1. Introduction
Influenza is a leading cause of lower respiratory tract infections (LRTIs) across all age groups, accounting for approximately 11.5% of all LRTI cases globally. In 2017 alone, the influenza virus was responsible for an estimated 54.5 million LRTI episodes, including 8.17 million severe cases worldwide [
1]. Beyond LRTIs, influenza contributes to a broader spectrum of disease burden, including cardiovascular events, exacerbation of chronic diseases, secondary bacterial infections, functional decline, and adverse pregnancy outcomes, especially among immunocompromised individuals [
2]. These complications significantly increase the risk of hospitalization and mortality, with approximately 145,000 influenza-related deaths reported globally each year [
1].
Vaccination remains the most effective strategy for preventing influenza infection. Current vaccine platforms include inactivated vaccines (whole-virus, split-virus, and subunit), live-attenuated vaccines, and recombinant protein vaccines [
3]. Among them, egg-based inactivated and live-attenuated vaccines are widely used but are unsuitable for individuals with egg allergies and may conflict with religious dietary restrictions [
3]. In contrast, recombinant vaccines such as Cadiflu-S [
4] and Flublok (also marketed as Supemtek Tetra) [
5] eliminate egg-derived components and enable faster, more scalable production. However, a key limitation of currently licensed recombinant influenza vaccines is the lack of an adjuvant.
Adjuvants have been shown to significantly enhance the immunogenicity of inactivated influenza vaccines, particularly in populations with weaker immune responses. For example, MF59-adjuvanted vaccines are widely approved for use in older adults aged ≥65 years to reduce the risk of severe influenza and its complications [
6,
7] and are also approved for children ≥6 months old in several countries, including Canada [
8]. Despite these benefits, no adjuvanted recombinant influenza vaccines are currently available.
Meanwhile, the effectiveness of seasonal influenza vaccines remains highly variable. Between the 2009–2010 pandemic and the 2019–2020 flu seasons, the overall vaccine effectiveness (VE) was moderate in the Southern Hemisphere (54%; 95% CI: 48–59%) but lower in the Northern Hemisphere (37%; 95% CI: 32–42%) [
9]. According to data from the U.S. Centers for Disease Control and Prevention (CDC), VE between 2009 and 2025 ranged from 19% to 60%. The lowest VE (19%) occurred during the 2014–2015 season, primarily due to antigenic drift in the circulating A/H3N2 strain that led to a mismatch with the vaccine strain [
10]. This antigenic drift is primarily driven by the lack of proofreading capability in the viral RNA-dependent RNA polymerase, leading to a high mutation rate [
11,
12]. Among the major influenza subtypes, A/H3N2 evolves the fastest, with an estimated adaptation rate of 5.7 × 10
−3 substitutions per codon per year, followed by A/H1N1 (3.2 × 10
−3) and the B/Victoria lineage (1.8 × 10
−3).
Taken together, these limitations underscore the urgent need for next-generation influenza vaccines that combine recombinant production platforms with potent adjuvants to enhance immunogenicity and provide broader, more durable protection against rapidly evolving influenza viruses. In this context, structural insights into influenza virions become particularly important. The viral envelope is densely packed with surface glycoproteins—approximately 300–400 HA and 40–50 neuraminidase (NA) molecules per 120 nm virion [
13]. Among these, HA plays a crucial role in mediating viral attachment to host sialic acid receptors [
14] and remains the primary antigenic target in influenza vaccine development [
15].
In this study, we developed a trivalent adjuvanted recombinant influenza virus-like particle vaccine (the a-RIV vaccine) based on AH1, AH3, and B/vic HA antigens and subsequently evaluated its immunogenicity, durability, and cross-reactivity in mice to assess its potential as a next-generation influenza vaccine candidate.
2. Materials and Methods
2.1. Cells and Virus
Sf9 cells were purchased from Gibco (Grand Island, NY, USA) and cultured in ExpiSfTM CD Medium, cells were maintained in shake flasks on a shaker platform in a non-humidified, non-CO2 cell culture incubator at 27 °C. Huh-7 and MDCK cells were maintained in Dulbecco’s Modified Eagle Medium (DMEM; Gibco) supplemented with 10% fetal bovine serum (FBS; Gibco) and 1% penicillin–streptomycin (Gibco), and cells were cultured at 37 °C in a humidified incubator with 5% CO2. Chicken and guinea pig red blood cells (RBCs) were obtained from SenBeiJia Biological Technology Co., Ltd. (Nanjing, China) and stored at 2–8 °C. Influenza virus strains were provided and preserved by the Changchun Institute of Biological Products Co., Ltd., (Changchun, China).
