Immune Responses to SARS-CoV-2 Variants WT and XBB.1.9: Assessing Vulnerabilities and Preparedness
Abstract
1. Introduction
2. Methods
2.1. Study Population and Setting
2.2. Neutralization Assay
2.3. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Zheng, Y.X.; Wang, L.; Kong, W.S.; Chen, H.; Wang, X.N.; Meng, Q.; Zhang, H.N.; Guo, S.J.; Jiang, H.W.; Tao, S.C. Nsp2 Has the Potential to Be a Drug Target Revealed by Global Identification of SARS-CoV-2 Nsp2-Interacting Proteins. Acta Biochim. Biophys. Sin. 2021, 53, 1134–1141. [Google Scholar] [CrossRef]
- Dousari, A.S.; Moghadam, M.T.; Satarzadeh, N. COVID-19 (Coronavirus Disease 2019): A New Coronavirus Disease. Infect. Drug Resist. 2020, 13, 2819–2828. [Google Scholar] [CrossRef] [PubMed]
- Velavan, T.P.; Meyer, C.G. The COVID-19 Epidemic. In Tropical Medicine and International Health; Blackwell Publishing Ltd.: Hoboken, NJ, USA, 2020; pp. 278–280. [Google Scholar] [CrossRef]
- Last, M. The First Wave of COVID-19 in Israel—Initial Analysis of Publicly Available Data. PLoS ONE 2020, 15, e0240393. [Google Scholar] [CrossRef] [PubMed]
- Chakraborty, C.; Bhattacharya, M.; Chopra, H.; Islam, M.A.; Saikumar, G.; Dhama, K. The SARS-CoV-2 Omicron Recombinant Subvariants XBB, XBB.1, and XBB.1.5 Are Expanding Rapidly with Unique Mutations, Antibody Evasion, and Immune Escape Properties–an Alarming Global Threat of a Surge in COVID-19 Cases Again? Int. J. Surg. 2023, 109, 1041–1043. [Google Scholar] [CrossRef] [PubMed]
- Lustig, Y.; Canetti, M.; Indenbaum, V.; Peretz, Y.; Weiss-Ottolenghi, Y.; Margalit, I.; Asraf, K.; Levin, T.; Zuckerman, N.; Tomer, E.; et al. SARS-CoV-2 IgG Levels as Predictors of XBB Variant Neutralization, Israel, 2022 and 2023. Emerg. Infect. Dis. 2024, 30, 1050. [Google Scholar] [CrossRef]
- Volz, E.; Mishra, S.; Chand, M.; Barrett, J.C.; Johnson, R.; Geidelberg, L.; Hinsley, W.R.; Laydon, D.J.; Dabrera, G.; O’Toole, Á.; et al. Assessing Transmissibility of SARS-CoV-2 Lineage B.1.1.7 in England. Nature 2021, 593, 266–269. [Google Scholar] [CrossRef]
- Tamura, T.; Ito, J.; Uriu, K.; Zahradnik, J.; Kida, I.; Anraku, Y.; Nasser, H.; Shofa, M.; Oda, Y.; Lytras, S.; et al. Virological Characteristics of the SARS-CoV-2 XBB Variant Derived from Recombination of Two Omicron Subvariants. Nat. Commun. 2023, 14, 2800. [Google Scholar] [CrossRef]
- Lasrado, N.; Collier, A.Y.; Miller, J.; Hachmann, N.P.; Liu, J.; Sciacca, M.; Wu, C.; Anand, T.; Bondzie, E.A.; Fisher, J.L.; et al. Waning Immunity Against XBB.1.5 Following Bivalent MRNA Boosters. bioRxiv 2023. [Google Scholar] [CrossRef]
- Haas, E.J.; McLaughlin, J.M.; Khan, F.; Angulo, F.J.; Anis, E.; Lipsitch, M.; Singer, S.R.; Mircus, G.; Brooks, N.; Smaja, M.; et al. Infections, Hospitalisations, and Deaths Averted via a Nationwide Vaccination Campaign Using the Pfizer–BioNTech BNT162b2 MRNA COVID-19 Vaccine in Israel: A Retrospective Surveillance Study. Lancet Infect. Dis. 2022, 22, 357–366. [Google Scholar] [CrossRef]
- Davis, C.; Logan, N.; Tyson, G.; Orton, R.; Harvey, W.T.; Perkins, J.S.; Mollett, G.; Blacow, R.M.; Peacock, T.P.; Barclay, W.S.; et al. Reduced Neutralisation of the Delta (B.1.617.2) SARS-CoV-2 Variant of Concern Following Vaccination. PLoS Pathog. 2021, 17, e1010022. [Google Scholar] [CrossRef]
- Higdon, M.M.; Baidya, A.; Walter, K.K.; Patel, M.K.; Issa, H.; Espié, E.; Feikin, D.R.; Knoll, M.D. Duration of Effectiveness of Vaccination against COVID-19 Caused by the Omicron Variant. Lancet Infect. Dis. 2022, 22, 1114–1116. [Google Scholar] [CrossRef] [PubMed]
