Fabrication and Characterization of Modified Graphene Oxide/PAN Hybrid Nanofiber Membrane
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Synthesis of Graphene Oxide (GO) and Modified GO by Polydiallyldimethylammonium (PDDA) (GP)
2.3. Fabrication of Polyacrylonitrile (PAN) and Graphene Oxide Modified by Polydiallyldimethylammonium Chloride/Polyacrylonitrile (GP/PAN) Hybrid Nanofiber Membranes via Electrospinning
2.4. Characterization of Synthesized Graphene Oxide GO and Graphene Oxide Modified by Polydiallyldimethylammonium Chloride/Polyacrylonitrile (GP/P) Hybrid Nanofiber Membranes
3. Results
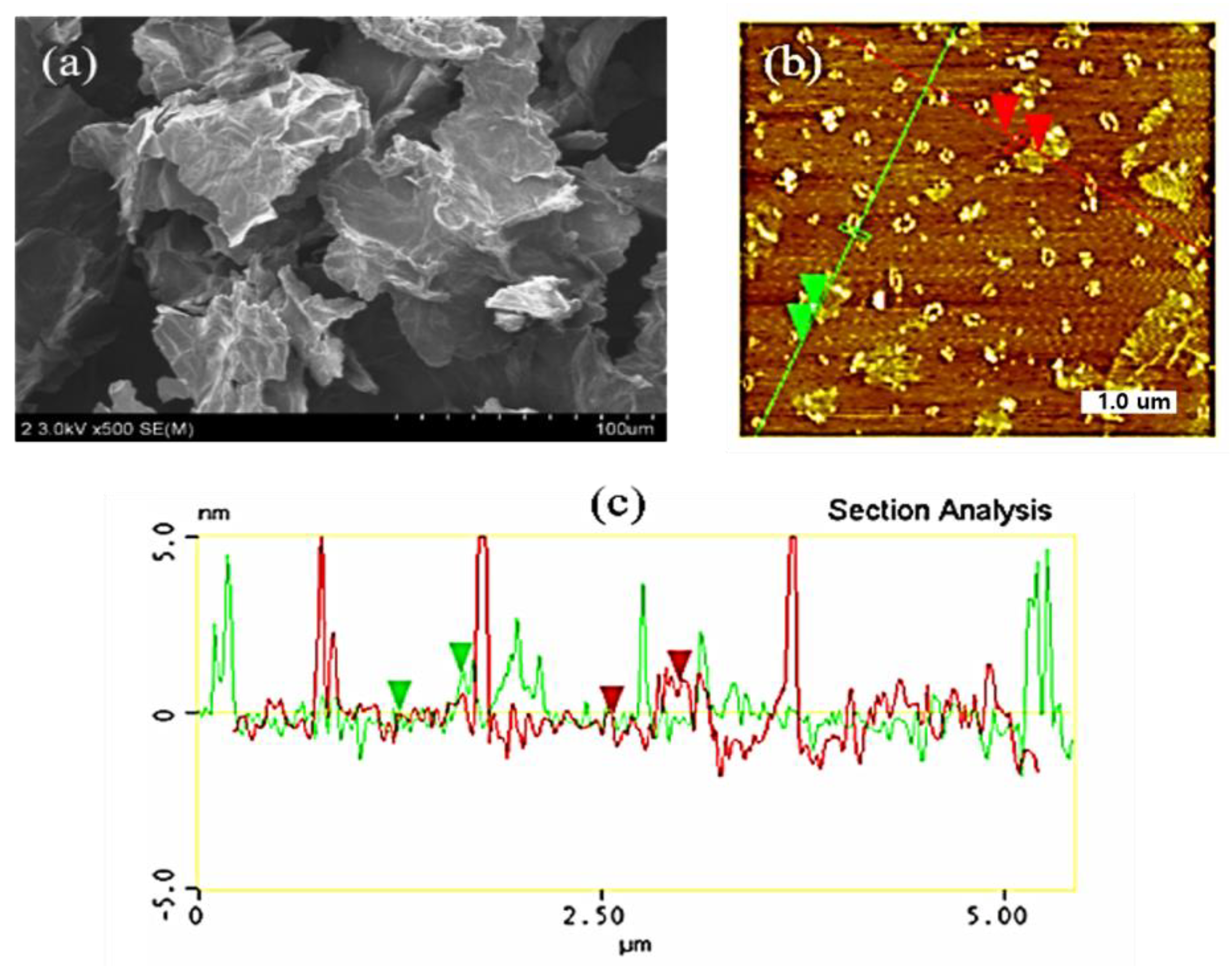
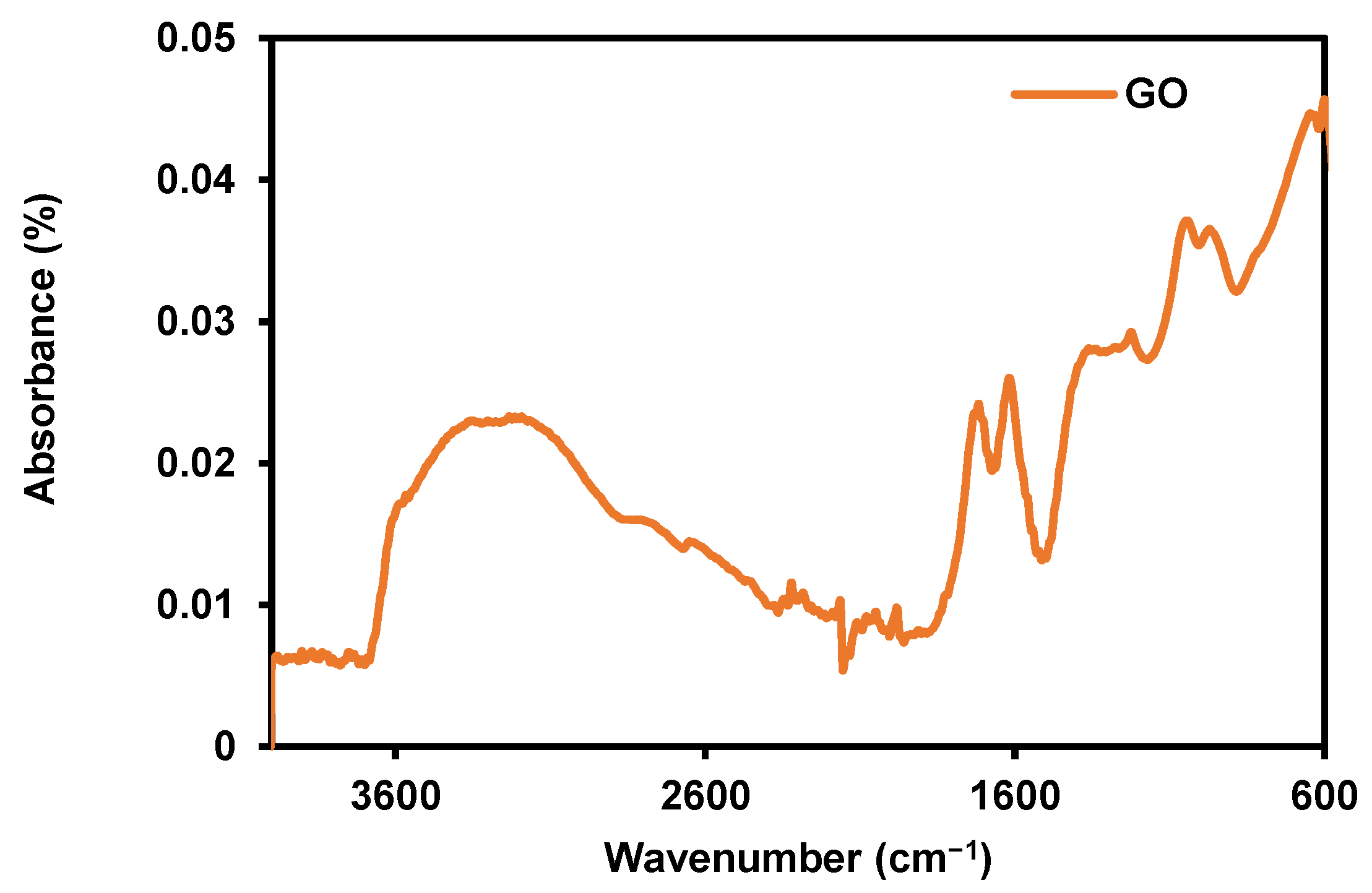
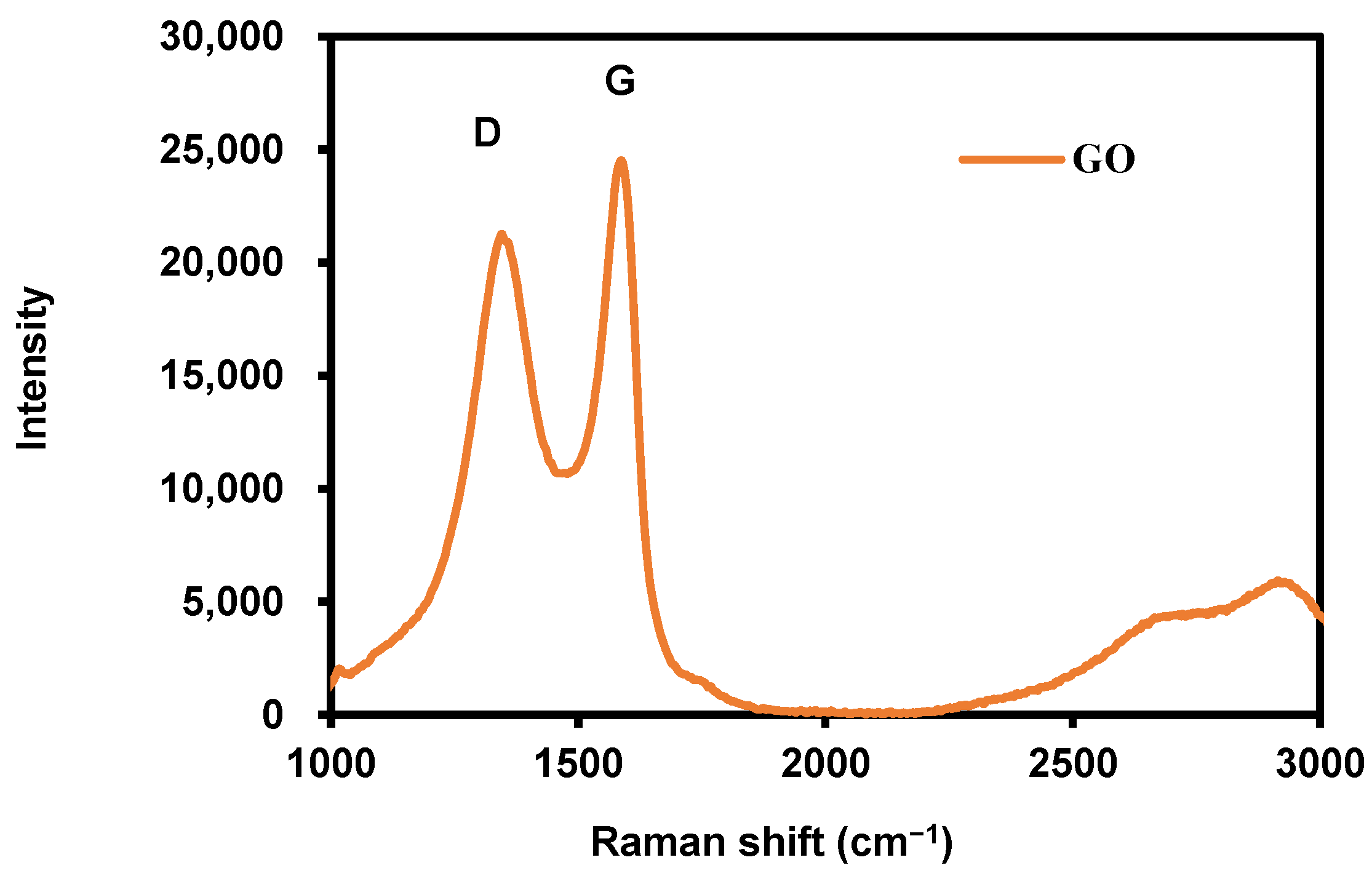
3.1. Characterization of Synthesized Graphene Oxide (GO)
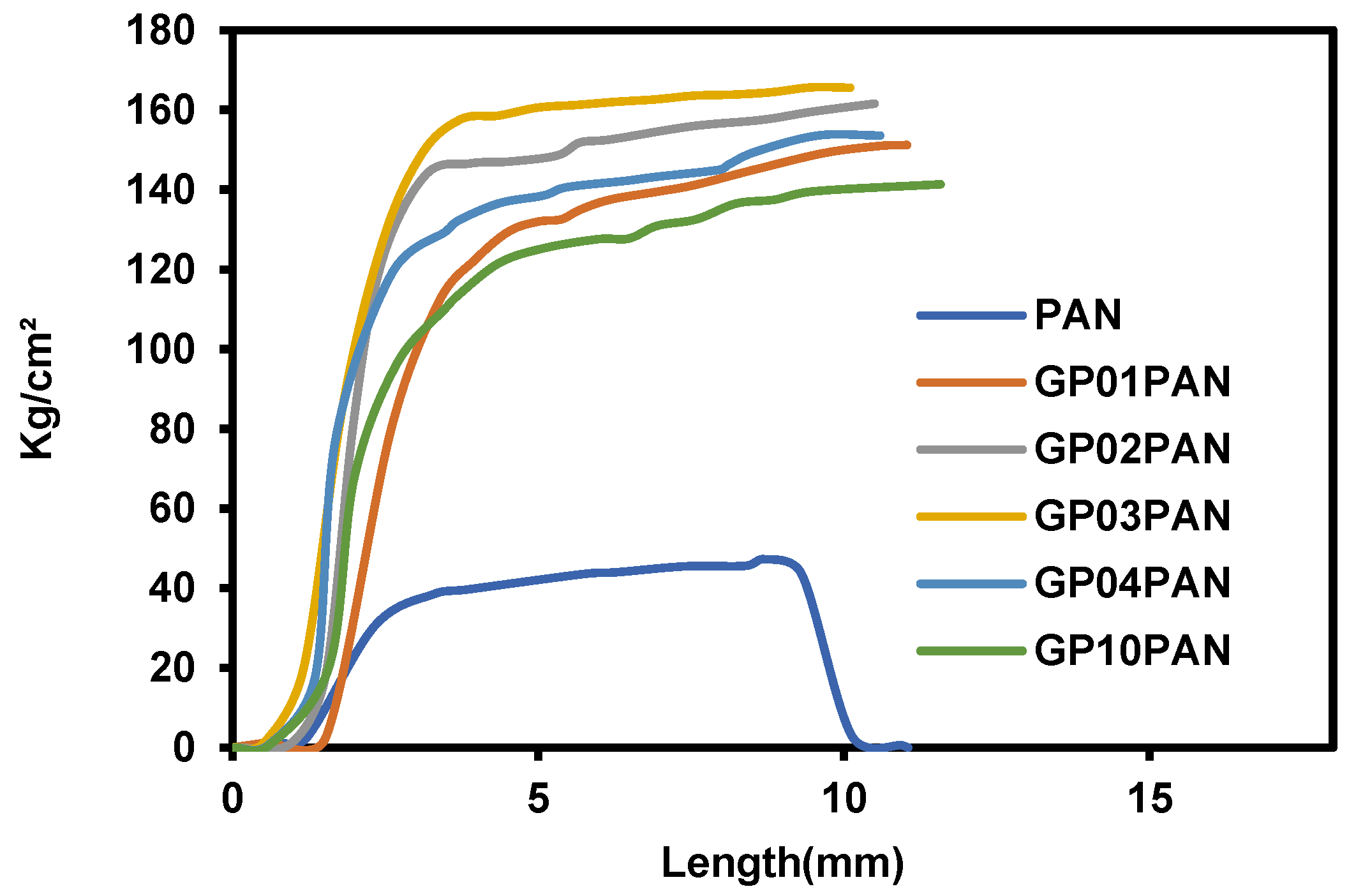
3.2. Characterization of Graphene Oxide Modified by Polydiallyldimethylammonium Chloride/Polyacrylonitrile (GP/PAN) Hybrid Nanofiber Membranes
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- MacDiarmid, A.G.; Jones, W.E., Jr.; Norris, I.D.; Gao, J.; Johnson, A.T., Jr.; Pinto, N.J.; Hone, J.; Han, B.; Ko, F.K.; Okuzaki, H.; et al. Electrostatically-generated nanofibers of electronic polymers. Synth. Met. 2001, 119, 27–30. [Google Scholar] [CrossRef]
