Soluble Cyanobacterial Carotenoprotein as a Robust Antioxidant Nanocarrier and Delivery Module
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Cloning, Protein Expression, and Purification
2.3. Lipid Bilayer Setup, Recording System, and Calculations
2.4. Measurement of the Membrane Boundary Potential
2.5. Confocal Microscopy of Unilamellar Vesicles
2.6. Differential Scanning Calorimetry
2.7. Thin Layer Chromatography of Carotenoids
2.8. Production of Liposomes with Carotenoids
2.9. Absorption Measurements
2.10. Raman Spectroscopy Measurements
2.11. Delivery of Carotenoids into Mammalian Cells by AnaCTDH
2.12. Antioxidant Activity of Carotenoids Delivered into Mammalian Cells by CTDH
2.13. Statistical Analysis
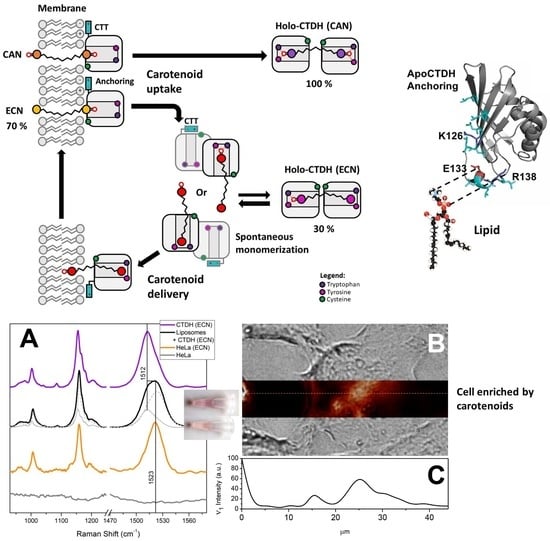
3. Results and Discussion
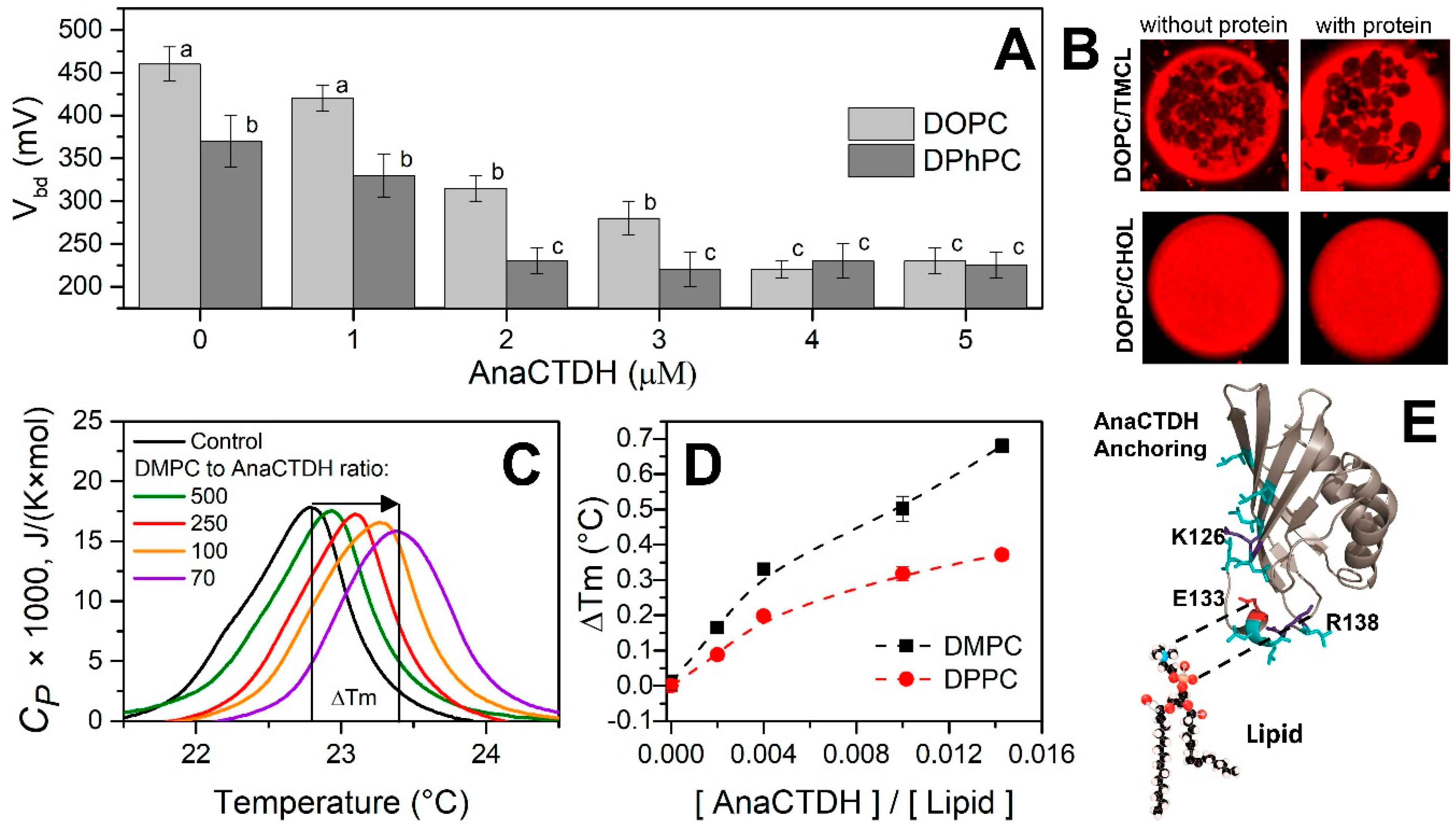
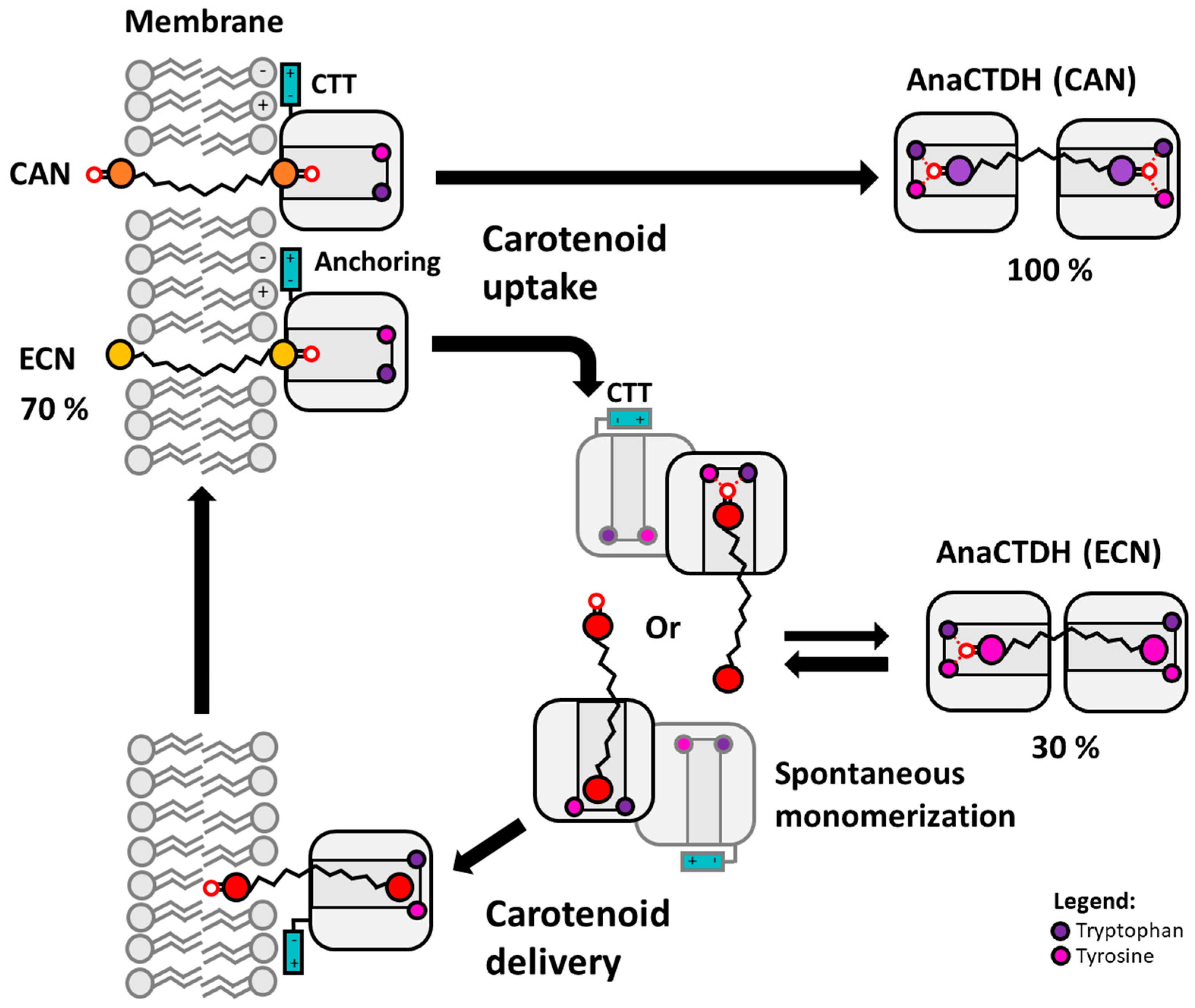
3.1. Direct Interaction of AnaCTDH Apoprotein with the Membranes
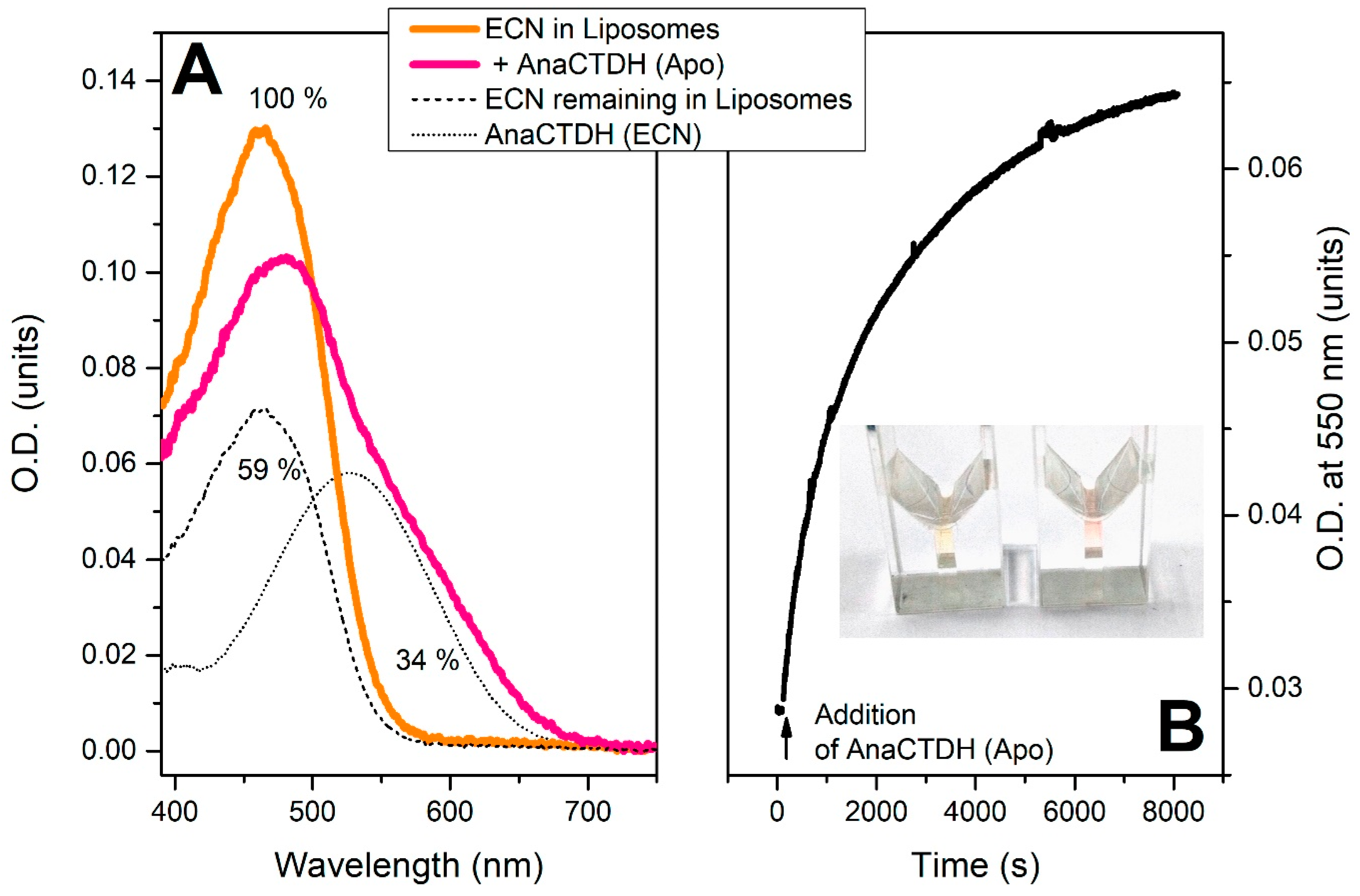
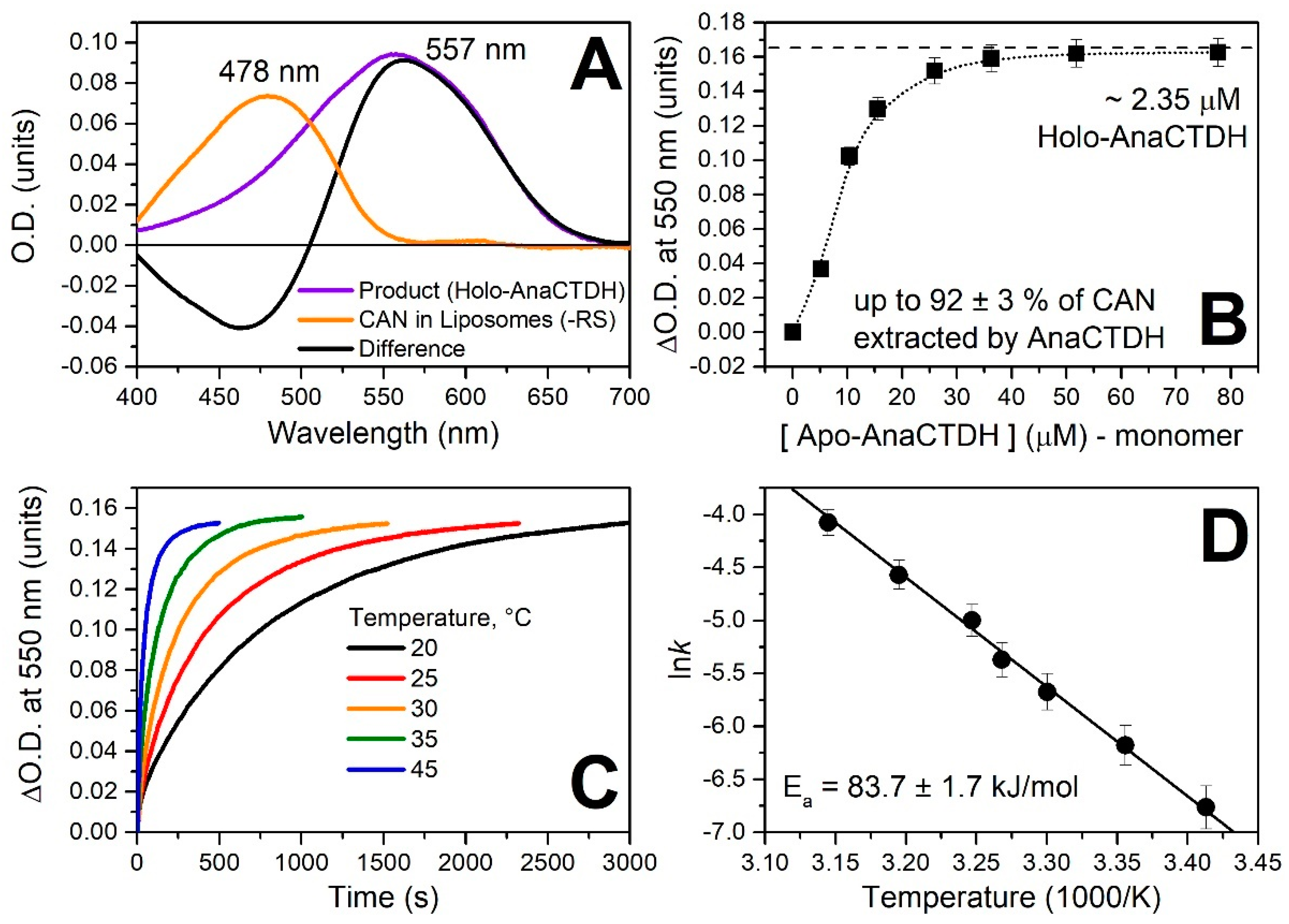
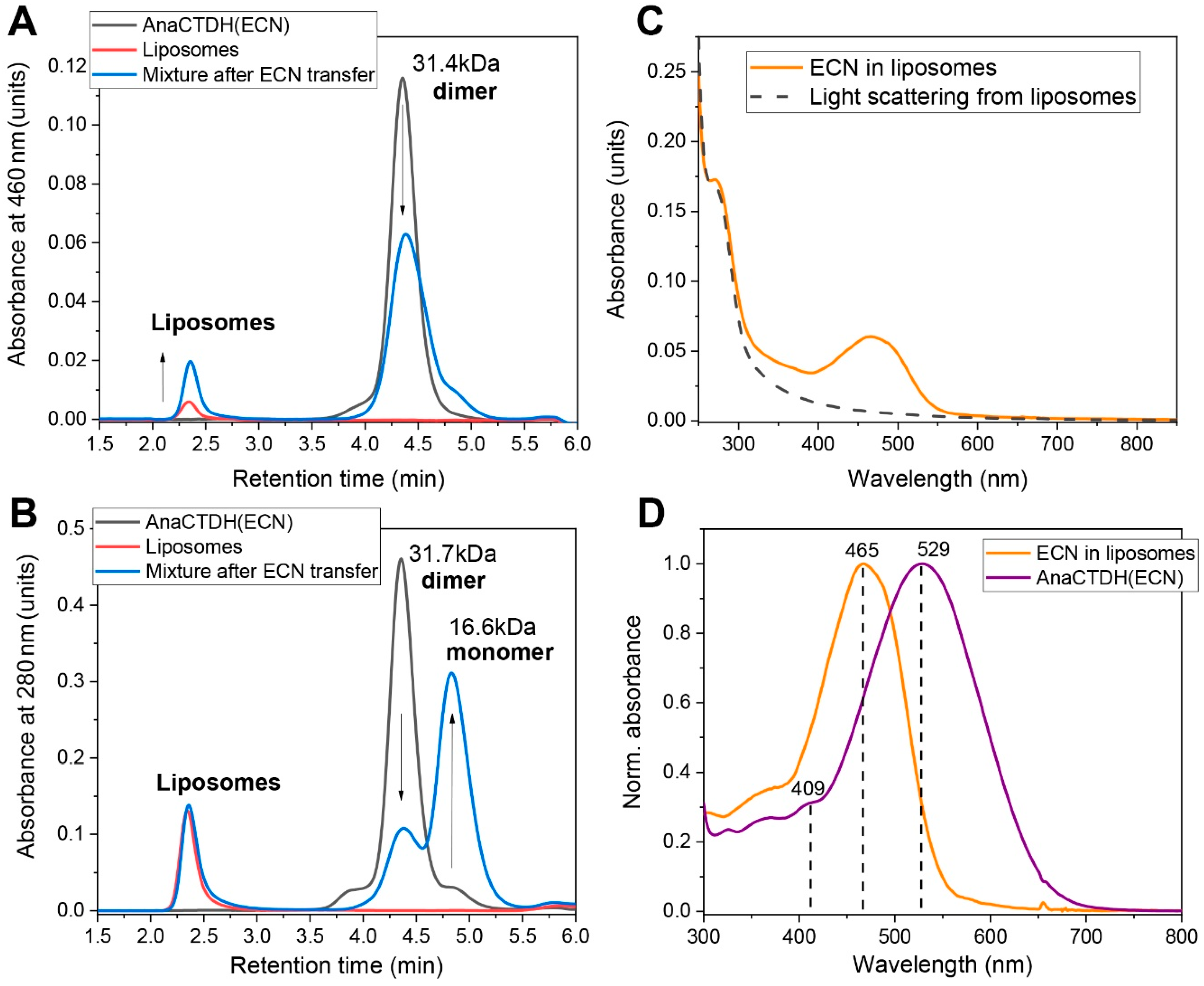
3.2. Carotenoid Uptake by the AnaCTDH Apoprotein from Liposomes
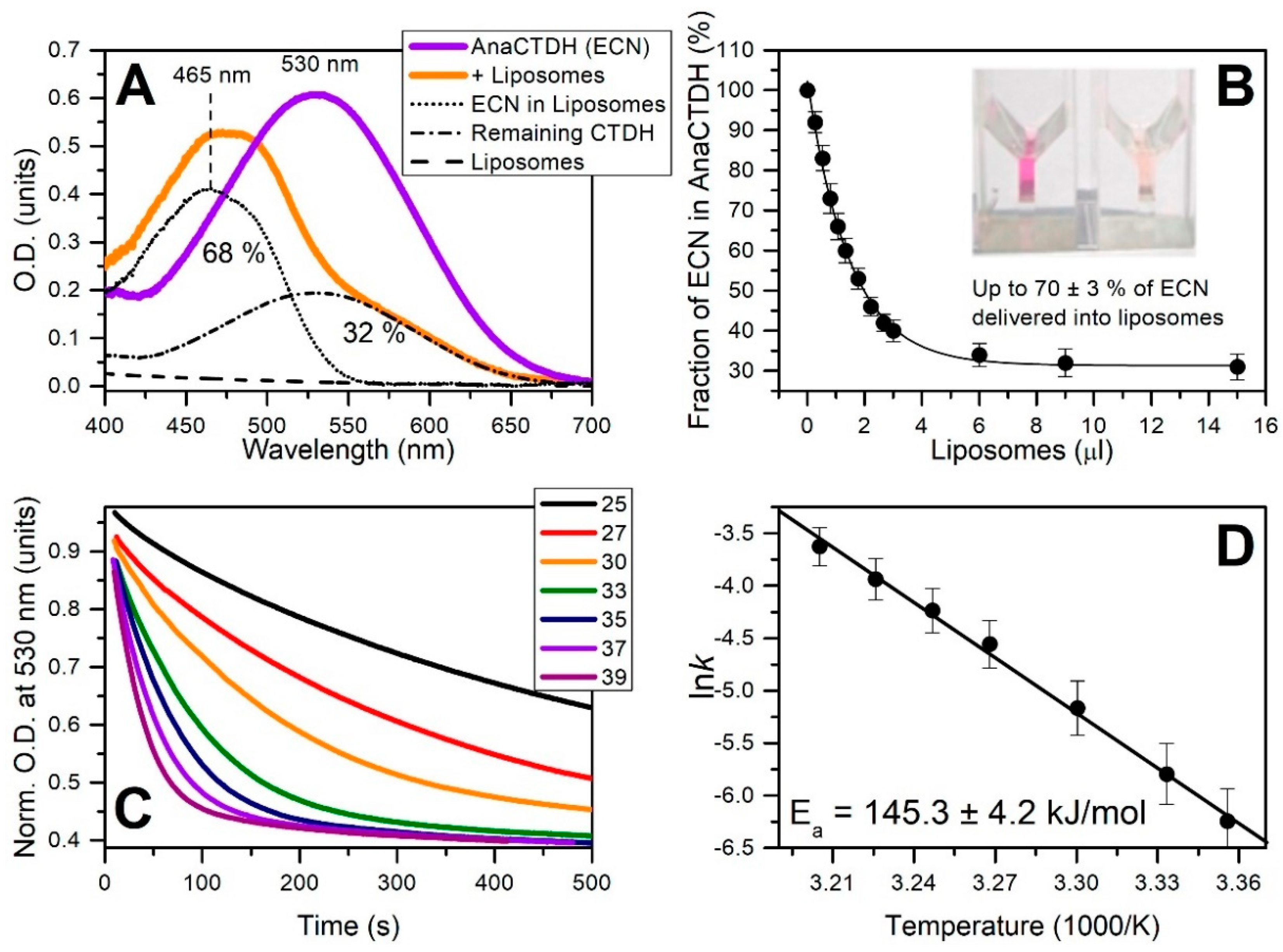
3.3. Carotenoid Delivery into Liposomes
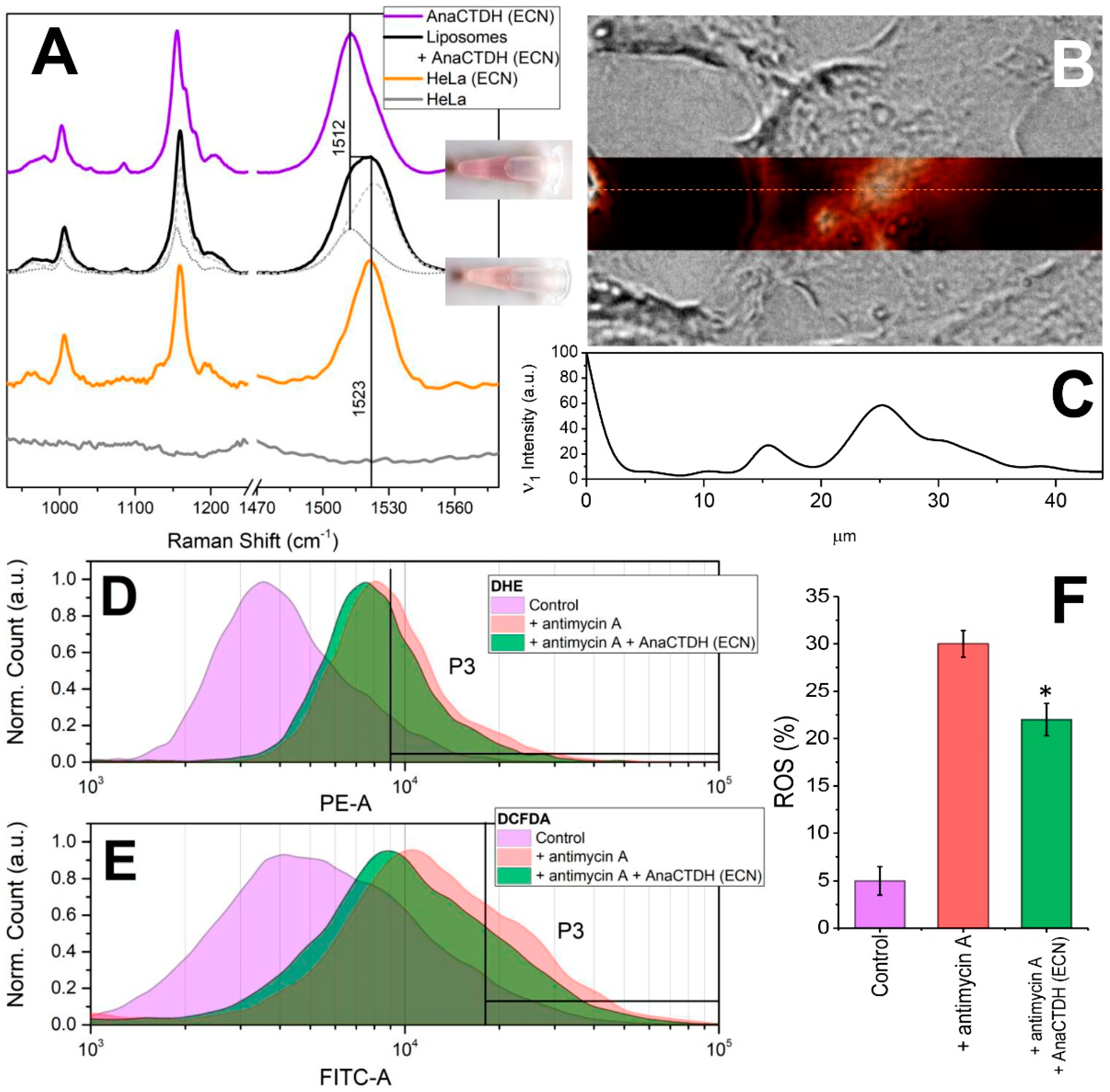
3.4. Carotenoid Delivery from AnaCTDH(ECN) Holoprotein into Mammalian Cells
3.5. Carotenoid Delivery to Mammalian Cells Alleviates Oxidative Stress
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
Appendix A
Spectroscopic Characterization of the AnaCTDH Apoprotein Species
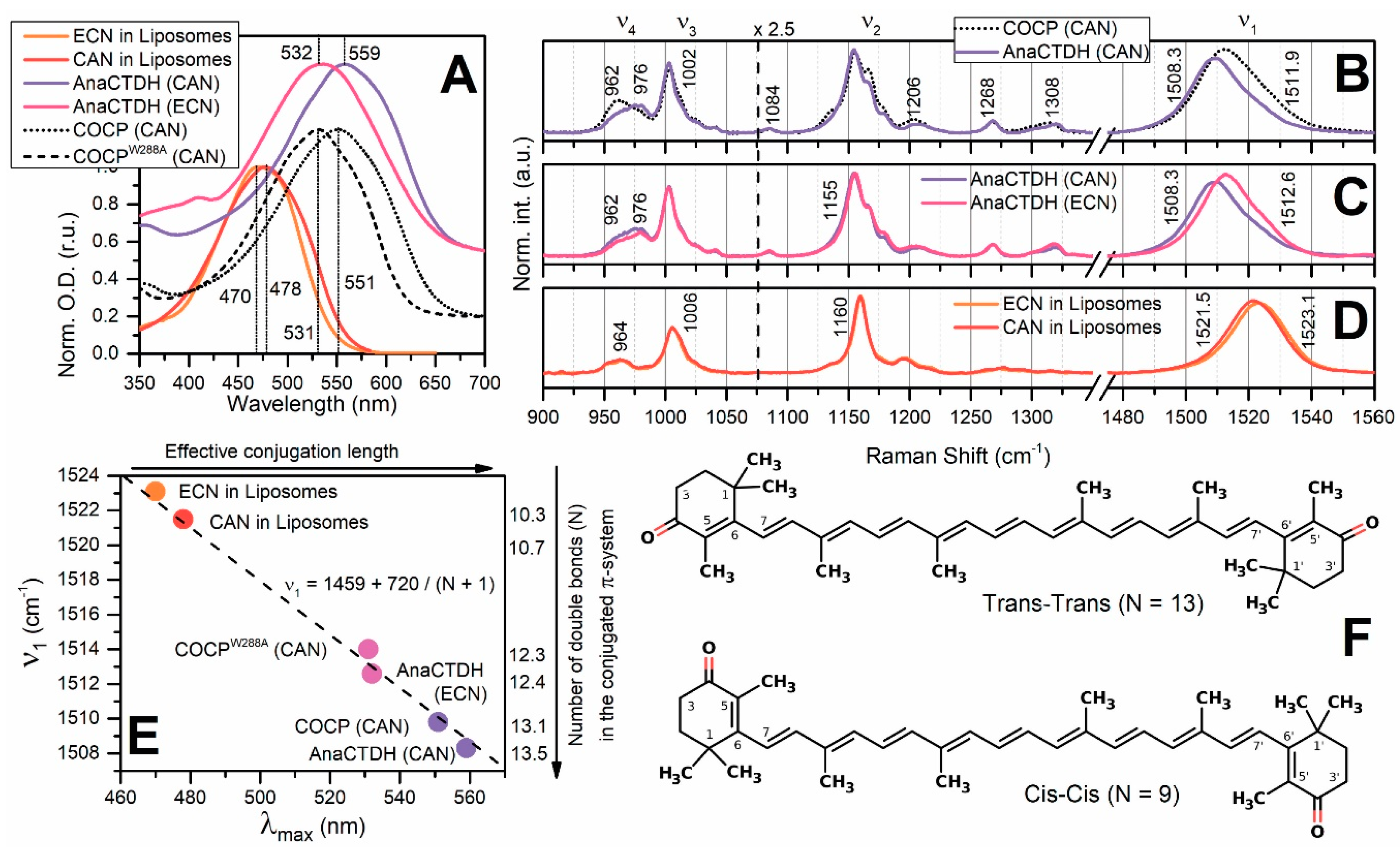
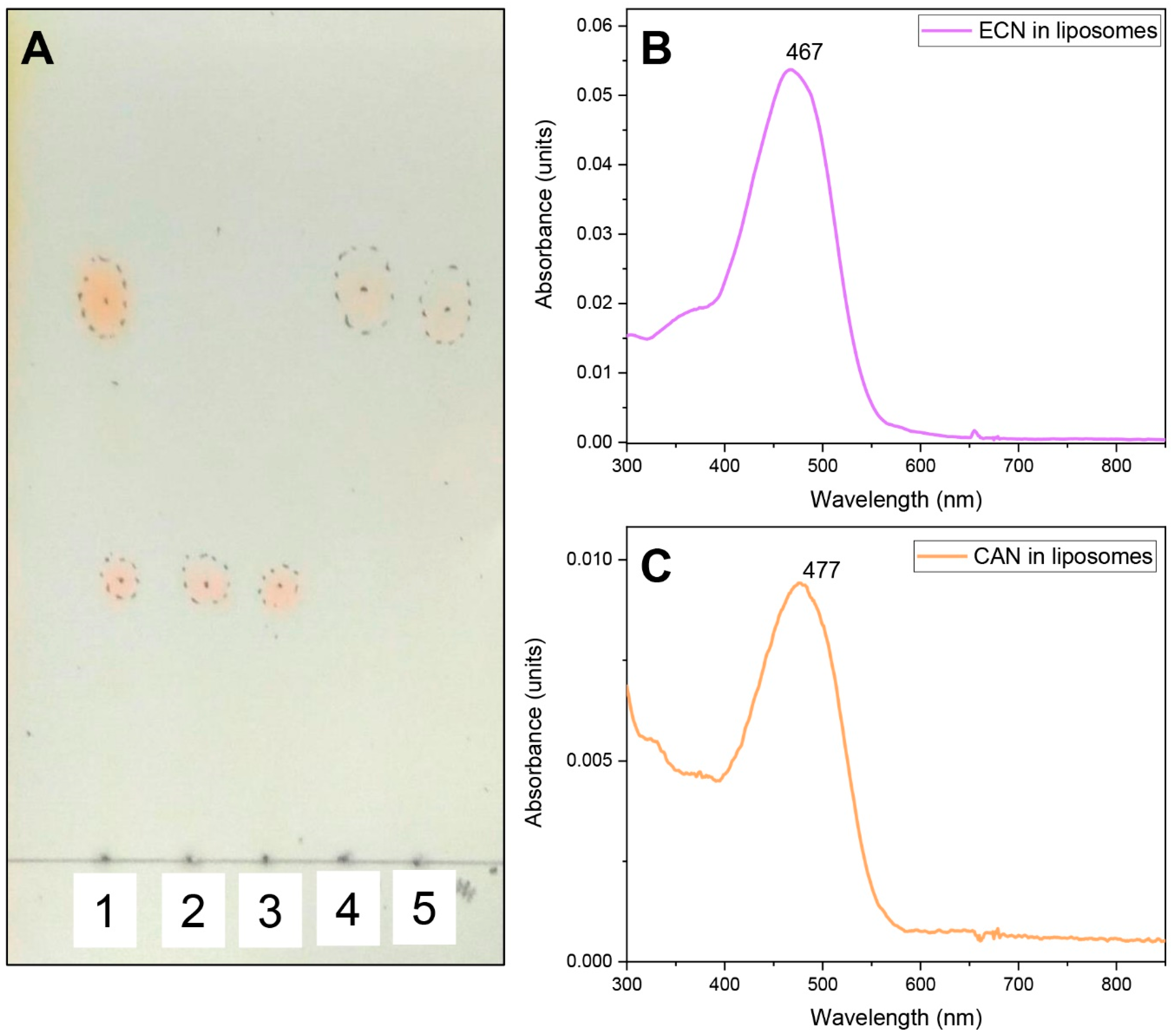
Echinenone Uptake from Liposomes
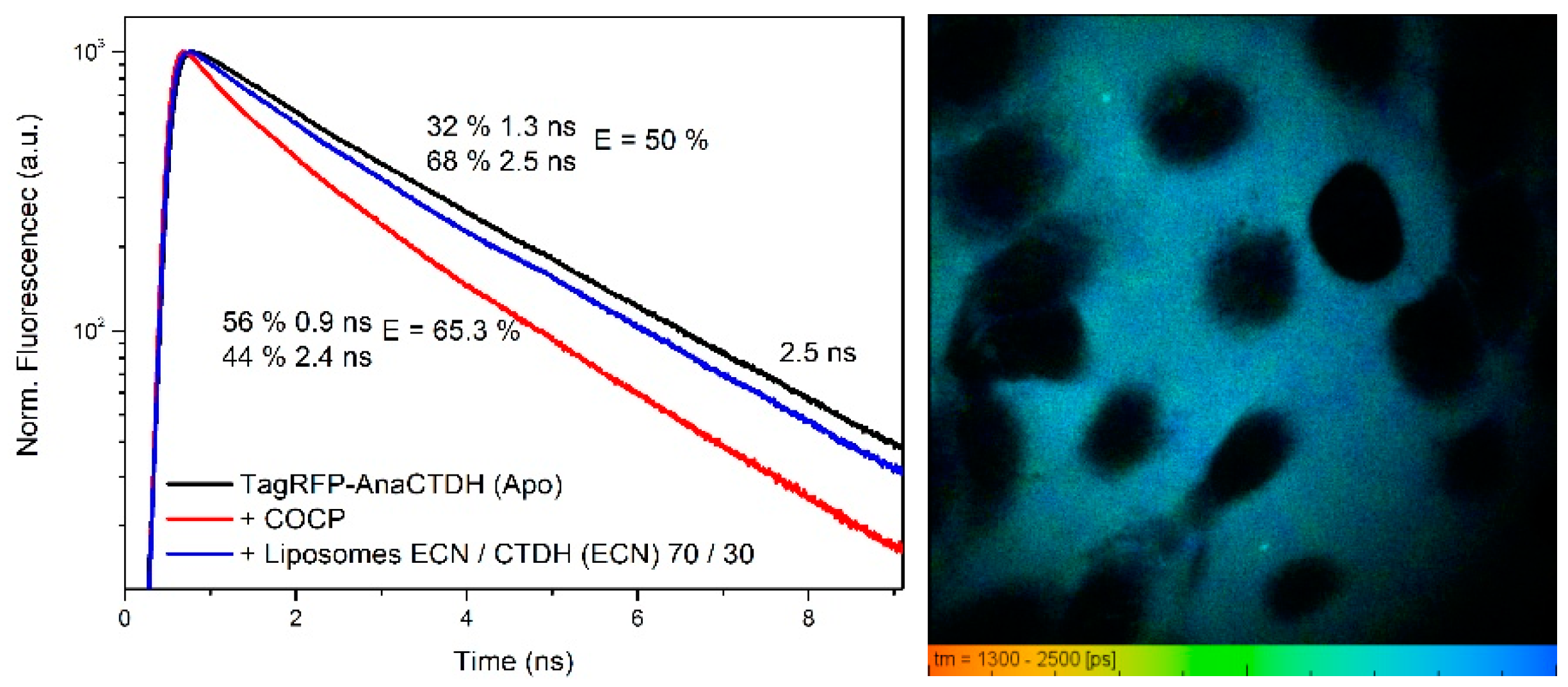
Carotenoid Delivery by AnaCTDH does not Require Internalization of the Protein
References
- Sies, H. Oxidative stress: Oxidants and antioxidants. Exp. Physiol. 1997, 82, 291–295. [Google Scholar] [CrossRef] [PubMed]
