Left Ventricle Phenotyping Utilizing Tissue Doppler Imaging in Premature Infants with Varying Severity of Bronchopulmonary Dysplasia
Abstract
:1. Introduction
2. Experimental Section
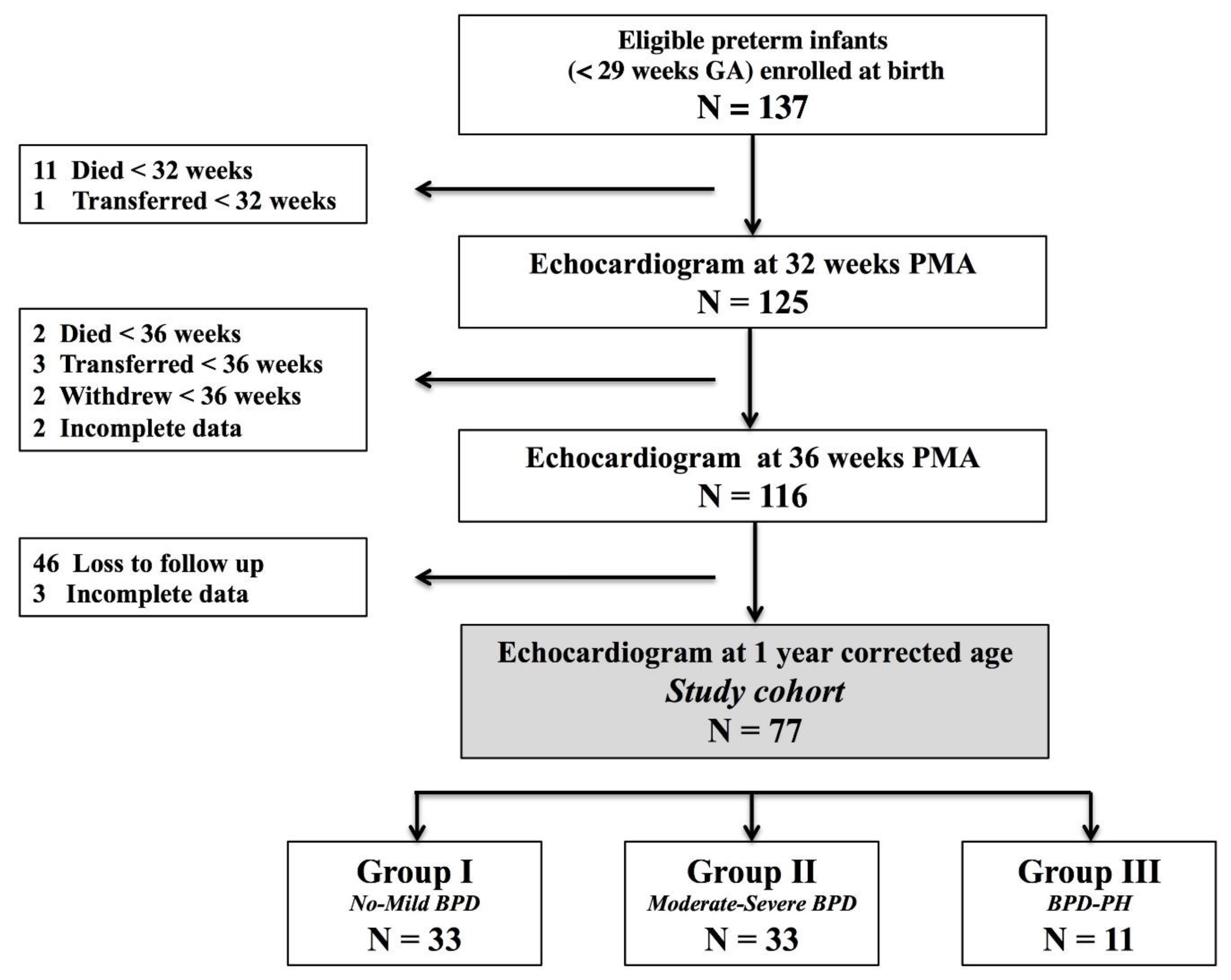
2.1. Design and Population
2.2. Patient Characteristics
2.3. Primary Exposure of BPD-PH
2.4. Echocardiography
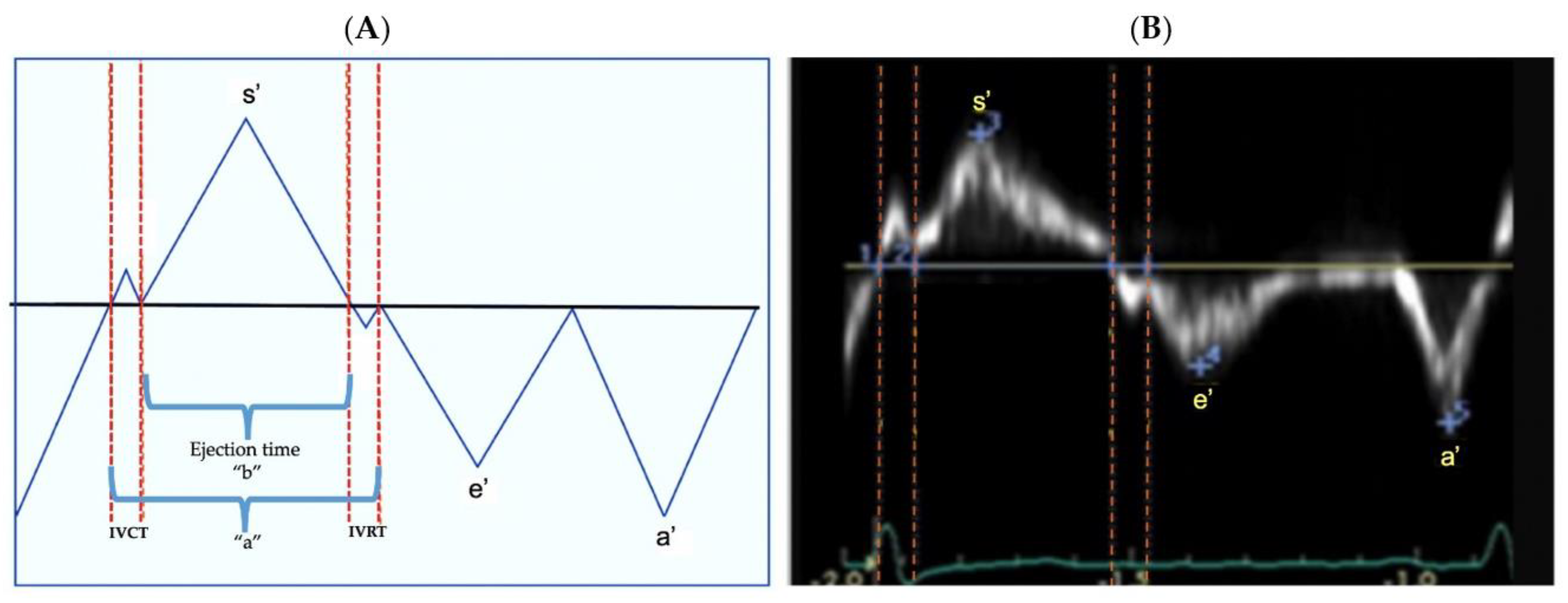
2.5. Tissue Doppler Imaging
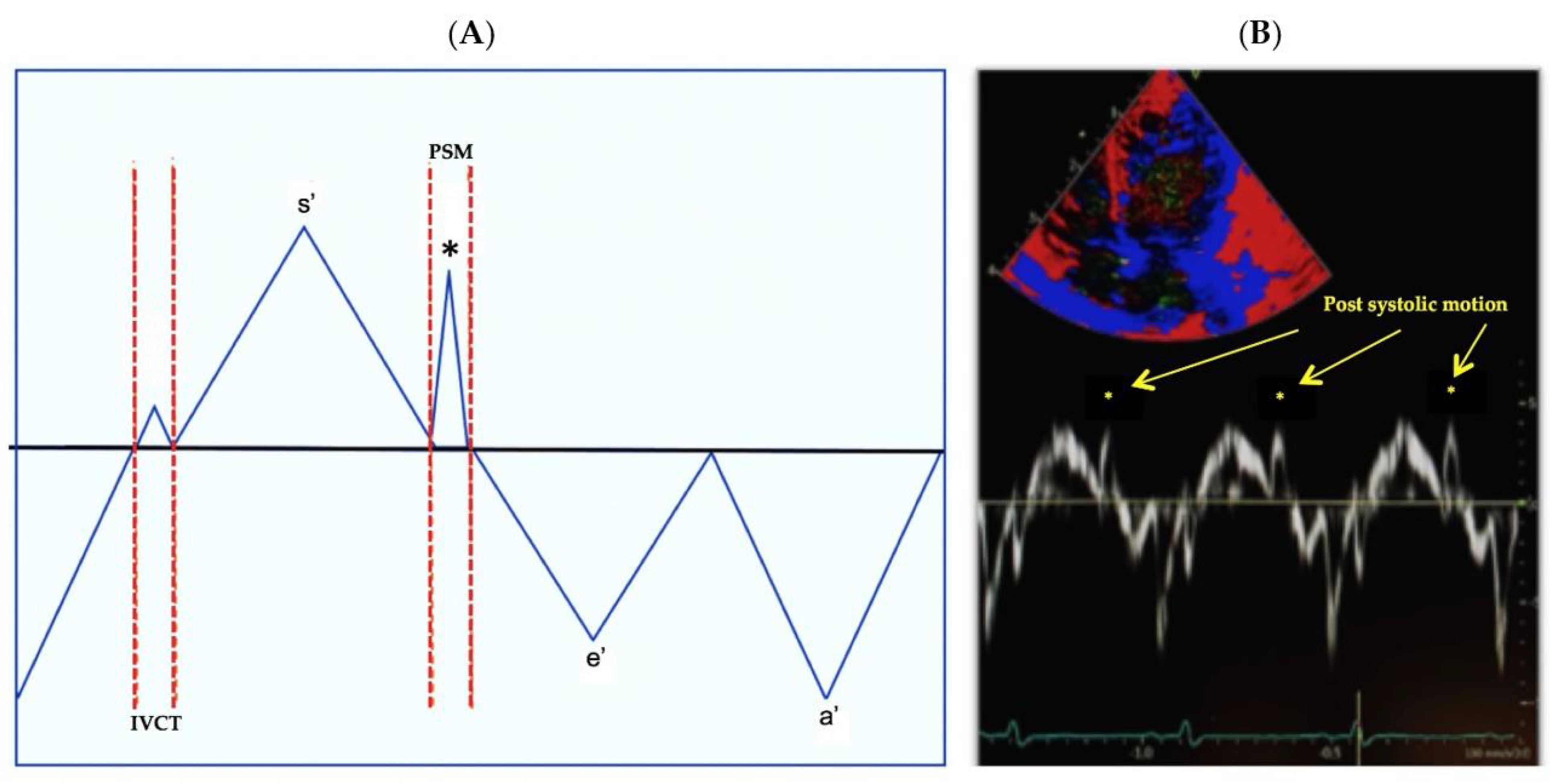
2.6. Main Outcome: Post Systolic Motion (PSM)
2.7. Statistical Analysis
3. Results
3.1. Patient Characteristics
3.2. Assessment of LV Tissue Doppler Indices
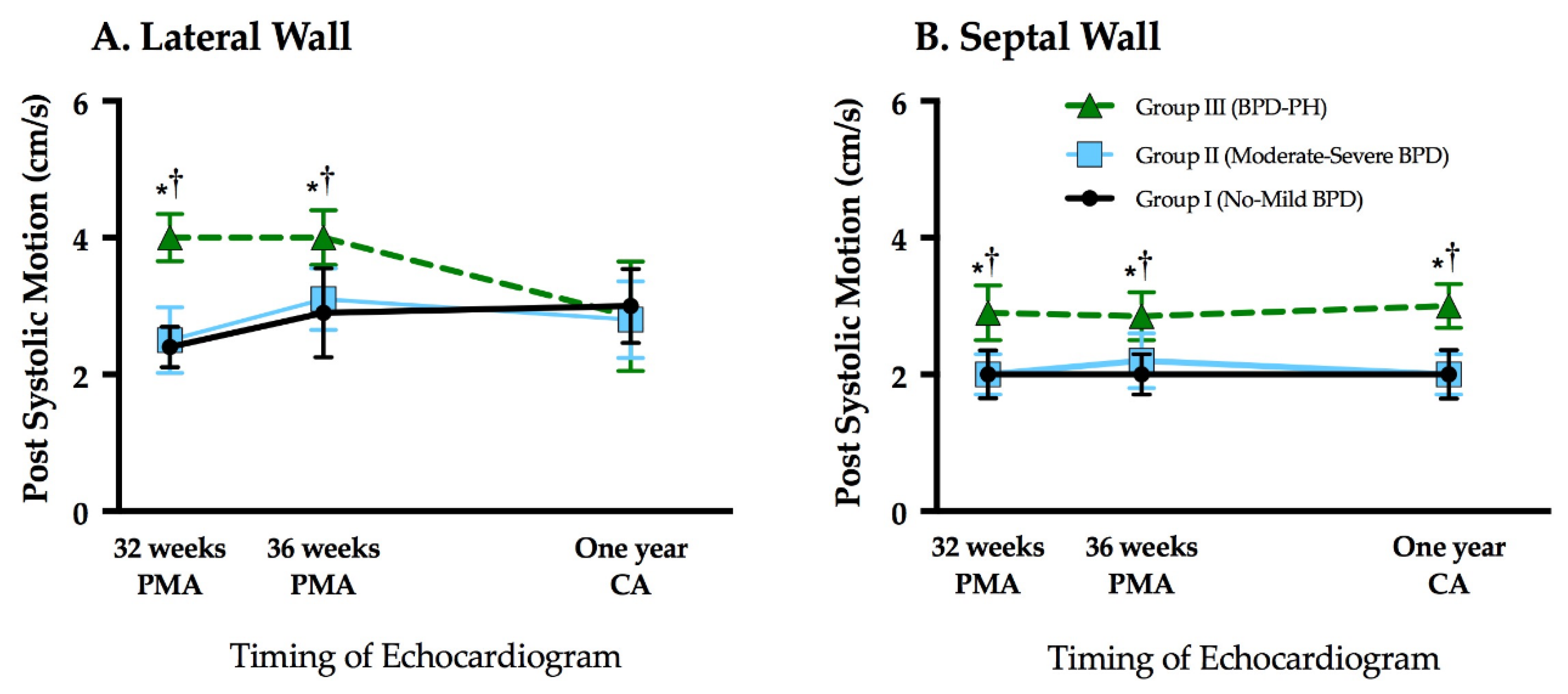
3.3. Assessment of Post Systolic Motion
3.4. Comparison of Tissue Doppler Indices between Term and Preterm Cohorts
3.5. Influence of Patent Ductus Arteriosus
4. Discussion
4.1. PSM in Neonates
4.2. Ventricular-Ventricular Interactions
4.3. TDI Measures at One Year of Age
4.4. Clinical Significance of PSM Evaluation in Neonates
4.5. Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Bensley, J.G.; Moore, L.; De Matteo, R.; Harding, R.; Black, M.J. Impact of preterm birth on the developing myocardium of the neonate. Pediatr. Res. 2018, 83, 880–888. [Google Scholar] [CrossRef] [PubMed]
- Aye, C.Y.L.; Lewandowski, A.J.; Lamata, P.; Upton, R.; Davis, E.; Ohuma, E.O.; Kenworthy, Y.; Boardman, H.; Wopperer, S.; Packham, A.; et al. Disproportionate cardiac hypertrophy during early postnatal development in infants born preterm. Pediatr. Res. 2017, 82, 36–46. [Google Scholar] [CrossRef] [PubMed]
- Burchert, H.; Lewandowski, A.J. Preterm Birth Is a Novel, Independent Risk Factor for Altered Cardiac Remodeling and Early Heart Failure: Is it Time for a New Cardiomyopathy? Curr. Treat. Options Cardiovasc. Med. 2019, 21, 8. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stoll, B.J.; Hansen, N.I.; Bell, E.F.; Walsh, M.C.; Carlo, W.A.; Shankaran, S.; Laptook, A.R.; Sánchez, P.J.; Van Meurs, K.P.; Wyckoff, M.H.; et al. Trends in Care Practices, Morbidity, and Mortality of Extremely Preterm Neonates, 1993–2012. JAMA 2015, 314, 1039–1051. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Northway, W.H.; Rosan, R.C.; Porter, D.Y. Pulmonary Disease Following Respirator Therapy of Hyaline-Membrane Disease. N. Engl. J. Med. 1967, 276, 357–368. [Google Scholar] [CrossRef]
- Arjaans, S.; Zwart, E.A.H.; Ploegstra, M.; Bos, A.F.; Kooi, E.M.W.; Hillege, H.L.; Berger, R.M.F. Identification of gaps in the current knowledge on pulmonary hypertension in extremely preterm infants: A systematic review and meta-analysis. Paediatr. Perinat. Epidemiol. 2018, 32, 258–267. [Google Scholar] [CrossRef]
- Mohlkert, L.; Hallberg, J.; Broberg, O.; Rydberg, A.; Halvorsen, C.P.; Liuba, P.; Fellman, V.; Domellöf, M.; Sjöberg, G.; Norman, M. The Preterm Heart in Childhood: Left Ventricular Structure, Geometry, and Function Assessed by Echocardiography in 6-Year-Old Survivors of Periviable Births. J. Am. Hearth Assoc. 2018, 7, e007742. [Google Scholar] [CrossRef] [Green Version]
- Bensley, J.G.; Stacy, V.K.; De Matteo, R.; Harding, R.; Black, M.J. Cardiac remodelling as a result of pre-term birth: Implications for future cardiovascular disease. Eur. Hearth J. 2010, 31, 2058–2066. [Google Scholar] [CrossRef]