- Huang, Z.M.; Zhang, Y.Z.; Kotaki, M.; Ramakrishna, S. A review on polymer nanofibers by electrospinning and their applications in nanocomposites. Compos. Sci. Technol. 2003, 63, 2223–2253. [Google Scholar] [CrossRef]
- Jacangelo, J.G.; Trussell, R.R.; Watson, M. Role of membrane technology in drinking water treatment in the United States. Desalination 1997, 113, 119–127. [Google Scholar] [CrossRef]
- Martin, C.R. Membrane-Based Synthesis of Nanomaterials. Chem. Mater. 1996, 8, 1739–1746. [Google Scholar] [CrossRef]
- Ma, P.X.; Zhang, R. Synthetic nano-scale fibrous extracellular matrix. J. Biomed. Mater. Res. 1999, 46, 60–72. [Google Scholar] [CrossRef] [Green Version]
- Whitesides, G.M.; Grzybowski, B. Self-assembly at all scales. Science 2002, 295, 2418–2421. [Google Scholar] [CrossRef] [PubMed]
- Yoon, K.; Hsiao, B.S.; Chu, B. Functional nanofibers for environmental applications. J. Mater. Chem. 2008, 18, 5326–5334. [Google Scholar] [CrossRef]
- Lu, X.; Wang, C.; Wei, Y. One-dimensional composite nanomaterials: Synthesis by electrospinning and their applications. Small 2009, 5, 2349–2370. [Google Scholar] [CrossRef]
- Greiner, A.; Wendorff, J.H. Electrospinning: A fascinating method for the preparation of ultrathin fibers. Angew. Chem. Int. Ed. Engl. 2007, 46, 5670–5703. [Google Scholar] [CrossRef]
