Prevalence of Chronic Obstructive Pulmonary Disease and Asthma in Polycythemia Vera and Essential Thrombocythemia and Its Prognostic Implications
Abstract
1. Introduction
2. Patients and Methods
2.1. Study Design and Patient Inclusion Criteria
2.2. Statistics
3. Results
3.1. Patient Characteristics and the Prevalence of COPD and Asthma
3.2. Survival Analyses
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Krecak, I.; Lucijanic, M.; Verstovsek, S. Advances in Risk Stratification and Treatment of Polycythemia Vera and Essential Thrombocythemia. Curr. Hematol. Malig. Rep. 2022, 17, 155–169. [Google Scholar] [CrossRef]
- Krecak, I.; Verstovsek, S.; Lucijanic, M. Optimization of cardiovascular risk factor management in patients with BCR::ABL1 negative chronic myeloproliferative neoplasms, current knowledge, and perspectives. Ann. Hematol. 2024, 103, 1513–1523. [Google Scholar] [CrossRef] [PubMed]
- Krecak, I.; Verstovsek, S.; Lucijanic, M. Reappraisal of cardiovascular risk factors in patients with chronic myeloproliferative neoplasms. Clin. Adv. Hematol. Oncol. 2023, 21, 541–548. [Google Scholar]
- Barbui, T.; Tefferi, A.; Vannucchi, A.M.; Passamonti, F.; Silver, R.T.; Hoffman, R.; Verstovsek, S.; Mesa, R.; Kiladjian, J.-J.; Hehlmann, R.; et al. Philadelphia chromosome-negative classical myeloproliferative neoplasms: Revised management recommendations from European LeukemiaNet. Leukemia 2018, 32, 1057–1069. [Google Scholar] [CrossRef] [PubMed]
- Marchetti, M.; Vannucchi, A.M.; Griesshammer, M.; Harrison, C.; Koschmieder, S.; Gisslinger, H.; Álvarez-Larrán, A.; De Stefano, V.; Guglielmelli, P.; Palandri, F.; et al. Appropriate management of polycythaemia vera with cytoreductive drug therapy: European LeukemiaNet 2021 recommendations. Lancet Haematol. 2022, 9, e301–e311. [Google Scholar] [CrossRef]
- Tefferi, A.; Barbui, T. Polycythemia vera and essential thrombocythemia: 2021 update on diagnosis, risk-stratification and management. Am. J. Hematol. 2020, 95, 1599–1613. [Google Scholar] [CrossRef]
- Boers, E.; Barrett, M.; Su, J.G.; Benjafield, A.V.; Sinha, S.; Kaye, L.; Zar, H.J.; Vuong, V.; Tellez, D.; Gondalia, R.; et al. Global Burden of Chronic Obstructive Pulmonary Disease Through 2050. JAMA Netw. Open 2023, 6, e2346598. [Google Scholar] [CrossRef]
- Adeloye, D.; Song, P.; Zhu, Y.; Campbell, H.; Sheikh, A.; Rudan, I. NIHR RESPIRE Global Respiratory Health Unit. Global, regional, and national prevalence of, and risk factors for, chronic obstructive pulmonary disease (COPD) in 2019: A systematic review and modelling analysis. Lancet Respir. Med. 2022, 10, 447–458. [Google Scholar] [CrossRef]
