Different Nitro-Oxidative Response of Odontarrhena lesbiaca Plants from Geographically Separated Habitats to Excess Nickel
Abstract
:1. Introduction
2. Materials and Methods
2.1. Plant Growing Conditions
2.2. Examination of the Root System Architecture
2.3. Analysis of Ni Content
2.4. Determination of the Root Apical Meristem Viability
2.5. Detection of ROS and RNS
2.6. Microscopy
2.7. Western Blot Detection of Nitrated Proteins
2.8. Statistical Analysis
3. Results and Discussion
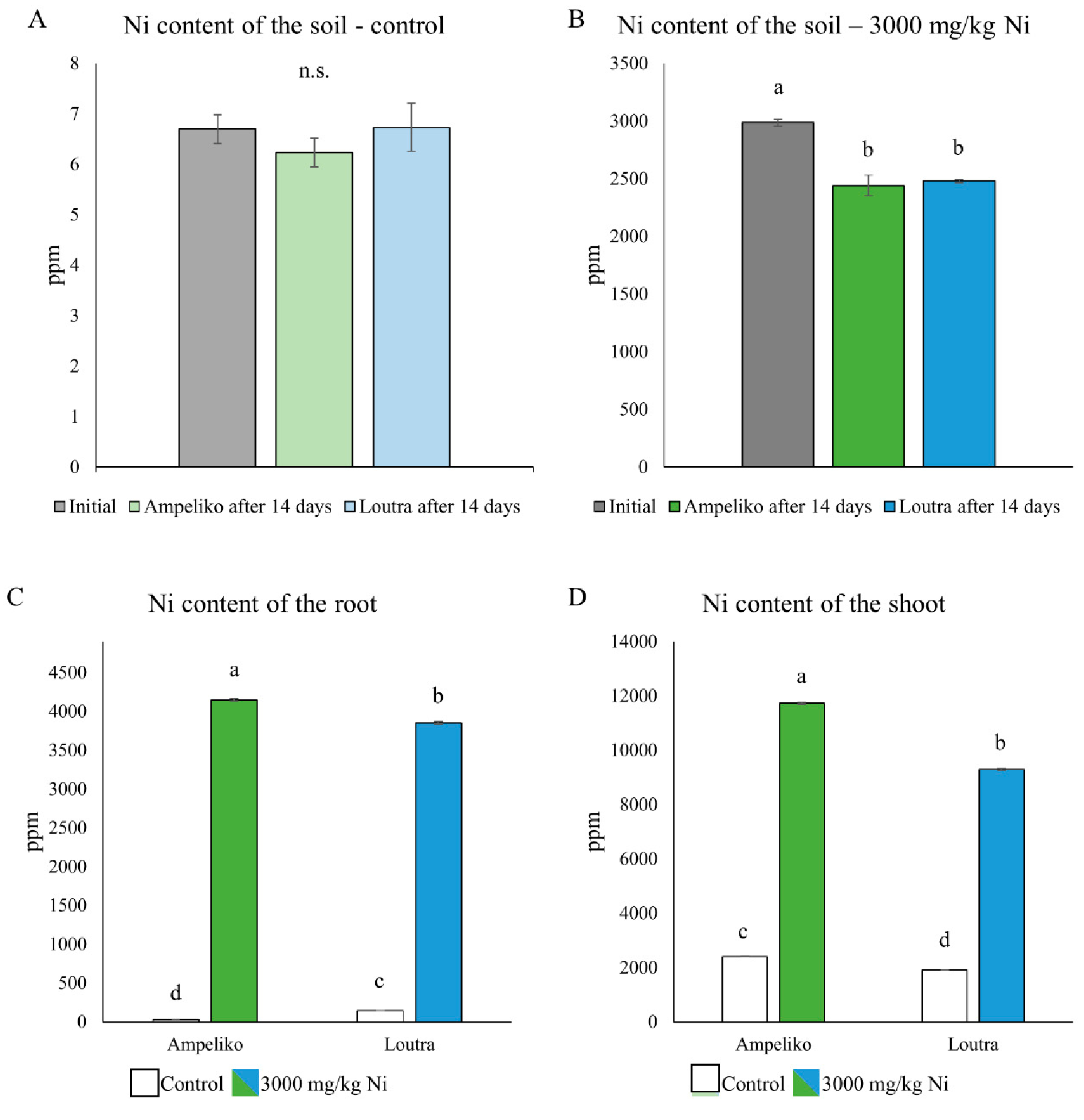
3.1. Changes in the Ni Content of the Soil and the Plants
3.2. Ni Induced Changes in the Root System Architecture
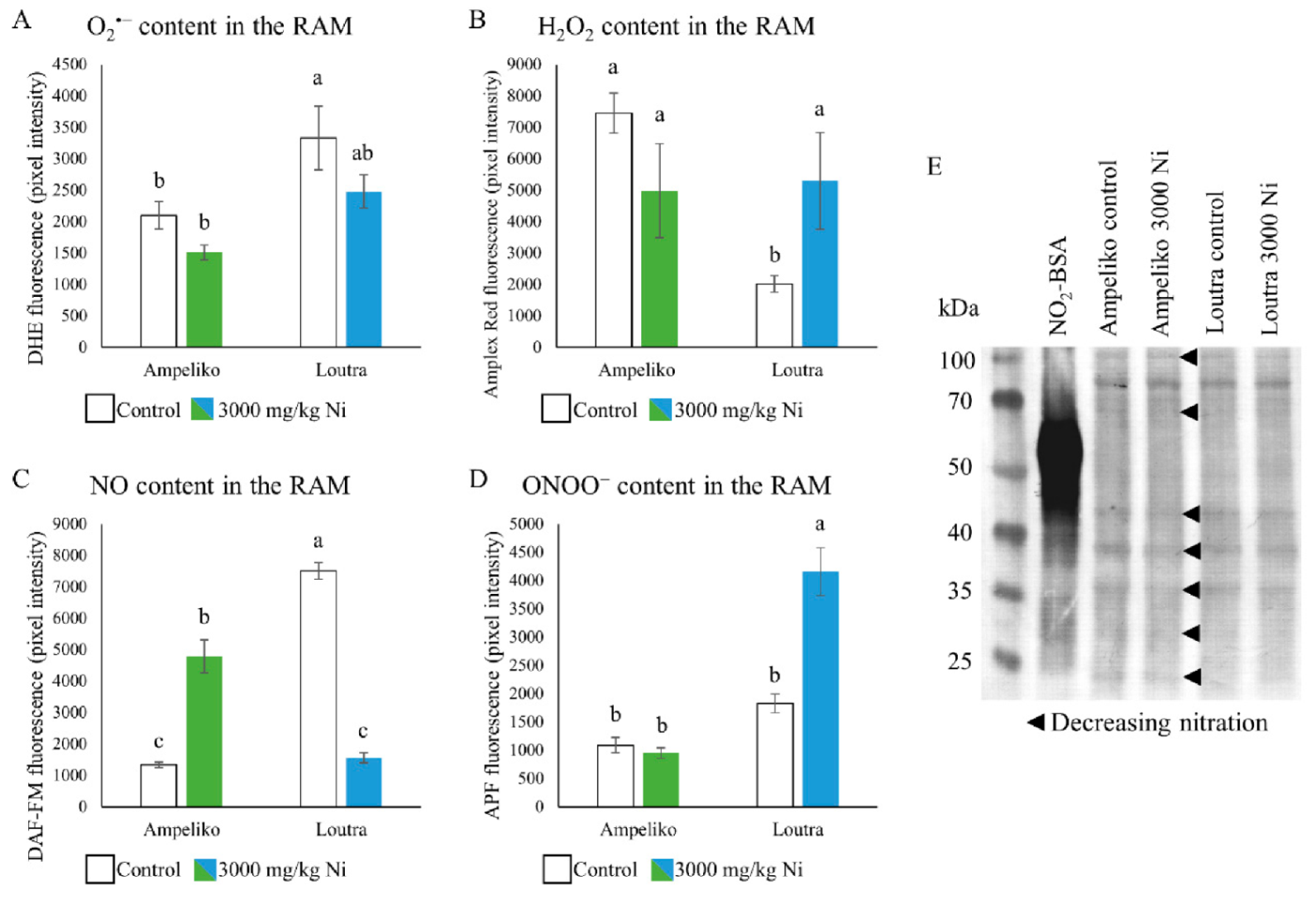
3.3. Differences in the Underlying Nitro-Oxidative Status
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Cempel, M.; Nikel, G. Nickel: A review of its sources and environmental toxicology. Pol. J. Environ. Stud. 2006, 15, 375–382. [Google Scholar]
- Shahzad, B.; Tanveer, M.; Rehman, A.; Cheema, S.; Fahad, S.; Rehman, S.; Sharma, A. Nickel; whether toxic or essential for plants and environment—A review. Plant Physiol. Biochem. 2018, 132, 641–651. [Google Scholar] [CrossRef]
- Alloway, B.J. Heavy Metals in Soils, 2nd ed.; Blackie Academic and Professional: London, UK, 1995. [Google Scholar] [CrossRef]
- Salt, D.; Kato, N.; Krämer, U.; Smith, R.; Raskin, I. The Role of Root Exudates in Nickel Hyperaccumulation and Tolerance in Accumulator and Nonaccumulator Species of Thlaspi. In Phytoremediation of Contaminated Soil and Water; Banuelos, G., Ed.; CRC Press: Boca Raton, FL, USA, 1999; pp. 191–202. [Google Scholar] [CrossRef]
- Kruckeberg, A.R. The Influences of Lithology on Plant Life. In Geology and Plant Life: The Effects of Landforms and Rock Type on Plants; University of Washington Press: Seattle, WA, USA, 2002; pp. 160–181. [Google Scholar]
- Kruckeberg, A.R. California Serpentines: Flora, Vegetation, Geology, Soils, and Management Problems; University of California Press: Berkeley, CA, USA, 1984; Volume 78. [Google Scholar]
- Kazakou, E.; Dimitrakopoulos, P.G.; Baker, A.J.M.; Reeves, R.D.; Troumbis, A.Y. Hypotheses, mechanisms and trade-offs of tolerance and adaptation to serpentine soils: From species to ecosystem level. Biol. Rev. 2008, 83, 495–508. [Google Scholar] [CrossRef] [PubMed]