- Carr, H.; Cnattingius, S.; Granath, F.; Ludvigsson, J.F.; Bonamy, A.-K.E. Preterm Birth and Risk of Heart Failure Up to Early Adulthood. J. Am. Coll. Cardiol. 2017, 69, 2634–2642. [Google Scholar] [CrossRef]
- Crump, C.; Howell, E.A.; Stroustrup, A.; McLaughlin, M.A.; Sundquist, J.; Sundquist, K. Association of Preterm Birth with Risk of Ischemic Heart Disease in Adulthood. JAMA Pediatr. 2019, 173, 736–743. [Google Scholar] [CrossRef]
- Celutkiene, J.; Sutherland, G.R.; Laucevicius, A.; Zakarkaite, D.; Rudys, A.; Grabauskiene, V. Is post-systolic motion the optimal ultrasound parameter to detect induced ischaemia during dobutamine stress echocardiography? Eur. Hearth J. 2004, 25, 932–942. [Google Scholar] [CrossRef]
- Citro, R.; Galderisi, M. Myocardial Postsystolic Motion in Ischemic and Not Ischemic Myocardium: The Clinical Value of Tissue Doppler. Echocardiography 2005, 22, 525–532. [Google Scholar] [CrossRef]
- Breatnach, C.R.; Levy, P.T.; James, A.T.; Franklin, O.; El-Khuffash, A. Novel Echocardiography Methods in the Functional Assessment of the Newborn Heart. Neonatology 2016, 110, 248–260. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yates, A.R.; Welty, S.E.; Gest, A.L.; Cua, C.L. Myocardial Tissue Doppler Changes in Patients with Bronchopulmonary Dysplasia. J. Pediatr. 2008, 152, 766–770.e1. [Google Scholar] [CrossRef] [PubMed]
- Yajamanyam, P.K.; Negrine, R.J.S.; Rasiah, S.V.; Zamora, J.; Ewer, A.K. Assessment of myocardial function in preterm infants with chronic lung disease using tissue Doppler imaging. Arch. Dis. Child. Fetal Neonatal Ed. 2016, 101, F527–F532. [Google Scholar] [CrossRef]
- Méndez-Abad, P.; Rodríguez, P.Z.; Lubián-López, S.; Benavente-Fernández, I. Myocardial Function Maturation in Very-Low-Birth-Weight Infants and Development of Bronchopulmonary Dysplasia. Front. Pediatr. 2020, 7. [Google Scholar] [CrossRef] [PubMed]
- Lehmann, G.C.; Levy, P.T.; Patel, M.D.; Sekarski, T.; Gu, H.; Choudhry, S.; Hamvas, A.; Singh, G.K. Maturational patterns of left ventricular rotational mechanics in pre-term infants through 1 year of age. Cardiol. Young 2020, 30, 1–9. [Google Scholar] [CrossRef]
- Levy, P.T.; El-Khuffash, A.; Patel, M.D.; Breatnach, C.R.; James, A.T.; Sanchez, A.A.; Abuchabe, C.; Rogal, S.R.; Holland, M.R.; McNamara, P.J.; et al. Maturational Patterns of Systolic Ventricular Deformation Mechanics by Two-Dimensional Speckle-Tracking Echocardiography in Preterm Infants over the First Year of Age. J. Am. Soc. Echocardiogr. 2017, 30, 685–698.e1. [Google Scholar] [CrossRef]
- Pryhuber, G.S.; Maitre, N.L.; Ballard, R.A.; Cifelli, D.; Davis, S.D.; Ellenberg, J.H.; Greenberg, J.M.; Kemp, J.; Mariani, T.J.; Panitch, H.; et al. Prematurity and respiratory outcomes program (PROP): Study protocol of a prospective multicenter study of respiratory outcomes of preterm infants in the United States. BMC Pediatr. 2015, 15, 37. [Google Scholar] [CrossRef] [Green Version]
