Neutralizing Antibodies against SARS-CoV-2, Anti-Ad5 Antibodies, and Reactogenicity in Response to Ad5-nCoV (CanSino Biologics) Vaccine in Individuals with and without Prior SARS-CoV-2
Abstract
:1. Introduction
2. Materials and Methods
2.1. Subjects and Sample Collection
2.2. Detection of IgG/IgM against SARS-CoV-2
2.3. Quantification of Neutralizing Antibodies
2.4. Quantification of Antibodies against Ad5
2.5. Statistical Analysis
3. Results
3.1. Description of Study Groups
3.2. Vaccine-Associated Side Effects
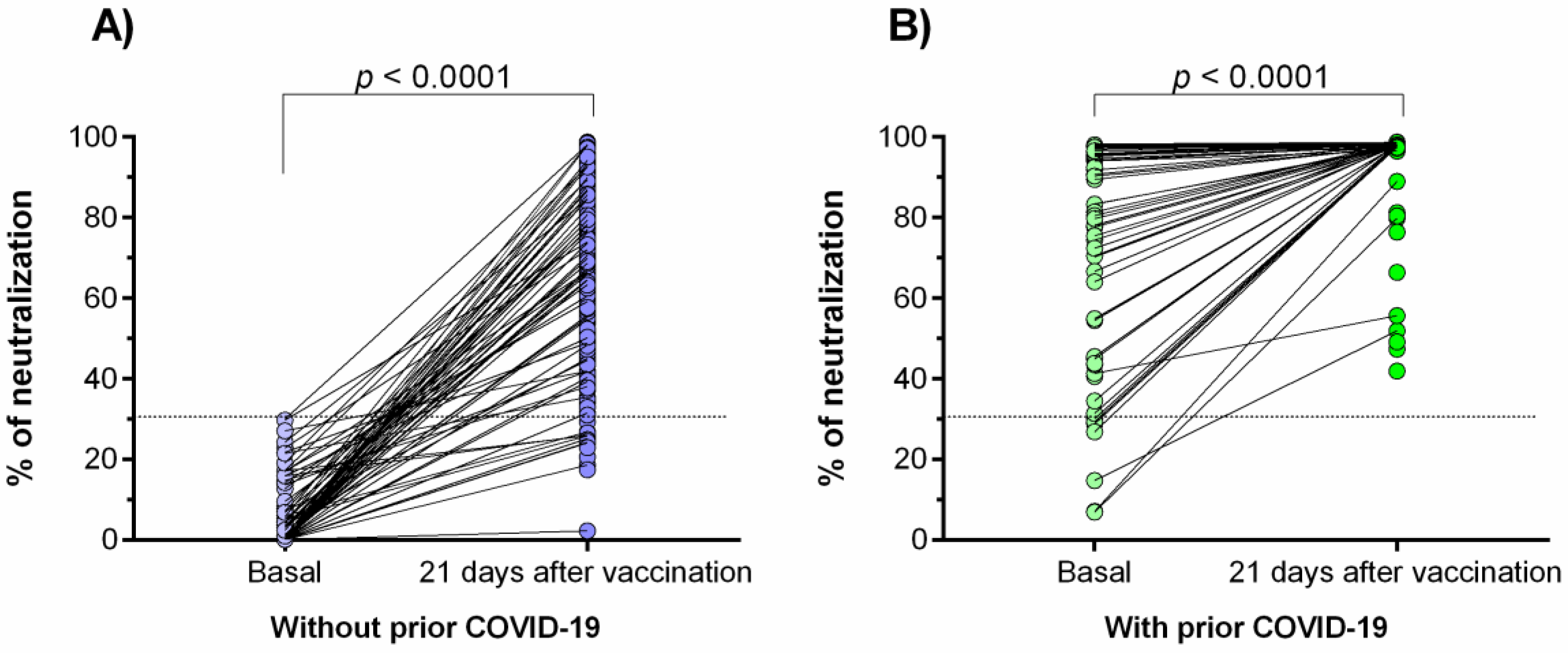
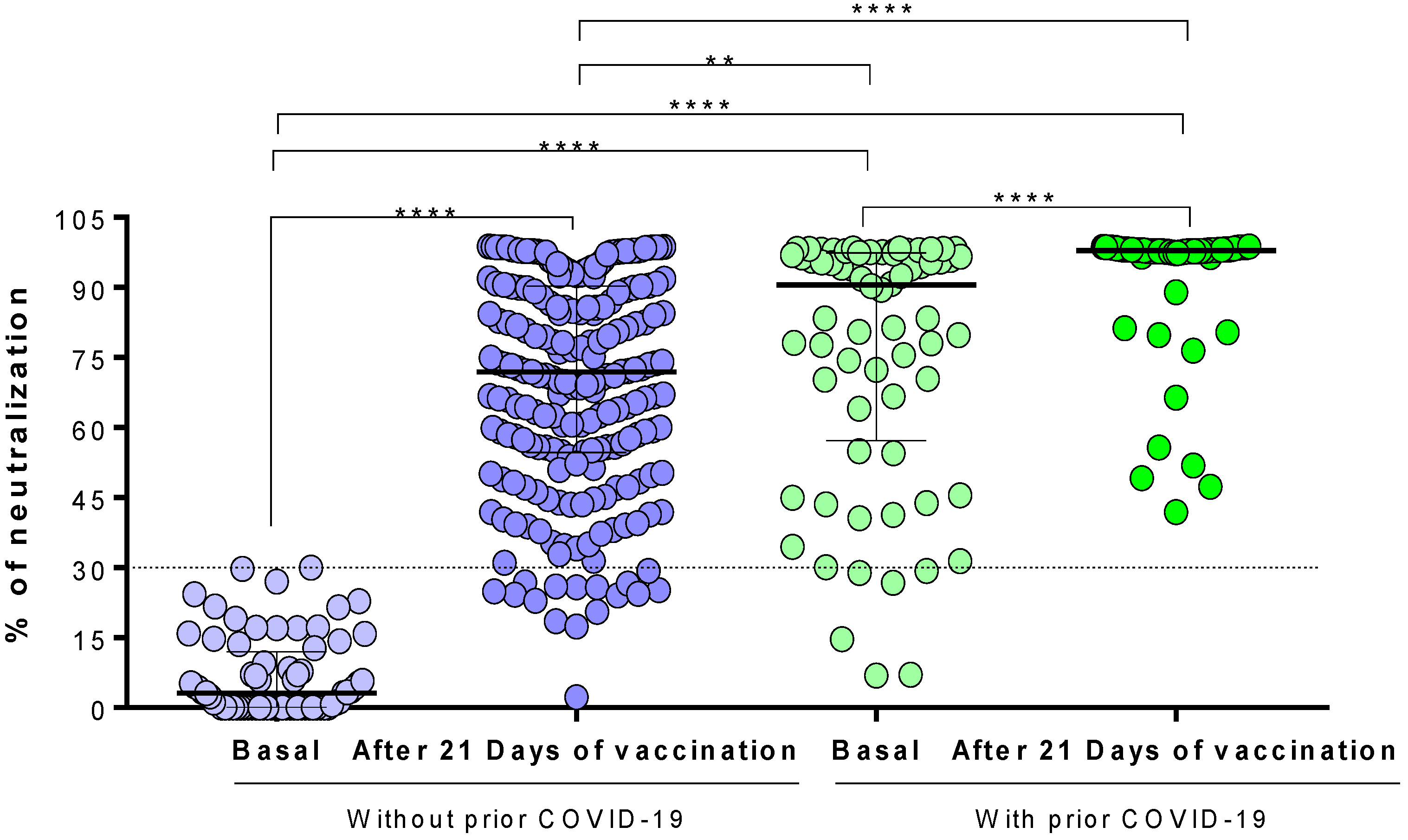
3.3. Generation of Neutralizing Antibodies in Response to the Ad5-nCoV Vaccine
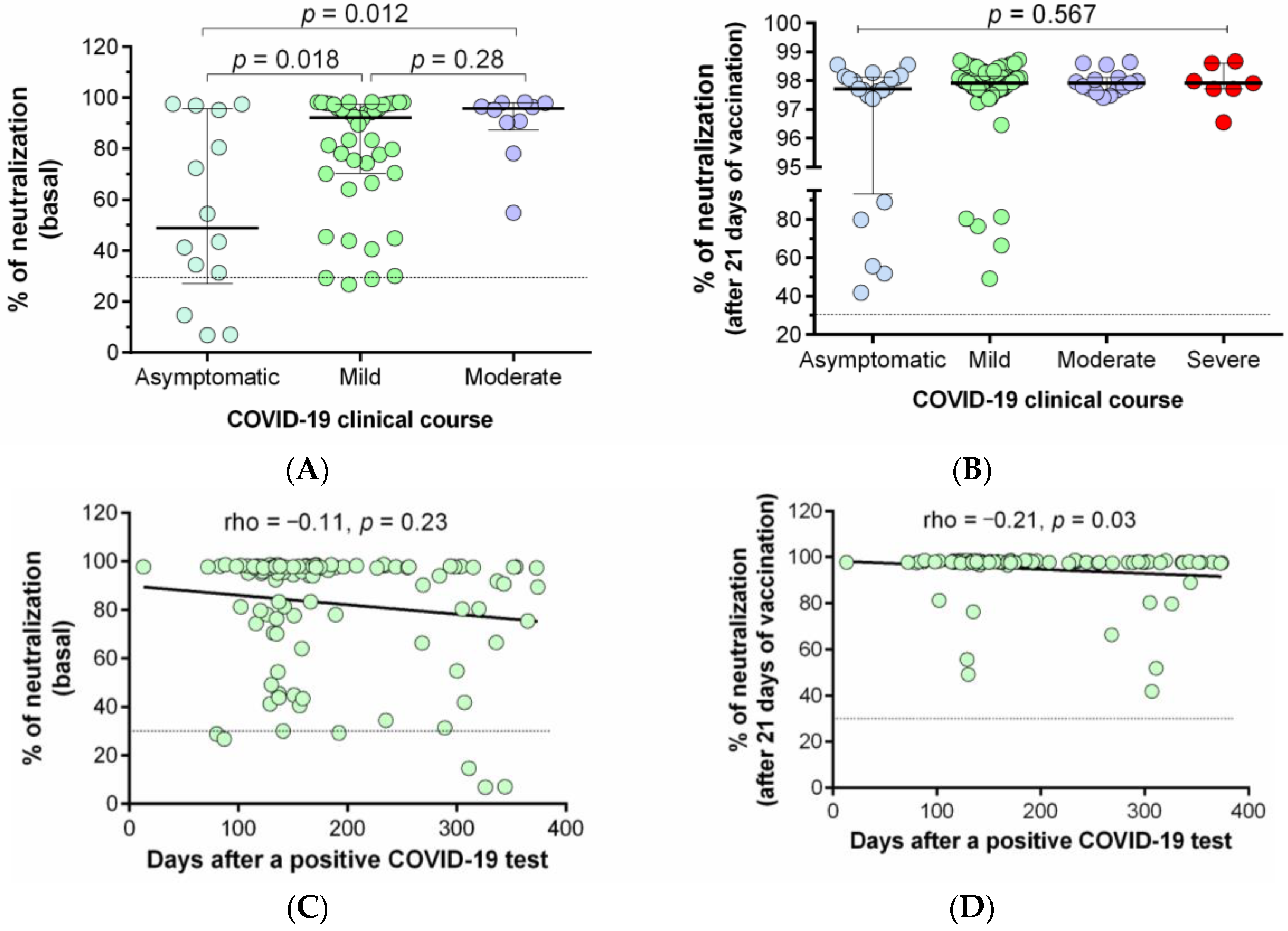
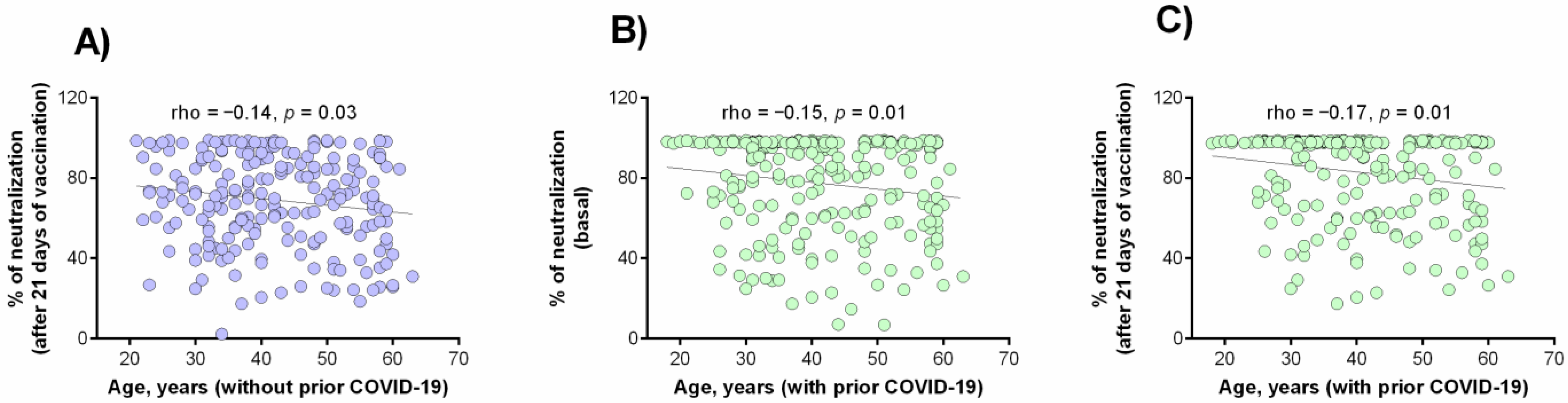
