Symptomatic Trends and Time to Recovery for Long COVID Patients Infected During the Omicron Phase
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design and Inclusion Criteria for Long COVID
2.2. Data Collection
2.3. Patient Classification and Data Analysis
2.4. Laboratory Examination
2.5. Assessment for Patients’ QOL and Mental Status
2.6. Statistical Analysis
2.7. Ethical Consideration
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
References
- Ely, E.W.; Brown, L.M.; Fineberg, H.V. National Academies of Sciences, Engineering; Medicine Committee on Examining the Working Definition for Long Covid. Long Covid Defined. N. Engl. J. Med. 2024, 391, 1746–1753. [Google Scholar] [CrossRef] [PubMed]
- Munipalli, B.; Seim, L.; Dawson, N.L.; Knight, D.; Dabrh, A.M.A. Post-acute sequelae of COVID-19 (PASC): A meta-narrative review of pathophysiology, prevalence, and management. SN Compr. Clin. Med. 2022, 4, 90. [Google Scholar] [CrossRef]
- Soriano, J.B.; Murthy, S.; Marshall, J.C.; Relan, P.; Diaz, J.V. A clinical case definition of post-COVID-19 condition by a Delphi consensus. Lancet Infect. Dis. 2022, 22, e102–e107. [Google Scholar] [CrossRef]
- Davis, H.E.; McCorkell, L.; Vogel, J.M.; Topol, E.J. Long COVID: Major findings, mechanisms and recommendations. Nat. Rev. Microbiol. 2023, 21, 133–146. [Google Scholar] [CrossRef] [PubMed]
- Proal, A.D.; Aleman, S.; Bomsel, M.; Brodin, P.; Buggert, M.; Cherry, S.; Chertow, D.S.; Davies, H.E.; Dupont, C.L.; Deeks, S.G.; et al. Targeting the SARS-CoV-2 reservoir in long COVID. Lancet Infect. Dis. 2025, 25, e294–e306. [Google Scholar] [CrossRef]
- Peluso, M.J.; Deeks, S.G. Mechanisms of long COVID and the path toward therapeutics. Cell 2024, 187, 5500–5529. [Google Scholar] [CrossRef]
- Matsuda, Y.; Sakurada, Y.; Otsuka, Y.; Tokumasu, K.; Nakano, Y.; Sunada, N.; Honda, H.; Hasegawa, T.; Takase, R.; Omura, D.; et al. Changes in Working Situations of Employed Long COVID Patients: Retrospective Study in Japanese Outpatient Clinic. J. Clin. Med. 2024, 13, 3809. [Google Scholar] [CrossRef]
- Ballouz, T.; Menges, D.; Anagnostopoulos, A.; Domenghino, A.; Aschmann, H.E.; Frei, A.; Fehr, J.S.; Puhan, M.A. Recovery and symptom trajectories up to two years after SARS-CoV-2 infection: Population based, longitudinal cohort study. BMJ 2023, 381, e074425. [Google Scholar] [CrossRef] [PubMed]
- Canas, L.S.; Molteni, E.; Deng, J.; Sudre, C.H.; Murray, B.; Kerfoot, E.; Antonelli, M.; Rjoob, K.; Capdevila Pujol, J.; Polidori, L.; et al. Profiling post-COVID-19 condition across different variants of SARS-CoV-2: A prospective longitudinal study in unvaccinated wild-type, unvaccinated alpha-variant, and vaccinated delta-variant populations. Lancet Digit. Health 2023, 5, e421–e434. [Google Scholar] [CrossRef]
- Whitaker, M.; Elliott, J.; Chadeau-Hyam, M.; Riley, S.; Darzi, A.; Cooke, G.; Ward, H.; Elliott, P. Persistent COVID-19 symptoms in a community study of 606,434 people in England. Nat. Commun. 2022, 13, 1957. [Google Scholar] [CrossRef]
- Yang, X.; Hou, C.; Shen, Y.; Zhang, M.; Zhang, K.; Wang, F.; Liu, Y.; Ma, X.; Cheng, L.; Kang, J.; et al. Two-Year Health Outcomes in Hospitalized COVID-19 Survivors in China. JAMA Netw. Open 2022, 5, e2231790. [Google Scholar] [CrossRef]
- Global Burden of Disease Long COVID, Collaborators; Hanson, S.W.; Abbafati, C.; Aerts, J.G.; Al-Aly, Z.; Ashbaugh, C.; Ballouz, T.; Blyuss, O.; Bobkova, P.; Bonsel, G.; et al. Estimated Global Proportions of Individuals with Persistent Fatigue, Cognitive, and Respiratory Symptom Clusters Following Symptomatic COVID-19 in 2020 and 2021. JAMA 2022, 328, 1604–1615. [Google Scholar] [CrossRef] [PubMed]