- GBD 2021 Asthma and Allergic Diseases Collaborators. Global, regional, and national burden of asthma and atopic dermatitis, 1990-2021, and projections to 2050: A systematic analysis of the Global Burden of Disease Study 2021. Lancet Respir. Med. 2025, 13, 425–446. [Google Scholar] [CrossRef]
- Chakaya, J.; Aït-Khaled, N. The Global Asthma Report 2022. Int. J. Tuberc. Lung Dis. 2022, 26 (Suppl. S1), 1–104. [Google Scholar] [PubMed]
- Polman, R.; Hurst, J.R.; Uysal, O.F.; Mandal, S.; Linz, D.; Simons, S. Cardiovascular disease and risk in COPD: A state of the art review. Expert Rev. Cardiovasc. Ther. 2024, 22, 177–191. [Google Scholar] [CrossRef]
- Pollevick, M.E.; Xu, K.Y.; Mhango, G.; Federmann, E.G.; Vedanthan, R.; Busse, P.; Holguin, F.; Federman, A.D.; Wisnivesky, J.P. The Relationship Between Asthma and Cardiovascular Disease: An Examination of the Framingham Offspring Study. Chest 2021, 159, 1338–1345. [Google Scholar] [CrossRef]
- Arber, D.A.; Orazi, A.; Hasserjian, R.; Thiele, J.; Borowitz, M.J.; Le Beau, M.M.; Bloomfield, C.D.; Cazzola, M.; Vardiman, J.W. The 2016 revision to the World Health Organization classification of myeloid neoplasms and acute leukemia. Blood 2016, 127, 2391–2405. [Google Scholar] [CrossRef]
- Vicari, A.M.; Ponzoni, M.; Alberetto, M.; Martani, C.; Pontiroli, A.E.; Folli, F. Erythrocytosis in a patient with chronic obstructive pulmonary disease. Haematologica 1998, 83, 183–186. [Google Scholar] [PubMed]
- Nguyen, E.; Harnois, M.; Busque, L.; Sirhan, S.; Assouline, S.; Chamaki, I.; Olney, H.; Mollica, L.; Szuber, N. Phenotypical differences and thrombosis rates in secondary erythrocytosis versus polycythemia vera. Blood Cancer J. 2021, 11, 75. [Google Scholar] [CrossRef]
- Wouters, H.J.C.M.; Mulder, R.; van Zeventer, I.A.; Schuringa, J.J.; van der Klauw, M.M.; van der Harst, P.; Diepstra, A.; Mulder, A.B.; Huls, G. Erythrocytosis in the general population: Clinical characteristics and association with clonal hematopoiesis. Blood Adv. 2020, 4, 6353–6363. [Google Scholar] [CrossRef] [PubMed]
- Krečak, I.; Holik, H.; Zekanović, I.; Morić Perić, M.; Marketin, T.; Coha, B.; Gverić-Krečak, V.; Vodanović, M.; Lucijanić, M. Thrombotic risk in secondary polycythemia resembles low-risk polycythemia vera and increases in specific subsets of patients. Thromb. Res. 2022, 209, 47–50. [Google Scholar] [CrossRef]
- Yeung, A.K.; Villacorta-Martin, C.; Hon, S.; Rock, J.R.; Murphy, G.J. Lung megakaryocytes display distinct transcriptional and phenotypic properties. Blood Adv. 2020, 4, 6204–6217. [Google Scholar] [CrossRef] [PubMed]