- Lannutti, J.; Reneker, D.; Ma, T.; Tomasko, D.; Farson, D. Electrospinning for tissue engineering scaffolds. Mater. Sci. Eng. C 2007, 27, 504–509. [Google Scholar] [CrossRef]
- Lee, S.; Kay Obendorf, S. Use of Electrospun Nanofiber Web for Protective Textile Materials as Barriers to Liquid Penetration. Text. Res. J. 2007, 77, 696–702. [Google Scholar] [CrossRef]
- Lee, S.H.; Ku, B.C.; Wang, X. Design, Synthesis and Electrospinning of a Novel Fluorescent Polymer for Optical Sensor Applications. Mater. Res. Soc. Symp. Proc. 2002, 708, 403–408. [Google Scholar] [CrossRef]
- Subbiah, T.; Bhat, G.S.; Tock, R.W.; Parameswaran, S.; Ramkumar, S.S. Electrospinning of Nanofibers. J. Appl. Polym. Sci. 2005, 96, 557–569. [Google Scholar] [CrossRef]
- Ahire, J.J.; Neveling, D.P.; Dicks, L.M.T. Polyacrylonitrile (PAN) nanofibres spun with copper nanoparticles: An anti-Escherichia coli membrane for water treatment. Appl. Microbiol. Biotechnol. 2018, 102, 7171–7181. [Google Scholar] [CrossRef] [PubMed]
- Dabirian, F.; Hosseini Ravandi, S.A.; Hashemi Sanatgar, R.; Hinestroza, J.P. Manufacturing of twisted continuous PAN nanofiber yarn by electrospinning process. Fibers Polym. 2011, 12, 610–615. [Google Scholar] [CrossRef]