3.4. Correlation between the Percentage of Neutralization with the Clinical and Demographic Variables
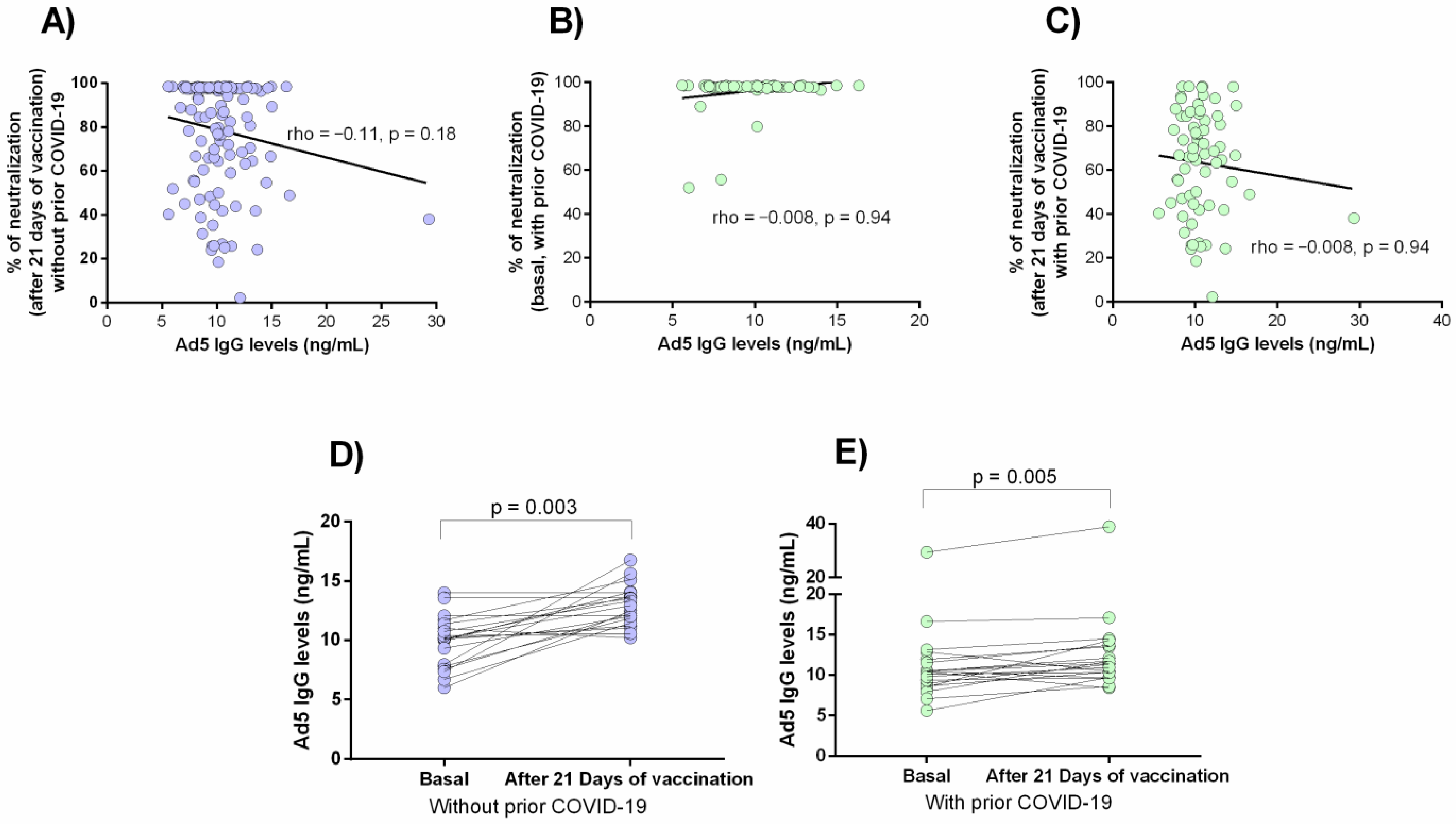
3.5. Anti-Ad5 Antibodies and Percentage of Neutralization
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- “COVID-19 Map”. Johns Hopkins Coronavirus Resource Center. Available online: https://coronavirus.jhu.edu/map.html (accessed on 31 July 2021).