- Mittler, R. Oxidative stress, antioxidants and stress tolerance. Trends Plant Sci. 2002, 7, 405–410. [Google Scholar] [CrossRef]
- Konold, P.E.; van Stokkum, I.H.M.; Muzzopappa, F.; Wilson, A.; Groot, M.L.; Kirilovsky, D.; Kennis, J.T.M. Photoactivation mechanism, timing of protein secondary structure dynamics and carotenoid translocation in the Orange Carotenoid Protein. J. Am. Chem. Soc. 2018, 10, 520–530. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vershinin, A. Biological functions of carotenoids-diversity and evolution. BioFactors 1999, 10, 99–104. [Google Scholar] [CrossRef] [PubMed]
- Chew, B.P.; Park, J.S.; Wong, M.W.; Wong, T.S. A comparison of the anticancer activities of dietary beta-carotene, canthaxanthin and astaxanthin in mice in vivo. Anticancer Res. 1999, 19, 1849–1853. [Google Scholar] [PubMed]
- Macdonald, K.; Holti, G.; Marks, J. Is there a place for beta-carotene/canthaxanthin in photochemotherapy for psoriasis? Dermatologica 1984, 169, 41–46. [Google Scholar] [CrossRef]
- Wennersten, G. Carotenoid treatment for light sensitivity: A reappraisal and six years’ experience. Acta Derm. Venereol. 1980, 60, 251–255. [Google Scholar]
- Beaulieu, R.A.; Warwar, R.E.; Buerk, B.M. Canthaxanthin retinopathy with visual loss: A case report and review. Case Rep. Ophthalmol. Med. 2013, 2013, 140901. [Google Scholar] [CrossRef] [Green Version]
- Bartley, G.E.; Scolnik, P.A. Plant carotenoids: Pigments for photoprotection, visual attraction, and human health. Plant Cell 1995, 7, 1027–1038. [Google Scholar] [CrossRef] [Green Version]
- Bjornson, L.K.; Kayden, H.J.; Miller, E.; Moshell, A.N. The transport of alpha-tocopherol and beta-carotene in human blood. J. Lipid Res. 1976, 17, 343–352. [Google Scholar]