- Reeves, R.D.; Baker, A.J.M.; Jaffré, T.; Erskine, P.; Echevarria, G.; van der Ent, A. A global database for plants that hyperaccumulate metal and metalloid trace elements. New Phytol. 2017, 218, 407–411. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Reeves, R.D.; van der Ent, A.; Baker, A.J.M. Global Distribution and Ecology of Hyperaccumulator Plants. In Agromining: Farming for Metals; van der Ent, A., Echevarria, G., Baker, A.J.M., Morel, J., Eds.; Springer: Cham, Switzerland, 2017; pp. 75–92. [Google Scholar] [CrossRef] [Green Version]
- Reeves, R.D. The hyperaccumulation of nickel by serpentine plants. In The Vegetation of Ultramafic (Serpentine) Soils; Baker, A.J.M., Proctor, J., Reeves, R.D., Eds.; Intercept Ltd.: Andover, MA, USA, 1992; pp. 253–277. [Google Scholar]
- Pollard, A.J.; Reeves, R.D.; Baker, A.J.M. Facultative hyperaccumulation of heavy metals and metalloids. Plant Sci. 2014, 217, 8–17. [Google Scholar] [CrossRef] [PubMed]
- Pollard, A.J.; Powell, K.; Harper, F.; Smith, J. The genetic basis of metal hyperaccumulation in plants. Crit. Rev. Plant Sci. 2002, 21, 539–566. [Google Scholar] [CrossRef]
- van der Ent, A.; Baker, A.J.M.; Reeves, R.D.; Pollard, A.J.; Schat, H. Hyperaccumulators of metal and metalloid trace elements: Facts and fiction. Plant Soil 2013, 362, 319–334. [Google Scholar] [CrossRef]
- Reeves, R.D.; Baker, A.J.M.; Becquer, T.; Echevarria, G.; Miranda, Z. The flora and biogeochemistry of the ultramafic soils of Goiás state, Brazil. Plant Soil 2007, 293, 107–119. [Google Scholar] [CrossRef]
- Mengoni, A.; Gonnelli, C.; Brocchini, E.; Galardi, F.; Pucci, S.; Gabbrielli, R.; Bazzicalupo, M. Chloroplast genetic diversity and biogeography in the serpentine endemic Ni-hyperaccumulator Alyssum bertolonii. New Phytol. 2003, 157, 349–356. [Google Scholar] [CrossRef]
- Galardi, F.; Mengoni, A.; Pucci, S.; Barletti, L.; Massi, L.; Barzanti, R.; Gabbrielli, R.; Gonnelli, C. Intra-specific differences in mineral element composition in the Ni-hyperaccumulator Alyssum bertolonii: A survey of populations in nature. Environ. Exp. Bot. 2007, 60, 50–56. [Google Scholar] [CrossRef]
- Adamidis, G.C.; Dimitrakopoulos, P.G.; Manolis, A.; Papageorgiou, A. Genetic diversity and population structure of the serpentine endemic Ni hyperaccumulator Alyssum lesbiacum. Plant Syst. Evol. 2014, 300, 2051–2060. [Google Scholar] [CrossRef]
- Bani, A.; Pavlova, D.; Echevarria, G.; Mullaj, A.; Reeves, R.D.; Morel, J.L.; Sulce, S. Nickel hyperaccumulation by the species of Alyssum and Thlaspi (Brassicaceae) from the ultramafic soils of the Balkans. Bot. Serbica 2010, 34, 3–14. [Google Scholar]