- Huang, C.; Wang, Y.; Li, X.; Ren, L.; Zhao, J.; Hu, Y.; Zhang, L.; Fan, G.; Xu, J.; Gu, X.; et al. Clinical Features of Patients Infected with 2019 Novel Coronavirus in Wuhan, China. Lancet Lond. Engl. 2020, 395, 497–506. [Google Scholar] [CrossRef] [Green Version]
- Netea, M.G.; Giamarellos-Bourboulis, E.J.; Domínguez-Andrés, J.; Curtis, N.; van Crevel, R.; van de Veerdonk, F.L.; Bonten, M. Trained Immunity: A Tool for Reducing Susceptibility to and the Severity of SARS-CoV-2 Infection. Cell 2020, 181, 969–977. [Google Scholar] [CrossRef] [PubMed]
- Funk, C.D.; Laferrière, C.; Ardakani, A. A Snapshot of the Global Race for Vaccines Targeting SARS-CoV-2 and the COVID-19 Pandemic. Front. Pharmacol. 2020, 11, 937. [Google Scholar] [CrossRef] [PubMed]
- Dan, J.M.; Mateus, J.; Kato, Y.; Hastie, K.M.; Yu, E.D.; Faliti, C.E.; Grifoni, A.; Ramirez, S.I.; Haupt, S.; Frazier, A.; et al. Immunological Memory to SARS-CoV-2 Assessed for up to 8 Months after Infection. Science 2021, 371, eabf4063. [Google Scholar] [CrossRef]
- Heaton, P.M. The Covid-19 Vaccine-Development Multiverse. N. Engl. J. Med. 2020, 383, 1986–1988. [Google Scholar] [CrossRef] [PubMed]
- Walls, A.C.; Park, Y.-J.; Tortorici, M.A.; Wall, A.; McGuire, A.T.; Veesler, D. Structure, Function, and Antigenicity of the SARS-CoV-2 Spike Glycoprotein. Cell 2020, 183, 1735. [Google Scholar] [CrossRef]
- Ye, T.; Zhong, Z.; García-Sastre, A.; Schotsaert, M.; De Geest, B.G. Current Status of COVID-19 (Pre)Clinical Vaccine Development. Angew. Chem. Int. Ed. 2020, 59, 18885–18897. [Google Scholar] [CrossRef]
- CanSino Biologics Inc. A Global Multicenter, Randomized, Double-Blind, Placebo -Controlled, Adaptive Designed Phase Ⅲ Clinical Trial to Evaluate the Efficacy, Safety and Immunogenicity of Ad5-NCoV in Adults 18 Years of Age and Older. clinicaltrials.gov; 2021. Available online: https://clinicaltrials.gov/ct2/show/record/NCT04526990 (accessed on 10 September 2021).
- CanSino: Ad5-NCoV–COVID19 Vaccine Tracker. Available online: https://covid19.trackvaccines.org/vaccines/2/ (accessed on 19 September 2021).
- Zhu, F.-C.; Guan, X.-H.; Li, Y.-H.; Huang, J.-Y.; Jiang, T.; Hou, L.-H.; Li, J.-X.; Yang, B.-F.; Wang, L.; Wang, W.-J.; et al. Immunogenicity and Safety of a Recombinant Adenovirus Type-5-Vectored COVID-19 Vaccine in Healthy Adults Aged 18 Years or Older: A Randomised, Double-Blind, Placebo-Controlled, Phase 2 Trial. Lancet Lond. Engl. 2020, 396, 479–488. [Google Scholar] [CrossRef]
- Wu, S.; Zhong, G.; Zhang, J.; Shuai, L.; Zhang, Z.; Wen, Z.; Wang, B.; Zhao, Z.; Song, X.; Chen, Y.; et al. A Single Dose of an Adenovirus-Vectored Vaccine Provides Protection against SARS-CoV-2 Challenge. Nat. Commun. 2020, 11, 4081. [Google Scholar] [CrossRef] [PubMed]
- Rogliani, P.; Chetta, A.; Cazzola, M.; Calzetta, L. SARS-CoV-2 Neutralizing Antibodies: A Network Meta-Analysis across Vaccines. Vaccines 2021, 9, 227. [Google Scholar] [CrossRef] [PubMed]
- Bull, J.J.; Nuismer, S.L.; Antia, R. Recombinant Vector Vaccine Evolution. PLoS Comput. Biol. 2019, 15, e1006857. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rhee, E.G.; Barouch, D.H. Adenoviruses. In Mandell, Douglas, and Bennett’s Principles and Practice of Infectious Diseases, 8th ed.; Bennett, J.E., Dolin, R., Blaser, M.J., Eds.; Elsevier: Philadelphia, PA, USA, 2014; pp. 1787–1793.e2. [Google Scholar]
- Schulick, A.H.; Vassalli, G.; Dunn, P.F.; Dong, G.; Rade, J.J.; Zamarron, C.; Dichek, D.A. Established Immunity Precludes Adenovirus-Mediated Gene Transfer in Rat Carotid Arteries. Potential for Immunosuppression and Vector Engineering to Overcome Barriers of Immunity. J. Clin. Investig. 1997, 99, 209–219. [Google Scholar] [CrossRef] [PubMed]
- Nwanegbo, E.; Vardas, E.; Gao, W.; Whittle, H.; Sun, H.; Rowe, D.; Robbins, P.D.; Gambotto, A. Prevalence of Neutralizing Antibodies to Adenoviral Serotypes 5 and 35 in the Adult Populations of The Gambia, South Africa, and the United States. Clin. Diagn. Lab. Immunol. 2004, 11, 351–357. [Google Scholar] [CrossRef] [Green Version]