- Williams, A.W.; Boileau, T.W.M.; Clinton, S.K.; Erdman, J.W. β-Carotene Stability and Uptake by Prostate Cancer Cells Are Dependent on Delivery Vehicle. Nutr. Cancer 2000, 36, 185–190. [Google Scholar] [CrossRef]
- Palozza, P.; Muzzalupo, R.; Trombino, S.; Valdannini, A.; Picci, N. Solubilization and stabilization of beta-carotene in niosomes: Delivery to cultured cells. Chem. Phys. Lipids 2006, 139, 32–42. [Google Scholar] [CrossRef] [PubMed]
- Kaur, I.P.; Kapila, M.; Agrawal, R. Role of novel delivery systems in developing topical antioxidants as therapeutics to combat photoageing. Ageing Res. Rev. 2007, 6, 271–288. [Google Scholar] [CrossRef] [PubMed]
- Hood, E.; Simone, E.; Wattamwar, P.; Dziubla, T.; Muzykantov, V. Nanocarriers for vascular delivery of antioxidants. Nanomedicine 2011, 6, 1257–1272. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tan, C.; Xue, J.; Lou, X.; Abbas, S.; Guan, Y.; Feng, B.; Zhang, X.; Xia, S. Liposomes as delivery systems for carotenoids: Comparative studies of loading ability, storage stability and in vitro release. Food Funct. 2014, 5, 1232–1240. [Google Scholar] [CrossRef] [PubMed]
- Slastnikova, T.A.; Rosenkranz, A.A.; Gulak, P.V.; Schiffelers, R.M.; Lupanova, T.N.; Khramtsov, Y.V.; Zalutsky, M.R.; Sobolev, A.S. Modular nanotransporters: A multipurpose in vivo working platform for targeted drug delivery. Int. J. Nanomed. 2012, 7, 467–482. [Google Scholar] [CrossRef] [Green Version]
- Raghavan, R.; Howell, R.W.; Zalutsky, M.R. A model for optimizing delivery of targeted radionuclide therapies into resection cavity margins for the treatment of primary brain cancers. Biomed. Phys. Eng. Express 2017, 3, 035005. [Google Scholar] [CrossRef] [Green Version]
- Slastnikova, T.A.; Rosenkranz, A.A.; Khramtsov, Y.V.; Karyagina, T.S.; Ovechko, S.A.; Sobolev, A.S. Development and evaluation of a new modular nanotransporter for drug delivery into nuclei of pathological cells expressing folate receptors. Drug. Des. Dev. Ther. 2017, 11, 1315–1334. [Google Scholar] [CrossRef] [Green Version]