- Kazakou, E.; Adamidis, G.C.; Baker, A.J.M.; Reeves, R.D.; Godino, M.; Dimitrakopoulos, P.G. Species adaptation in serpentine soils in Lesbos Island (Greece): Metal hyperaccumulation and tolerance. Plant Soil 2010, 332, 369–385. [Google Scholar] [CrossRef]
- Dixon, N.; Gazzola, C.; Blakeley, R.; Zerner, B. Jack bean urease (EC 3.5.1.5). Metalloenzyme. Simple biological role for nickel. J. Am. Chem. Soc. 1975, 97, 4131–4133. [Google Scholar] [CrossRef]
- Shi, G.; Cai, Q. Leaf plasticity in peanut (Arachis hypogaea L.) in response to heavy metal stress. Environ. Exp. Bot. 2009, 67, 112–117. [Google Scholar] [CrossRef]
- Saad, R.; Kobaissi, A.; Robin, C.; Echevarria, G.; Benizri, E. Nitrogen fixation and growth of Lens culinaris as affected by nickel availability: A pre-requisite for optimization of agromining. Environ. Exp. Bot. 2016, 131, 1–9. [Google Scholar] [CrossRef]
- Halliwell, B.; Gutteridge, J. Oxygen toxicity, oxygen radicals, transition metals and disease. Biochem. J. 1984, 219, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Gajewska, E.; Sklodowska, M.; Slaba, M.; Mazur, J. Effect of nickel on antioxidative enzyme activities, proline and chlorophyll contents in wheat shoots. Biol. Plant. 2006, 50, 653–659. [Google Scholar] [CrossRef]
- Khaliq, A.; Ali, S.; Hameed, A.; Farooq, M.; Farid, M.; Shakoor, M.; Mahmood, K.; Ishaque, W.; Rizwan, M. Silicon alleviates nickel toxicity in cotton seedlings through enhancing growth, photosynthesis, and suppressing Ni uptake and oxidative stress. Arch. Agron. Soil Sci. 2015, 62, 633–647. [Google Scholar] [CrossRef]
- Gajewska, E.; Skłodowska, M. Effect of nickel on ROS content and antioxidative enzyme activities in wheat leaves. BioMetals 2006, 20, 27–36. [Google Scholar] [CrossRef]
- Kazemi, N.; Khavari-Nejad, R.; Fahimi, H.; Saadatmand, S.; Nejad-Sattari, T. Effects of exogenous salicylic acid and nitric oxide on lipid peroxidation and antioxidant enzyme activities in leaves of Brassica napus L. under nickel stress. Sci. Hortic. 2010, 126, 402–407. [Google Scholar] [CrossRef]
- Boominathan, R.; Doran, P. Ni-induced oxidative stress in roots of the Ni hyperaccumulator, Alyssum bertolonii. New Phytol. 2002, 156, 205–215. [Google Scholar] [CrossRef]
- Kolbert, Z.; Oláh, D.; Molnár, Á.; Szőllősi, R.; Erdei, L.; Ördög, A. Distinct redox signalling and nickel tolerance in Brassica juncea and Arabidopsis thaliana. Ecotoxicol. Environ. Saf. 2020, 189, 109989. [Google Scholar] [CrossRef] [PubMed]
- Molassiotis, A.; Fotopoulos, V. Oxidative and nitrosative signaling in plants. Plant Signal. Behav. 2011, 6, 210–214. [Google Scholar] [CrossRef] [Green Version]