- Ejaz, H.; Alsrhani, A.; Zafar, A.; Javed, H.; Junaid, K.; Abdalla, A.E.; Abosalif, K.O.A.; Ahmed, Z.; Younas, S. COVID-19 and Comorbidities: Deleterious Impact on Infected Patients. J. Infect. Public Health 2020, 13, 1833–1839. [Google Scholar] [CrossRef]
- Richardson, S.; Hirsch, J.S.; Narasimhan, M.; Crawford, J.M.; McGinn, T.; Davidson, K.W.; The Northwell COVID-19 Research Consortium; Barnaby, D.P.; Becker, L.B.; Chelico, J.D.; et al. Presenting Characteristics, Comorbidities, and Outcomes Among 5700 Patients Hospitalized With COVID-19 in the New York City Area. JAMA 2020, 323, 2052–2059. [Google Scholar] [CrossRef]
- Sanyaolu, A.; Okorie, C.; Marinkovic, A.; Patidar, R.; Younis, K.; Desai, P.; Hosein, Z.; Padda, I.; Mangat, J.; Altaf, M. Comorbidity and Its Impact on Patients with COVID-19. SN Compr. Clin. Med. 2020, 2, 1–8. [Google Scholar] [CrossRef]
- Levaillant, M.; Lièvre, G.; Baert, G. Ending Diabetes in Mexico. Lancet Lond. Engl. 2019, 394, 467–468. [Google Scholar] [CrossRef] [Green Version]
- Hoertel, N.; Sánchez-Rico, M.; Vernet, R.; Beeker, N.; Jannot, A.-S.; Neuraz, A.; Salamanca, E.; Paris, N.; Daniel, C.; Gramfort, A.; et al. Association between Antidepressant Use and Reduced Risk of Intubation or Death in Hospitalized Patients with COVID-19: Results from an Observational Study. Mol. Psychiatry 2021, 1–14. [Google Scholar] [CrossRef]
- Rabeea, S.A.; Merchant, H.A.; Khan, M.U.; Kow, C.S.; Hasan, S.S. Surging Trends in Prescriptions and Costs of Antidepressants in England amid COVID-19. Daru 2021, 29, 217–221. [Google Scholar] [CrossRef]
- Carpinteiro, A.; Edwards, M.J.; Hoffmann, M.; Kochs, G.; Gripp, B.; Weigang, S.; Adams, C.; Carpinteiro, E.; Gulbins, A.; Keitsch, S.; et al. Pharmacological Inhibition of Acid Sphingomyelinase Prevents Uptake of SARS-CoV-2 by Epithelial Cells. Cell Rep. Med. 2020, 1, 100142. [Google Scholar] [CrossRef]
- Hojyo, S.; Uchida, M.; Tanaka, K.; Hasebe, R.; Tanaka, Y.; Murakami, M.; Hirano, T. How COVID-19 Induces Cytokine Storm with High Mortality. Inflamm. Regen. 2020, 40, 37. [Google Scholar] [CrossRef]
- Lenze, E.J.; Mattar, C.; Zorumski, C.F.; Stevens, A.; Schweiger, J.; Nicol, G.E.; Miller, J.P.; Yang, L.; Yingling, M.; Avidan, M.S.; et al. Fluvoxamine vs Placebo and Clinical Deterioration in Outpatients With Symptomatic COVID-19: A Randomized Clinical Trial. JAMA 2020, 324, 2292–2300. [Google Scholar] [CrossRef]
- Zhu, F.-C.; Li, Y.-H.; Guan, X.-H.; Hou, L.-H.; Wang, W.-J.; Li, J.-X.; Wu, S.-P.; Wang, B.-S.; Wang, Z.; Wang, L.; et al. Safety, Tolerability, and Immunogenicity of a Recombinant Adenovirus Type-5 Vectored COVID-19 Vaccine: A Dose-Escalation, Open-Label, Non-Randomised, First-in-Human Trial. Lancet Lond. Engl. 2020, 395, 1845–1854. [Google Scholar] [CrossRef]
- Schmidt, T.; Klemis, V.; Schub, D.; Mihm, J.; Hielscher, F.; Marx, S.; Abu-Omar, A.; Ziegler, L.; Guckelmus, C.; Urschel, R.; et al. Immunogenicity and Reactogenicity of Heterologous ChAdOx1 NCoV-19/MRNA Vaccination. Nat. Med. 2021, 27, 1530–1535. [Google Scholar] [CrossRef]
- Logunov, D.Y.; Dolzhikova, I.V.; Shcheblyakov, D.V.; Tukhvatulin, A.I.; Zubkova, O.V.; Dzharullaeva, A.S.; Kovyrshina, A.V.; Lubenets, N.L.; Grousova, D.M.; Erokhova, A.S.; et al. Safety and Efficacy of an RAd26 and RAd5 Vector-Based Heterologous Prime-Boost COVID-19 Vaccine: An Interim Analysis of a Randomised Controlled Phase 3 Trial in Russia. Lancet 2021, 397, 671–681. [Google Scholar] [CrossRef]
- Bae, S.; Lee, Y.W.; Lim, S.Y.; Lee, J.H.; Lim, J.S.; Lee, S.; Park, S.; Kim, S.K.; Lim, Y.J.; Kim, E.O.; et al. Adverse Reactions Following the First Dose of ChAdOx1 NCoV-19 Vaccine and BNT162b2 Vaccine for Healthcare Workers in South Korea. J. Korean Med. Sci. 2021, 36, e115. [Google Scholar] [CrossRef]