- He, J.H.; Wan, Y.Q.; Yu, J.Y. Effect of concentration on electrospun polyacrylonitrile (PAN) nanofibers. Fibers Polym. 2008, 9, 140–142. [Google Scholar] [CrossRef]
- Hou, C.; Yang, H.; Xu, Z.-L.; Wei, Y. Preparation of PAN/PAMAM blend nanofiber mats as efficient adsorbent for dye removal. Fibers Polym. 2015, 16, 1917–1924. [Google Scholar] [CrossRef]
- Wu, M.; Wang, Q.; Li, K.; Wu, Y.; Liu, H. Optimization of stabilization conditions for electrospun polyacrylonitrile nanofibers. Polym. Degrad. Stab. 2012, 97, 1511–1519. [Google Scholar] [CrossRef]
- Wang, T.; Kumar, S. Electrospinning of polyacrylonitrile nanofibers. J. Appl. Polym. Sci. 2006, 102, 1023–1029. [Google Scholar] [CrossRef]
- Hosseini Ravandi, S.A.; Mehrara, S.; Sadrjahani, M.; Khodaparast Haghi, A. Tunable wicking behavior via titanium oxide embedded in polyacrylonitrile nanofiber strings of yarn. Polym. Bull. 2019. [Google Scholar] [CrossRef]
- Kancheva, M.; Toncheva, A.; Paneva, D.; Manolova, N.; Rashkov, I.; Markova, N. Materials from Nanosized ZnO and Polyacrylonitrile: Properties Depending on the Design of Fibers (Electrospinning or Electrospinning/Electrospraying). J. Inorg. Organomet. Polym. 2017, 27, 912–922. [Google Scholar] [CrossRef]