- Corpas, F.; Barroso, J. Nitro-oxidative stress vs oxidative or nitrosative stress in higher plants. New Phytol. 2013, 199, 633–635. [Google Scholar] [CrossRef]
- Corpas, F.; Palma, J.; del Río, L.; Barroso, J. Protein tyrosine nitration in higher plants grown under natural and stress conditions. Front. Plant Sci. 2013, 4, 29. [Google Scholar] [CrossRef] [Green Version]
- Greenacre, S.; Ischiropoulos, H. Tyrosine nitration: Localisation, quantification, consequences for protein function and signal transduction. Free Radic. Res. 2001, 34, 541–581. [Google Scholar] [CrossRef]
- Radi, R. Nitric oxide, oxidants, and protein tyrosine nitration. Proc. Natl. Acad. Sci. USA 2004, 101, 4003–4008. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Galetskiy, D.; Lohscheider, J.; Kononikhin, A.; Popov, I.; Nikolaev, E.; Adamska, I. Phosphorylation and nitration levels of photosynthetic proteins are conversely regulated by light stress. Plant Mol. Biol. 2011, 77, 461–473. [Google Scholar] [CrossRef]
- Gzyl, J.; Izbiańska, K.; Floryszak-Wieczorek, J.; Jelonek, T.; Arasimowicz-Jelonek, M. Cadmium affects peroxynitrite generation and tyrosine nitration in seedling roots of soybean (Glycine max L.). Environ. Exp. Bot. 2016, 131, 155–163. [Google Scholar] [CrossRef]
- Feigl, G.; Lehotai, N.; Molnár, Á.; Ördög, A.; Rodríguez-Ruiz, M.; Palma, J.; Corpas, F.; Erdei, L.; Kolbert, Z. Zinc induces distinct changes in the metabolism of reactive oxygen and nitrogen species (ROS and RNS) in the roots of two Brassica species with different sensitivity to zinc stress. Ann. Bot. 2014, 116, 613–625. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Feigl, G.; Kolbert, Z.; Lehotai, N.; Molnár, Á.; Ördög, A.; Bordé, Á.; Laskay, G.; Erdei, L. Different zinc sensitivity of Brassica organs is accompanied by distinct responses in protein nitration level and pattern. Ecotoxicol. Environ. Saf. 2016, 125, 141–152. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Feigl, G.; Czifra, Á.; Molnár, Á.; Bodor, A.; Kovács, E.; Perei, K.; Jebet, V.; Kolbert, Z. Reorganization of protein tyrosine nitration pattern indicates the relative tolerance of Brassica napus (L.) over Helianthus annuus (L.) to combined heavy metal treatment. Plants 2020, 9, 902. [Google Scholar] [CrossRef] [PubMed]
- Strid, A.; Tan, K. Flora Hellenica; A.R.G. Gantner Verlag K.G.: Ruggell, Liechtenstein, 2002; Volume 2. [Google Scholar]