- Keller, R.L.; Feng, R.; DeMauro, S.B.; Ferkol, T.; Hardie, W.; Rogers, E.E.; Stevens, T.P.; Voynow, J.A.; Bellamy, S.L.; Shaw, P.A.; et al. Prematurity and Respiratory Outcomes Program. Bronchopulmonary Dysplasia and Perinatal Characteristics Predict 1-Year Respiratory Outcomes in Newborns Born at Extremely Low Gestational Age: A Prospective Cohort Study. J. Pediatr. 2017, 187, 89–97. [Google Scholar] [CrossRef]
- Papile, L.-A.; Burstein, J.; Burstein, R.; Koffler, H. Incidence and evolution of subependymal and intraventricular hemorrhage: A study of infants with birth weights less than 1500 gm. J. Pediatr. 1978, 92, 529–534. [Google Scholar] [CrossRef]
- Higgins, R.D.; Jobe, A.H.; Koso-Thomas, M.; Bancalari, E.; Viscardi, R.M.; Hartert, T.V.; Ryan, R.M.; Kallapur, S.G.; Steinhorn, R.H.; Konduri, G.G.; et al. Bronchopulmonary Dysplasia: Executive Summary of a Workshop. J. Pediatr. 2018, 197, 300–308. [Google Scholar] [CrossRef] [PubMed]
- Poindexter, B.B.; Feng, R.; Schmidt, B.; Aschner, J.L.; Ballard, R.A.; Hamvas, A.; Reynolds, A.M.; Shaw, P.A.; Jobe, A.H. Comparisons and Limitations of Current Definitions of Bronchopulmonary Dysplasia for the Prematurity and Respiratory Outcomes Program. Ann. Am. Thorac. Soc. 2015, 12, 1822–1830. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mourani, P.M.; Sontag, M.K.; Younoszai, A.; Miller, J.I.; Kinsella, J.P.; Baker, C.D.; Poindexter, B.B.; Ingram, D.A.; Abman, S.H. Early Pulmonary Vascular Disease in Preterm Infants at Risk for Bronchopulmonary Dysplasia. Am. J. Respir. Crit. Care Med. 2015, 191, 87–95. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Levy, P.T.; Patel, M.D.; Choudhry, S.; Hamvas, A.; Singh, G.K. Evidence of Echocardiographic Markers of Pulmonary Vascular Disease in Asymptomatic Infants Born Preterm at One Year of Age. J. Pediatr. 2018, 197, 48–56. [Google Scholar] [CrossRef] [PubMed]
- Lang, R.M.; Badano, L.P.; Mor-Avi, V.; Afilalo, J.; Armstrong, A.; Ernande, L.; Flachskampf, F.A.; Foster, E.; Goldstein, S.A.; Kuznetsova, T.; et al. Recommendations for Cardiac Chamber Quantification by Echocardiography in Adults: An Update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. J. Am. Soc. Echocardiogr. 2015, 28, 1–39. [Google Scholar] [CrossRef] [Green Version]
- Bussmann, N.; Breatnach, C.; Levy, P.T.; McCallion, N.; Franklin, O.; El-Khuffash, A. Early diastolic dysfunction and respiratory morbidity in premature infants: An observational study. J. Perinatol. 2018, 38, 1205–1211. [Google Scholar] [CrossRef]
- Tei, C.; Ling, L.H.; Hodge, D.O.; Bailey, K.R.; Oh, J.K.; Rodeheffer, R.J.; Tajik, A.J.; Seward, J.B. New index of combined systolic and diastolic myocardial performance: A simple and reproducible measure of cardiac function—A study in normals and dilated cardiomyopathy. J. Cardiol. 1995, 26, 357–366. [Google Scholar]
- Roberson, D.A.; Cui, W. Right Ventricular Tei Index in Children: Effect of Method, Age, Body Surface Area, and Heart Rate. J. Am. Soc. Echocardiogr. 2007, 20, 764–770. [Google Scholar] [CrossRef]
- Galderisi, M.; Cicala, S.; Sangiorgi, G.; Caso, P.; De Divitiis, O. Tissue Doppler-Derived Postsystolic Motion in a Patient with Left Bundle Branch Block: A Sign of Myocardial Wall Asynchrony. Echocardiography 2002, 19, 79–81. [Google Scholar] [CrossRef]