- Meierkord, A.; Schulze, D.; Gertler, M.; Seybold, J.; Mall, M.A.; Kurth, T.; Mockenhaupt, F.P.; Theuring, S. Post-infection symptoms up to 24 months after COVID-19: A matched cohort study in Berlin, Germany. Front. Public Health 2025, 13, 1513664. [Google Scholar] [CrossRef]
- Al-Aly, Z.; Rosen, C.J. Long Covid and Impaired Cognition—More Evidence and More Work to Do. N. Engl. J. Med. 2024, 390, 858–860. [Google Scholar] [CrossRef] [PubMed]
- Tsampasian, V.; Elghazaly, H.; Chattopadhyay, R.; Debski, M.; Naing, T.K.P.; Garg, P.; Clark, A.; Ntatsaki, E.; Vassiliou, V.S. Risk Factors Associated with Post-COVID-19 Condition: A Systematic Review and Meta-analysis. JAMA Intern. Med. 2023, 183, 566–580. [Google Scholar] [CrossRef] [PubMed]
- Wong, M.C.; Huang, J.; Wong, Y.Y.; Wong, G.L.; Yip, T.C.; Chan, R.N.; Chau, S.W.; Ng, S.C.; Wing, Y.K.; Chan, F.K. Epidemiology, Symptomatology, and Risk Factors for Long COVID Symptoms: Population-Based, Multicenter Study. JMIR Public Health Surveill. 2023, 9, e42315. [Google Scholar] [CrossRef]
- Kamalakkannan, A.; Prgomet, M.; Thomas, J.; Pearce, C.; McGuire, P.; Mackintosh, F.; Georgiou, A. Factors associated with general practitioner-led diagnosis of long COVID: An observational study using electronic general practice data from Victoria and New South Wales, Australia. Med. J. Aust. 2024, 221 (Suppl. S9), S18–S22. [Google Scholar] [CrossRef]
- Dehlia, A.; Guthridge, M.A. The persistence of myalgic encephalomyelitis/chronic fatigue syndrome (ME/CFS) after SARS-CoV-2 infection: A systematic review and meta-analysis. J. Infect. 2024, 89, 106297. [Google Scholar] [CrossRef]
- Wood, M.S.; Halmer, N.; Bertolli, J.; Amsden, L.B.; Nugent, J.R.; Lin, J.S.; Rothrock, G.; Nadle, J.; Chai, S.J.; Cope, J.R.; et al. Impact of COVID-19 on myalgic encephalomyelitis/chronic fatigue syndrome-like illness prevalence: A cross-sectional survey. PLoS ONE 2024, 19, e0309810. [Google Scholar] [CrossRef]
- Vernon, S.D.; Zheng, T.; Do, H.; Marconi, V.C.; Jason, L.A.; Singer, N.G.; Natelson, B.H.; Sherif, Z.A.; Bonilla, H.F.; Taylor, E.; et al. Incidence and Prevalence of Post-COVID-19 Myalgic Encephalomyelitis: A Report from the Observational RECOVER-Adult Study. J. Gen. Intern. Med. 2025, 40, 1085–1094. [Google Scholar] [CrossRef]
- Morita, S.; Tokumasu, K.; Otsuka, Y.; Honda, H.; Nakano, Y.; Sunada, N.; Sakurada, Y.; Matsuda, Y.; Soejima, Y.; Ueda, K.; et al. Phase-dependent trends in the prevalence of myalgic encephalomyelitis/chronic fatigue syndrome (ME/CFS) related to long COVID: A criteria-based retrospective study in Japan. PLoS ONE 2024, 19, e0315385. [Google Scholar] [CrossRef] [PubMed]
- Kato, Y. Case Management of COVID-19 (Secondary Version). JMA J. 2021, 4, 191–197. [Google Scholar] [CrossRef]
- De Vries, J.; Michielsen, H.; Van Heck, G.L.; Drent, M. Measuring fatigue in sarcoidosis: The Fatigue Assessment Scale (FAS). Br. J. Health Psychol. 2004, 9, 279–291. [Google Scholar] [CrossRef]
- Tokumasu, K.; Matsuki, N.; Fujikawa, H.; Sakamoto, Y.; Otsuka, F. Reliability and Validity of the Japanese Version of the Fatigue Assessment Scale. Intern. Med. 2025, 64, 658–663. [Google Scholar] [CrossRef] [PubMed]
- Shiroiwa, T.; Ikeda, S.; Noto, S.; Igarashi, A.; Fukuda, T.; Saito, S.; Shimozuma, K. Comparison of Value Set Based on DCE and/or TTO Data: Scoring for EQ-5D-5L Health States in Japan. Value Health 2016, 19, 648–654. [Google Scholar] [CrossRef] [PubMed]
- Zung, W.W. A Self-Rating Depression Scale. Arch. Gen. Psychiatry 1965, 12, 63–70. [Google Scholar] [CrossRef]
- ISO 15189; Medical Laboratories. Japan Accreditation Board (JAB): Tokyo, Japan, 2016.