- Brooks, R.R.; Morrison, R.S.; Reeves, R.D.; Dudley, T.R.; Akmans, Y. Hyperaccumulation of nickel by Alyssum Linnaeus (Cruciferae). Proc. R. Soc. Lond. Ser. B. Biol. Sci. 1979, 203, 387–403. [Google Scholar] [CrossRef]
- Adamidis, G.C.; Aloupi, M.; Kazakou, E.; Dimitrakopoulos, P.G. Intra-specific variation in Ni tolerance, accumulation and translocation patterns in the Ni-hyperaccumulator Alyssum lesbiacum. Chemosphere 2014, 95, 496–502. [Google Scholar] [CrossRef] [PubMed]
- van der Ent, A.; Callahan, D.; Noller, B.; Mesjasz-Przybylowicz, J.; Przybylowicz, W.; Barnabas, A.; Harris, H. Nickel biopathways in tropical nickel hyperaccumulating trees from Sabah (Malaysia). Sci. Rep. 2017, 7. [Google Scholar] [CrossRef]
- Ingle, R.; Mugford, S.; Rees, J.; Campbell, M.; Smith, J. Constitutively High expression of the histidine biosynthetic pathway contributes to nickel tolerance in hyperaccumulator plants. Plant Cell 2005, 17, 2089–2106. [Google Scholar] [CrossRef] [Green Version]
- Ingle, R.; Fricker, M.; Smith, J. Evidence for nickel/proton antiport activity at the tonoplast of the hyperaccumulator plant Alyssum lesbiacum. Plant Biol. 2008, 10, 746–753. [Google Scholar] [CrossRef]
- Stefanatou, A.; Dimitrakopoulos, P.G.; Aloupi, M.; Adamidis, G.C.; Nakas, G.; Petanidou, T. From bioaccumulation to biodecumulation: Nickel movement from Odontarrhena lesbiaca (Brassicaceae) individuals into consumers. Sci. Total Environ. 2020, 747, 141197. [Google Scholar] [CrossRef]
- Adamidis, G.C.; Kazakou, E.; Aloupi, M.; Dimitrakopoulos, P.G. Is it worth hyperaccumulating Ni on non-serpentine soils? Decomposition dynamics of mixed-species litters containing hyperaccumulated Ni across serpentine and non-serpentine environments. Ann. Bot. 2016, 117, 1241–1248. [Google Scholar] [CrossRef] [Green Version]
- Adamidis, G.C.; Kazakou, E.; Baker, A.J.M.; Reeves, R.D.; Dimitrakopoulos, P.G. The effect of harsh abiotic conditions on the diversity of serpentine plant communities on Lesbos Island, an eastern Mediterranean island. Plant Ecol. Divers. 2014, 7, 433–444. [Google Scholar] [CrossRef]
- Feigl, G.; Molnár, Á.; Szőllősi, R.; Ördög, A.; Törőcsik, K.; Oláh, D.; Bodor, A.; Perei, K.; Kolbert, Z. Zinc-induced root architectural changes of rhizotron-grown B. napus correlate with a differential nitro-oxidative response. Nitric Oxide 2019, 90, 55–65. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schindelin, J.; Arganda-Carreras, I.; Frise, E.; Kaynig, V.; Longair, M.; Pietzsch, T.; Preibisch, S.; Rueden, C.; Saalfeld, S.; Schmid, B.; et al. Fiji: An open-source platform for biological-image analysis. Nat. Methods 2012, 9, 676–682. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ladányi, Z.; Csányi, K.; Farsang, A.; Perei, K.; Bodor, A.; Kézér, A.; Barta, K.; Babcsányi, I. Impact of Low-Dose Municipal Sewage Sludge Compost Treatments on the Nutrient and the Heavy Metal Contents in a Chernozem Topsoil Near Újkígyós, Hungary: A 5-Year Comparison. J. Environ. Geogr. 2020, 13, 25–30. [Google Scholar] [CrossRef]
- Lehotai, N.; Kolbert, Z.; Peto, A.; Feigl, G.; Ordog, A.; Kumar, D.; Tari, I.; Erdei, L. Selenite-induced hormonal and signalling mechanisms during root growth of Arabidopsis thaliana L. J. Exp. Bot. 2012, 63, 5677–5687. [Google Scholar] [CrossRef] [Green Version]
- Sun, Y.; Zhou, Q.; An, J.; Liu, W.; Liu, R. Chelator-enhanced phytoextraction of heavy metals from contaminated soil irrigated by industrial wastewater with the hyperaccumulator plant (Sedum alfredii Hance). Geoderma 2009, 150, 106–112. [Google Scholar] [CrossRef]
- Rezvani, M.; Zaefarian, F. Bioaccumulation and translocation factors of cadmium and lead in Aeluropus littoralis. Aust. J. Agric. Eng. 2011, 2, 114–119. [Google Scholar]
- Pető, A.; Lehotai, N.; Feigl, G.; Tugyi, N.; Ördög, A.; Gémes, K.; Tari, I.; Erdei, L.; Kolbert, Z. Nitric oxide contributes to copper tolerance by influencing ROS metabolism in Arabidopsis. Plant Cell Rep. 2013, 32, 1913–1923. [Google Scholar] [CrossRef]
- Kolbert, Z.; Pető, A.; Lehotai, N.; Feigl, G.; Ördög, A.; Erdei, L. In vivo and in vitro studies on fluorophore-specificity. Acta Biol. Szeged. 2012, 56, 37–41. [Google Scholar]
- Chaki, M.; Valderrama, R.; Fernández-Ocaña, A.; Carreras, A.; López-Jaramillo, J.; Luque, F.; Palma, J.; Pedrajas, J.; Begara-Morales, J.; Sánchez-Calvo, B.; et al. Protein targets of tyrosine nitration in sunflower (Helianthus annuus L.) hypocotyls. J. Exp. Bot. 2009, 60, 4221–4234. [Google Scholar] [CrossRef]
- Kolbert, Z.; Molnár, Á.; Szőllősi, R.; Feigl, G.; Erdei, L.; Ördög, A. Nitro-Oxidative stress correlates with Se tolerance of Astragalus species. Plant Cell Physiol. 2018, 59, 1827–1843. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bradford, M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
- Küpper, H.; Lombi, E.; Zhao, F.; Wieshammer, G.; McGrath, S. Cellular compartmentation of nickel in the hyperaccumulators Alyssum lesbiacum, Alyssum bertolonii and Thlaspi goesingense. J. Exp. Bot. 2001, 52, 2291–2300. [Google Scholar] [CrossRef] [Green Version]
