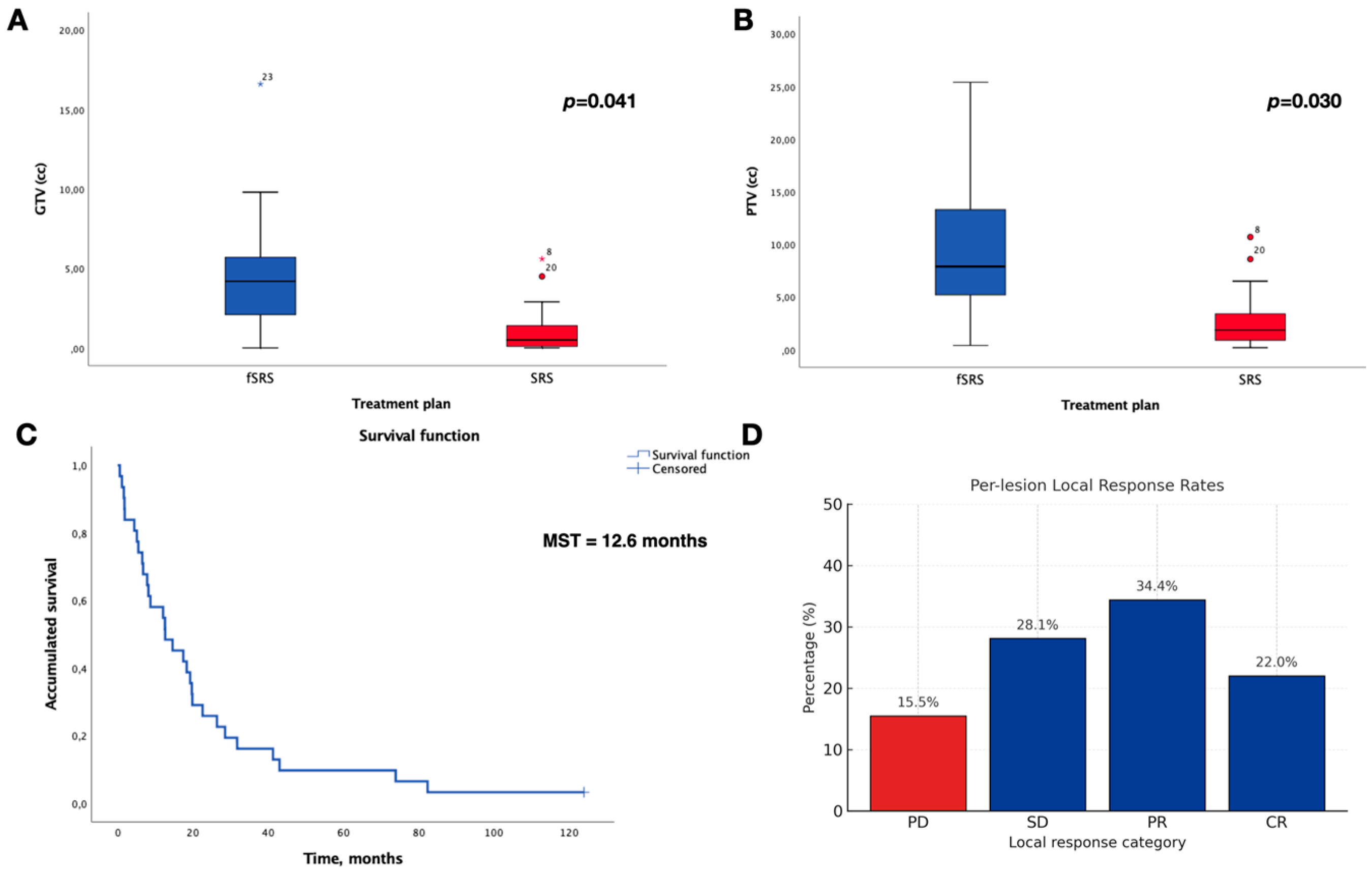
3.2. Local Control and Overall Survival
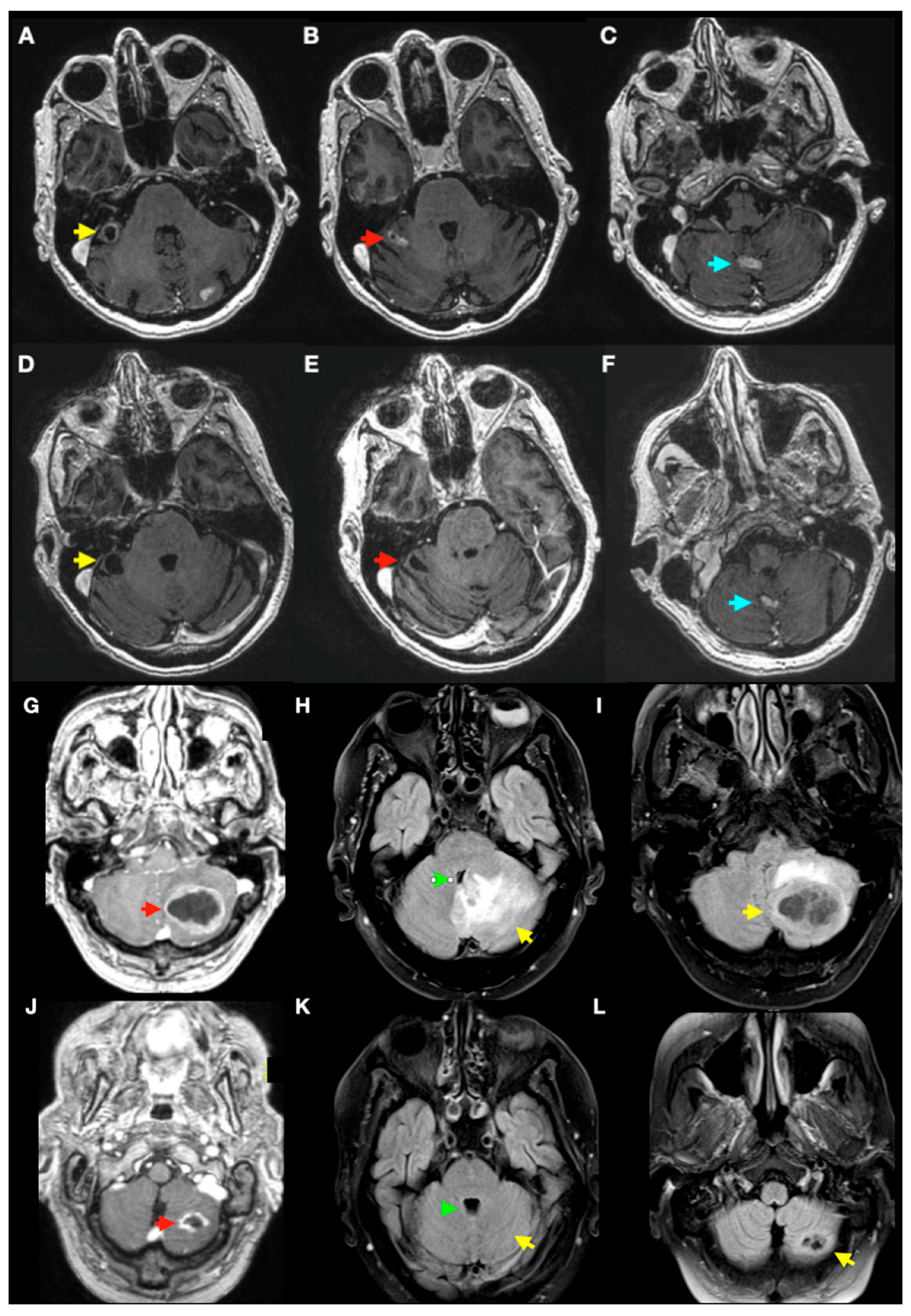
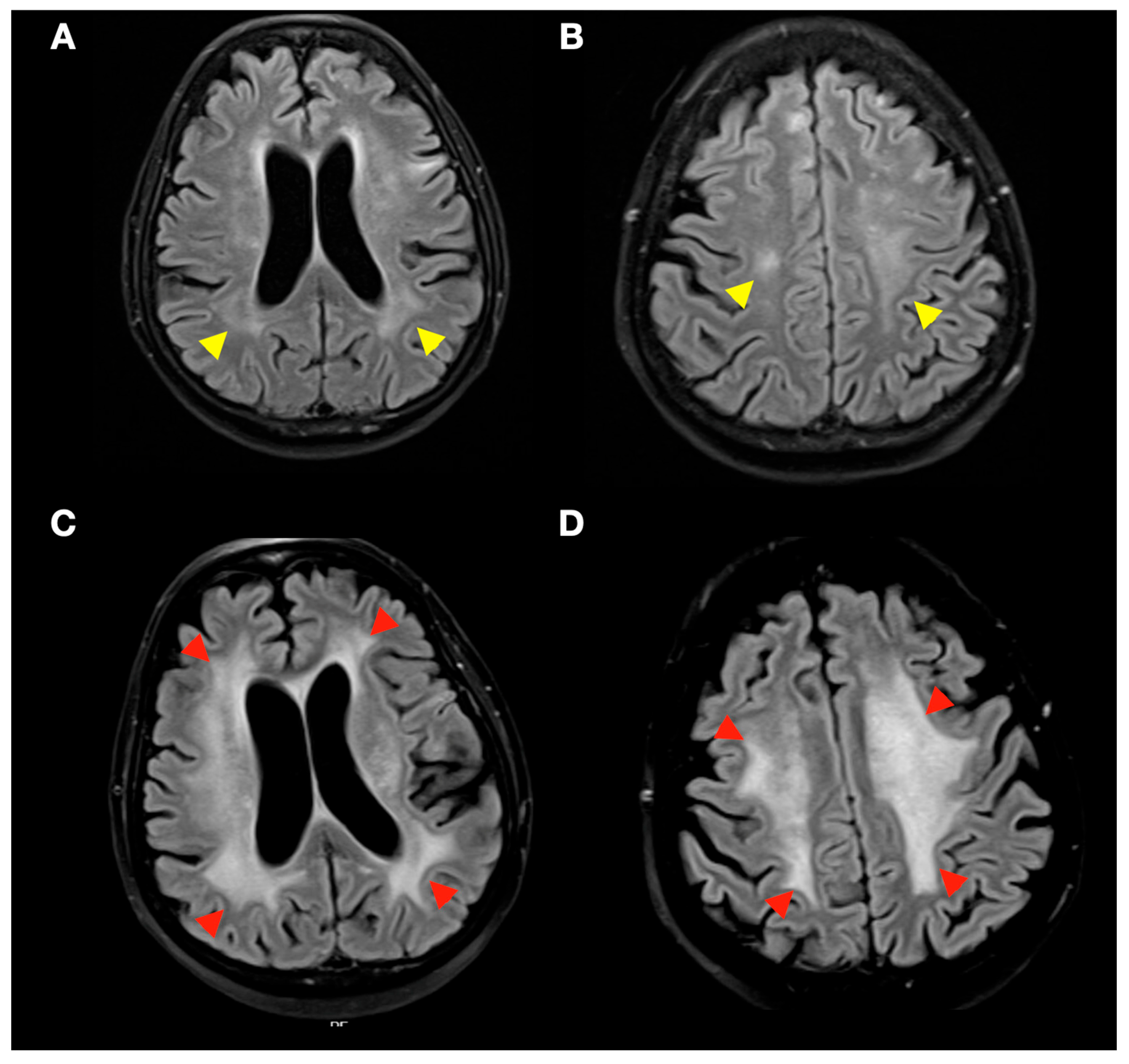
3.3. Radiological Complications of SRS Treatment
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| BED | Biologically effective dose |
| BM(s) | Brain Metastasis(es) |
| CBCT | Cone-Beam Computed Tomography |
| CI | Confidence Interval |
| CR | Complete Response |
| CSF | Cerebrospinal Fluid |
| EVD | External Ventricular Drainage |
| fSRS | Fractionated Stereotactic Radiosurgery |
| GKRS | Gamma Knife Radiosurgery |
| GTV | Gross Tumour Volume |
| HA (HyperArc) | Varian HyperArc VMAT platform |
| HR | Hazard Ratio |
| IQR | Interquartile Range |
| KPS | Karnofsky Performance Status |
| LINAC | Linear Accelerator |
| MRI | Magnetic Resonance Imaging |
| OS | Overall Survival |
| PD | Progressive Disease |
| PFBM(s) | Posterior Fossa Brain Metastasis(es) |
| PR | Partial Response |
| PTV | Planning Target Volume |
| RA (RapidArc) | Varian RapidArc VMAT delivery |
| SD | Stable Disease |
| SE | Standard Error |
| SRS | Stereotactic Radiosurgery |
| VMAT | Volumetric Modulated Arc Therapy |
| WBRT | Whole-Brain Radiotherapy |
References
- Franchino, F.; Rudà, R.; Soffietti, R. Mechanisms and Therapy for Cancer Metastasis to the Brain. Front. Oncol. 2018, 8, 161. [Google Scholar] [CrossRef]
- Linskey, M.E.; Andrews, D.W.; Asher, A.L.; Burri, S.H.; Kondziolka, D.; Robinson, P.D.; Ammirati, M.; Cobbs, C.S.; Gaspar, L.E.; Loeffler, J.S.; et al. The role of stereotactic radiosurgery in the management of patients with newly diagnosed brain metastases: A systematic review and evidence-based clinical practice guideline. J. Neurooncol. 2010, 96, 45–68. [Google Scholar] [CrossRef]