- Yamamoto, Y.; Otsuka, Y.; Tokumasu, K.; Sunada, N.; Nakano, Y.; Honda, H.; Sakurada, Y.; Hasegawa, T.; Hagiya, H.; Otsuka, F. Utility of Serum Ferritin for Predicting Myalgic Encephalomyelitis/Chronic Fatigue Syndrome in Patients with Long COVID. J. Clin. Med. 2023, 12, 4737. [Google Scholar] [CrossRef]
- Demko, Z.O.; Yu, T.; Mullapudi, S.K.; Varela Heslin, M.G.; Dorsey, C.A.; Payton, C.B.; Tornheim, J.A.; Blair, P.W.; Mehta, S.H.; Thomas, D.L.; et al. Two-Year Longitudinal Study Reveals That Long COVID Symptoms Peak and Quality of Life Nadirs at 6–12 Months Postinfection. Open Forum Infect. Dis. 2024, 11, ofae027. [Google Scholar] [CrossRef]
- Davis, H.E.; Assaf, G.S.; McCorkell, L.; Wei, H.; Low, R.J.; Re’em, Y.; Redfield, S.; Austin, J.P.; Akrami, A. Characterizing long COVID in an international cohort: 7 months of symptoms and their impact. EClinicalMedicine 2021, 38, 101019. [Google Scholar] [CrossRef]
- Perlis, R.H.; Santillana, M.; Ognyanova, K.; Safarpour, A.; Lunz Trujillo, K.; Simonson, M.D.; Green, J.; Quintana, A.; Druckman, J.; Baum, M.A.; et al. Prevalence and Correlates of Long COVID Symptoms Among US Adults. JAMA Netw. Open 2022, 5, e2238804. [Google Scholar] [CrossRef]
- Fischer, A.; Zhang, L.; Elbeji, A.; Wilmes, P.; Snoeck, C.J.; Larche, J.; Oustric, P.; Ollert, M.; Fagherazzi, G. Trajectories of persisting COVID-19 symptoms up to 24 months after acute infection: Findings from the Predi-Covid cohort study. BMC Infect. Dis. 2025, 25, 603. [Google Scholar] [CrossRef]
