Rhabdomyolysis Following Ad26.COV2.S COVID-19 Vaccination
Abstract
:1. Introduction
2. Case Report
3. Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Greinacher, A.; Thiele, T.; Warkentin, T.E.; Weisser, K.; Kyrle, P.A.; Eichinger, S. Thrombotic Thrombocytopenia after ChAdOx1 nCov-19 Vaccination. N. Engl. J. Med. 2021, 384, 2092–2101. [Google Scholar] [CrossRef] [PubMed]
- Bos, R.; Rutten, L.; van der Lubbe, J.E.; Bakkers, M.J.; Hardenberg, G.; Wegmann, F.; Zuijdgeest, D.; De Wilde, A.H.; Koornneef, A.; Verwillingen, A.; et al. Ad26 vector-based COVID-19 vaccine encoding a prefusion-stabilized SARS-CoV-2 Spike immunogen induces potent humoral and cellular immune responses. NPJ Vaccines 2020, 5, 91. [Google Scholar] [CrossRef]
- Sadoff, J.; Le Gars, M.; Shukarev, G.; Heerwegh, D.; Truyers, C.; de Groot, A.M.; Stoop, J.; Tete, S.; Van Damme, W.; Leroux-Roels, I.; et al. Interim Results of a Phase 1–2a Trial of Ad26.COV2.S Covid-19 Vaccine. N. Engl. J. Med. 2021, 384, 1824–1835. [Google Scholar] [CrossRef]
- Sadoff, J.; Gray, G.; Vandebosch, A.; Cárdenas, V.; Shukarev, G.; Grinsztejn, B.; Goepfert, P.A.; Truyers, C.; Fennema, H.; Spiessens, B.; et al. Safety and Efficacy of Single-Dose Ad26.COV2.S Vaccine against Covid-19. N. Engl. J. Med. 2021, 384, 2187–2201. [Google Scholar] [CrossRef] [PubMed]
- Bosch, X.; Poch, E.; Grau, J.M. Rhabdomyolysis and Acute Kidney Injury. N. Engl. J. Med. 2009, 361, 62–72. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Giannoglou, G.D.; Chatzizisis, Y.S.; Misirli, G. The syndrome of rhabdomyolysis: Pathophysiology and diagnosis. Eur. J. Intern. Med. 2007, 18, 90–100. [Google Scholar] [CrossRef] [PubMed]
- Wrogemann, K.; Pena, S. Mitochondrial Calcium Overload: A General Mechanism for Cell-Necrosis in Muscle Diseases. Lancet 1976, 307, 672–674. [Google Scholar] [CrossRef]
- Vanholder, R.; Sever, M.S.; Erek, E.; Lameire, N. Rhabdomyolysis. J. Am. Soc. Nephrol. 2000, 11, 1553–1561. [Google Scholar] [CrossRef]
- Warren, J.; Blumbergs, P.C.; Thompson, P.D. Rhabdomyolysis: A review. Muscle Nerve 2002, 25, 332–347. [Google Scholar] [CrossRef]