- Lucijanic, M.; Krecak, I.; Soric, E.; Sedinic, M.; Sabljic, A.; Derek, L.; Jaksic, O.; Kusec, R. Thrombocytosis in COVID-19 patients without myeloproliferative neoplasms is associated with better prognosis but higher rate of venous thromboembolism. Blood Cancer J. 2021, 11, 189. [Google Scholar] [CrossRef]
- Harrison, M.T.; Short, P.; Williamson, P.A.; Singanayagam, A.; Chalmers, J.D.; Schembri, S. Thrombocytosis is associated with increased short and long term mortality after exacerbation of chronic obstructive pulmonary disease: A role for antiplatelet therapy? Thorax 2014, 69, 609–615. [Google Scholar] [CrossRef]
- Sørensen, A.L.; Knudsen, T.A.; Skov, V.; Kjaer, L.; Holm, N.; Ellervik, C.; Hasselbalch, H.C. Smoking impairs molecular response, and reduces overall survival in patients with chronic myeloproliferative neoplasms: A retrospective cohort study. Br. J. Haematol. 2021, 193, 83–92. [Google Scholar] [CrossRef]
- Kyriakopoulos, C.; Chronis, C.; Papapetrou, E.; Tatsioni, A.; Gartzonika, K.; Tsaousi, C.; Gogali, A.; Katsanos, C.; Vaggeli, A.; Tselepi, C.; et al. Prothrombotic state in patients with stable COPD: An observational study. ERJ Open Res. 2021, 7, 00297-2021. [Google Scholar] [CrossRef] [PubMed]
- Tuleta, I.; Skowasch, D.; Aurich, F.; Eckstein, N.; Schueler, R.; Pizarro, C.; Schahab, N.; Nickenig, G.; Schaefer, C.; Pingel, S. Asthma is associated with atherosclerotic artery changes. PLoS ONE 2017, 12, e0186820. [Google Scholar] [CrossRef]
- Meng, K.; Zhang, X.; Dai, H. Obstructive Airway Disease is Associated with Increased Cardiovascular Disease Risk Independent of Phenotype: Evidence from Two Nationwide Population-Based Studies. Int. J. Chronic Obstr. Pulm. Dis. 2025, 20, 1435–1446. [Google Scholar] [CrossRef]
- Morgan, A.D.; Herrett, E.; De Stavola, B.L.; Smeeth, L.; Quint, J.K. COPD disease severity and the risk of venous thromboembolic events: A matched case-control study. Int. J. Chronic Obstr. Pulm. Dis. 2016, 11, 899–908. [Google Scholar] [CrossRef] [PubMed]
- Portegies, M.L.; Lahousse, L.; Joos, G.F.; Hofman, A.; Koudstaal, P.J.; Stricker, B.H.; Brusselle, G.G.; Ikram, M.A. Chronic Obstructive Pulmonary Disease and the Risk of Stroke. The Rotterdam Study. Am. J. Respir. Crit. Care Med. 2016, 193, 251–258. [Google Scholar] [CrossRef]
- Ambrosetti, M.; Ageno, W.; Spanevello, A.; Salerno, M.; Pedretti, R.F. Prevalence and prevention of venous thromboembolism in patients with acute exacerbations of COPD. Thromb. Res. 2003, 112, 203–207. [Google Scholar] [CrossRef]