- Sudre, C.H.; Murray, B.; Varsavsky, T.; Graham, M.S.; Penfold, R.S.; Bowyer, R.C.; Pujol, J.C.; Klaser, K.; Antonelli, M.; Canas, L.S.; et al. Attributes and predictors of long COVID. Nat. Med. 2021, 27, 626–631. [Google Scholar] [CrossRef]
- Sakurada, Y.; Matsuda, Y.; Motohashi, K.; Hasegawa, T.; Otsuka, Y.; Nakano, Y.; Tokumasu, K.; Yamamoto, K.; Sunada, N.; Honda, H.; et al. Clinical characteristics of female long COVID patients with menstrual symptoms: A retrospective study from a Japanese outpatient clinic. J. Psychosom. Obstet. Gynaecol. 2024, 45, 2305899. [Google Scholar] [CrossRef]
- Wong, A.C.; Devason, A.S.; Umana, I.C.; Cox, T.O.; Dohnalova, L.; Litichevskiy, L.; Perla, J.; Lundgren, P.; Etwebi, Z.; Izzo, L.T.; et al. Serotonin reduction in post-acute sequelae of viral infection. Cell 2023, 186, 4851–4867.e20. [Google Scholar] [CrossRef] [PubMed]
- Eugene, D.; Nothling, J.; Tarsitani, L.; Palantza, C.; Papola, D.; Barbui, C.; Bryant, R.; Panter-Brick, C.; Hall, B.J.; Lam, A.I.F.; et al. Mental health during the COVID-19 pandemic: An international comparison of gender-related home and work-related responsibilities, and social support. Arch. Womens Ment. Health 2025, 28, 359–374. [Google Scholar] [CrossRef]
- Flor, L.S.; Friedman, J.; Spencer, C.N.; Cagney, J.; Arrieta, A.; Herbert, M.E.; Stein, C.; Mullany, E.C.; Hon, J.; Patwardhan, V.; et al. Quantifying the effects of the COVID-19 pandemic on gender equality on health, social, and economic indicators: A comprehensive review of data from March, 2020, to September, 2021. Lancet 2022, 399, 2381–2397. [Google Scholar] [CrossRef]