- Tenforde, M.W.; Weber, Z.A.; Natarajan, K.; Klein, N.P.; Kharbanda, A.B.; Stenehjem, E.; Embi, P.J.; Reese, S.E.; Naleway, A.L.; Grannis, S.J.; et al. Early Estimates of Bivalent MRNA Vaccine Effectiveness in Preventing COVID-19-Associated Emergency Department or Urgent Care Encounters and Hospitalizations Among Immunocompetent Adults-VISION Network, Nine States, September-November 2022. Morb. Mortal. Wkly. Rep. 2022, 71, 1616–1624. Available online: https://www.cdc.gov/mmwr/volumes/71/wr/mm715152e1.htm?utm_medium=email&utm_source=transaction (accessed on 5 November 2025).
- Zou, J.; Kurhade, C.; Patel, S.; Kitchin, N.; Tompkins, K.; Cutler, M.; Cooper, D.; Yang, Q.; Cai, H.; Muik, A.; et al. Neutralization of BA.4–BA.5, BA.4.6, BA.2.75.2, BQ.1.1, and XBB.1 with Bivalent Vaccine. N. Engl. J. Med. 2023, 388, 854–857. [Google Scholar] [CrossRef] [PubMed]
- Lin, D.-Y.; Du, Y.; Xu, Y.; Paritala, S.; Donahue, M.; Maloney, P. Durability of XBB.1.5 Vaccines against Omicron Subvariants. N. Engl. J. Med. 2024, 390, 2124–2127. [Google Scholar] [CrossRef]
- Lavery, B.P. Updated 2024–2025 MRNA COVID-19 Vaccines Approved and Granted EUA by FDA. 2025; pp. 1–5. Available online: https://www.fda.gov/news-events/press-announcements/fda-approves-and-authorizes-updated-mrna-covid-19-vaccines-better-protect-against-currently (accessed on 5 November 2025).
- Kliker, L.; Zuckerman, N.; Atari, N.; Barda, N.; Gilboa, M.; Nemet, I.; Elkader, B.A.; Fratty, I.S.; Jaber, H.; Mendelson, E.; et al. COVID-19 Vaccination and BA.1 Breakthrough Infection Induce Neutralising Antibodies Which Are Less Efficient against BA.4 and BA.5 Omicron Variants, Israel, March to June 2022. Eurosurveillance 2022, 27, 2200559. [Google Scholar] [CrossRef]
- Atari, N.; Kliker, L.; Zuckerman, N.; Elkader, B.A.; Weiss-Ottolenghi, Y.; Mendelson, E.; Kreiss, Y.; Regev-Yochay, G.; Mandelboim, M. Omicron BA.2.75 Variant Is Efficiently Neutralised Following BA.1 and BA.5 Breakthrough Infection in Vaccinated Individuals, Israel, June to September 2022. Eurosurveillance 2022, 27, 2200785. [Google Scholar] [CrossRef]
- Peled, Y.; Afek, A.; Patel, J.K.; Raanani, E.; Segev, A.; Ram, E.; Fardman, A.; Beigel, R.; Jurkowicz, M.; Atari, N.; et al. Sixth Monovalent XBB.1.5 Vaccine Elicits Robust Immune Response against Emerging SARS-CoV-2 Variants in Heart Transplant Recipients. J. Hear. Lung Transplant. 2024, 43, 1188–1192. [Google Scholar] [CrossRef]
- Bar-On, Y.M.; Goldberg, Y.; Mandel, M.; Bodenheimer, O.; Freedman, L.; Kalkstein, N.; Mizrahi, B.; Alroy-Preis, S.; Ash, N.; Milo, R.; et al. Protection of BNT162b2 Vaccine Booster against Covid-19 in Israel. N. Engl. J. Med. 2021, 385, 1393–1400. [Google Scholar] [CrossRef]
- Cui, T.; Su, X.; Sun, J.; Liu, S.; Huang, M.; Li, W.; Luo, C.; Cheng, L.; Wei, R.; Song, T.; et al. Dynamic Immune Landscape in Vaccinated-BA.5-XBB.1.9.1 Reinfections Revealed a 5-Month Protection-Duration against XBB Infection and a Shift in Immune Imprinting. eBioMedicine 2024, 99, 104903. [Google Scholar] [CrossRef]
- Tseng, H.F.; Ackerson, B.K.; Sy, L.S.; Tubert, J.E.; Luo, Y.; Qiu, S.; Lee, G.S.; Bruxvoort, K.J.; Ku, J.H.; Florea, A.; et al. MRNA-1273 Bivalent (Original and Omicron) COVID-19 Vaccine Effectiveness against COVID-19 Outcomes in the United States. Nat. Commun. 2023, 14, 5851. [Google Scholar] [CrossRef]
- Central Bureau of Statistics. Characterisation and Classification of Geographical Units by the Socio-Economic Level of the Population 2019; Special Publication No. 1903; Central Bureau of Statistics: Jerusalem, Israel, 2021.