- Ramasamy, M.N.; Minassian, A.M.; Ewer, K.J.; Flaxman, A.L.; Folegatti, P.M.; Owens, D.R.; Voysey, M.; Aley, P.K.; Angus, B.; Babbage, G.; et al. Safety and Immunogenicity of ChAdOx1 NCoV-19 Vaccine Administered in a Prime-Boost Regimen in Young and Old Adults (COV002): A Single-Blind, Randomised, Controlled, Phase 2/3 Trial. Lancet 2020, 396, 1979–1993. [Google Scholar] [CrossRef]
- Dorrington, M.G.; Bowdish, D.M.E. Immunosenescence and Novel Vaccination Strategies for the Elderly. Front. Immunol. 2013, 4, 171. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yu, S.; Chen, K.; Fang, L.; Mao, H.; Lou, X.; Li, C.; Zhang, Y. Comparison and Analysis of Neutralizing Antibody Levels in Serum after Inoculating with SARS-CoV-2, MERS-CoV, or SARS-CoV Vaccines in Humans. Vaccines 2021, 9, 588. [Google Scholar] [CrossRef] [PubMed]
- Folegatti, P.M.; Ewer, K.J.; Aley, P.K.; Angus, B.; Becker, S.; Belij-Rammerstorfer, S.; Bellamy, D.; Bibi, S.; Bittaye, M.; Clutterbuck, E.A.; et al. Safety and Immunogenicity of the ChAdOx1 NCoV-19 Vaccine against SARS-CoV-2: A Preliminary Report of a Phase 1/2, Single-Blind, Randomised Controlled Trial. Lancet 2020, 396, 467–478. [Google Scholar] [CrossRef]
- van Aalst, M.; Langedijk, A.C.; Spijker, R.; de Bree, G.J.; Grobusch, M.P.; Goorhuis, A. The Effect of Immunosuppressive Agents on Immunogenicity of Pneumococcal Vaccination: A Systematic Review and Meta-Analysis. Vaccine 2018, 36, 5832–5845. [Google Scholar] [CrossRef] [PubMed]
- Zimmermann, P.; Curtis, N. Factors That Influence the Immune Response to Vaccination. Clin. Microbiol. Rev. 2019, 32, e00084-18. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ebinger, J.E.; Fert-Bober, J.; Printsev, I.; Wu, M.; Sun, N.; Prostko, J.C.; Frias, E.C.; Stewart, J.L.; Van Eyk, J.E.; Braun, J.G.; et al. Antibody Responses to the BNT162b2 MRNA Vaccine in Individuals Previously Infected with SARS-CoV-2. Nat. Med. 2021, 27, 1–4. [Google Scholar] [CrossRef] [PubMed]
- Van Gils, M.J.; van Willigen, H.D.; Wynberg, E.; Han, A.X.; van der Straten, K.; Verveen, A.; Lebbink, R.; Dijkstra, M.; Burger, J.A.; Oomen, M.; et al. Single-Dose SARS-CoV-2 Vaccine in a Prospective Cohort of COVID-19 Patients. medRxiv 2021. [Google Scholar] [CrossRef]
- Goel, R.R.; Apostolidis, S.A.; Painter, M.M.; Mathew, D.; Pattekar, A.; Kuthuru, O.; Gouma, S.; Hicks, P.; Meng, W.; Rosenfeld, A.M.; et al. Distinct Antibody and Memory B Cell Responses in SARS-CoV-2 Naïve and Recovered Individuals Following MRNA Vaccination. Sci. Immunol. 2021, 6, eabi6950. [Google Scholar] [CrossRef]
- Krammer, F.; Srivastava, K.; The PARIS Team; Simon, V. Robust Spike Antibody Responses and Increased Reactogenicity in Seropositive Individuals after a Single Dose of SARS-CoV-2 MRNA Vaccine. medRxiv 2021. [Google Scholar] [CrossRef]
- Dispinseri, S.; Secchi, M.; Pirillo, M.F.; Tolazzi, M.; Borghi, M.; Brigatti, C.; De Angelis, M.L.; Baratella, M.; Bazzigaluppi, E.; Venturi, G.; et al. Neutralizing Antibody Responses to SARS-CoV-2 in Symptomatic COVID-19 Is Persistent and Critical for Survival. Nat. Commun. 2021, 12, 2670. [Google Scholar] [CrossRef]
- Garcia-Beltran, W.F.; Lam, E.C.; Astudillo, M.G.; Yang, D.; Miller, T.E.; Feldman, J.; Hauser, B.M.; Caradonna, T.M.; Clayton, K.L.; Nitido, A.D.; et al. COVID-19-Neutralizing Antibodies Predict Disease Severity and Survival. Cell 2021, 184, 476–488. [Google Scholar] [CrossRef]
- Anichini, G.; Terrosi, C.; Gandolfo, C.; Gori Savellini, G.; Fabrizi, S.; Miceli, G.B.; Cusi, M.G. SARS-CoV-2 Antibody Response in Persons with Past Natural Infection. N. Engl. J. Med. 2021, 385, 90–92. [Google Scholar] [CrossRef]
- Morales-Núñez, J.J.; Muñoz-Valle, J.F.; Meza-López, C.; Wang, L.-F.; Machado Sulbarán, A.C.; Torres-Hernández, P.C.; Bedolla-Barajas, M.; De la O-Gómez, B.; Balcázar-Félix, P.; Hernández-Bello, J. Neutralizing Antibodies Titers and Side Effects in Response to BNT162b2 Vaccine in Healthcare Workers with and without Prior SARS-CoV-2 Infection. Vaccines 2021, 9, 742. [Google Scholar] [CrossRef]