- Kim, V.; Goel, N.; Gangar, J.; Zhao, H.; Ciccolella, D.E.; Silverman, E.K.; Crapo, J.D.; Criner, G.J.; the COPD Gene Investigators. Risk Factors for Venous Thromboembolism in Chronic Obstructive Pulmonary Disease. Chronic Obstr. Pulm. Dis. 2014, 1, 239–249. [Google Scholar] [CrossRef]
- Chung, W.S.; Lin, C.L.; Ho, F.M.; Li, R.Y.; Sung, F.C.; Kao, C.H.; Yeh, J.J. Asthma increases pulmonary thromboembolism risk: A nationwide population cohort study. Eur. Respir. J. 2014, 43, 801–807. [Google Scholar] [CrossRef]
- Barbui, T.; De Stefano, V.; Ghirardi, A.; Masciulli, A.; Finazzi, G.; Vannucchi, A.M. Different effect of hydroxyurea and phlebotomy on prevention of arterial and venous thrombosis in Polycythemia Vera. Blood Cancer J. 2018, 8, 124. [Google Scholar] [CrossRef] [PubMed]
- Montani, D.; Thoré, P.; Mignard, X.; Jaïs, X.; Boucly, A.; Jevnikar, M.; Seferian, A.; Jutant, E.-M.; Cottin, V.; Fadel, E.; et al. Clinical Phenotype and Outcomes of Pulmonary Hypertension Associated with Myeloproliferative Neoplasms: A Population-based Study. Am. J. Respir. Crit. Care Med. 2023, 208, 600–612. [Google Scholar] [CrossRef]
- Leiva, O.; Ren, S.; Neuberg, D.; Bhatt, A.; Jenkins, A.; Rosovsky, R.; Leaf, R.K.; Goodarzi, K.; Hobbs, G. Pulmonary hypertension is associated with poor cardiovascular and hematologic outcomes in patients with myeloproliferative neoplasms and cardiovascular disease. Int. J. Hematol. 2023, 117, 90–99. [Google Scholar] [CrossRef] [PubMed]
- Dransfield, M.T.; Criner, G.J.; Halpin, D.M.G.; Han, M.K.; Hartley, B.; Kalhan, R.; Lange, P.; Lipson, D.A.; Martinez, F.J.; Midwinter, D.; et al. Time-Dependent Risk of Cardiovascular Events Following an Exacerbation in Patients With Chronic Obstructive Pulmonary Disease: Post Hoc Analysis from the IMPACT Trial. J. Am. Heart Assoc. 2022, 11, e024350. [Google Scholar] [CrossRef] [PubMed]
- Kunisaki, K.M.; Dransfield, M.T.; Anderson, J.A.; Brook, R.D.; Calverley, P.M.A.; Celli, B.R.; Crim, C.; Hartley, B.F.; Martinez, F.J.; Newby, D.E.; et al. Exacerbations of Chronic Obstructive Pulmonary Disease and Cardiac Events. A Post Hoc Cohort Analysis from the SUMMIT Randomized Clinical Trial. Am. J. Respir. Crit. Care Med. 2018, 198, 51–57. [Google Scholar] [CrossRef] [PubMed]
| Variable | Overall (n = 246) | Non COPD-Asthma (n = 226, 91.6%) | COPD/Asthma (n = 20, 8.1%) | p Value * |
|---|---|---|---|---|
| Sex, female | 137 (55.7%) | 127 (56.2%) | 10 (50%) | 0.593 |
| Age, years (median, range) | 68 (20–91) | 68 (20–91) | 68.5 (53.84) | 0.379 |
| Year of MPN diagnosis | 0.596 | |||
| 1997–2009 | 48 (19.5%) | 45 (93.7%) | 3 (6.2%) | |
| 2010–2023 | 198 (80.5%) | 181 (91.4%) | 17 (8.6%) | |