- Lei, L.; Li, H.; Yan, F.; Xiao, Y. Hyperlipidemia impaired innate immune response to periodontal pathogen porphyromonas gingivalis in apolipoprotein E knockout mice. PLoS ONE 2013, 8, e71849. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; Lin, G.; You, X.; Lei, L.; Li, Y.; Lin, M.; Luo, K.; Yan, F. Hyperlipidemia causes changes in inflammatory responses to periodontal pathogen challenge: Implications in acute and chronic infections. Arch. Oral. Biol. 2014, 59, 1075–1084. [Google Scholar] [CrossRef]
- Kim, D.; Chung, H.; Lee, J.E.; Kim, J.; Hwang, J.; Chung, Y. Immunologic Aspects of Dyslipidemia: A Critical Regulator of Adaptive Immunity and Immune Disorders. J. Lipid Atheroscler. 2021, 10, 184–201. [Google Scholar] [CrossRef] [PubMed]
- Xu, E.; Xie, Y.; Al-Aly, Z. Risks and burdens of incident dyslipidaemia in long COVID: A cohort study. Lancet Diabetes Endocrinol. 2023, 11, 120–128. [Google Scholar] [CrossRef]
- Fujita, K.; Otsuka, Y.; Sunada, N.; Honda, H.; Tokumasu, K.; Nakano, Y.; Sakurada, Y.; Obika, M.; Hagiya, H.; Otsuka, F. Manifestation of Headache Affecting Quality of Life in Long COVID Patients. J. Clin. Med. 2023, 12, 3533. [Google Scholar] [CrossRef] [PubMed]
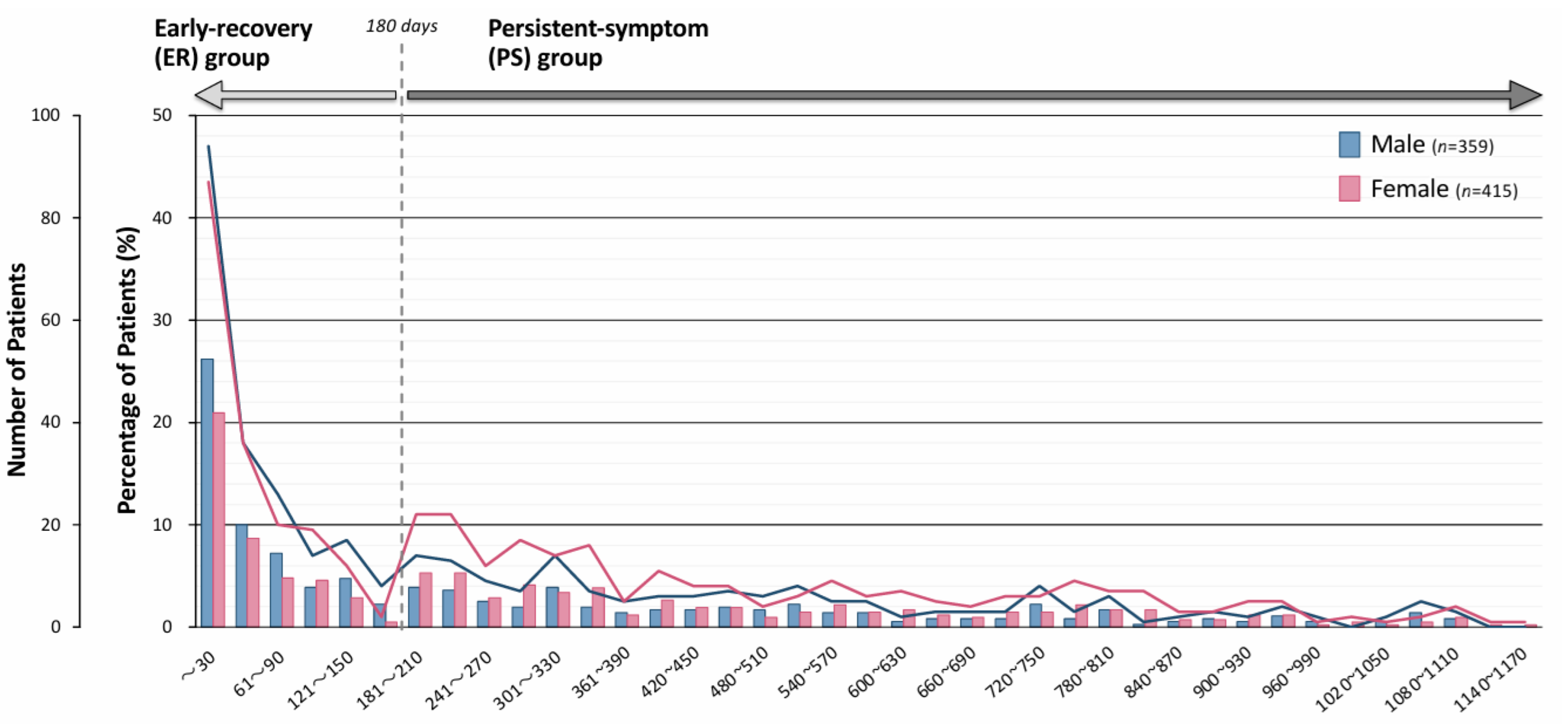
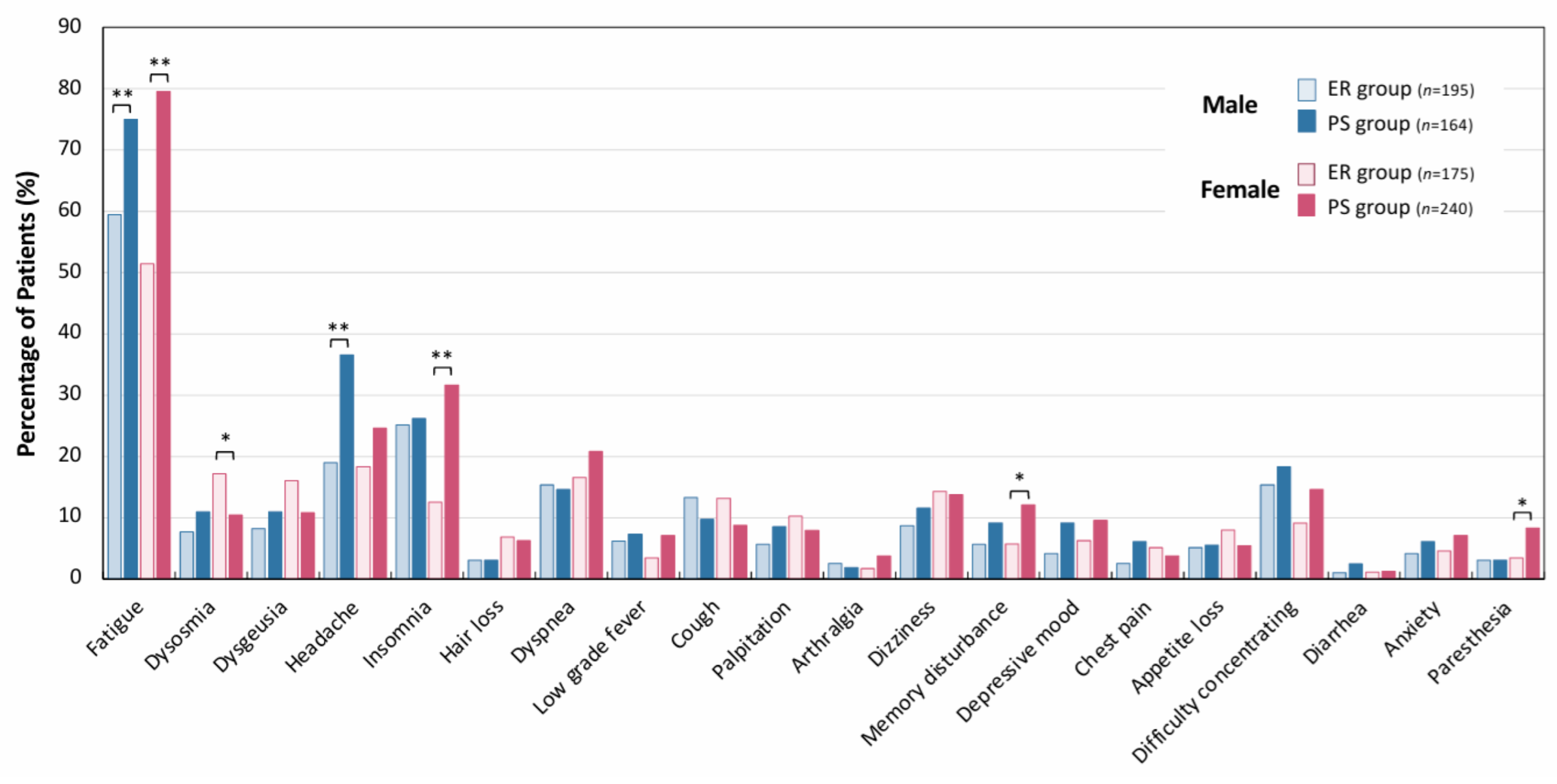
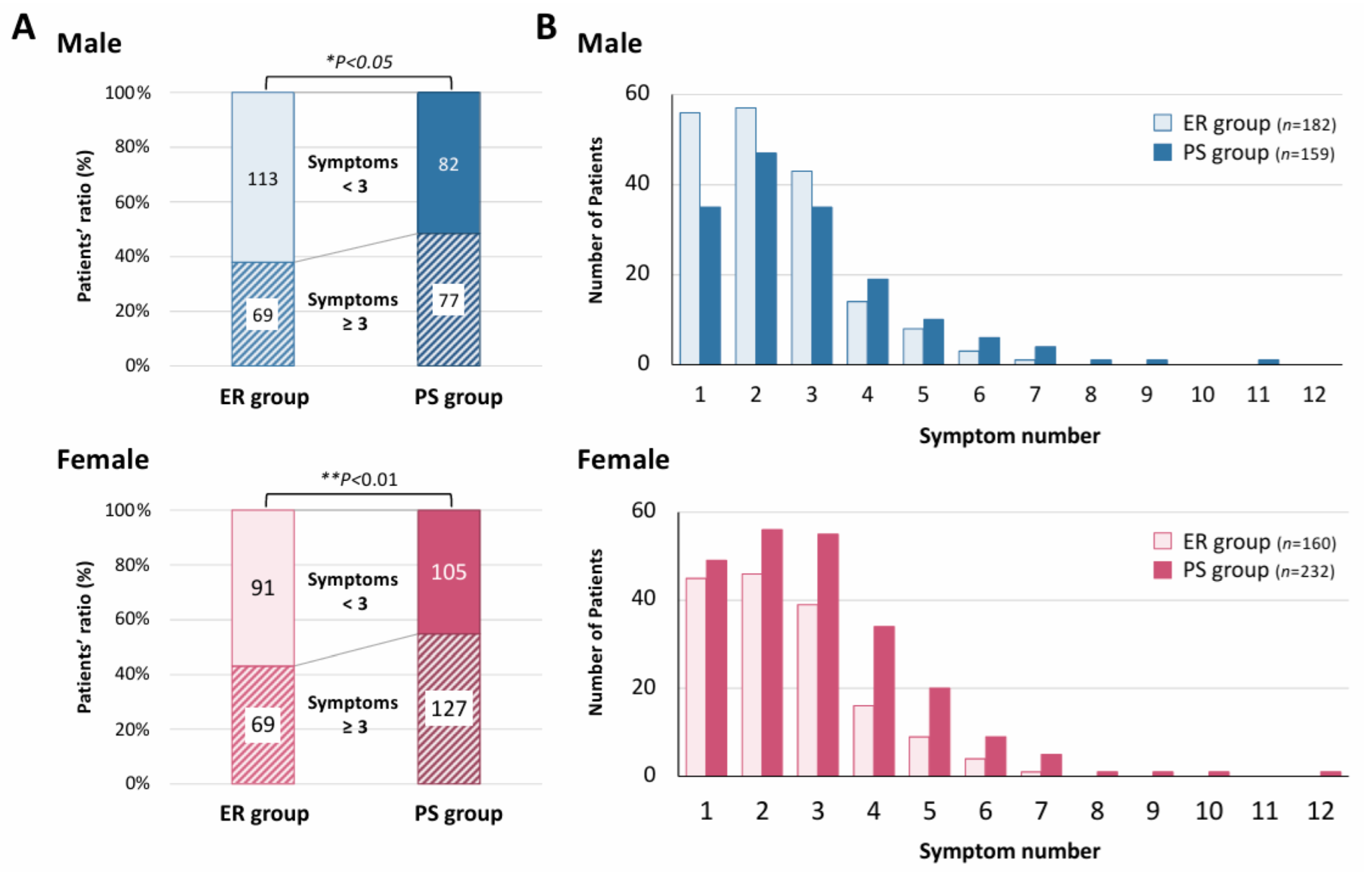

| Duration of Outpatient Treatment | Early Recovery (ER) Group (n = 370) | Persistent-Symptom (PS) Group (n = 404) | p-Value | |||
|---|---|---|---|---|---|---|
| Duration (median days, [IQR]) | 33 | [1–72] | 437 | [280–707.5] | <0.01 (a)** | |
| Number of patients (%) | 370 | (47.8) | 404 | (52.2) | ||