- Holt, S.; Moore, K.P. Pathogenesis and treatment of renal dysfunction in rhabdomyolysis. Intensiv. Care Med. 2001, 27, 803–811. [Google Scholar] [CrossRef]
- Melli, G.; Chaudhry, V.; Cornblath, D.R. Rhabdomyolysis: An evaluation of 475 hospitalized patients. Medicine 2005, 84, 377–385. [Google Scholar] [CrossRef] [PubMed]
- Ward, M.M. Factors Predictive of Acute Renal Failure in Rhabdomyolysis. Arch. Intern. Med. 1988, 148, 1553. [Google Scholar] [CrossRef] [PubMed]
- Zager, R.A. Studies of mechanisms and protective maneuvers in myoglobinuric acute renal injury. Lab. Investig. 1989, 60, 619–629. [Google Scholar] [PubMed]
- Knochel, J.P. Rhabdomyolysis and Myoglobinuria. Annu. Rev. Med. 1982, 33, 435–443. [Google Scholar] [CrossRef]
- Nassar, M.; Chung, H.; Dhayaparan, Y.; Nyein, A.; Acevedo, B.J.; Chicos, C.; Zheng, D.; Barras, M.; Mohamed, M.; Alfishawy, M.; et al. COVID-19 vaccine induced rhabdomyolysis: Case report with literature review. Diabetes Metab. Syndr. 2021, 15, 102170. [Google Scholar] [CrossRef] [PubMed]
- Mack, M.; Nichols, L.; Guerrero, D.M. Rhabdomyolysis Secondary to COVID-19 Vaccination. Cureus 2021, 13. [Google Scholar] [CrossRef]
- Tan, A.; Stepien, K.M.; Narayana, S.T.K. Carnitine palmitoyltransferase II deficiency and post-COVID vaccination rhabdomyolysis. QJM Int. J. Med. 2021. [Google Scholar] [CrossRef]
- Callado, R.B.; Carneiro, T.G.P.; Parahyba, C.C.D.C.; Lima, N.; Junior, G.S.; Daher, E. Rhabdomyolysis secondary to influenza A H1N1 vaccine resulting in acute kidney injury. Travel Med. Infect. Dis. 2013, 11, 130–133. [Google Scholar] [CrossRef]
- Konrad, R.J.; Goodman, D.B.P.; Davis, W.L. Tumor Necrosis Factor and Coxsackie B4 Rhabdomyolysis. Ann. Intern. Med. 1993, 119, 861. [Google Scholar] [CrossRef]
- Jin, M.; Tong, Q. Rhabdomyolysis as Potential Late Complication Associated with COVID-19. Emerg. Infect. Dis. 2020, 26, 1618–1620. [Google Scholar] [CrossRef]
- Chedid, N.R.; Udit, S.; Solhjou, Z.; Patanwala, M.Y.; Sheridan, A.M.; Barkoudah, E. COVID-19 and Rhabdomyolysis. J. Gen. Intern. Med. 2020, 35, 3087–3090. [Google Scholar] [CrossRef] [PubMed]
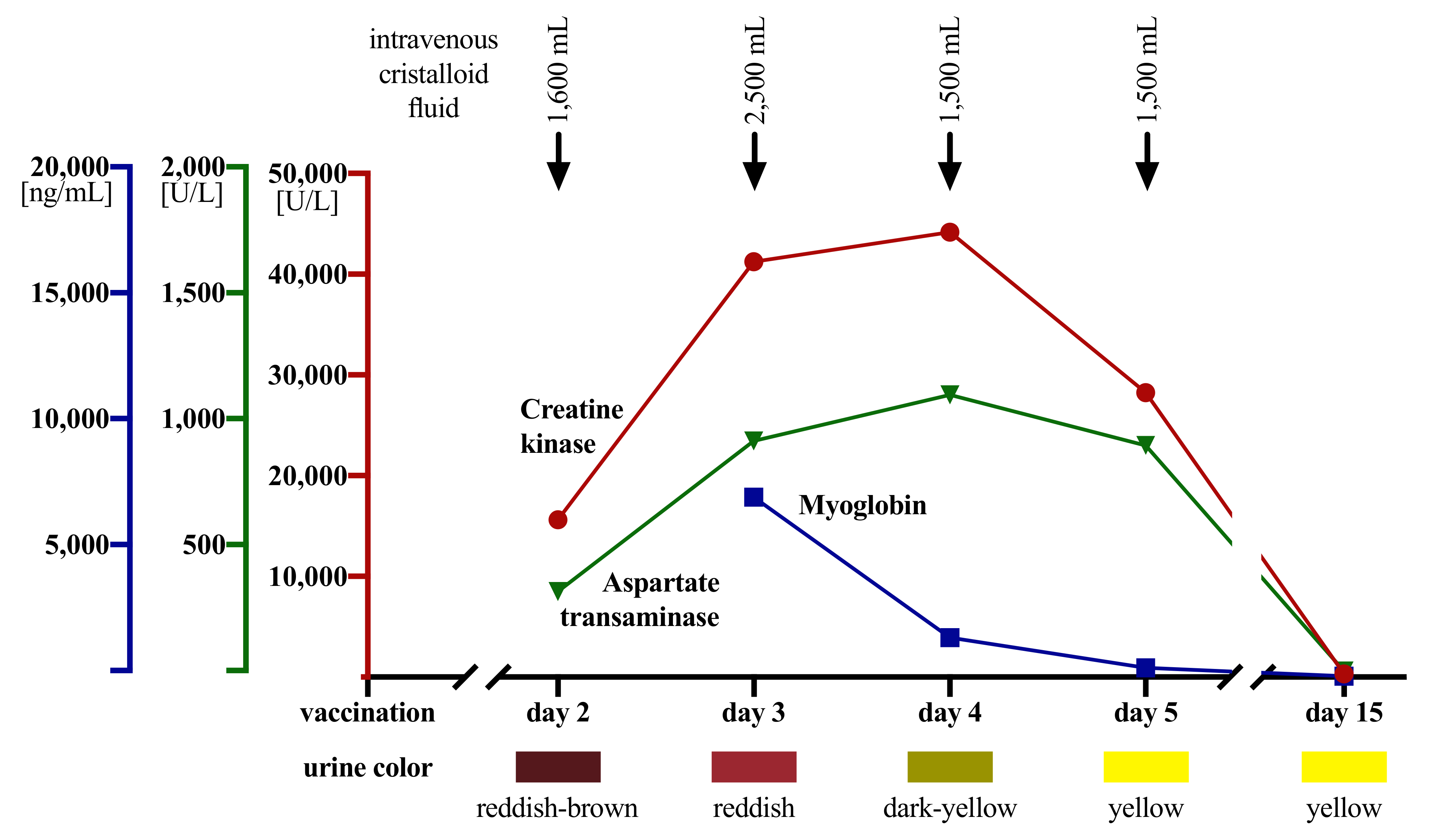