- Kirilovsky, D. The photoactive orange carotenoid protein and photoprotection in cyanobacteria. Adv. Exp. Med. Biol. 2010, 675, 139–159. [Google Scholar] [CrossRef]
- Leverenz, R.L.; Sutter, M.; Wilson, A.; Gupta, S.; Thurotte, A.; de Carbon, C.B.; Petzold, C.J.; Ralston, C.; Perreau, F.; Kirilovsky, D. A 12 Å carotenoid translocation in a photoswitch associated with cyanobacterial photoprotection. Science 2015, 348, 3. [Google Scholar] [CrossRef] [Green Version]
- Maksimov, E.G.; Shirshin, E.A.; Sluchanko, N.N.; Zlenko, D.V.; Parshina, E.Y.; Tsoraev, G.V.; Klementiev, K.E.; Budylin, G.S.; Schmitt, F.J.; Friedrich, T.; et al. The Signaling State of Orange Carotenoid Protein. Biophys. J. 2015, 109, 595–607. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sluchanko, N.N.; Slonimskiy, Y.B.; Maksimov, E.G. Features of Protein-Protein Interactions in the Cyanobacterial Photoprotection Mechanism. Biochemistry 2017, 82, 1592–1614. [Google Scholar] [CrossRef] [PubMed]
- Sedoud, A.; Lopez-Igual, R.; Ur Rehman, A.; Wilson, A.; Perreau, F.; Boulay, C.; Vass, I.; Krieger-Liszkay, A.; Kirilovsky, D. The Cyanobacterial Photoactive Orange Carotenoid Protein Is an Excellent Singlet Oxygen Quencher. Plant. Cell 2014, 26, 1781–1791. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Slonimskiy, Y.B.; Muzzopappa, F.; Maksimov, E.G.; Wilson, A.; Friedrich, T.; Kirilovsky, D.; Sluchanko, N.N. Light-controlled carotenoid transfer between water-soluble proteins related to cyanobacterial photoprotection. FEBS J. 2019, 286, 1908–1924. [Google Scholar] [CrossRef]
- de Carbon, C.B.; Thurotte, A.; Wilson, A.; Perreau, F.; Kirilovsky, D. Biosynthesis of soluble carotenoid holoproteins in Escherichia coli. Sci. Rep. 2015, 5, 9085. [Google Scholar] [CrossRef]