- Sánchez-Villalobos, J.M.; Aledo-Serrano, Á.; Villegas-Martínez, I.; Shaikh, M.F.; Alcaraz, M. Epilepsy treatment in neuro-oncology: A rationale for drug choice in common clinical scenarios. Front. Pharmacol. 2022, 13, 991244. [Google Scholar] [CrossRef] [PubMed]
- Chang, E.L.; Wefel, J.S.; Hess, K.R.; Allen, P.K.; Lang, F.F.; Kornguth, D.G.; Arbuckle, R.B.; Swint, J.M.; Shiu, A.S.; Maor, M.H.; et al. Neurocognition in patients with brain metastases treated with radiosurgery or radiosurgery plus whole-brain irradiation: A randomised controlled trial. Lancet Oncol. 2009, 10, 1037–1044. [Google Scholar] [CrossRef]
- Brown, P.D.; Jaeckle, K.; Ballman, K.V.; Farace, E.; Cerhan, J.H.; Anderson, S.K.; Carrero, X.W.; Barker, F.G.; Deming, R.; Burri, S.H.; et al. Effect of Radiosurgery Alone vs Radiosurgery With Whole Brain Radiation Therapy on Cognitive Function in Patients With 1 to 3 Brain Metastases. JAMA 2016, 316, 401–409. [Google Scholar] [CrossRef] [PubMed]
- Xue, J.; LaCouture, T.; Goldman, H.; Gault, W.; Chen, Y.; Potrebko, P.; Croce, R.; Kubicek, G. Implication of Biological Doses in Whole Brain Radiation and Radiosurgery of Multiple Metastases. Int. J. Radiat. Oncol. 2013, 87, S565–S566. [Google Scholar] [CrossRef]
- Xue, J.; LaCouture, T.; Grimm, J.; Goldman, H.W.; Ibbott, G.S.; Yorke, E.; Kubicek, G.J. Overview of dosimetric and biological perspectives on radiosurgery of multiple brain metastases in comparison with whole brain radiotherapy. J. Radiosurg. SBRT 2015, 3, 271. [Google Scholar] [PubMed]
- Tang, K.; Zhang, N.; Yuan, X.; Qian, Z.; Li, Y.; Feng, X. Conservation of pyramidal tract in radiosurgery for brain metastases of lung adenocarcinoma: Three-dimensional analysis of biologically effective dose. Radiother. Oncol. 2023, 179, 109451. [Google Scholar] [CrossRef]
- Sánchez-Villalobos, J.M.; Serna-Berna, A.; Salinas-Ramos, J.; Escolar-Pérez, P.P.; Andreu-Gálvez, M.; Martínez-Alonso, E.; Pérez-Vicente, J.A.; Alcaraz, M. Volumetric Modulated Arc Therapy for Radiosurgery of Brain Metastases: A Single-Center Study. Appl. Sci. 2023, 13, 10097. [Google Scholar] [CrossRef]