- Buckberg, G.; Hoffman, J.I.E.; Mahajan, A.; Saleh, S.; Coghlan, C. Cardiac mechanics revisited: The relationship of cardiac architecture to ventricular function. Circulation 2008, 118, 2571–2587. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Breatnach, C.R.; Forman, E.; Foran, A.; Monteith, C.; McSweeney, L.; Malone, F.; McCallion, N.; Franklin, O.; El-Khuffash, A. Left ventricular rotational mechanics in infants with hypoxic ischemic encephalopathy and preterm infants at 36 weeks postmenstrual age: A comparison with healthy term controls. Echocardiography 2017, 34, 232–239. [Google Scholar] [CrossRef] [PubMed]
- Patel, N.; Mills, J.F.; Cheung, M.M.H. Assessment of Right Ventricular Function Using Tissue Doppler Imaging in Infants with Pulmonary Hypertension. Neonatology 2009, 96, 193–199. [Google Scholar] [CrossRef]
- Streeter, D.D.; Spotnitz, H.M.; Patel, D.P.; Ross, J.; Sonnenblick, E.H. Fiber Orientation in the Canine Left Ventricle during Diastole and Systole. Circ. Res. 1969, 24, 339–347. [Google Scholar] [CrossRef] [Green Version]
- Di Maria, M.V.; Younoszai, A.K.; Sontag, M.K.; Miller, J.I.; Poindexter, B.B.; Ingram, D.A.; Abman, S.H.; Mourani, P.M. Maturational Changes in Diastolic Longitudinal Myocardial Velocity in Preterm Infants. J. Am. Soc. Echocardiogr. 2015, 28, 1045–1052. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lewandowski, A.J.; Bradlow, W.M.; Augustine, D.; Davis, E.F.; Francis, J.; Singhal, A.; Lucas, A.; Neubauer, S.; McCormick, K.; Leeson, P. Right Ventricular Systolic Dysfunction in Young Adults Born Preterm. Circulation 2013, 128, 713–720. [Google Scholar] [CrossRef] [Green Version]
- Goss, K.N.; Beshish, A.G.; Barton, G.P.; Haraldsdottir, K.; Levin, T.S.; Tetri, L.H.; Battiola, T.J.; Mulchrone, A.M.; Pegelow, D.F.; Palta, M.; et al. Early Pulmonary Vascular Disease in Young Adults Born Preterm. Am. J. Respir. Crit. Care Med. 2018, 198, 1549–1558. [Google Scholar] [CrossRef]
- Burkett, D.A.; Slorach, C.; Patel, S.S.; Redington, A.N.; Ivy, D.D.; Mertens, L.; Younoszai, A.K.; Friedberg, M.K. Impact of Pulmonary Hemodynamics and Ventricular Interdependence on Left Ventricular Diastolic Function in Children with Pulmonary Hypertension. Circ. Cardiovasc. Imaging 2016, 9, 9. [Google Scholar] [CrossRef] [Green Version]
- Sehgal, A.; Steenhorst, J.J.; McLennan, D.I.; Merkus, D.; Ivy, D.; McNamara, P.J. The Left Heart, Systemic Circulation, and Bronchopulmonary Dysplasia: Relevance to Pathophysiology and Therapeutics. J. Pediatr. 2020, 225, 13–22. [Google Scholar] [CrossRef]
- Cabrita, I.Z.; Ruisanchez, C.; Grapsa, J.; Dawson, D.; North, B.; Pinto, F.J.; Gibbs, J.S.R.; Nihoyannopoulos, P. Validation of the isovolumetric relaxation time for the estimation of pulmonary systolic arterial blood pressure in chronic pulmonary hypertension. Eur. Hearth J.Cardiovasc. Imaging 2012, 14, 51–55. [Google Scholar] [CrossRef] [Green Version]
- Driessen, M.M.P.; Leiner, T.; Sieswerda, G.T.; Van Dijk, A.P.J.; Post, M.C.; Friedberg, M.K.; Mertens, L.; Doevendans, P.A.; Snijder, R.J.; Hulzebos, E.H.; et al. RV adaptation to increased afterload in congenital heart disease and pulmonary hypertension. PLoS ONE 2018, 13, e0205196. [Google Scholar] [CrossRef] [PubMed]