- Punginelli, C.; Wilson, A.; Routaboul, J.M.; Kirilovsky, D. Influence of zeaxanthin and echinenone binding on the activity of the orange carotenoid protein. Biochim. Biophys. Acta 2009, 1787, 280–288. [Google Scholar] [CrossRef] [Green Version]
- Muzzopappa, F.; Wilson, A.; Yogarajah, V.; Cot, S.; Perreau, F.; Montigny, C.; Bourcier de Carbon, C.; Kirilovsky, D. Paralogs of the C-Terminal Domain of the Cyanobacterial Orange Carotenoid Protein Are Carotenoid Donors to Helical Carotenoid Proteins. Plant. Physiol. 2017, 175, 1283–1303. [Google Scholar] [CrossRef]
- Harris, D.; Wilson, A.; Muzzopappa, F.; Sluchanko, N.N.; Friedrich, T.; Maksimov, E.G.; Kirilovsky, D.; Adir, N. Structural rearrangements in the C-terminal domain homolog of Orange Carotenoid Protein are crucial for carotenoid transfer. Commun. Biol. 2018, 1, 125. [Google Scholar] [CrossRef] [Green Version]
- Maksimov, E.G.; Moldenhauer, M.; Shirshin, E.A.; Parshina, E.A.; Sluchanko, N.N.; Klementiev, K.E.; Tsoraev, G.V.; Tavraz, N.N.; Willoweit, M.; Schmitt, F.J.; et al. A comparative study of three signaling forms of the orange carotenoid protein. Photosynth. Res. 2016, 130, 389–401. [Google Scholar] [CrossRef]
- Montal, M.; Mueller, P. Formation of bimolecular membranes from lipid monolayers and a study of their electrical properties. Proc. Natl. Acad. Sci. USA 1972, 69, 3561–3566. [Google Scholar] [CrossRef] [Green Version]
- Andersen, O.S.; Finkelstein, A.; Katz, I.; Cass, A. Effect of phloretin on the permeability of thin lipid membranes. J. Gen. Physiol. 1976, 67, 749–771. [Google Scholar] [CrossRef] [PubMed]