| Screening Visit | Day 2 (After Vaccination) | Day 3 | Day 4 | Day 5 | Day 15 | |
|---|---|---|---|---|---|---|
| Lab parameter | ||||||
| CK (<190 U/L) | 163 | 15,638 | 41,260 | 44,180 | 28,260 | 347 |
| GOT (<50 U/L) | 15 | 340 | 938 | 1121 | 920 | 24 |
| GPT (<50 U/L) | 21 | 70 | 237 | 309 | 354 | 51 |
| LDH (<250 U/L) | 163 | 428 | 784 | 875 | 608 | 211 |
| Myoglobin (23–72 ng/mL) | ND | ND | 7146 | 1576 | 371 | 50 |
| Creatinine (0.7–1.2 mg/dL) | 0.96 | 1.06 | 0.93 | 0.82 | 0.92 | 0.97 |
| Urinary dipstick | ||||||
| color | reddish-brown | reddish | dark yellow | yellow | yellow | |
| pH (4.5–7.4) | 5 | 6 | 6 | NA | 7 | |
| blood (hemoglobin *) | positive | positive | negative | negative | negative | |
| Pain (NRS) | 0 | 10 | 6–7 | 5 | 2 | 0 |
| Case Report | Vaccine | Age, Sex | Time to Symptom Onset | Peak CK Level | Acute Kidney Failure | Outcome | Note |
|---|---|---|---|---|---|---|---|
| Tan et al., 2021 | ChAdOx1 nCoV-19 (AstraZeneca) | 27, m | 5 h | 250,000 U/L | no | recovered | known CPT2 deficiency |
| Mack et al., 2021 | mRNA-1273 (Moderna) | 80, m | 2 days | 6546 U/L | no | recovered | - |
| Nassar et al., 2021 | BNT161b2 mRNA (Pfizer/BioNTech) | 21, m | 1 day | >22,000 U/L | no | recovered | - |
| VAERS ID | Age, Sex | Time to Symptom Onset | Peak CK Level [U/L] | Acute Kidney Failure | Outcome | Note |
|---|---|---|---|---|---|---|
| 1177103-1 | 47, m | 1 day | 1700 | yes | recovered | sepsis with unknown source of infection |
| 1211823-1 | 37, f | 6 days | >32,000 | no | recovered | - |
| 1223359-1 | 30, m | 5 days | 95,694 | no | recovered | notable recent moderate exercise an over-ingestion of gabapentin; under methadone, quetiapine, lithium, gabapentin, nicotine vape |
| 1225589-1 | 22, f | 7 days | 127,768 | no | recovered | HIIT training, not more strenuous than usual, NKDA |
| 1227789-1 | unknown, f | < 1 day | unknown | no | recovered | no rhabdomyolysis |
| 1282506-1 | 86, m | 4 weeks | unknown | no | recovered | ischemic stroke, likely traumatic rhabdomyolysis |
| 1286545-1 | 81, f | 21 days | unknown | no | recovered | evidence of rhabdomyolysis/traumatic rhabdomyolysis? |