| Age years, median [IQR] | 40 | [24–53] | 42 | [28–52] | 0.4055 (a) | |
| Gender, n (%) | ||||||
| Male | 195 | (52.7) | 164 | (40.6) | <0.01 (b)** | |
| Female | 175 | (47.3) | 240 | (59.4) | ||
| Interval (infection to the visit), median [IQR] | 103 | [65–167] | 99 | [62–189] | 0.8824 (a) | |
| Smoking habits, n (%) | 89 | (24.3) | 107 | (26.8) | 0.626 (b) | |
| Alcohol habits, n (%) | 100 | (27.4) | 90 | (22.6) | 0.127 (b) | |
| BMI, median [IQR] | 22.5 | [20.0–25.8] | 21.7 | [19.4–25.3] | 0.1215 (a) | |
| Severity of acute condition, n (%) | ||||||
| Mild (%) | 360 | (97.3) | 393 | (97.3) | 0.997 (b) | |
| Moderate (%) | 9 | (2.4) | 10 | (2.5) | ||
| Severe (%) | 1 | (0.2) | 1 | (0.25) | ||
| Vaccinations, number (%) | ||||||
| 0 (%) | 82 | (22.6) | 95 | (23.7) | 0.719 (b) | |
| ≥1~7 (%) | 281 | (77.4) | 306 | (76.3) | ||
| 0~1 (%) | 84 | (23.1) | 100 | (24.9) | 0.562 (b) | |
| ≥2~7 (%) | 279 | (76.9) | 301 | (75.1) | ||
| 0~2 (%) | 192 | (52.9) | 200 | (49.9) | 0.405 (b) | |
| ≥3~7 (%) | 171 | (47.1) | 201 | (50.1) | ||
| 0~3 (%) | 287 | (79.1) | 312 | (77.8) | 0.673 (b) | |
| ≥4~7 (%) | 76 | (20.9) | 89 | (22.2) | ||
| Male Patients | (ER) Group (n = 195) | (PS) Group (n = 164) | p-Value | |||
|---|---|---|---|---|---|---|
| Reference Range | Median [IQR] | n | Median [IQR] | n | ||
| Hb (g/dL) | 13.7–16.8 | 15.4 [14.7–16.0] | 160 | 15.4 [14.4–16.1] | 158 | 0.4276 |
| Alb (g/dL) | 4.1–5.1 | 4.6 [4.3–4.8] | 156 | 4.6 [4.3–4.8] | 157 | 0.9530 |
| AST (U/L) | 13–30 | 19 [16–25] | 159 | 21 [17–27] | 158 | 0.1730 |
| ALT (U/L) | 10–42 | 21 [14–31] | 159 | 24 [16–40] | 158 | 0.0599 |