- Palau-Caballero, G.; Walmsley, J.; Van Empel, V.P.M.; Lumens, J.; Delhaas, T. Why septal motion is a marker of right ventricular failure in pulmonary arterial hypertension: Mechanistic analysis using a computer model. Am. J. Physiol. Circ. Physiol. 2017, 312, H691–H700. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Poon, C.Y.; Wilson, D.G.; Joshi, S.; Fraser, A.G.; Kotecha, S. Longitudinal evaluation of myocardial function in preterm infants with respiratory distress syndrome. Echocardiography 2019, 36, 1713–1726. [Google Scholar] [CrossRef]
- Telles, F.; McNamara, N.; Nanayakkara, S.; Doyle, M.P.; Williams, M.; Yaeger, L.; Marwick, T.H.; Leeson, P.; Levy, P.T.; Lewandowski, A.J. Changes in the Preterm Heart from Birth to Young Adulthood: A Meta-analysis. Pediatrics 2020, 146, e20200146. [Google Scholar] [CrossRef]
- Choi, S.-H.; Eun, L.Y.; Kim, N.K.; Jung, J.W.; Choi, J.Y. Myocardial Tissue Doppler Velocity in Child Growth. J. Cardiovasc. Ultrasound 2016, 24, 40–47. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Alp, H.; Karaarslan, S.; Baysal, T.; Çimen, D.; Örs, R.; Oran, B. Normal values of left and right ventricular function measured by M-mode, pulsed doppler and Doppler tissue imaging in healthy term neonates during a 1-year period. Early Hum. Dev. 2012, 88, 853–859. [Google Scholar] [CrossRef] [PubMed]
- Levy, P.T.; Jain, A.; Nawaytou, H.; Teitel, D.; Keller, R.; Fineman, J.; Steinhorn, R.; Abman, S.H.; McNamara, P.J. Risk Assessment and Monitoring of Chronic Pulmonary Hypertension in Premature Infants. J. Pediatr. 2020, 217, 199–209.e4. [Google Scholar] [CrossRef] [PubMed]
- Thomas, L.; Baczynski, M.; Deshpande, P.; Kharrat, A.; Joye, S.; Zhu, F.; Ibarra-Rios, D.; Shah, P.S.; Mertens, L.; Jankov, R.P.; et al. Multicentre prospective observational study exploring the predictive value of functional echocardiographic indices for early identification of preterm neonates at risk of developing chronic pulmonary hypertension secondary to chronic neonatal lung disease. BMJ Open 2021, 11, e044924. [Google Scholar] [CrossRef] [PubMed]
- Rios, D.R.; Martins, F.D.F.; El-Khuffash, A.; Weisz, D.E.; Giesinger, R.E.; McNamara, P.J. Early Role of the Atrial-Level Communication in Premature Infants with Patent Ductus Arteriosus. J. Am. Soc. Echocardiogr. 2021, 43, 423–432. [Google Scholar] [CrossRef]
- Czernik, C.; Rhode, S.; Metze, B.; Schmalisch, G.; Bührer, C. Persistently Elevated Right Ventricular Index of Myocardial Performance in Preterm Infants with Incipient Bronchopulmonary Dysplasia. PLoS ONE 2012, 7, e38352. [Google Scholar] [CrossRef] [Green Version]
- Sehgal, A.; Malikiwi, A.; Paul, E.; Tan, K.; Menahem, S. Right Ventricular Function in Infants with Bronchopulmonary Dysplasia: Association with Respiratory Sequelae. Neonatology 2016, 109, 289–296. [Google Scholar] [CrossRef] [PubMed]
- Murase, M.; Morisawa, T.; Ishida, A. Serial assessment of right ventricular function using tissue Doppler imaging in preterm infants within 7 days of life. Early Hum. Dev. 2015, 91, 125–130. [Google Scholar] [CrossRef] [PubMed]