- Sunderland, G.J.; Jenkinson, M.D.; Zakaria, R. Surgical management of posterior fossa metastases. J. Neurooncol. 2016, 130, 535–542. [Google Scholar] [CrossRef]
- Hill, C.; Trifiletti, D.M.; Romano, K.D.; Showalter, T.N.; Sheehan, J.P. Stereotactic radiosurgery for cerebellar metastases and the risk of obstructive hydrocephalus. Appl. Radiat. Oncol. 2017, 60, 17–23. [Google Scholar] [CrossRef]
- Voong, K.R.; Farnia, B.; Wang, Q.; Luo, D.; McAleer, M.F.; Rao, G.; Guha-Thakurta, N.; Likhacheva, A.; Ghia, A.J.; Brown, P.D.; et al. Gamma knife stereotactic radiosurgery in the treatment of brainstem metastases: The MD Anderson experience. Neuro-Oncol. Pract. 2015, 2, 40. [Google Scholar] [CrossRef]
- Muhsen, B.A.; Joshi, K.C.; Lee, B.S.; Thapa, B.; Borghei-Razavi, H.; Jia, X.; Barnett, G.H.; Chao, S.T.; Mohammadi, A.M.; Suh, J.H.; et al. The effect of Gamma Knife radiosurgery on large posterior fossa metastases and the associated mass effect from peritumoral edema. J. Neurosurg. 2020, 134, 466–474. [Google Scholar] [CrossRef] [PubMed]
- Matsunaga, S.; Shuto, T.; Kobayashi, N.; Murata, H.; Yamamoto, T. Acute Management of Gamma Knife Radiosurgery for Asymptomatic Obstructive Hydrocephalus Associated with Posterior Fossa Metastases. World Neurosurg. 2020, 144, e714–e722. [Google Scholar] [CrossRef] [PubMed]
- Sinclair, G.; Benmakhlouf, H.; Martin, H.; Brigui, M.; Maeurer, M.; Dodoo, E. The role of radiosurgery in the acute management of fourth ventricle compression due to brain metastases. Surg. Neurol. Int. 2018, 9, 112. [Google Scholar] [CrossRef]
- Rangwala, S.D.; Han, J.S.; Strickland, B.; Yu, C.; Ye, J.C.; Zada, G. Stereotactic radiosurgery for fourth ventricle brain metastases: Tumor control outcomes and the need for CSF diversion. Patient series. J. Neurosurg. Case Lessons 2024, 8, 1–8. [Google Scholar] [CrossRef]