2.2. Construct Design and Protein Expression
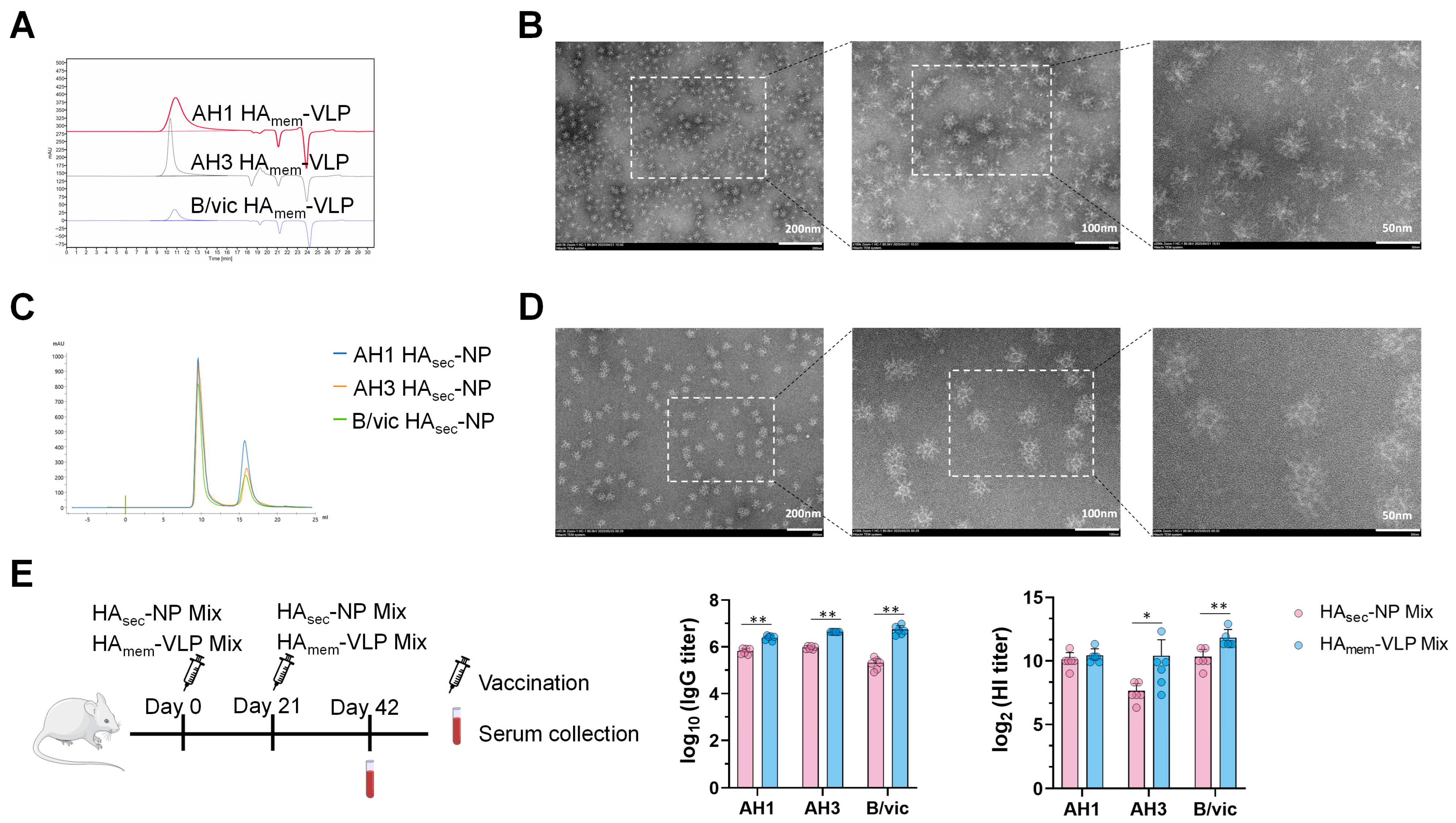
The codon-optimized nucleotide sequences of full-length AH1 HA, AH3 HA, and B/vic HA, along with their secreted variants carrying a C-terminal Spytag, a foldon domain, and a His tag, were synthesized by GenScript (China). For the membrane-anchored HA (HAmem) antigens, the native signal peptide was replaced with the sequence MPLYKLLNVLWLVAVSNA, followed by the full-length HA amino acid sequence, including the ectodomain, transmembrane, and cytoplasmic domains. The expressed regions correspond to residues A17–I566 for A/Wisconsin/67/2022 (H1N1), Q17–I566 for A/Massachusetts/18/2022 (H3N2), and D16–L582 for B/Austria/1359417/2021 (B/Victoria). Retaining the transmembrane and cytoplasmic domains enables insertion of HA trimers into the insect cell membrane, where they associate through hydrophobic interactions and oligomerization. Upon detergent-mediated membrane disruption, these membrane-bound HA trimers self-associate to form nanoparticle-like aggregates (typically 20–40 nm in diameter, comprising approximately 5–20 trimers), mimicking the surface organization of influenza virions. For the secreted HA (HAsec) constructs, the transmembrane and cytoplasmic regions were removed, and the signal peptide was replaced with MVSAIVLYVLLAAAAHSAFA to facilitate secretion. The expressed ectodomains spanned A17–V520 (H1N1), Q17–V521 (H3N2), and D16–S535 (B/Victoria). Because these constructs lack the membrane anchor, the trimeric HA proteins are released into the culture supernatant upon expression and subsequently purified from the medium.