- Yar, A.; Haspulat, B.; Üstün, T.; Eskizeybek, V.; Avcı, A.; Kamış, H. Achour, S., Electrospun TiO2/ZnO/PAN hybrid nanofiber membranes with efficient photocatalytic activity. RSC Adv. 2017, 7, 29806–29814. [Google Scholar] [CrossRef]
- Wang, C.; Li, Y.; Ding, G.; Xie, X.; Jiang, M. Preparation and characterization of graphene oxide/poly(vinyl alcohol) composite nanofibers via electrospinning. J. Appl. Polym. Sci. 2013, 127, 3026–3032. [Google Scholar] [CrossRef]
- Cassagneau, T.; Guerin, F.; Fendler, J.H. Preparation and Characterization of Ultrathin Films Layer-by-Layer Self-Assembled from Graphite Oxide Nanoplatelets and Polymers. Langmuir 2000, 16, 7318–7324. [Google Scholar] [CrossRef]
- Hou, H.; Hu, X.; Liu, X.; Hu, W.; Meng, R.; Li, L. Sulfonated graphene oxide with improved ionic performances. Ionics 2015, 21, 1919–1923. [Google Scholar] [CrossRef]
- Lee, J.; Yoon, J.; Kim, J.H.; Lee, T.; Byun, H. Electrospun PAN–GO composite nanofibers as water purification membranes. J. Appl. Polym. Sci. 2017, 135, 45858–45866. [Google Scholar] [CrossRef]
- Stankovich, S.; Piner, R.D.; Nguyen, S.; Ruoff, R.S. Synthesis and exfoliation of isocyanate-treated graphene oxide nanoplatelets. Carbon 2006, 44, 3342–3347. [Google Scholar] [CrossRef]