- Hendrich, A.B. Flavonoid-membrane interactions: Possible consequences for biological effects of some polyphenolic compounds. Acta Pharmacol. Sin. 2006, 27, 27–40. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Juhasz, J.; Davis, J.H.; Sharom, F.J. Fluorescent probe partitioning in giant unilamellar vesicles of ‘lipid raft’ mixtures. Biochem. J. 2010, 430, 415–423. [Google Scholar] [CrossRef] [PubMed]
- Hsieh, L.K.; Lee, T.-C.; Chichester, C.O.; Simpson, K.L. Biosynthesis of Carotenoids in Brevibacterium sp. KY-4313. J. Bacteriol. 1974, 118, 385–393. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shafaa, M.W.; Diehl, H.A.; Socaciu, C. The solubilisation pattern of lutein, zeaxanthin, canthaxanthin and beta-carotene differ characteristically in liposomes, liver microsomes and retinal epithelial cells. Biophys. Chem. 2007, 129, 111–119. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Alexandre, A.; Lehninger, A.L. Bypasses of the antimycin a block of mitochondrial electron transport in relation to ubisemiquinone function. Biochim. Biophys. Acta 1984, 767, 120–129. [Google Scholar] [CrossRef]
- Harris, D.; Muzzopappa, F.; Glaser, F.; Wilson, A.; Kirilovsky, D.; Adir, N. Structural dynamics in the C terminal domain homolog of orange carotenoid Protein reveals residues critical for carotenoid uptake. Biochim. Biophys. Acta 2020, 1861, 148214. [Google Scholar] [CrossRef] [PubMed]
- Moldenhauer, M.; Sluchanko, N.N.; Buhrke, D.; Zlenko, D.V.; Tavraz, N.N.; Schmitt, F.-J.; Hildebrandt, P.; Maksimov, E.G.; Friedrich, T. Assembly of photoactive orange carotenoid protein from its domains unravels a carotenoid shuttle mechanism. Photosynth. Res. 2017, 133, 327–341. [Google Scholar] [CrossRef]
- Maksimov, E.G.; Sluchanko, N.N.; Slonimskiy, Y.B.; Slutskaya, E.A.; Stepanov, A.V.; Argentova-Stevens, A.M.; Shirshin, E.A.; Tsoraev, G.V.; Klementiev, K.E.; Slatinskaya, O.V.; et al. The photocycle of orange carotenoid protein conceals distinct intermediates and asynchronous changes in the carotenoid and protein components. Sci. Rep. 2017, 7, 15548. [Google Scholar] [CrossRef] [Green Version]