Each HA gene was subsequently cloned into the pOET5.1 transfer plasmid (OET, Oxford, UK). These recombinant plasmids were then co-transfected with flashBAC™ baculovirus genomic DNA (OET) into Sf9 cells using baculoFECTIN II transfection reagent (Mirus Bio, Madison, WI, USA). Following a 5-day incubation, the culture supernatants were harvested to collect the recombinant baculoviruses. These recombinant viruses were subsequently used to infect Sf9 cells at a multiplicity of infection (MOI) of 1. Infected cells were cultured at 27 °C for 3–4 days, after which they were harvested by centrifugation at 2000× g for 30 min. Expression of full-length HA led to the formation of HA membrane protein virus-like particle (HAmem-VLP), whereas secreted HA variant (HAsec) was released into the culture supernatant. For HAmem-VLPs, the supernatants were discarded and the cell pellets were collected for subsequent protein purification. For HAsec proteins, the cell pellets were discarded, and the supernatants were used for downstream purification.
2.3. Protein Purification
Purification of AH3 HAmem-VLP: Following the harvest, Sf9 cells were washed with PBS and lysed using a 1% NP-9 solution to extract AH3 HA membrane protein. The resulting lysate was clarified by centrifugation, and the supernatant was subjected to initial purification using a Diamond MMC Mustang ion-exchange chromatography column (Bestchrom, Shanghai, China). The elution buffer consisted of 20 mM phosphate buffer (PB), 5% glycerol, 0.3% NP-9, 0.05% cysteine, and 120 mM NaCl at a pH of 6.5. The eluted AH3 HA protein was subsequently adjusted to pH 7.0 and further purified using a ceramic hydroxyapatite (CHT) chromatography column (NanoMicro, Suzhou, China). The CHT elution buffer comprised 40 mM PB, 5% glycerol, 0.05% polysorbate 20 (Tween-20), 0.05% cysteine, and 200 mM NaCl at pH 7.0. The final purified AH3 HAmem-VLP was buffer-exchanged into PBS using ultrafiltration tube (Merck, Shanghai, China) and stored at 4 °C.
Purification of AH1 HAmem-VLP: Sf9 cells were washed with PBS and lysed in 1% Triton X-100 to extract AH1 HA membrane protein. The clarified lysate was loaded onto a Diamond MMC Mustang ion-exchange chromatography column. The elution buffer contained 20 mM PB, 5% glycerol, 0.1% Triton X-100, and 160 mM NaCl (pH 6.5). Then the eluted sample was adjusted to pH 7.0 for further purification using a CHT chromatography column. Elution was performed using a linear salt gradient with 10 mM PB, 5% glycerol, 0.05% Tween-20, and 0–1000 mM NaCl at pH 7.4. The final purified AH1 HAmem-VLP was subjected to buffer exchange using ultrafiltration into PBS and subsequently stored at 4 °C.
Purification of B/vic HAmem-VLP: After washing Sf9 cells, the B/vic HA membrane protein was extracted using a 1% NP-9 solution. The clarified supernatant was initially purified with a Nanogel 50Q HC ion-exchange chromatography column (NanoMicro). The partially purified protein was adjusted to pH 6.5 before undergoing a second purification step using a CHT chromatography column. The CHT elution buffer consisted of 20 mM PB, 210 mM NaCl, 0.05% Tween-20, at pH 7.4. The purified B/vic HAmem-VLP was buffer-exchanged into PBS via ultrafiltration and stored at 4 °C.