| PV ET | 154 (62.6%) 92(37.4%) | 140 (61.9%) 86 (38.1%) | 14 (70%) 6 (30%) | 0.476 |
| Palpable splenomegaly | 49 (19.9%) | 45 (19.9%) | 4 (20%) | 0.992 |
| Constitutional symptoms | 128 (52%) | 110 (48.7%) | 18 (90%) | 0.001 |
| JAK2-V617F Calreticulin | 189 (76.8%) 16 (6.5%) | 171 (75.7%) 15 (6.6%) | 18 (90%) 1 (5%) | 0.146 0.708 |
| History of thrombosis | 63 (25.6%) | 58 (25.7%) | 5 (25%) | 0.948 |
| High-risk disease ** | 196 (79.7%) | 181 (80.1%) | 15 (75%) | 0.588 |
| Hydroxyurea | 170 (69.1%) | 156 (69%) | 14 (70%) | 0.928 |
| Aspirin | 201 (81.7%) | 186 (82.3%) | 15 (75%) | 0.419 |
| Warfarin | 38 (15.4%) | 34 (15%) | 4 (20%) | 0.557 |
| Arterial hypertension | 184 (74.8%) | 169 (74.8%) | 15 (75%) | 0.982 |
| Hyperlipidemia | 92 (37.4%) | 85 (37.6%) | 7 (35%) | 0.817 |
| Smoking (current/previous vs. never) | 58 (23.6%) | 50 (22.1%) | 9 (45%) | 0.021 |
| Total leukocytes, ×109/L (median, range) | 9.6 (1.5–26.2) | 9.5 (1.5–26.2) | 10.4 (4.5–20.7) | 0.163 |
| Granulocytes, ×109/L (median, range) | 7.1 (0.34–20.6) | 6.69(0.34–20.6) | 7.64 (4.82–17.78) | 0.278 |
| Lymphocytes. ×109/L (median, range) | 2.01 (0.15–5.47) | 1.97 (0.15–5.02) | 2.2 (1.41–5.47) | 0.062 |
| Erythrocytes, ×1012/L (median, range) | 5.47 (1.56–21) | 5.49 (1.56–21) | 5.54 (2.9–9.5) | 0.804 |
| Platelets, ×109/L (median, range) | 570 (104–3211) | 570 (104–3211) | 595 (261–1055) | 0.673 |
| Hemoglobin, g/L (median, range) | 157 (148–229) | 157 (148–229) | 159 (115–195) | 0.855 |
| Hematocrit, % (median, range) | 48.3 (34–90) | 48.3 (34–90) | 50.2 (37.9–62) | 0.534 |
| Variable | Overall Thrombosis (n = 40) | Arterial Thrombosis (n = 30) | Venous Thrombosis (n = 10) | Interaction Between COPD/Asthma and Overall Thrombotic Risk |
|---|---|---|---|---|
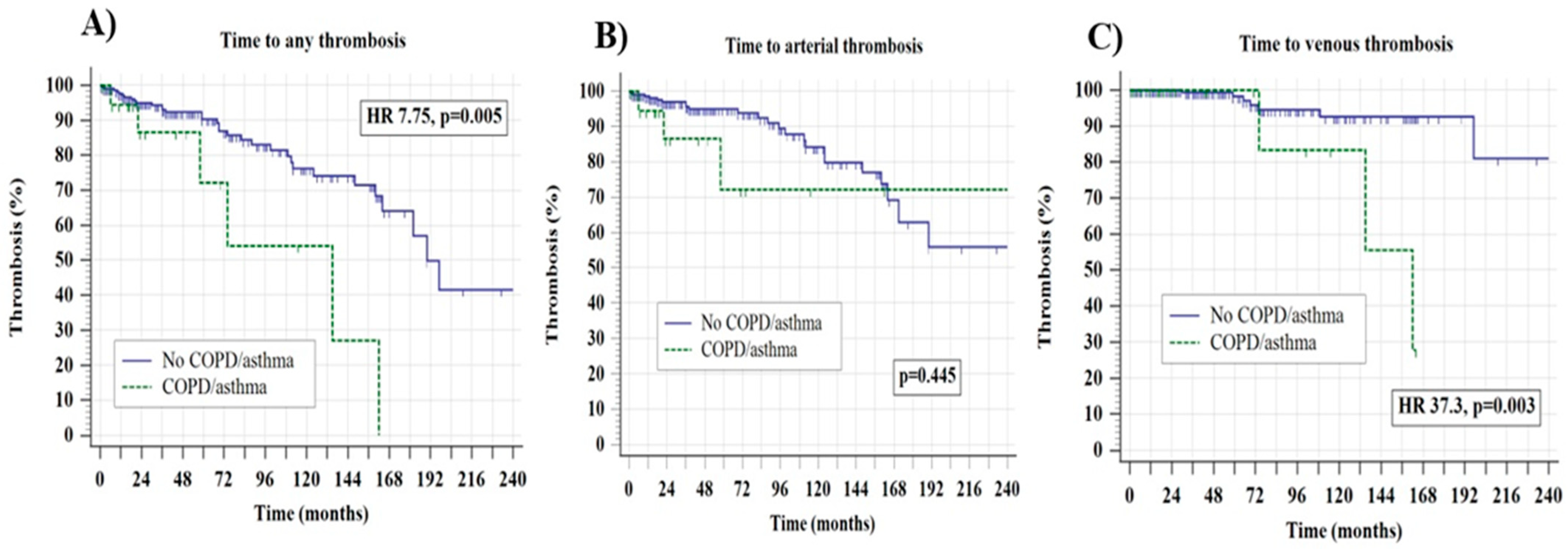
| COPD/asthma | HR 7.75, 95 CI 1.8–29.5, p = 0.005 | HR 1.77, 95% CI 0.40–7.78, p = 0.445 | HR 37.3, 95% CI 3.2–43.6, p = 0.003 | - |
| Female sex | HR 1.03, 95% CI 0.55–1.94 p = 0.903 | HR 1.64, 95% CI 0.76–3.52, p = 0.201 | HR 0.41, 95% CI 0.11–1.45, p = 0.169 | HR 3.94, 95% CI 1.01–11.02, interaction p = 0.047 |
| Age > 60 years | HR 2.15, 95% CI 1.1–4.2, p = 0.026 | HR 2.83, 95% CI 1.25–6.4, p = 0.012 | HR 0.93, 95% CI 0.24–3.59, p = 0.927 | HR 2.9, 95% CI 0.85–9.44, interaction p = 0.088 |
| ET phenotype | HR 1.08, 95% CI 0.56–2.07, p = 0.805 | HR 1.55, 95% CI 0.70–3.41, p = 0.275 | HR 0.66, 95% CI 0.18–2.85, p = 0.529 | HR 7.1, 95% CI 15.3–16.7, interaction p = 0.007 |
| JAK2 vs. other/negative mutations | HR 1.68, 95% CI 0.82–3.44, p = 0.153 | HR 2.50, 95% CI 1.1–5.9, p = 0.037 | HR 1.62, 95% CI 0.39, 6.7, p = 0.503 | HR 4.17, 95% CI 1.04–6.9, interaction p = 0.041 |