| 1293926-1 | 71, f | 28 days | unknown | no | recovered with permanent disability | ischemic stroke, likely traumatic rhabdomyolysis; concomitant simvastatin treatment |
| 1316930-1 | 21, f | 3 days | 204,900 | no | recovered | - |
| 1336145-1 | 37, f | 17 days | 744,000 | yes | unknown | CT scan suggested myositis in her masseter muscles, transfer to ICU, intubated, RRT |
| 1345707-1 | unknown | unknown | >500,000 | no | unknown | - |
| 1371923-1 | 49, m | 8 days | unknown | no | recovered | - |
| 1388426-1 | 61, m | 19 days | unknown | no | unknown | de novo Guillain-Barré syndrome, rhabdomyolysis due to statin intake |
| 1391630-1 | 86, m | 3 days | unknown | yes | fatal | sepsis, bilateral pneumonia, acute kidney injury; PMHx tumor in lungs, AFIB, hyperlipidemia |
| 1423267-1 | 72, f | 28 days | unknown | unknown | recovered with permanent disability | sepsis, UTI, pneumonia, SVT, NSTEMI; under atorvastatin |
| 1430442-1 | unknown | unknown | ~750,000 | yes | recovered with permanent disability | INT, tracheostomy, 19 days in ICU; myoglobin > 5000 |
| 1441330-1 | 61, f | 19 days | >15,000 | no | recovered | current COVID-19 infection, statin-induced rhabdomyolysis |
| 1450792-1 | 57, f | 4 days | unknown | unknown | unknown | hospitalized, unknown days in hospital |
| 1512116-1 | 41, f | 6 days | 16,000 | no | recovered | crossfit as possible confounding error |
| 1519231-1 | 30, m | 8 days | unknown | unknown | unknown | under citalopram |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gelbenegger, G.; Cacioppo, F.; Firbas, C.; Jilma, B. Rhabdomyolysis Following Ad26.COV2.S COVID-19 Vaccination. Vaccines 2021, 9, 956. https://doi.org/10.3390/vaccines9090956
Gelbenegger G, Cacioppo F, Firbas C, Jilma B. Rhabdomyolysis Following Ad26.COV2.S COVID-19 Vaccination. Vaccines. 2021; 9(9):956. https://doi.org/10.3390/vaccines9090956
Chicago/Turabian StyleGelbenegger, Georg, Filippo Cacioppo, Christa Firbas, and Bernd Jilma. 2021. "Rhabdomyolysis Following Ad26.COV2.S COVID-19 Vaccination" Vaccines 9, no. 9: 956. https://doi.org/10.3390/vaccines9090956