- Compton, O.C.; Dikin, D.A.; Putz, K.W.; Brinson, L.C.; Nguyen, S.T. Electrically Conductive “Alkylated” Graphene Paper via Chemical Reduction of Amine-Functionalized Graphene Oxide Paper. Adv. Mater. 2010, 22, 892–896. [Google Scholar] [CrossRef]
- Pierini, F.; Lanzi, M.; Nakielski, P.; Pawłowska, S.; Zembrzycki, K.; Kowalewski, T.A. Electrospun poly(3-hexylthiophene)/poly(ethylene oxide)/graphene oxide composite nanofibers: Effects of graphene oxide reduction. Polym. Adv. Technol. 2016, 27, 1465–1475. [Google Scholar] [CrossRef]
- Jang, W.; Yun, J.; Jeon, K.; Byun, H. PVdF/graphene oxide hybrid membranes via electrospinning for water treatment applications. RSC Adv. 2015, 5, 46711–46717. [Google Scholar] [CrossRef]
- Jang, W.; Yun, J.; Byun, H. Preparation of PAN Nanofiber Composite Membrane with Fe3O4 Functionalized Graphene Oxide and its Application as a Water Treatment Membrane. Membr. J. 2014, 24, 151–157. [Google Scholar] [CrossRef]
- Wang, Q.; Du, Y.; Feng, Q.; Huang, F.; Lu, K.; Liu, J.; Wei, Q. Nanostructures and surface nanomechanical properties of polyacrylonitrile/graphene oxide composite nanofibers by electrospinning. J. Appl. Polym. Sci. 2013, 128, 1152–1157. [Google Scholar] [CrossRef]