- Zafarullah, M.; Li, W.Q.; Sylvester, J.; Ahmad, M. Molecular mechanisms of N-acetylcysteine actions. CMLS—Cell Mol. Life Sci. 2003, 60, 6–20. [Google Scholar] [CrossRef]
- Wegmann, J.; Krucker, M.; Bachmann, S.; Fischer, G.; Zeeb, D.; Lienau, A.; Glaser, T.; Runge, F.; Luddecke, E.; Albert, K. Characterization of lycopene nanoparticles combining solid-state and suspended-state NMR spectroscopy. J. Agric. Food Chem. 2002, 50, 7510–7514. [Google Scholar] [CrossRef]
- Rehman, A.; Tong, Q.; Jafari, S.M.; Assadpour, E.; Shehzad, Q.; Aadil, R.M.; Iqbal, M.W.; Rashed, M.M.A.; Mushtaq, B.S.; Ashraf, W. Carotenoid-loaded nanocarriers: A comprehensive review. Adv. Colloid Interface Sci. 2020, 275, 102048. [Google Scholar] [CrossRef] [PubMed]
- Polyakov, N.E.; Leshina, T.V.; Salakhutdinov, N.F.; Kispert, L.D. Host−Guest Complexes of Carotenoids with β-Glycyrrhizic Acid. J. Phys. Chem. B 2006, 110, 6991–6998. [Google Scholar] [CrossRef]
- Polyakov, N.E.; Kispert, L.D. Water soluble biocompatible vesicles based on polysaccharides and oligosaccharides inclusion complexes for carotenoid delivery. Carbohydr. Polym. 2015, 128, 207–219. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Washington, K.S.; Bashur, C.A. Delivery of Antioxidant and Anti-inflammatory Agents for Tissue Engineered Vascular Grafts. Front. Pharm. 2017, 8, 659. [Google Scholar] [CrossRef] [Green Version]
- Maksimov, E.G.; Sluchanko, N.N.; Slonimskiy, Y.B.; Mironov, K.S.; Klementiev, K.E.; Moldenhauer, M.; Friedrich, T.; Los, D.A.; Paschenko, V.Z.; Rubin, A.B. The unique protein-to-protein carotenoid transfer mechanism. Biophys. J. 2017, 113, 402–414. [Google Scholar] [CrossRef] [PubMed]