- Bassal, R.; Cohen, D.; Green, M.S.; Keinan-Boker, L. The Israel National Sera Bank: Methods, Representativeness, and Challenges. Int. J. Environ. Res. Public Health 2021, 18, 2280. [Google Scholar] [CrossRef]
- Uraki, R.; Ito, M.; Kiso, M.; Yamayoshi, S.; Iwatsuki-Horimoto, K.; Sakai-Tagawa, Y.; Imai, M.; Koga, M.; Yamamoto, S.; Adachi, E.; et al. Antiviral Efficacy against and Replicative Fitness of an XBB.1.9.1 Clinical Isolate. iScience 2023, 26, 108147. [Google Scholar] [CrossRef]
- Rosen, B.; Waitzberg, R.; Israeli, A. Israel’s Rapid Rollout of Vaccinations for COVID-19. Isr. J. Health Policy Res. 2021, 10, 6. [Google Scholar] [CrossRef] [PubMed]
- Andrews, N.; Stowe, J.; Kirsebom, F.; Toffa, S.; Sachdeva, R.; Gower, C.; Ramsay, M.; Lopez Bernal, J. Effectiveness of COVID-19 Booster Vaccines against COVID-19-Related Symptoms, Hospitalization and Death in England. Nat. Med. 2022, 28, 831–837. [Google Scholar] [CrossRef] [PubMed]
- Burki, T.K. Fourth Dose of COVID-19 Vaccines in Israel. Lancet Respir. Med. 2022, 10, e19. [Google Scholar] [CrossRef] [PubMed]
- Valanparambil, R.M.; Lai, L.; Johns, M.A.; Davis-Gardner, M.; Linderman, S.L.; McPherson, T.O.; Chang, A.; Akhtar, A.; Gamarra, E.L.B.; Matia, H.; et al. BA.5 Bivalent Booster Vaccination Enhances Neutralization of XBB.1.5, XBB.1.16 and XBB.1.9 Variants in Patients with Lung Cancer. npj Vaccines 2023, 8, 179. [Google Scholar] [CrossRef]
- Ministry of Health’s World of Data. Available online: https://datadashboard.health.gov.il/portal/dashboard/corona (accessed on 5 November 2025).