- Ke, H.; Pang, Z.; Xu, Y.; Chen, X.; Fu, J.; Cai, Y.; Huang, F.; Wei, Q. Graphene oxide improved thermal and mechanical properties of electrospun methyl stearate/polyacrylonitrile form-stable phase change composite nanofibers. J. Therm. Anal. Calorim. 2014, 117, 109–122. [Google Scholar] [CrossRef]
- Hu, M.; Mi, B. Enabling Graphene Oxide Nanosheets as Water Separation Membranes. Environ. Sci. Technol. 2013, 47, 3715–3723. [Google Scholar] [CrossRef] [PubMed]
- Ramakrishnan, S.; Dhakshnamoorthy, M.; Jelmy, E.J.; Vasanthakumari, R.; Kothurkar, N.K. Synthesis and characterization of graphene oxide–polyimide nanofiber composites. RSC Adv. 2014, 4, 9743–9749. [Google Scholar] [CrossRef]
- Park, Y.H.; Nam, S.Y. Characterization of Water Treatment Membrane Using Various Hydrophilic Coating Materials. Membr. J. 2017, 27, 60–67. [Google Scholar] [CrossRef]
- Chen, J.; Yao, B.; Li, C.; Shi, G.Q. An improved Hummers method for eco-friendly synthesis of graphene oxide. Carbon 2013, 64, 225–229. [Google Scholar] [CrossRef]
- Tan, C.; Huang, X.; Zhang, H. Synthesis and applications of graphene-based noble metal nanostructures. Mater. Today 2013, 16, 29–36. [Google Scholar] [CrossRef]
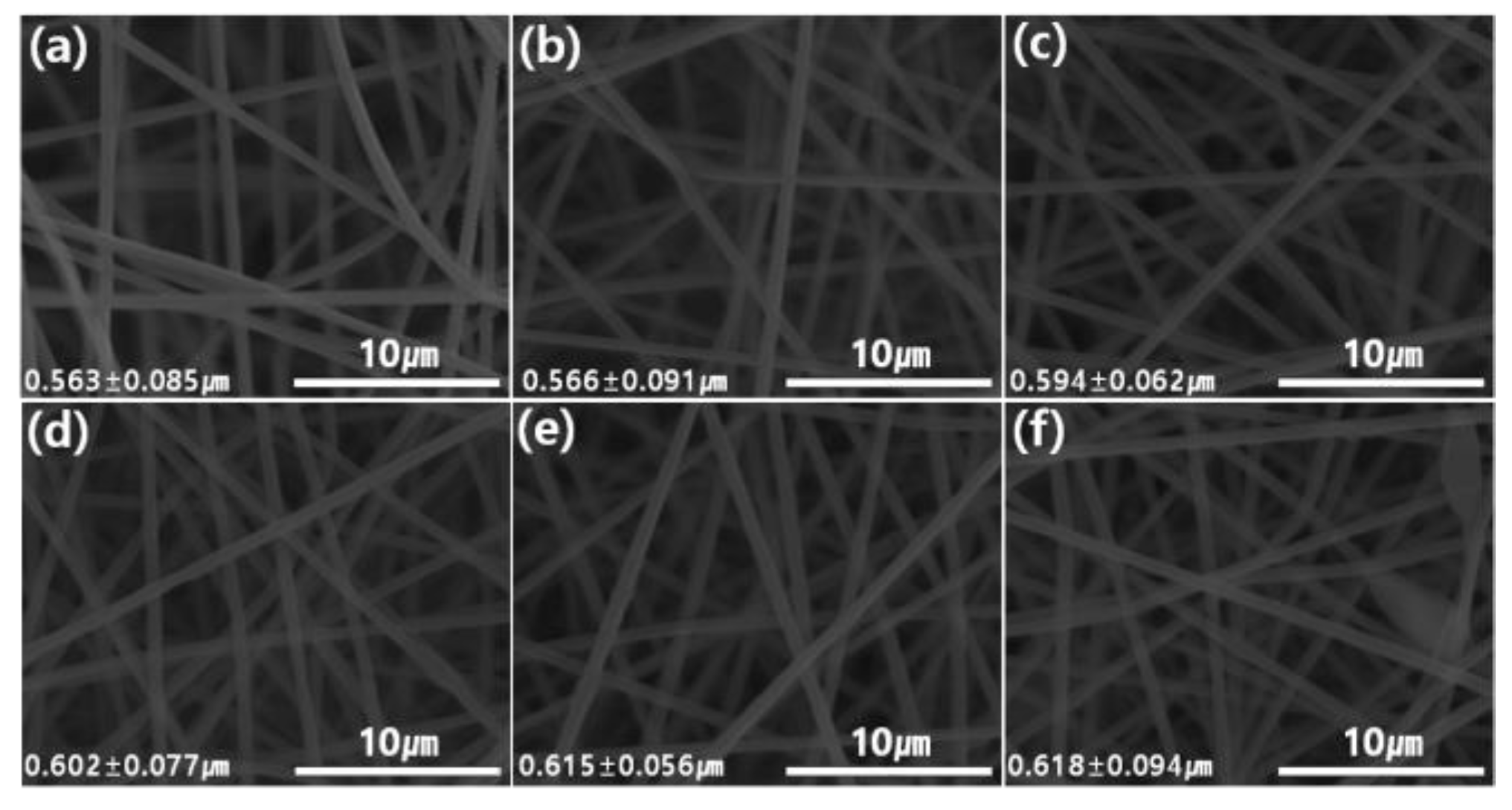
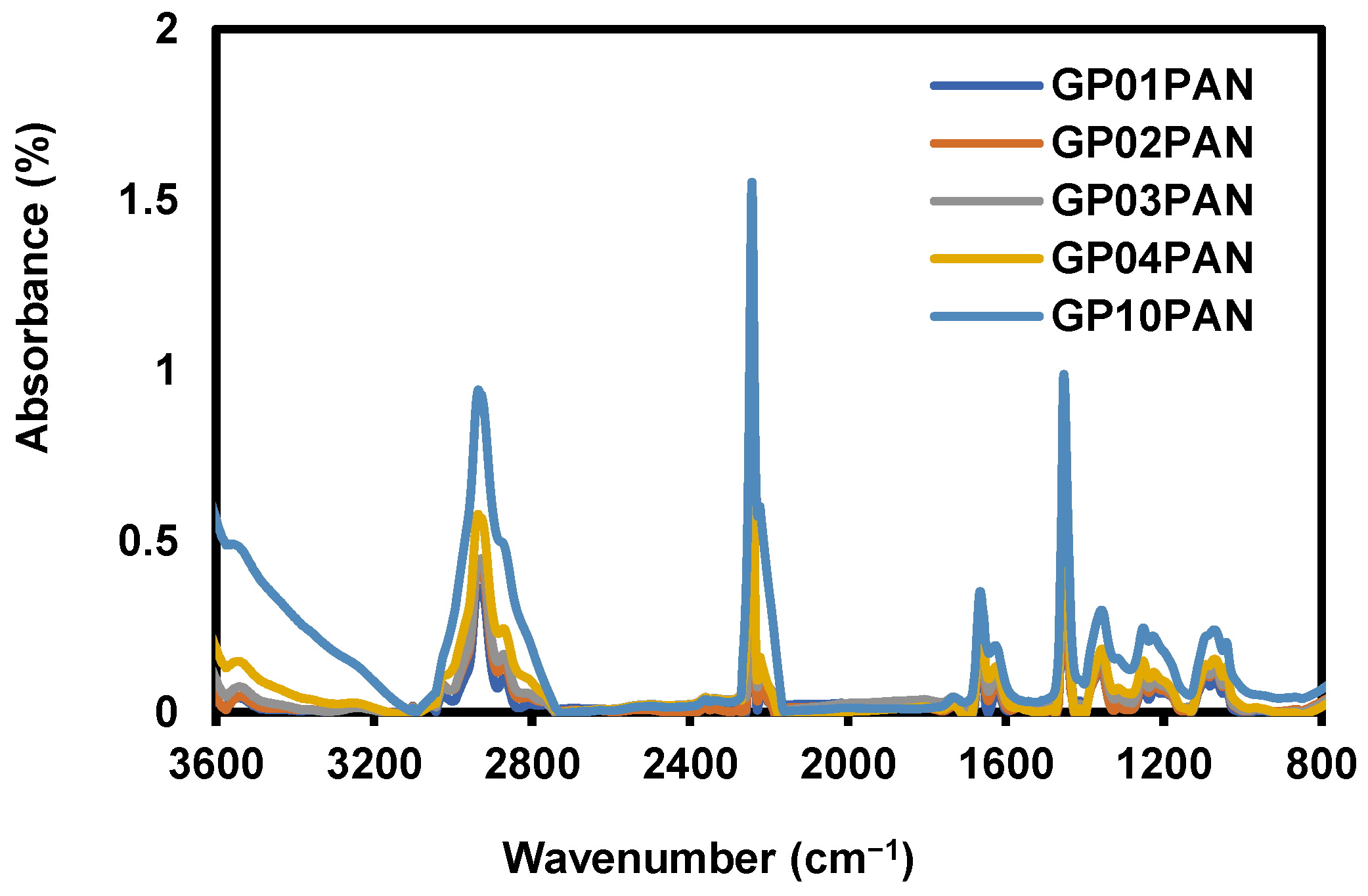
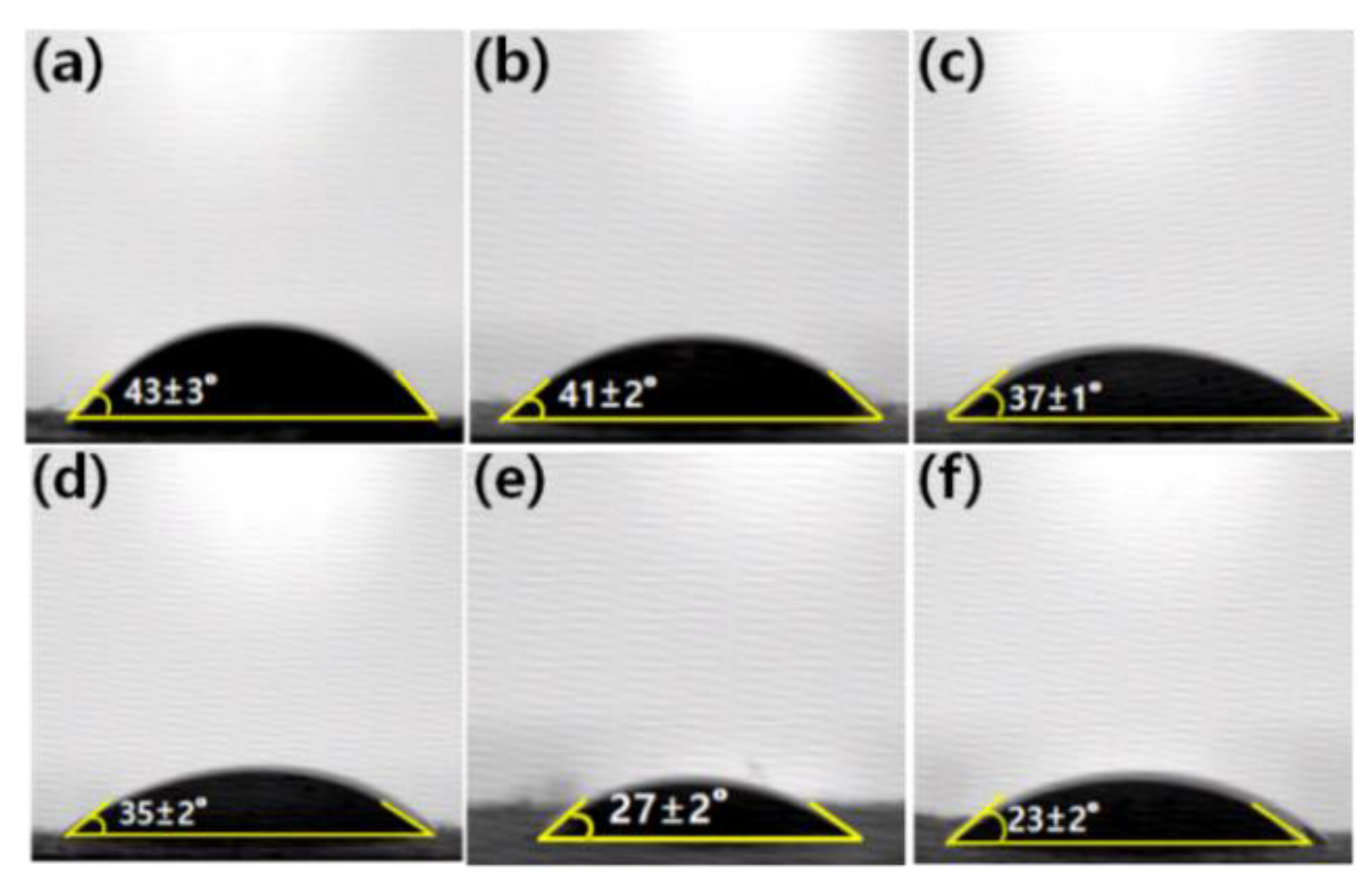
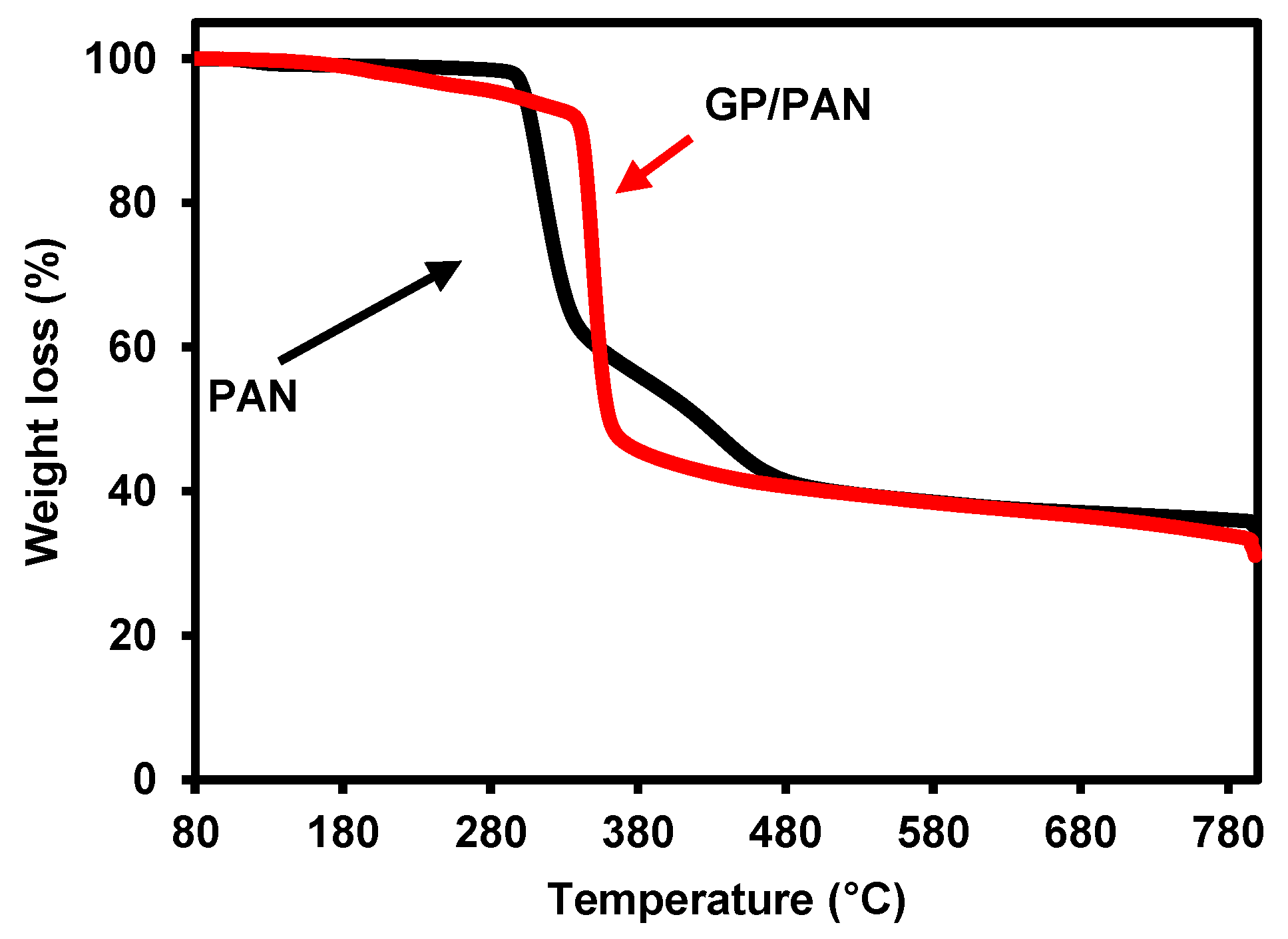