- Barlow-Pay, F.; Htut, T.W.; Khezrian, M.; Myint, P.K. Systematic Review of Immunosuppressant Guidelines in the COVID-19 Pandemic. Ther. Adv. Drug Saf. 2021, 12, 2042098620985687. [Google Scholar] [CrossRef] [PubMed]
- Spiera, R.; Jinich, S.; Jannat-Khah, D. Rituximab, but not other antirheumatic therapies, is associated with impaired serological response to SARS- CoV-2 vaccination in patients with rheumatic diseases. Ann. Rheum. Dis. 2021, 80, 1357–1359. [Google Scholar] [CrossRef]
- Deepak, P.; Kim, W.; Paley, M.A.; Yang, M.; Carvidi, A.B.; El-Qunni, A.A.; Haile, A.; Huang, K.; Kinnett, B.; Liebeskind, M.J.; et al. Glucocorticoids and B Cell Depleting Agents Substantially Impair Immunogenicity of MRNA Vaccines to SARS-CoV-2. medRxiv 2021. [Google Scholar] [CrossRef]
- Sprent, J.; King, C. COVID-19 Vaccine Side Effects: The Positives about Feeling Bad. Sci. Immunol. 2021, 6. [Google Scholar] [CrossRef] [PubMed]
- Jackson, L.A.; Anderson, E.J.; Rouphael, N.G.; Roberts, P.C.; Makhene, M.; Coler, R.N.; McCullough, M.P.; Chappell, J.D.; Denison, M.R.; Stevens, L.J.; et al. An MRNA Vaccine against SARS-CoV-2—Preliminary Report. N. Engl. J. Med. 2020, 383, 1920–1931. [Google Scholar] [CrossRef] [PubMed]
- Connors, J.; Bell, M.R.; Marcy, J.; Kutzler, M.; Haddad, E.K. The Impact of Immuno-Aging on SARS-CoV-2 Vaccine Development. GeroScience 2021, 43, 31–51. [Google Scholar] [CrossRef]
- Cox, L.S.; Bellantuono, I.; Lord, J.M.; Sapey, E.; Mannick, J.B.; Partridge, L.; Gordon, A.L.; Steves, C.J.; Witham, M.D. Tackling Immunosenescence to Improve COVID-19 Outcomes and Vaccine Response in Older Adults. Lancet Healthy Longev. 2020, 1, e55–e57. [Google Scholar] [CrossRef]
- Pawelec, G.; McElhaney, J. Unanticipated Efficacy of SARS-CoV-2 Vaccination in Older Adults. Immun. Ageing 2021, 18, 7. [Google Scholar] [CrossRef]
- Coughlan, L. Factors Which Contribute to the Immunogenicity of Non-Replicating Adenoviral Vectored Vaccines. Front. Immunol. 2020, 11, 909. [Google Scholar] [CrossRef]
- Rauch, S.; Jasny, E.; Schmidt, K.E.; Petsch, B. New Vaccine Technologies to Combat Outbreak Situations. Front. Immunol. 2018, 9, 1963. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Galipeau, Y.; Greig, M.; Liu, G.; Driedger, M.; Langlois, M.-A. Humoral Responses and Serological Assays in SARS-CoV-2 Infections. Front. Immunol. 2020, 11, 3382. [Google Scholar] [CrossRef] [PubMed]
- Meyer, B.; Reimerink, J.; Torriani, G.; Brouwer, F.; Godeke, G.-J.; Yerly, S.; Hoogerwerf, M.; Vuilleumier, N.; Kaiser, L.; Eckerle, I.; et al. Validation and Clinical Evaluation of a SARS-CoV-2 Surrogate Virus Neutralisation Test (SVNT). Emerg. Microbes. Infect. 2020, 9, 2394–2403. [Google Scholar] [CrossRef] [PubMed]
- Sahin, U.; Muik, A.; Vogler, I.; Derhovanessian, E.; Kranz, L.M.; Vormehr, M.; Quandt, J.; Bidmon, N.; Ulges, A.; Baum, A.; et al. BNT162b2 Induces SARS-CoV-2-Neutralising Antibodies and T Cells in Humans. medRxiv 2020. [Google Scholar] [CrossRef]
| Immunized with Ad5-nCoV Vaccine | p-Value | ||
|---|---|---|---|
| without Prior COVID-19 n = 229 | with Prior COVID-19 n = 117 | ||
| Age (years), mean±SD | 41.6 ± 10.6 | 39.8 ± 10.9 | 0.128 |
| Gender, n,(%) | |||
| Female | 166 (72.5) | 79 (67.5) | 0.338 |
| Male | 63 (27.5) | 38 (32.5) | |
| Days of the last positive PCR test for COVID-19 median(IQR) | - | 158 (130–241) | - |
| Comorbidities, n,(%) | |||
| None | 101 (44.1) | 50 (42.7) | 0.80 |
| Overweight/obesity | 75 (32.8) | 41 (35.0) | 0.67 |
| Allergic diseases | 32 (14.0) | 13 (11.1) | 0.45 |
| SAH | 24 (10.5) | 9 (7.7) | 0.39 |
| Diabetes | 15 (6.6) | 5 (4.3) | 0.39 |
| Hypothyroidism | 8 (3.5) | 2 (1.7) | 0.35 |
| Autoimmune diseases | 8 (3.5) | 1 (0.9) | 0.14 |