- Oda, Y.; Kumagai, Y.; Kanai, M.; Iwama, Y.; Okura, I.; Minamida, T.; Yagi, Y.; Kurosawa, T.; Greener, B.; Zhang, Y.; et al. Immunogenicity and Safety of a Booster Dose of a Self-Amplifying RNA COVID-19 Vaccine (ARCT-154) versus BNT162b2 MRNA COVID-19 Vaccine: A Double-Blind, Multicentre, Randomised, Controlled, Phase 3, Non-Inferiority Trial. Lancet Infect. Dis. 2024, 24, 351–360. [Google Scholar] [CrossRef]
- Saban, M.; Myers, V.; Ben-Shetrit, S.; Wilf-Miron, R. Socioeconomic Gradient in COVID-19 Vaccination: Evidence from Israel. Int. J. Equity Health 2021, 20, 242. [Google Scholar] [CrossRef]
- Shkalim Zemer, V.; Grossman, Z.; Cohen, H.A.; Hoshen, M.; Gerstein, M.; Yosef, N.; Cohen, M.; Ashkenazi, S. Acceptance Rates of COVID-19 Vaccine Highlight the Need for Targeted Public Health Interventions. Vaccines 2022, 10, 1167. [Google Scholar] [CrossRef]
- Waitzberg, R.; Davidovitch, N.; Leibner, G.; Penn, N.; Brammli-Greenberg, S. Israel’s Response to the COVID-19 Pandemic: Tailoring Measures for Vulnerable Cultural Minority Populations. Int. J. Equity Health 2020, 19, 71. [Google Scholar] [CrossRef]
- Muhsen, K.; Green, M.S.; Soskolne, V.; Neumark, Y. Inequalities in Non-Communicable Diseases between the Major Population Groups in Israel: Achievements and Challenges. Lancet 2017, 389, 2531–2541. [Google Scholar] [CrossRef]
- Aga, A.M.; Mulugeta, D.; Gebreegziabxier, A.; Zeleke, G.T.; Girmay, A.M.; Tura, G.B.; Ayele, A.; Mohammed, A.; Belete, T.; Taddele, T.; et al. Genome Diversity of SARS-CoV-2 Lineages Associated with Vaccination Breakthrough Infections in Addis Ababa, Ethiopia. BMC Infect. Dis. 2025, 25, 738. [Google Scholar] [CrossRef]

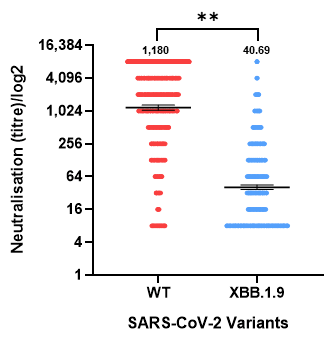
| Group | Number of Participants (%) | GMT (WT) | GMT (XBB.1.9) | p-Value * | |
|---|---|---|---|---|---|
| Total participants | 1140 (100) | 1180 | 40.69 | <0.001 | |
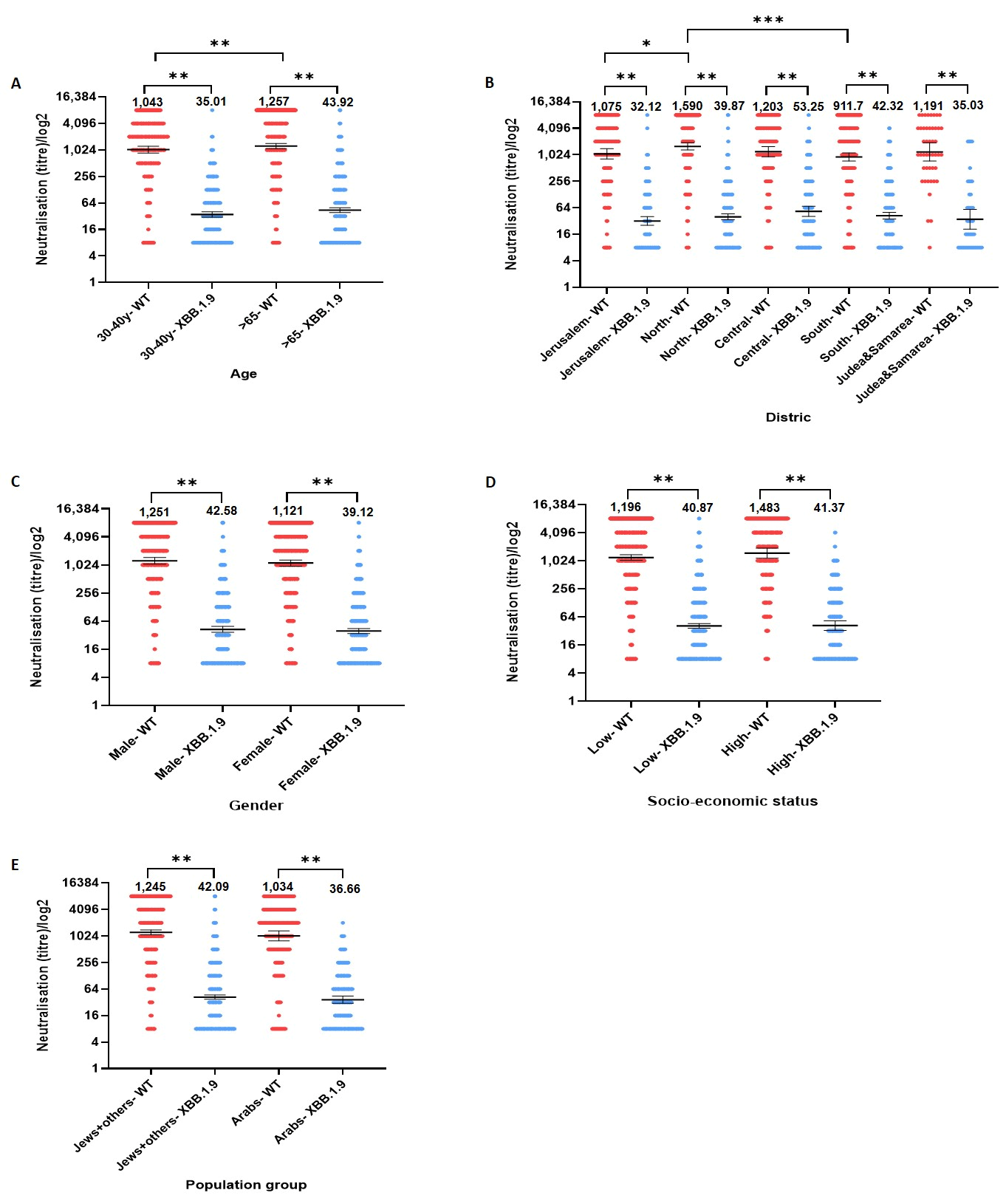
| Gender | Male | 529 (46.4) | 1251 | 42.58 | <0.001 |