| History of thrombosis | HR 0.72, 95% CI 0.32–1.58, p = 0.419 | HR 0.80, 95% CI 0.30–2.13, p = 0.665 | HR 0.39, 95% CI 0.07–2.1, p = 0.278 | HR 1.48, 95% CI 0.58–10.1, interaction p = 0.223 |
| High-risk disease * | HR 1.83, 95% CI 0.92–3.72 p = 0.092 | HR 1.55, 95% CI 0.65–3.68, p = 0.315 | HR 3.01, 95% CI 0.73–12.4, p = 0.126 | HR 3.43, 95% CI 0.94–7.63, interaction p = 0.063 |
| Hydroxyurea | HR 1.43, 95% CI 0.69–2.9, p = 0.339 | HR 1.07, 95% CI 0.44–2.62, p = 0.870 | No events | HR 4.67, 95% CI 1.10–7.43, interaction p = 0.030 |
| Aspirin | HR 0.67, 95% CI 0.32–1.42, p = 0.302 | HR 0.68, 95% CI 0.27–1.69, p = 0.411 | HR 0.93, 95% CI 0.23–3,70, p = 0.922 | HR 0.04, 95% CI 0.29–4.8, interaction p = 0.827 |
| Cardiovascular risk factors ** | HR 0.78, 95% CI 0.35–1.73, p = 0.554 | HR 0.70, 95% CI 0.28–1.75, p = 0.453 | HR 0.72, 95% CI 1.6–3.1, p = 0.663 | HR 8.1, 95% CI 1.55–10.72, interaction p = 0.004 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Krecak, I.; Lekovic, D.; Arsenovic, I.; Dabcevic, N.; Ivankovic, I.; Holik, H.; Zekanovic, I.; Moric Peric, M.; Matic, A.A.; Bogdanovic, A.; et al. Prevalence of Chronic Obstructive Pulmonary Disease and Asthma in Polycythemia Vera and Essential Thrombocythemia and Its Prognostic Implications. J. Clin. Med. 2025, 14, 8416. https://doi.org/10.3390/jcm14238416
Krecak I, Lekovic D, Arsenovic I, Dabcevic N, Ivankovic I, Holik H, Zekanovic I, Moric Peric M, Matic AA, Bogdanovic A, et al. Prevalence of Chronic Obstructive Pulmonary Disease and Asthma in Polycythemia Vera and Essential Thrombocythemia and Its Prognostic Implications. Journal of Clinical Medicine. 2025; 14(23):8416. https://doi.org/10.3390/jcm14238416
Chicago/Turabian StyleKrecak, Ivan, Danijela Lekovic, Isidora Arsenovic, Nina Dabcevic, Iva Ivankovic, Hrvoje Holik, Ivan Zekanovic, Martina Moric Peric, Andrea Anic Matic, Andrija Bogdanovic, and et al. 2025. "Prevalence of Chronic Obstructive Pulmonary Disease and Asthma in Polycythemia Vera and Essential Thrombocythemia and Its Prognostic Implications" Journal of Clinical Medicine 14, no. 23: 8416. https://doi.org/10.3390/jcm14238416
APA StyleKrecak, I., Lekovic, D., Arsenovic, I., Dabcevic, N., Ivankovic, I., Holik, H., Zekanovic, I., Moric Peric, M., Matic, A. A., Bogdanovic, A., Skelin, M., & Lucijanic, M. (2025). Prevalence of Chronic Obstructive Pulmonary Disease and Asthma in Polycythemia Vera and Essential Thrombocythemia and Its Prognostic Implications. Journal of Clinical Medicine, 14(23), 8416. https://doi.org/10.3390/jcm14238416