- Yoon, J.; Cao, X.; Zhou, Q.; Ma, L. Accumulation of Pb, Cu, and Zn in native plants growing on a contaminated Florida site. Sci. Total Environ. 2006, 368, 456–464. [Google Scholar] [CrossRef] [PubMed]
- Rizwan, M.; Mostofa, M.; Ahmad, M.; Imtiaz, M.; Mehmood, S.; Adeel, M.; Dai, Z.; Li, Z.; Aziz, O.; Zhang, Y.; et al. Nitric oxide induces rice tolerance to excessive nickel by regulating nickel uptake, reactive oxygen species detoxification and defense-related gene expression. Chemosphere 2018, 191, 23–35. [Google Scholar] [CrossRef]
- Mihailovic, N.; Drazic, G. Incomplete alleviation of nickel toxicity in bean by nitric oxide supplementation. Plant Soil Environ. 2011, 57, 396–401. [Google Scholar] [CrossRef] [Green Version]
- Kotapati, K.; Palaka, B.; Ampasala, D. Alleviation of nickel toxicity in finger millet (Eleusine coracana L.) germinating seedlings by exogenous application of salicylic acid and nitric oxide. Crop J. 2017, 5, 240–250. [Google Scholar] [CrossRef] [Green Version]
- Castillo, M.; Lozano-Juste, J.; González-Guzmán, M.; Rodriguez, L.; Rodriguez, P.; León, J. Inactivation of PYR/PYL/RCAR ABA receptors by tyrosine nitration may enable rapid inhibition of ABA signaling by nitric oxide in plants. Sci. Signal. 2015, 8, ra89. [Google Scholar] [CrossRef]
- Van Hoewyk, D.; Taskin, M.; Yaprak, A.; Turgay, O.; Ergul, A. Profiling of proteasome activity in Alyssum species on serpentine soils in Turkey reveals possible insight into nickel tolerance and accumulation. Plant Physiol. Biochem. 2018, 124, 184–189. [Google Scholar] [CrossRef]

© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Feigl, G.; Varga, V.; Molnár, Á.; Dimitrakopoulos, P.G.; Kolbert, Z. Different Nitro-Oxidative Response of Odontarrhena lesbiaca Plants from Geographically Separated Habitats to Excess Nickel. Antioxidants 2020, 9, 837. https://doi.org/10.3390/antiox9090837
Feigl G, Varga V, Molnár Á, Dimitrakopoulos PG, Kolbert Z. Different Nitro-Oxidative Response of Odontarrhena lesbiaca Plants from Geographically Separated Habitats to Excess Nickel. Antioxidants. 2020; 9(9):837. https://doi.org/10.3390/antiox9090837
Chicago/Turabian StyleFeigl, Gábor, Viktória Varga, Árpád Molnár, Panayiotis G. Dimitrakopoulos, and Zsuzsanna Kolbert. 2020. "Different Nitro-Oxidative Response of Odontarrhena lesbiaca Plants from Geographically Separated Habitats to Excess Nickel" Antioxidants 9, no. 9: 837. https://doi.org/10.3390/antiox9090837