| Female | 611 (53.6) | 1121 | 39.12 | <0.001 | |
| Age group | Age 30–39 | 385 (33.77) | 1043 | 35.01 | <0.001 |
| Age 65+ | 755 (66.23) | 1257 | 43.92 | <0.001 | |
| Socioeconomic status | Low SES | 782 (68.6) | 1196 | 40.87 | <0.001 |
| High SES | 189 (16.58) | 1483 | 41.37 | <0.001 | |
| Population group | Jews and others | 855 (75) | 1245 | 42.09 | <0.001 |
| Arabs | 214 (18.77) | 1034 | 36.66 | <0.001 | |
| District | Jerusalem | 184 (16.14) | 1075 | 32.12 | <0.001 |
| North | 372 (32.63) | 1590 | 39.87 | <0.001 | |
| Central | 181 (15.88) | 1203 | 53.25 | <0.001 | |
| South | 352 (30.88) | 911.7 | 42.32 | <0.001 | |
| Judea & Samaria | 46 (4.04) | 1191 | 35.03 | <0.001 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kliker, L.; Mandelboim, M.; Jurkowicz, M.; Zuckerman, N.S.; Tomer, E.; Lustig, Y.; Keinan-Boker, L.; Indenbaum, V.; Bassal, R. Immune Responses to SARS-CoV-2 Variants WT and XBB.1.9: Assessing Vulnerabilities and Preparedness. Vaccines 2025, 13, 1167. https://doi.org/10.3390/vaccines13111167
Kliker L, Mandelboim M, Jurkowicz M, Zuckerman NS, Tomer E, Lustig Y, Keinan-Boker L, Indenbaum V, Bassal R. Immune Responses to SARS-CoV-2 Variants WT and XBB.1.9: Assessing Vulnerabilities and Preparedness. Vaccines. 2025; 13(11):1167. https://doi.org/10.3390/vaccines13111167
Chicago/Turabian StyleKliker, Limor, Michal Mandelboim, Menucha Jurkowicz, Neta S. Zuckerman, Enosh Tomer, Yaniv Lustig, Lital Keinan-Boker, Victoria Indenbaum, and Ravit Bassal. 2025. "Immune Responses to SARS-CoV-2 Variants WT and XBB.1.9: Assessing Vulnerabilities and Preparedness" Vaccines 13, no. 11: 1167. https://doi.org/10.3390/vaccines13111167
APA StyleKliker, L., Mandelboim, M., Jurkowicz, M., Zuckerman, N. S., Tomer, E., Lustig, Y., Keinan-Boker, L., Indenbaum, V., & Bassal, R. (2025). Immune Responses to SARS-CoV-2 Variants WT and XBB.1.9: Assessing Vulnerabilities and Preparedness. Vaccines, 13(11), 1167. https://doi.org/10.3390/vaccines13111167