| Dermatitis | 3 (1.3) | 3 (2.6) | 0.39 |
| Dyslipidemia | 4 (1.7) | 2 (1.7) | 0.98 |
| Heart diseases | 4 (1.7) | 1 (0.9) | 0.51 |
| Treatment, n,(%) | |||
| Antihypertensive | 25 (10.9) | 10 (8.5) | 0.49 |
| Antidepressants | 23 (10.0) | 2 (1.7) | 0.005 |
| Hormonal | 20 (8.7) | 7 (6.0) | 0.368 |
| Hypoglycemic agents | 17 (7.4) | 6 (5.1) | 0.41 |
| Hypolipidemic agents | 3 (1.3) | 4 (3.4) | 0.18 |
| NSAIDs | 7 (3.1) | 3 (2.6) | 0.79 |
| Antihistamines | 3 (1.3) | 2 (1.7) | 0.76 |
| Immunosuppressants | 4 (1.7) | 1 (1.7) | 0.51 |
| Side Effects | Immunized with Vaccine Ad5-nCoV | p-Value | |
|---|---|---|---|
| without Prior COVID-19 n = 229 | with Prior COVID-19 n = 117 | ||
| At least onemedian(Q25–Q75) | 160 (69.9) | 86 (73.5) | 0.482 |
| Number of symptoms, mean±SD | 2.28 ± 2.321 | 2.85 ± 2.964 | 0.054 |
| Headache n,(%) | 107 (46.7) | 58 (49.6) | 0.617 |
| Myalgia n,(%) | 82 (35.8) | 52 (44.4) | 0.119 |
| Fatigue n,(%) | 92 (40.2) | 48 (41) | 0.879 |
| Shivers n,(%) | 54 (23.6) | 34 (29.1) | 0.270 |
| Fever n,(%) | 48 (21) | 33 (28.2) | 0.133 |
| Arthralgia n,(%) | 45 (19.7) | 33 (28.2) | 0.072 |
| Irritability n,(%) | 29 (12.7) | 13 (11.1) | 0.677 |
| Abdominal pain n,(%) | 11 (4.8) | 10 (8.5) | 0.169 |
| Odynophagia n,(%) | 8 (3.5) | 10 (8.5) | 0.002 |
| Rhinorrhea n,(%) | 15 (6.6) | 6 (5.1) | 0.601 |
| Cough n,(%) | 9 (3.9) | 3 (2.6) | 0.513 |
| Dizziness n,(%) | 6 (2.6) | 3 (2.6) | 0.975 |
| Conjunctivitis n,(%) | 4 (1.7) | 3 (2.6) | 0.611 |
| Application-site pain n,(%) | 3 (1.3) | 3 (2.6) | 0.399 |
| Vomit n,(%) | 5 (2.2) | 2 (1.7) | 0.768 |
| Percentage of Neutralization, Median (Q25–Q75) (n = 346) | p-Value | |
|---|---|---|
| Comorbidity | ||
| With autoimmunity (n = 9) | 60.58 (24.98–88.85) | 0.053 |
| Without autoimmunity (n = 337) | 87.90 (62.49–97.79) | |
| Treatment | ||
| Use of antidepressants (n = 25) | 72.65 (39.43–87.77) | 0.017 |
| No use of antidepressants (n = 321) | 89.61 (61.64–97.81) | |
| Use of immunosuppressants (n = 5) | 23.52 (22.85–28.5) | 0.018 |
| No use of immunosuppressants (n = 341) | 86.76 (61.16–97.78) | |
| Side-effects to Ad5-nCoV vaccine | ||
| With fever (n = 81) | 96.20 (73.47–97.96) | 0.008 |
| Without fever (n = 265) | 83.60 (56.51–97.75) | |
| With shivers (n = 88) | 91.03 (73.96–97.94) | 0.038 |
| Without shivers (n = 258) | 84.41 (57.08–97.76) | |
| With arthralgia (n = 78) | 95.79 (73.34–97.99) | 0.009 |
| Without arthralgia (n = 268) | 83.60 (58.29–97.72) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hernández-Bello, J.; Morales-Núñez, J.J.; Machado-Sulbarán, A.C.; Díaz-Pérez, S.A.; Torres-Hernández, P.C.; Balcázar-Félix, P.; Gutiérrez-Brito, J.A.; Lomelí-Nieto, J.A.; Muñoz-Valle, J.F. Neutralizing Antibodies against SARS-CoV-2, Anti-Ad5 Antibodies, and Reactogenicity in Response to Ad5-nCoV (CanSino Biologics) Vaccine in Individuals with and without Prior SARS-CoV-2. Vaccines 2021, 9, 1047. https://doi.org/10.3390/vaccines9091047
Hernández-Bello J, Morales-Núñez JJ, Machado-Sulbarán AC, Díaz-Pérez SA, Torres-Hernández PC, Balcázar-Félix P, Gutiérrez-Brito JA, Lomelí-Nieto JA, Muñoz-Valle JF. Neutralizing Antibodies against SARS-CoV-2, Anti-Ad5 Antibodies, and Reactogenicity in Response to Ad5-nCoV (CanSino Biologics) Vaccine in Individuals with and without Prior SARS-CoV-2. Vaccines. 2021; 9(9):1047. https://doi.org/10.3390/vaccines9091047
Chicago/Turabian StyleHernández-Bello, Jorge, José Javier Morales-Núñez, Andrea Carolina Machado-Sulbarán, Saúl Alberto Díaz-Pérez, Paola Carolina Torres-Hernández, Paulina Balcázar-Félix, Jesús Alberto Gutiérrez-Brito, José Alvaro Lomelí-Nieto, and José Francisco Muñoz-Valle. 2021. "Neutralizing Antibodies against SARS-CoV-2, Anti-Ad5 Antibodies, and Reactogenicity in Response to Ad5-nCoV (CanSino Biologics) Vaccine in Individuals with and without Prior SARS-CoV-2" Vaccines 9, no. 9: 1047. https://doi.org/10.3390/vaccines9091047