- Samanci, Y.; Aydin, S.; Düzkalir, A.H.; Askeroglu, M.O.; Peker, S. Upfront frameless hypofractionated gamma knife radiosurgery for large posterior Fossa metastases. Neurosurg. Rev. 2025, 48, 418. [Google Scholar] [CrossRef]
- Sánchez-Villalobos, J.M.; Serna-Berna, A.; Salinas-Ramos, J.; Escolar-Pérez, P.P.; Martínez-Alonso, E.; Achel, D.G.; Alcaraz, M. Volumetric modulated arc radiosurgery for brain metastases from breast cancer: A single-center study. Colomb. Med. 2021, 52, e2004567. [Google Scholar] [CrossRef] [PubMed]
- Qian, J.M.; Mahajan, A.; Yu, J.B.; Tsiouris, A.J.; Goldberg, S.B.; Kluger, H.M.; Chiang, V.L.S. Comparing available criteria for measuring brain metastasis response to immunotherapy. J. Neurooncol. 2017, 132, 479–485. [Google Scholar] [CrossRef]
- Izawa, M.; Chernov, M.; Hayashi, M.; Kubota, Y.; Kasuya, H.; Hori, T. Fatal intratumoral hemorrhage immediately after gamma knife radiosurgery for brain metastases: Case report. Minim. Invasive Neurosurg. 2006, 49, 251–254. [Google Scholar] [CrossRef]
- Lehrer, E.J.; Snyder, M.H.; Desai, B.D.; Li, C.E.; Narayan, A.; Trifiletti, D.M.; Schlesinger, D.; Sheehan, J.P. Clinical and radiographic adverse events after Gamma Knife radiosurgery for brainstem lesions: A dosimetric analysis. Radiother. Oncol. 2020, 147, 200–209. [Google Scholar] [CrossRef]
- Shimizu, Y.; Miyamori, T.; Yamano, J. Hydrocephalus after Gamma Knife Radiosurgery for Schwannoma. Asian J. Neurosurg. 2019, 14, 487. [Google Scholar] [CrossRef]
- Thombre, B.; Sadashiva, N.; Krishnan, J.B.; Prabhuraj, A.R.; Rao, K.N.; Arima, A. Symptomatic Post-Radiosurgery Intratumoral Hemorrhage in a Case of Vestibular Schwannoma: A Case Report and Review of the Literature. Stereotact. Funct. Neurosurg. 2020, 97, 399–403. [Google Scholar] [CrossRef]