- Mitrowska, K.; Vincent, U.; von Holst, C. Separation and quantification of 15 carotenoids by reversed phase high performance liquid chromatography coupled to diode array detection with isosbestic wavelength approach. J. Chromatogr. A 2012, 1233, 44–53. [Google Scholar] [CrossRef] [PubMed]
- Weesie, R.J.; Merlin, J.C.; Lugtenburg, J.; Britton, G.; Jansen, F.J.; Cornard, J.P. Semiempirical and Raman spectroscopic studies of carotenoids. Biospectroscopy 1999, 5, 19–33. [Google Scholar] [CrossRef]
- Maksimov, E.G.; Yaroshevich, I.A.; Tsoraev, G.V.; Sluchanko, N.N.; Slutskaya, E.A.; Shamborant, O.G.; Bobik, T.V.; Friedrich, T.; Stepanov, A.V. A genetically encoded fluorescent temperature sensor derived from the photoactive Orange Carotenoid Protein. Sci. Rep. 2019, 9, 8937. [Google Scholar] [CrossRef] [PubMed] [Green Version]
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Maksimov, E.G.; Zamaraev, A.V.; Parshina, E.Y.; Slonimskiy, Y.B.; Slastnikova, T.A.; Abdrakhmanov, A.A.; Babaev, P.A.; Efimova, S.S.; Ostroumova, O.S.; Stepanov, A.V.; et al. Soluble Cyanobacterial Carotenoprotein as a Robust Antioxidant Nanocarrier and Delivery Module. Antioxidants 2020, 9, 869. https://doi.org/10.3390/antiox9090869
Maksimov EG, Zamaraev AV, Parshina EY, Slonimskiy YB, Slastnikova TA, Abdrakhmanov AA, Babaev PA, Efimova SS, Ostroumova OS, Stepanov AV, et al. Soluble Cyanobacterial Carotenoprotein as a Robust Antioxidant Nanocarrier and Delivery Module. Antioxidants. 2020; 9(9):869. https://doi.org/10.3390/antiox9090869
Chicago/Turabian StyleMaksimov, Eugene G., Alexey V. Zamaraev, Evgenia Yu. Parshina, Yury B. Slonimskiy, Tatiana A. Slastnikova, Alibek A. Abdrakhmanov, Pavel A. Babaev, Svetlana S. Efimova, Olga S. Ostroumova, Alexey V. Stepanov, and et al. 2020. "Soluble Cyanobacterial Carotenoprotein as a Robust Antioxidant Nanocarrier and Delivery Module" Antioxidants 9, no. 9: 869. https://doi.org/10.3390/antiox9090869