- Ciccone, M.M.; Scicchitano, P.; Zito, A.; Gesualdo, M.; Sassara, M.; Calderoni, G.; Di Mauro, F.; Ladisa, G.; Di Mauro, A.; Laforgia, N. Different functional cardiac characteristics observed in term/preterm neonates by echocardiography and tissue doppler imaging. Early Hum. Dev. 2011, 87, 555–558. [Google Scholar] [CrossRef] [PubMed]
| Group I No-Mild BPD (n = 33) | Group II Mod-Sev BPD (n = 33) | Group III BPD-PH (n = 11) | F-Ratio p Value | |
|---|---|---|---|---|
| Gestational age (weeks) | 27 (26–28) | 26 (25–27) ∞ | 26 (25–27) * | <0.01 |
| Birth weight (grams) | 950 (818–1070) | 890 (670–985) | 920 (860–1030) | 0.58 |
| Female, No. (%) | 19 (58%) | 17 (52%) | 2 (19%) * | 0.02 |
| Infant Race, No. (%) | 0.14 | |||
| White | 13 (39%) | 15 (45%) | 4 (36%) | |
| Black | 20 (61%) | 18 (55%) | 7 (64%) | |
| Antenatal Steroids—No. (%) | 29 (88%) | 23 (70%) | 8 (73%) | 0.51 |
| Surfactant therapy (Yes) | 33 (100%) | 33 (100%) | 11 (100%) | >0.99 |
| Cesarean section | 24 (73%) | 22 (67%) | 4 (36%) | |
| Maternal complications, No. (%) | ||||
| Gestational Diabetes Mellitus | 1 (3%) | 3 (10%) | 0 | 0.35 |
| PROM | 5 (15%) | 2 (6%) | 2 (18%) | 0.61 |
| Chorioamnionitis | 3 (10%) | 3 (10%) | 1 (9%) | 0.65 |
| Pre-eclampsia | 4 (12%) | 5 (15%) | 3 (27%) | 0.78 |
| Postnatal complications, No. (%) | ||||
| Necrotizing enterocolitis | 2 (6%) | 3 (9%) | 1 (9%) | 0.65 |
| ROP threshold (>Stage 2) | 9 (27%) | 13 (39%) | 4 (36%) | 0.15 |
| IVH (Grade 3 or 4) | 5 (15%) | 6 (18%) | 6 (54%) *,† | 0.02 |
| Presence of PDA at 36 weeks | 3 (9%) | 4 (12%) | 1 (9%) | 0.23 |
| Postnatal steroid use | 3 (6%) | 17 (51%) ∞ | 4 (36%) * | <0.01 |
| Mechanical ventilation days | 2 (1–5) | 18 (2–30) ∞ | 18 (2–41) * | <0.01 |
| Total oxygen days (NICU) | 46 (28–76) | 98 (88–115) ∞ | 97 (66–118) * | <0.01 |
| Length of stay (NICU) | 81 (69–101) | 113 (91–120) ∞ | 101 (66–119) * | <0.01 |
| Patient | GA | BPD Severity | PH * | Designated Criteria for Diagnosis of PH | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| PDA Shunt ** | PFO/ASD Shunt ** | VSD Shunt ** | RVSP∞ > 40 mmHg | RVSP/sBP > 0.5 | RV Morphology Changes † | Septal Wall Flattening | ||||
| 1 | 28 | Mild | Yes | No | No | No | Yes | Yes | No | Moderate |
| 2 | 27 | Mild | Yes | No | No | No | Yes | Yes | No | None |
| 3 | 25 | Mild | Yes | No | No | No | Yes | Yes | No | Moderate |
| 4 | 25 | Mild | Yes | No | No | No | Yes | Yes | No | None |
| 5 | 27 | Moderate | Yes | No | No | No | Yes | Yes | No | None |
| 6 | 24 | Moderate | Yes | No | No | No | Yes | Yes | No | None |
| 7 | 27 | Moderate | Yes | No | No | No | Yes | Yes | No | None |
| 8 | 27 | Moderate | Yes | No | No | No | Yes | Yes | No | Moderate |
| 9 | 25 | Moderate | Yes | No | No | No | Yes | Yes | No | None |
| 10 | 25 | Severe | Yes | No | No | No | Yes | Yes | Yes | Moderate |
| 11 | 26 | Severe | Yes | No | No | No | Yes | Yes | No | Moderate |
| Group I No-Mild BPD (n = 33) | Group II Mod-Sev BPD (n = 33) | Group III BPD-PH (n = 11) | F-Ratio p Value | |
|---|---|---|---|---|
| 32 weeks PMA | ||||
| LV e’ (cm/s) | 4.0 (4.0–5.0) | 4.0 (3.0–4.0) | 3.0 (3.0–4.0) | 0.19 |
| LV a’ (cm/s) | 8.0 (7.0–9.0) | 7.0 (6.0–9.0) | 9.0 (8.0–10.0) | 0.23 |