- Bird, C.M.; Burgess, N. The hippocampus and memory: Insights from spatial processing. Nat. Rev. Neurosci. 2008, 9, 182–194. [Google Scholar] [CrossRef]
- Ohira, S.; Ueda, Y.; Akino, Y.; Hashimoto, M.; Masaoka, A.; Hirata, T.; Miyazaki, M.; Koizumi, M.; Teshima, T. HyperArc VMAT planning for single and multiple brain metastases stereotactic radiosurgery: A new treatment planning approach. Radiat. Oncol. 2018, 13, 13. [Google Scholar] [CrossRef]
- Vergalasova, I.; Liu, H.; Alonso-Basanta, M.; Dong, L.; Li, J.; Nie, K.; Shi, W.; Teo, B.K.K.; Yu, Y.; Yue, N.J.; et al. Multi-Institutional Dosimetric Evaluation of Modern Day Stereotactic Radiosurgery (SRS) Treatment Options for Multiple Brain Metastases. Front. Oncol. 2019, 9, 483. [Google Scholar] [CrossRef]
- Goldberg, S.B.; Gettinger, S.N.; Mahajan, A.; Chiang, A.C.; Herbst, R.S.; Sznol, M.; Tsiouris, A.J.; Cohen, J.; Vortmeyer, A.; Jilaveanu, L.; et al. A Phase II trial of pembrolizumab for patients with melanoma or non-small cell lung cancer and untreated brain metastases. Lancet. Oncol. 2016, 17, 976–983. [Google Scholar] [CrossRef]
- Eisenhauer, E.A.; Therasse, P.; Bogaerts, J.; Schwartz, L.H.; Sargent, D.; Ford, R.; Dancey, J.; Arbuck, S.; Gwyther, S.; Mooney, M.; et al. New response evaluation criteria in solid tumours: Revised RECIST guideline (version 1.1). Eur. J. Cancer 2009, 45, 228–247. [Google Scholar] [CrossRef] [PubMed]
- Trifiletti, D.M.; Lee, C.C.; Kano, H.; Cohen, J.; Janopaul-Naylor, J.; Alonso-Basanta, M.; Lee, J.Y.K.; Simonova, G.; Liscak, R.; Wolf, A.; et al. Stereotactic Radiosurgery for Brainstem Metastases: An International Cooperative Study to Define Response and Toxicity. Int. J. Radiat. Oncol. Biol. Phys. 2016, 96, 280. [Google Scholar] [CrossRef] [PubMed]