| Sample Name | GP (wt.%) | PAN (wt.%) | DMF (wt.%) |
|---|---|---|---|
| PAN | 0 | 10 | 90.0 |
| GP01PAN | 0.1 | 10 | 89.9 |
| GP02PAN | 0.2 | 10 | 89.8 |
| GP03PAN | 0.3 | 10 | 89.7 |
| GP04PAN | 0.4 | 10 | 89.6 |
| GP10PAN | 1.0 | 10 | 89.0 |
| Sample Name | Biggest Pore Size (nm) | Smallest Pore Size (nm) | Average Pore Size (nm) | Thickness (µm) |
|---|---|---|---|---|
| PAN | 459.5 | 169.5 | 213.3 | 77 ± 3 |
| GP01PAN | 474.2 | 171.2 | 226.3 | 79 ± 2 |
| GP02PAN | 484.5 | 175.5 | 232.7 | 81 ± 2 |
| GP03PAN | 473.3 | 183.2 | 243.5 | 83 ± 3 |
| GP04PAN | 482.2 | 194.3 | 245.5 | 84 ± 2 |
| GP10PAN | 485.5 | 243.6 | 269.3 | 88 ± 3 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hou, J.; Yun, J.; Byun, H. Fabrication and Characterization of Modified Graphene Oxide/PAN Hybrid Nanofiber Membrane. Membranes 2019, 9, 122. https://doi.org/10.3390/membranes9090122
Hou J, Yun J, Byun H. Fabrication and Characterization of Modified Graphene Oxide/PAN Hybrid Nanofiber Membrane. Membranes. 2019; 9(9):122. https://doi.org/10.3390/membranes9090122
Chicago/Turabian StyleHou, Jian, Jaehan Yun, and Hongsik Byun. 2019. "Fabrication and Characterization of Modified Graphene Oxide/PAN Hybrid Nanofiber Membrane" Membranes 9, no. 9: 122. https://doi.org/10.3390/membranes9090122