| LV s’ (cm/s) | 5.0 (4.0–5.0) | 5.0 (4.0–5.0) | 5.0 (4.0–5.0) | 0.98 |
| LV e’/a’ | 0.57 (0.42–0.68) | 0.44 (0.37–0.62) | 0.46 (0.44–0.62) | 0.45 |
| LV IVRT (ms) | 48 (45–58) | 52 (45–55) | 68 (60–69) *,† | <0.01 |
| LV MPI | 0.49 (0.43–0.58) | 0.52 (0.44–0.65) | 0.51 (0.43–0.57) | 0.32 |
| LV PSM (cm/s) | 2.5 (2.0–3.0) | 2.5 (2.0–3.0) | 4.0 (3.0–5.0) *,† | <0.01 |
| 36 weeks PMA | ||||
| LV e’ (cm/s) | 4.0 (4.0–6.0) | 4.0 (3.0–4.0) | 3.0 (3.0–5.0) | 0.14 |
| LV a’ (cm/s) | 8.0 (7.0–10.0) | 9.0 (7.0–10.0) | 7.0 (5.0 –10.0) | 0.56 |
| LV s’ (cm/s) | 5.0 (4.0–5.0) | 5.0 (4.0–6.0) | 5.0 (4.0 –5.0) | 0.80 |
| LV e’/a’ | 0.57 (0.50–0.67) | 0.46 (0.34–0.50) | 0.50 (0.40–0.60) | 0.67 |
| LV IVRT (ms) | 52 (47–58) | 54 (47–61) | 67 (61–74) | <0.01 |
| LV MPI | 0.54 (0.45–0.64) | 0.56 (0.49–0.62) | 0.52 (0.50–0.64) | 0.42 |
| LV PSM (cm/s) | 3.0 (2.0–3.0) | 3.0 (2.0–4.0) | 4.0 (2.8–4.5) *,† | <0.01 |
| One year CA | ||||
| LV e’ (cm/s) | 12.0 (11.0–13.0) | 10.0 (8.0–13.0) | 12.0 (9.0–15.0)) | 0.4 |
| LV a’ (cm/s) | 7.0 (6.0–8.0) | 7.0 (6.0–8.0) | 8.0 (6.0–0.0) | 0.93 |
| LV s’ (cm/s) | 7.0 (6.0–8.0) | 7.0 (6.0–8.0) | 7.0 (6.0–8.0) | 0.20 |
| LV e’/a’ | 1.44 (1.31–1.85) | 1.44 (1.31–1.85) | 1.38 (1.33–1.88) | 0.67 |
| LV IVRT (ms) | 50 (45–57) | 49 (44–53) | 52 (48–57) | 0.43 |
| LV MPI | 0.42 (0.38–0.46) | 0.43 (0.39–0.48) | 0.43 (0.39–0.51) | 0.64 |
| LV PSM (cm/s) | 3.0 (2.0–4.0) | 3.0 (2.0–3.0) | 3.0 (2.0–3.0) | 0.56 |
| Group I No-Mild BPD (n = 33) | Group II Mod-Sev BPD (n = 33) | Group III BPD-PH (n = 11) | F-Ratio p Value | |
|---|---|---|---|---|
| 32 weeks PMA | ||||
| Septal e’ (cm/s) | 4.0 (4.0–5.0) | 4.0 (3.0–4.0) | 5.0 (4.0–5.0) | 0.40 |
| Septal a’ (cm/s) | 7.0 (6.0–8.0) | 6.0 (6.0–7.0) | 8.0 (6.0–8.0) | 0.42 |
| Septal s’ (cm/s) | 4.0 (4.0–5.0) | 4.0 (4.0–5.0) | 4.0 (4.0–4.0) | 0.27 |
| Septal e’/a’ | 0.56 (0.50–0.68) | 0.57 (0.50–0.67) | 0.65 (0.63–0.70) | 0.34 |
| Septal IVRT (ms) | 45 (41–53) | 46 (40–53) | 52 (43–61) *,† | <0.01 |
| Septal MPI | 0.43 (0.39–0.50) | 0.46 (0.41–0.50) | 0.46 (0.37–0.53) | 0.45 |
| Septal PSM (cm/s) | 2.0 (2.0–2.0) | 2.0 (2.0–2.0) | 2.5 (2.0–3.0) *,† | <0.01 |
| 36 weeks PMA | ||||
| Septal e’ (cm/s) | 5.0 (3.0–5.0) | 4.0 (3.0–4.0) | 4.0 (4.0–5.0) | 0.34 |
| Septal a’ (cm/s) | 7.0 (6.0–8.0) | 7.0 (5.0–7.0) | 8.0 (7.0–9.0) | 0.65 |
| Septal s’ (cm/s) | 5.0 (4.0–5.0) | 5.0 (4.0–5.0) | 5.0 (4.0–6.0) | 0.39 |
| Septal e’/a’ | 0.63 (0.45–0.71) | 0.57 (0.43–0.71) | 0.56 (0.49–0.70) | 0.67 |
| Septal IVRT (ms) | 46 (40–53) | 46 (41–54) | 52 (45–58) *,† | <0.01 |
| Septal MPI | 0.44 (0.41–0.49) | 0.44 (0.40–0.51) | 0.44 (0.37–0.51) | 0.74 |
| Septal PSM (cm/s) | 2.0 (2.0–3.0) | 2.0 (2.0–2.0) | 2.5 (2.0–3.3) *,† | <0.01 |
| One year CA | ||||
| Septal e’ (cm/s) | 10.0 (10.0–11.0) | 10.0 (9.0–11.0) | 10.0 (8.0–11.0) | 0.14 |
| Septal a’ (cm/s) | 8.0 (6.0–9.0) | 9.0 (7.0–10.0) | 7.0 (6.0–9.0) | 0.24 |
| Septal s’ (cm/s) | 7.0 (6.0–8.0) | 7.0 (6.0–8.0) | 7.0 (6.0–7.0) | 0.29 |
| Septal e’/a’ | 1.4 (1.13–1.74) | 1.26 (0.88–1.67) | 1.25 (1.03– 1.86) | 0.67 |
| Septal IVRT (ms) | 51 (46–54) | 50 (44–54) | 58 (44–64) *,† | <0.01 |
| Septal MPI | 0.40 (0.35–0.44) | 0.40 (0.37–0.43) | 0.38 (0.33–0.45) | 0.50 |