- Patel, A.; Mohammadi, H.; Dong, T.; Shiue, K.R.Y.; Frye, D.; Le, Y.; Ansari, S.; Watson, G.A.; Miller, J.C.; Lautenschlaeger, T. Brainstem metastases treated with Gamma Knife stereotactic radiosurgery: The Indiana University Health experience. CNS Oncol. 2018, 7, 15–23. [Google Scholar] [CrossRef] [PubMed]
- Ehret, F.; Rueß, D.; Blanck, O.; Fichte, S.; Chatzikonstantinou, G.; Wolff, R.; Mose, L.; Mose, S.; Fortmann, T.; Lehrke, R.; et al. Stereotactic radiosurgery and radiotherapy for brainstem metastases: An international multicenter analysis. Int. J. Cancer 2024, 155, 916–924. [Google Scholar] [CrossRef] [PubMed]
- Li, F.; Mail, N.; Stefania diMayorca, M.; McCaw, T.J.; Ozhasoglu, C.; Lalonde, R.; Chang, J.; Huq, M.S. Single isocenter HyperArc treatment of multiple intracranial metastases: Targeting accuracy. J. Appl. Clin. Med. Phys. 2023, 25, e14234. [Google Scholar] [CrossRef] [PubMed]
| Age, Years | Median (IQR) | 58 | (12) |
| Sex, n (%) | Female | 14 | (45.2) |
| Male | 17 | (54.8) | |
| Primary Tumour, n (%) | Total cohort | 31 | (100) |
| Non-small cell lung carcinoma | 27 | (58) | |
| Small cell lung carcinoma | 1 | (3.2) | |
| Breast | 10 | (32.2) | |
| Remainder | 2 | (6.6) | |
| KPS Score, n (%) | 70–100 | 24 | (77.4) |
| <70 | 7 | (22.6) | |
| Overall Survival, Months | Median (CI 95%) | 12.6 | (3–22.1) |
| Extracranial Metastases, n (%) | Yes | 21 | (67.7) |
| No | 10 | (32.3) | |
| Radiosurgery Treatment, n (%) | Total BMs treated | 39 | (100) |
| SRS | 30 | (76.9) | |
| Fractionated SRS (fSRS) | 9 | (23.1) | |
| Previous Treatment, n (%) * | None | 20 | (64.5) |
| WBRT | 10 | (32.3) | |
| Surgery | 1 | (3.2) | |
| Posterior Treatment, n (%) * | None | 18 | (58.1) |
| WBRT | 6 | (19.4) | |
| Single or Fractionated SRS | 10 | (32.3) | |
| Surgery | 0 | (0.0) | |
| Patients with PFBMs ± Supratentorial BMs, n(%) | Only PFBMs Treated | 8 | (25.8) |
| PFBMs + Supratentorial BMs | 23 | (74.2) | |