| CRE (mg/dL) | 0.65–1.07 | 0.84 [0.76–0.92] | 159 | 0.82 [0.75–0.91] | 158 | 0.4294 |
| LDL-C (mg/dL) | 65–163 | 111 [92–136] | 148 | 124 [100–151] | 151 | <0.01 ** |
| Ferritin (ng/mL) | 39.9–465 | 200 [117–308] | 157 | 210 [129–338] | 158 | 0.6949 |
| CRP (mg/dL) | <0.15 | 0.05 [0.02–0.11] | 161 | 0.06 [0.03–0.13] | 158 | 0.0776 |
| TSH (mIU/L) | 0.61–4.23 | 1.52 [1.00–2.09] | 158 | 1.48 [0.97–2.29] | 152 | 0.8791 |
| FT4 (ng/dL) | 0.97–1.69 | 1.35 [1.22–1.48] | 158 | 1.3 [1.16–1.46] | 152 | 0.1252 |
| ACTH (pg/mL) | 7.2–63.3 | 25.8 [19.1–38.9] | 155 | 23.7 [17.9–33.6] | 155 | 0.0952 |
| Cortisol (μg/dL) | 7.1–19.6 | 7.4 [5.2–11.0] | 155 | 7.2 [5.1–10.1] | 155 | 0.5111 |
| Female Patients | ER Group (n = 175) | PS Group (n = 240) | p-Value | |||
| Reference range | Median [IQR] | n | Median [IQR] | n | ||
| Hb (g/dL) | 11.6–14.8 | 13.55 [12.9–14.3] | 142 | 13.50 [12.8–14.1] | 221 | 0.2225 |
| Alb (g/dL) | 4.1–5.1 | 4.4 [4.2–4.6] | 138 | 4.4 [4.2–4.6] | 221 | 0.7997 |
| AST (U/L) | 13–30 | 18 [15–23] | 140 | 18 [15–21] | 220 | 0.6029 |
| ALT (U/L) | 7–23 | 16 [11–23] | 140 | 14 [11–21] | 220 | 0.1753 |
| CRE (mg/dL) | 0.65–1.07 | 0.62 [0.56–0.72] | 139 | 0.60 [0.54–0.67] | 220 | <0.01 ** |
| LDL-C (mg/dL) | 65–163 | 111 [92–135] | 131 | 120 [97–143] | 212 | <0.05 * |
| Ferritin (ng/mL) | 6.2–138 | 71.4 [36.9–150] | 137 | 67.9 [29.0–125] | 220 | 0.1332 |
| CRP (mg/dL) | <0.15 | 0.05 [0.02–0.16] | 141 | 0.05 [0.02–0.10] | 221 | 0.6109 |
| TSH (mIU/L) | 0.61–4.23 | 1.42 [0.89–2.24] | 136 | 1.52 [1.04–2.07] | 218 | 0.8126 |