Purification of AH3, AH1 and B/vic HAsec Proteins and Preparation of HAsec nanoparticles (HAsec-NPs): The cell culture supernatants were first filtered through a 0.22 μm membrane and then purified on an ÄKTA system using prepacked HisTrap Excel and Superdex 200 Increase 10/300 GL columns, with buffer exchange into 20 mM Tris-HCl, 150 mM NaCl, pH 7.4, to obtain highly purified AH3, AH1, and B/vic HAsec proteins. These HAsec proteins were individually mixed with mi3 at a fixed mess ratio (mess ratio of HAsec: mi3 = 1:6) and incubated for 24 h at 22 °C to prepare nanoparticles. The resulting mixtures were subsequently separated on a Superose 6 Increase 10/300 GL column to isolate HAsec-NPs from unbound HAsec proteins. Finally, HAsec-NPs were stored at –80 °C. All columns and the ÄKTA system were purchased from Cytiva (Hauppauge, NY, USA).
As previously described [
16,
17], the mi3 scaffold is a self-assembling 60-mer nanoparticle derived from 2-keto-3-deoxy-6-phosphogluconate (KDPG) aldolase. To enable site-specific antigen display via the SpyTag/SpyCatcher isopeptide conjugation system, the Catcher domain was genetically fused to the mi3 subunit using a flexible linker. The Catcher-mi3 construct was cloned into the pET30a vector and expressed in
Escherichia coli BL21 (DE3) cells cultured in a 5 L bioreactor. Following expression, the bacterial cells were lysed using an AH-PILOT high-pressure homogenizer (ATS Engineering Ltd., Cambridge, Ontario, Canada), and the clarified lysate was purified using a multi-step protocol based on Good Manufacturing Practice (GMP)–compliant procedures. This purification strategy ensured high yield and structural integrity of the assembled 60-mer nanoparticles.
HAmem-VLPs and HAsec-NPs were evaluated by transmission electron microscopy (TEM). The samples were adsorbed onto freshly glow-discharged carbon-coated copper grids (EMCN Co.) for 1 min at room temperature. The grids were negatively stained with 2% phosphotungstic acid (Rhawn, R128447) for 60 s, air-dried, and examined using a Hitachi HT7800 (Hitachi, Chiyoda-ku, Tokyo, Japan) transmission electron microscope operating at 80–120 kV
2.4. Preparation of AS01-like Adjuvant and Formulation of Adjuvanted Vaccine
A measured amount of cholesterol (Avanti Polar Lipids, Alabaster, AL, USA), 1,2-dioleoyl-sn-glycero-3-phosphocholine (DOPC; Avanti Polar Lipids, Alabaster, AL, USA), and monophosphoryl lipid A (MPL; Sigma-Aldrich, St. Louis, MO, USA) was dissolved in isopropanol (Sinopharm Chemical Reagent Co., Ltd., Shanghai, China) and evaporated under reduced pressure using a RE-52AA rotary evaporator (Yarong Biochemical Instrument Factory, Shanghai, China) to form a lipid film. The lipid film was subsequently hydrated with sterile Milli-Q water (Millipore, Billerica, MA, USA) to generate a coarse liposome suspension, which was then processed with an Avestin EmulsiFlex-C3 high-pressure homogenizer (Avestin, Ottawa, Ontario, Canada) to reduce particle size and produce homogeneous liposomes. QS-21 (Desert King, San Diego, CA, USA) was then added to the liposome suspension to obtain the final adjuvant formulation, designated AS01-like adjuvant. The final composition of AS01-like adjuvant consisted of 4 mg/mL DOPC, 1 mg/mL cholesterol, and 0.2 mg/mL each of QS-21 and MPL.
For vaccine formulation, AS01-like adjuvant was mixed at equal volume with a trivalent recombinant HA protein preparation (comprising AH1, AH3, and B/vic antigens). Mice were immunized intramuscularly with 100 μL of the adjuvanted vaccine.
2.5. Animal Assay
Female BALB/c mice (6–8 weeks old) were purchased from Beijing Vital River Laboratory Animal Technology Co., Ltd. (Beijing, China), and housed at Forevergen Biotechnology Co., Ltd. (Guangzhou, China), where all animal experiments were conducted. All procedures were approved by the Institutional Animal Care and Use Committee (IACUC) of Forevergen Biotechnology (approval No. IACUC-AEWC-F2401021) and were performed in strict accordance with the 3Rs principles (Replacement, Reduction, and Refinement).