| Gross Tumour Volume, cc (GTV) | SRS, Median (IQR) | 0.5 | (1.3) |
| fSRS, Median (IQR) | 4.2 | (6.6) | |
| Planning Target Volume, cc (PTV) | SRS, Median (IQR) | 1.9 | (2.7) |
| fSRS, Median (IQR) | 7.9 | (13.7) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sánchez-Villalobos, J.M.; Serna-Berna, A.; Salinas-Ramos, J.; Escolar-Pérez, P.P.; Luengo-Gil, G.; Andreu-Gálvez, M.; Martínez-Alonso, E.; Alcaraz, M. Safety and Effectiveness of Volumetric Modulated Arc Therapy-Based Stereotactic Radiosurgery for Posterior Fossa Brain Metastases: A Single-Centre Experience. J. Clin. Med. 2025, 14, 8540. https://doi.org/10.3390/jcm14238540
Sánchez-Villalobos JM, Serna-Berna A, Salinas-Ramos J, Escolar-Pérez PP, Luengo-Gil G, Andreu-Gálvez M, Martínez-Alonso E, Alcaraz M. Safety and Effectiveness of Volumetric Modulated Arc Therapy-Based Stereotactic Radiosurgery for Posterior Fossa Brain Metastases: A Single-Centre Experience. Journal of Clinical Medicine. 2025; 14(23):8540. https://doi.org/10.3390/jcm14238540
Chicago/Turabian StyleSánchez-Villalobos, José Manuel, Alfredo Serna-Berna, Juan Salinas-Ramos, Pedro Pablo Escolar-Pérez, Ginés Luengo-Gil, Marina Andreu-Gálvez, Emma Martínez-Alonso, and Miguel Alcaraz. 2025. "Safety and Effectiveness of Volumetric Modulated Arc Therapy-Based Stereotactic Radiosurgery for Posterior Fossa Brain Metastases: A Single-Centre Experience" Journal of Clinical Medicine 14, no. 23: 8540. https://doi.org/10.3390/jcm14238540
APA StyleSánchez-Villalobos, J. M., Serna-Berna, A., Salinas-Ramos, J., Escolar-Pérez, P. P., Luengo-Gil, G., Andreu-Gálvez, M., Martínez-Alonso, E., & Alcaraz, M. (2025). Safety and Effectiveness of Volumetric Modulated Arc Therapy-Based Stereotactic Radiosurgery for Posterior Fossa Brain Metastases: A Single-Centre Experience. Journal of Clinical Medicine, 14(23), 8540. https://doi.org/10.3390/jcm14238540