| FT4 (ng/dL) | 0.97–1.69 | 1.22 [1.105–1.36] | 136 | 1.28 [1.13–1.38] | 218 | 0.4130 |
| ACTH (pg/mL) | 7.2–63.3 | 18.2 [11.5–26.0] | 138 | 16.4 [12.0–22.9] | 220 | 0.1640 |
| Cortisol (μg/dL) | 7.1–19.6 | 8.1 [5.7–10.7] | 138 | 7.3 [5.4–10.1] | 220 | 0.2564 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Akiyama, H.; Sakurada, Y.; Honda, H.; Matsuda, Y.; Otsuka, Y.; Tokumasu, K.; Nakano, Y.; Takase, R.; Omura, D.; Ueda, K.; et al. Symptomatic Trends and Time to Recovery for Long COVID Patients Infected During the Omicron Phase. J. Clin. Med. 2025, 14, 4918. https://doi.org/10.3390/jcm14144918
Akiyama H, Sakurada Y, Honda H, Matsuda Y, Otsuka Y, Tokumasu K, Nakano Y, Takase R, Omura D, Ueda K, et al. Symptomatic Trends and Time to Recovery for Long COVID Patients Infected During the Omicron Phase. Journal of Clinical Medicine. 2025; 14(14):4918. https://doi.org/10.3390/jcm14144918
Chicago/Turabian StyleAkiyama, Hiroshi, Yasue Sakurada, Hiroyuki Honda, Yui Matsuda, Yuki Otsuka, Kazuki Tokumasu, Yasuhiro Nakano, Ryosuke Takase, Daisuke Omura, Keigo Ueda, and et al. 2025. "Symptomatic Trends and Time to Recovery for Long COVID Patients Infected During the Omicron Phase" Journal of Clinical Medicine 14, no. 14: 4918. https://doi.org/10.3390/jcm14144918
APA StyleAkiyama, H., Sakurada, Y., Honda, H., Matsuda, Y., Otsuka, Y., Tokumasu, K., Nakano, Y., Takase, R., Omura, D., Ueda, K., & Otsuka, F. (2025). Symptomatic Trends and Time to Recovery for Long COVID Patients Infected During the Omicron Phase. Journal of Clinical Medicine, 14(14), 4918. https://doi.org/10.3390/jcm14144918