2.6. Enzyme-Linked Immunosorbent Assay (ELISA)
Serum samples collected from immunized animals were analyzed for anti-HA total IgG endpoint titers using an indirect ELISA. Briefly, 96 well Nunc MaxiSorp plates (Thermo Fisher Scientific, Waltham, MA, USA) were coated with vaccine-matched recombinant HA proteins from Sino Biological corresponding to AH1 (catalog #40940-V08H7), AH3 (catalog #40992-V08H), and B/Victoria (catalog #40862-V08H) for experiments shown in
Figure 1,
Figure 2 and
Figure 3. Each antigen was applied at 50 ng per well in 50 μL PBS and incubated at 2–8 °C for at least 12 h. For the cross-reactivity analysis (
Figure 4), additional heterologous HA proteins were coated under identical conditions. These included AH1 antigens (catalog #11085-V08B and #11683-V08H) and AH3 antigens (catalog #40868-V08B and #40555-V08B), also obtained from Sino Biological. Following coating, plates were washed twice with PBS containing 0.05% Tween-20 (PBST) and blocked with 200 μL/well of Blocker Casein in PBS (Thermo Fisher Scientific) for 1 h at room temperature (RT). After two additional washes with PBST, serially diluted serum samples were added and incubated for 1 h at RT. Plates were then incubated with HRP-conjugated goat anti-mouse IgG (1:5000 dilution; Abcam, Cambridge, UK) for 1 h at RT, followed by six washes with PBST. TMB substrate (InnoReagents, Huzhou, China) was added and incubated for 10 min at RT, after which the reaction was stopped by the addition of 1 M HCl. Absorbance was measured at 450 nm with a reference wavelength of 620 nm. The endpoint titer was defined as the reciprocal of the highest serum dilution yielding an optical density above the established cutoff. Pre-immune sera from the same animals were used as baseline negative control.
2.7. Hemagglutination Inhibition (HI) Assay
HI titers were determined using standardized influenza virus antigens obtained from the National Institute for Biological Standards and Control (NIBSC, Potters Bar, Hertfordshire, UK). For
Figure 1,
Figure 2 and
Figure 3, the following standard reagents were used: 22/320 (A/H1N1), 23/220 (A/H3N2), and 21/316 (B/Victoria). For the cross-reactivity analysis shown in
Figure 4, l antigens were used: 22/320, 22/100, and 18/238 for AH1; 23/220, 21/314, 21/100, and 19/316 for AH3 Before testing, immune sera were treated with receptor destroying enzyme (RDE II; Denka Seiken, Tokyo, Japan) according to the manufacturer’s instructions. Treated sera were serially twofold diluted in PBS (Gibco), starting at a 1:10 dilution, and incubated with four hemagglutination units (HAU) of influenza antigen for 1 h at room temperature. Subsequently, a 1% suspension of chicken RBCs was added to each well. For A/H3N2 strains, guinea pig RBCs were used instead. Plates were incubated for 30–60 min at RT. The HI titer was defined as the highest serum dilution that completely inhibited hemagglutination, as determined by visual inspection of the last well showing a distinct RBC button without agglutination.
2.8. Pseudovirus Neutralizing Assay (PN)
Pseudoviruses encoding influenza HA, NA, and firefly luciferase were provided by the National Institutes for Food and Drug Control (NIFDC, Beijing, China). Serum samples were heat inactivated at 56 °C for 30 min and then serially diluted in 3-fold steps using DMEM containing 2% FBS. The diluted sera were mixed with a fixed amount of pseudovirus and incubated at 37 °C for 1 h. Following incubation, 100 μL of the serum–virus mixture was added to 96-well plates pre-seeded with 2 × 104 Huh-7 cells per well. Cells were then incubated at 37 °C in a humidified atmosphere containing 5% CO2 for 24 h. Finally, luciferase activity was measured to assess infection levels. The 50% pseudovirus neutralization titer was calculated using the Reed–Muench method and expressed as the reciprocal of the serum dilution that inhibited 50% of the luciferase signal. Pre-immune sera from the same animals were used as baseline negative control.
2.9. Microneutralization Assay (MN)
Heat inactivated serum samples were serially diluted in DMEM containing 1% BSA. A volume of 50 μL of each serum dilution was mixed with an equal volume of influenza virus containing 100 TCID
50, and the mixtures were incubated in 96-well plates at 37 °C for 1 h. Subsequently, 1.5 × 10
4 MDCK cells were added to each well, and plates were incubated at 37 °C with 5% CO
2 for 18–22 h. After incubation, the cells were washed with PBS and fixed with cold acetone (pre-chilled at 4 °C) at room temperature for 10 min. Following removal of the fixative, 100 μL of mouse anti-influenza nucleoprotein monoclonal antibody (1:4000 dilution) was added to each well and incubated at room temperature for 1 h. After PBS washes, 100 μL of HRP-conjugated goat anti-mouse IgG (1:4000 dilution) was added and incubated for another hour at room temperature. Plates were then washed five times with PBS, and 100 μL of substrate solution was added to each well and incubated for 10 min for color development. The enzymatic reaction was terminated by adding 1 M sulfuric acid, and absorbance was measured at 450 nm with a reference wavelength of 620 nm using a microplate reader and the neutralizing antibody titer was calculated according to previously described methods [
18]. Pre-immune sera from the same animals were used as baseline negative control.