| Septal PSM (cm/s) | 2.0 (2.0–3.0) | 2.0 (2.0–3.0) | 3.0 (2.0–3.0) *,† | <0.01 |
| Group I No-Mild BPD (n = 33) | Group II Mod-Sev BPD (n = 33) | Group III BPD-PH (n = 11) | Term Control (n = 50) | F-Ratio p Value | |
|---|---|---|---|---|---|
| LV medial (Septal) wall | |||||
| Septal e’ (cm/s) | 10.0 (10.0–11.0) | 10.0 (9.0–11.0) | 10.0 (8.0–11.0) | 11.0 (9.0–12.0) | 0.11 |
| Septal a’ (cm/s) | 8.0 (6.0–9.0) | 9.0 (7.0–10.0) | 7.0 (6.0–9.0) | 7.4 (6.3–9.5) | 0.29 |
| Septal s’ (cm/s) | 7.0 (6.0–8.0) | 7.0 (6.0–8.0) | 7.0 (6.0–7.0) | 7.9 (6.5–9.0) | 0.24 |
| Septal e’/a’ | 1.4 (1.13–1.74) | 1.26 (0.88–1.67) | 1.25 (1.03–1.86) | 1.40 (1.23–1.66) | 0.27 |
| Septal IVRT (ms) | 51 (46–54) | 50 (44–54) | 58 (44–64) *,† | 46 (40–54) c | <0.01 |
| Septal MPI | 0.40 (0.35–0.44) | 0.40 (0.37–0.43) | 0.38 (0.33–0.45) | 0.35 (0.31–0.43) | 0.30 |
| Septal PSM (cm/s) | 2.0 (2.0–3.0) | 2.0 (2.0–3.0) | 3.0 (2.0–3.0) *† | NR | NA |
| LV lateral wall | |||||
| LV e’ (cm/s) | 12.0 (11.0–13.0) | 10.0 (8.0–13.0) | 12.0 (9.0–15.0) | 12.9 (11.0–15.0) | <0.01 |
| LV a’ (cm/s) | 7.0 (6.0–8.0) | 7.0 (6.0–8.0) | 8.0 (6.0–0.0) | 9.0 (7.0–10.0) | 0.23 |
| LV s’ (cm/s) | 7.0 (6.0–8.0) | 7.0 (6.0–8.0) | 7.0 (6.0–8.0) | 8.5 (7.0–10.0) a,b,c | <0.01 |
| LV e’/a’ | 1.44 (1.31–1.85) | 1.44 (1.31–1.85) | 1.38 (1.33–1.88) | 1.28 (113–1.68) a,b,c | <0.01 |
| LV IVRT (ms) | 50 (45–57) | 49 (44–53) | 52 (48–57) | 44 (38–51) a,b,c | <0.01 |
| LV MPI | 0.42 (0.38–0.46) | 0.43 (0.39–0.48) | 0.43 (0.39–0.51) | 0.36 (0.31–0.49) a,b,c | <0.01 |
| LV PSM (cm/s) | 3.0 (2.0–4.0) | 3.0 (2.0–3.0) | 3.0 (2.0–3.0) | NR | NA |
| Preterm | Term | |||||
|---|---|---|---|---|---|---|
| Torres * | Poon 2019 [43] ** | Torres | Alp 2012 [46] | Choi 2016 [45] | Poon 2019 [43] | |
| LV a’ (cm/s) | 11.8 (1.7) | 10.8 (1.2) | 12.8 (1.2) | 13.3 (0.9) | 11.3 (1.5) | 11.7 (2.1) |
| LV a’ (cm/s) | 7.2 (1.6) | 3.6 (1.0) | 8.2 (1.3) | 11.1 (1.2) | 6.2 (1.3) | 3.7 (1.6) |
| LV s’ (cm/s) | 6.5 (0.8) | 4.7 (1.0) | 8.4 (0.9) | 9.9 (0.8) | 7.0 (0.79) | 4.8 (1.0) |
| LV e’/a’ | 1.6 (0.3) | NR | 1.3 (0.2) | 1.2 (0.1) | NR | 1.4 (0.2) |
| LV IVRT (s) | 50.2 (7.4) | NR | 45 (3) | NR | NR | NR |
| LV PSM (cm/s) | 3.0 (1.0) | NR | NR | NR | NR | NR |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Torres, E.; Levy, P.T.; El-Khuffash, A.; Gu, H.; Hamvas, A.; Singh, G.K. Left Ventricle Phenotyping Utilizing Tissue Doppler Imaging in Premature Infants with Varying Severity of Bronchopulmonary Dysplasia. J. Clin. Med. 2021, 10, 2211. https://doi.org/10.3390/jcm10102211
Torres E, Levy PT, El-Khuffash A, Gu H, Hamvas A, Singh GK. Left Ventricle Phenotyping Utilizing Tissue Doppler Imaging in Premature Infants with Varying Severity of Bronchopulmonary Dysplasia. Journal of Clinical Medicine. 2021; 10(10):2211. https://doi.org/10.3390/jcm10102211
Chicago/Turabian StyleTorres, Eunice, Philip T. Levy, Afif El-Khuffash, Hongjie Gu, Aaron Hamvas, and Gautam K. Singh. 2021. "Left Ventricle Phenotyping Utilizing Tissue Doppler Imaging in Premature Infants with Varying Severity of Bronchopulmonary Dysplasia" Journal of Clinical Medicine 10, no. 10: 2211. https://doi.org/10.3390/jcm10102211