2.10. Enzyme-Linked Immunospot Assay (ELISpot)
Following euthanasia, spleens were aseptically harvested from mice and mechanically dissociated to generate single cell suspensions. The suspensions were passed through a 70 μm cell strainer to remove debris. Red blood cells were lysed using in-house prepared Ammonium-Chloride-Potassium lysis buffer, and viable splenocytes were counted before the assay. The ELISpot assay was performed using the Mouse IFN-γ/IL-4 ELISpotPRO kit (Mabtech, Nacka, Sweden) according to the manufacturer’s instructions.
Briefly, pre-coated 96-well ELISpot plates were washed with PBS and blocked with 100 μL of α-MEM medium (Gibco) supplemented with 10% fetal bovine serum for 1–2 h at room temperature. After blocking, 2.5 × 105 splenocytes per well were stimulated in a total volume of 100 μL with peptide pools at a final concentration of 0.25 μg/mL, the cells were separately stimulated with AH1 HA-specific peptides (56 overlapping 20-mer peptides with 10 amino acid overlaps, GenScript), AH3 HA-specific peptides (55 × 20-mer, GenScript), or B/vic HA-specific peptides (54 × 20-mer, GenScript). Then cells were incubated for 40 h at 37 °C in a humidified 5% CO2 incubator. After incubation, plates were washed with PBS and sequentially incubated with biotinylated detection antibodies against IFN-γ and IL-4 (Mabtech) for 2 h at room temperature, followed by streptavidin–alkaline phosphatase conjugate (Mabtech) for 1 h. Spot development was performed using BCIP/NBT-plus substrate for 8–10 min, after which the reaction was stopped by rinsing with distilled water. Plates were air dried and analyzed using an automated ELISpot reader (AID, Straßberg, Germany) to quantify cytokine secreting cells.
2.11. Statistical Analysis
Statistical analyses were performed using GraphPad Prism software (version 9.5.1). Comparisons between two groups were conducted using the nonparametric Mann–Whitney test. A p-value of <0.05 was considered statistically significant. For clarity, non-significant (ns) comparisons were not shown.
4. Discussion
In this study, we first generated HA
mem-VLPs and HA
sec-NPs using the baculovirus–insect cell expression system, with HA sequences based on the WHO-recommended strains for the 2024–2025 influenza season. The morphology and nanoparticle size distribution of HA
mem-VLPs and HA
sec-NPs were consistent with previous reports [
25,
26], confirming the successful assembly of these particles into virus-like particles and nanoparticles. Compared with the HA
sec-NP mix, the HA
mem-VLP mix induced stronger antibody responses in mice, which may be attributed to the fact that HA
mem-VLP more closely resembles the native HA conformation on the influenza virus surface and that HA
mem-VLP, unlike HA
sec-NP, does not contain the mi3 nanoparticle carrier, thereby focusing the immune response exclusively on HA. Therefore, we focused subsequent studies on the immunogenicity of the adjuvanted HA
mem-VLP mix vaccine, which we designated as a-RIV.
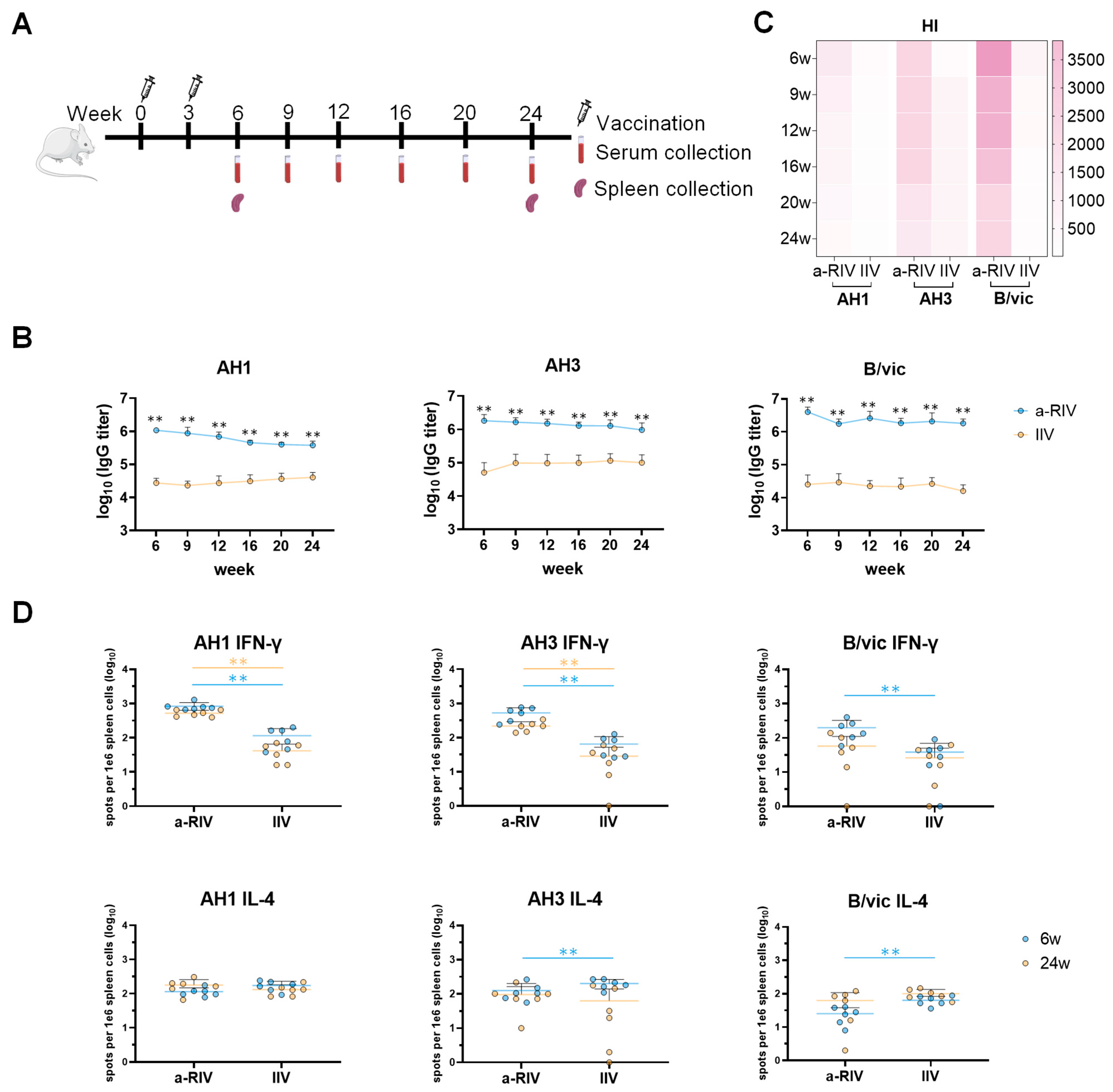
Immunogenicity evaluation in mice revealed that the a-RIV elicited significantly higher HA-specific binding antibody titers, HI titers, and T cell responses compared with both the licensed inactivated influenza vaccine and the recombinant protein vaccine Flublok. Notably, the a-RIV demonstrated enhanced durability and breadth of immune responses, maintaining elevated antibody and T cell levels for at least six months post-immunization—substantially outperforming IIV in this regard.
The a-RIV vaccine induced strong antibody responses not only against the homologous vaccine strains but also against multiple heterologous A/H1N1 and A/H3N2 strains from 2019 to 2025. ELISA results showed sustained high IgG binding titers across these drifted strains, while responses from IIV declined for earlier isolates. This was corroborated by consistently higher HI, pseudovirus neutralization, and microneutralization titers in the a-RIV group, confirming functional cross-reactivity. These findings suggest that a-RIV elicits antibodies capable of recognizing conserved epitopes across antigenically diverse strains. The improved performance of a-RIV likely results from multiple synergistic factors. Structurally, recombinant HA often exists as uncleaved HA0, preserving unique prefusion epitopes that can induce broadly cross-reactive and protective antibodies, particularly those targeting the conserved HA stem region. In addition, Hemagglutinin produced in the baculovirus–insect cell system carries mainly high-mannose, non-sialylated N-glycans, whereas HA expressed in human cells contains complex, branched, and sialylated glycans. These structural differences can influence antigenic properties by reducing glycan shielding and thereby exposing conserved epitopes, including those in the HA stem region [
5,
27]. Although insect-type glycans differ from native human patterns, they generally maintain correct folding and trimeric conformation, and the reduced glycan masking may help broaden cross-reactive antibody responses. Therefore, the simplified glycosylation of Sf9-derived HA may actually favor the induction of broad protective immunity. Furthermore, the use of the baculovirus–insect cell recombinant protein platform provides significant advantages over traditional egg- or cell-based vaccines. Unlike these conventional approaches, the recombinant protein platform does not rely on live influenza virus. It can be rapidly initiated by cloning the wild-type HA gene, thereby shortening the manufacturing timeline to 2–3 months, which is critical for seasonal updates and pandemic preparedness. Moreover, recombinant HA avoids the adaptive mutations introduced during viral propagation in eggs or mammalian cells, ensuring sequence fidelity to circulating strains and reducing the risk of antigenic mismatch.
Beyond the expression platform, the superior immunogenicity of a-RIV may also be attributed to its adjuvant formulation. Previous studies have demonstrated that oil-in-water adjuvants such as MF59 and saponin-based adjuvants like Matrix-M™ can enhance both innate and adaptive immune responses by promoting antigen uptake, dendritic cell activation, and recruitment of inflammatory cells [
28,
29,
30,
31,
32,
33]. Matrix-M™, for example, has shown promising results in enhancing antibody responses and CD4
+ T cell immunity in the context of NanoFlu—a novel adjuvanted recombinant influenza vaccine currently in late-stage clinical trials [
34]. Likewise, the AS01 adjuvant system—comprising MPL, QS-21, and liposomes—has been widely validated in malaria, RSV, and herpes zoster vaccines and is known to promote robust germinal center responses, CD4
+ T cell polyfunctionality, and CD8
+ T cell cross-presentation [
35,
36,
37,
38]. Unlike MF59 and Matrix-M™, AS01 engages TLR4-dependent signaling via MPL and synergistic saponin-induced antigen presentation to trigger a potent Th1-polarized immune profile with strong polyfunctional CD4
+ T-cell responses and elicit long-lasting T-cell and antibody immunity [
35]. So the AS01-like adjuvant was explored for the next generation of flu vaccine prototypes requiring strong T-cell responses.
Supplementary Figure S3 demonstrates that the AS01-like formulation elicited significantly higher IgG titers, HI titers, and IFN-γ–secreting splenocytes (as measured by ELISpot) than the MF59-like adjuvant or unadjuvanted controls.
The hemagglutination inhibition assay remains the standard immunological correlate of protection for influenza, with an HI titer ≥40 generally associated with ~50% protection [
39]. However, increasing evidence underscores the critical role of T cell–mediated immunity in influenza protection. Antigen-specific CD4
+ T cells contribute by secreting cytokines such as IFN-γ, which support CD8
+ T cell activation and memory maintenance [
21,
40], and in experimental human infections, pre-existing CD4
+ T cells have been associated with reduced viral shedding and milder disease [
41]. CD8
+ cytotoxic T lymphocytes (CTLs) eliminate virus-infected cells by perforin/granzyme B release and Fas-mediated apoptosis [
42], and they also produce IFN-γ to suppress viral replication [
43]. Thus, an optimal influenza vaccine should elicit both humoral and cellular immunity. In addition to the strong IFN-γ responses observed, our ELISpot data demonstrated significant secretion of IL-4, particularly in response to AH1 HA antigens. IL-4, a canonical Th2 cytokine, plays a central role in B cell activation, class-switch recombination (particularly to IgG1), and the formation of germinal centers. These processes are essential for the generation of long-lived plasma cells and high-affinity antibody responses. a-RIV induced a balanced Th1/Th2 response (co-induction of IFN-γ and IL-4), which is advantageous for combined cellular and humoral immunity, despite eliciting IL-4 levels similar to non-adjuvanted IIV or Flublok.
To the best of our knowledge, this study represents one of the first direct comparisons between a membrane-anchored trivalent recombinant influenza virus-like particle (RIV) vaccine and a secreted HA–nanoparticle (HAsec-NP) vaccine, both produced via the baculovirus–insect cell expression system. This head-to-head comparison helps elucidate the relative immunogenicity of membrane-bound HA presented in VLP form versus scaffolded HA on the mi3 nanoparticle platform. In addition, this study includes a novel application of an AS01-like adjuvant in combination with a Flublok-like recombinant HA vaccine. While Flublok has been previously evaluated without adjuvants, the incorporation of an AS01-like adjuvant—known for its capacity to elicit potent Th1-biased cellular responses—has not been extensively explored in this context. Furthermore, we believe this work provides valuable comparative data by evaluating an adjuvanted trivalent recombinant HA VLP vaccine (a-RIV) alongside two licensed influenza vaccines: the inactivated influenza vaccine (IIV) and non-adjuvanted recombinant Flublok. Conducted under controlled conditions in a murine model, these comparisons offer new insights into the potential immunological advantages and durability of the a-RIV platform.