Pharmacological Activation of Sirt1 Ameliorates Cisplatin-Induced Acute Kidney Injury by Suppressing Apoptosis, Oxidative Stress, and Inflammation in Mice
Abstract
:1. Introduction
2. Materials and Methods
2.1. Animals and Drug Treatment
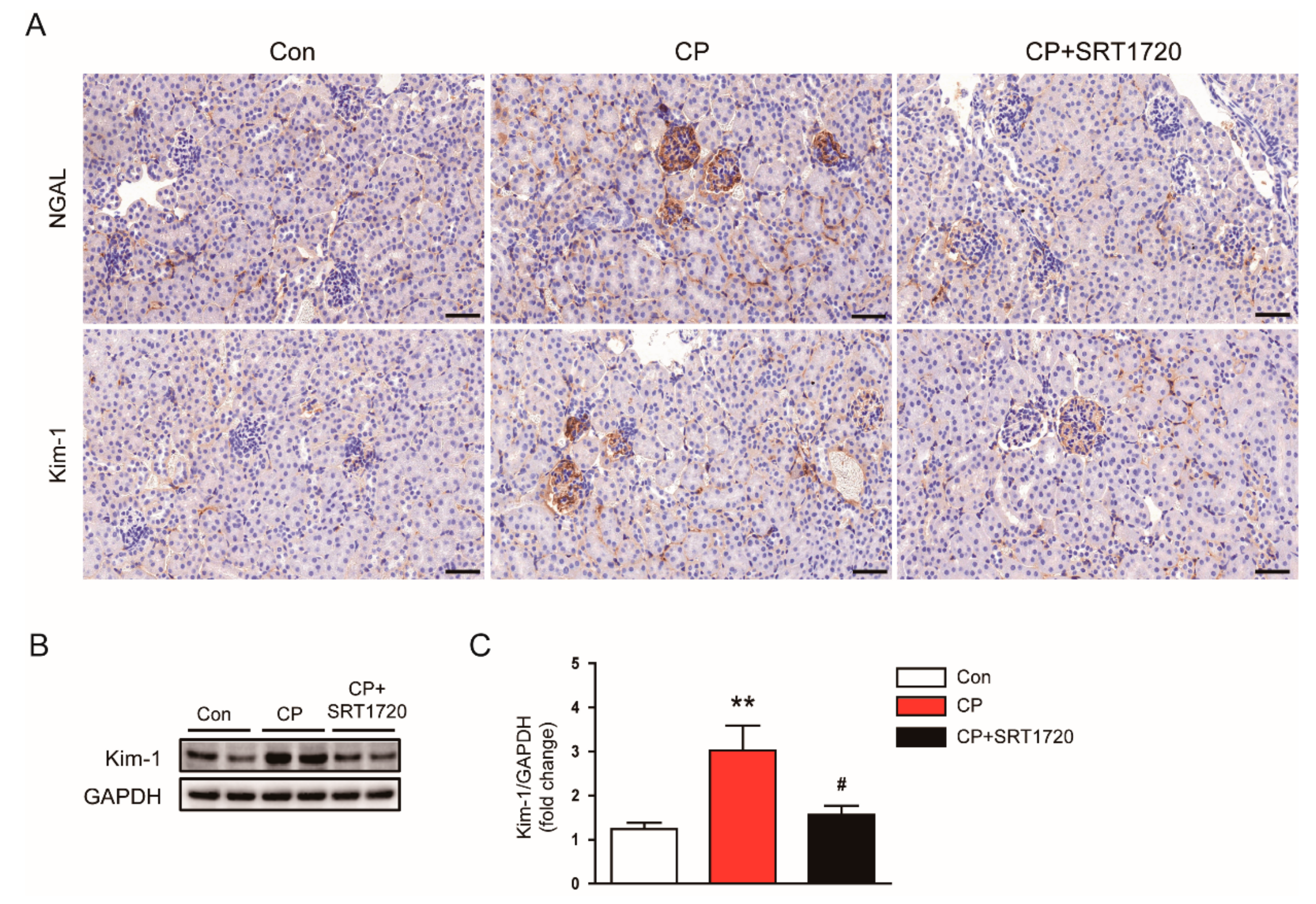
2.2. Histology and Immunohistochemistry
2.3. Evaluation of Renal Function and Oxidative Stress
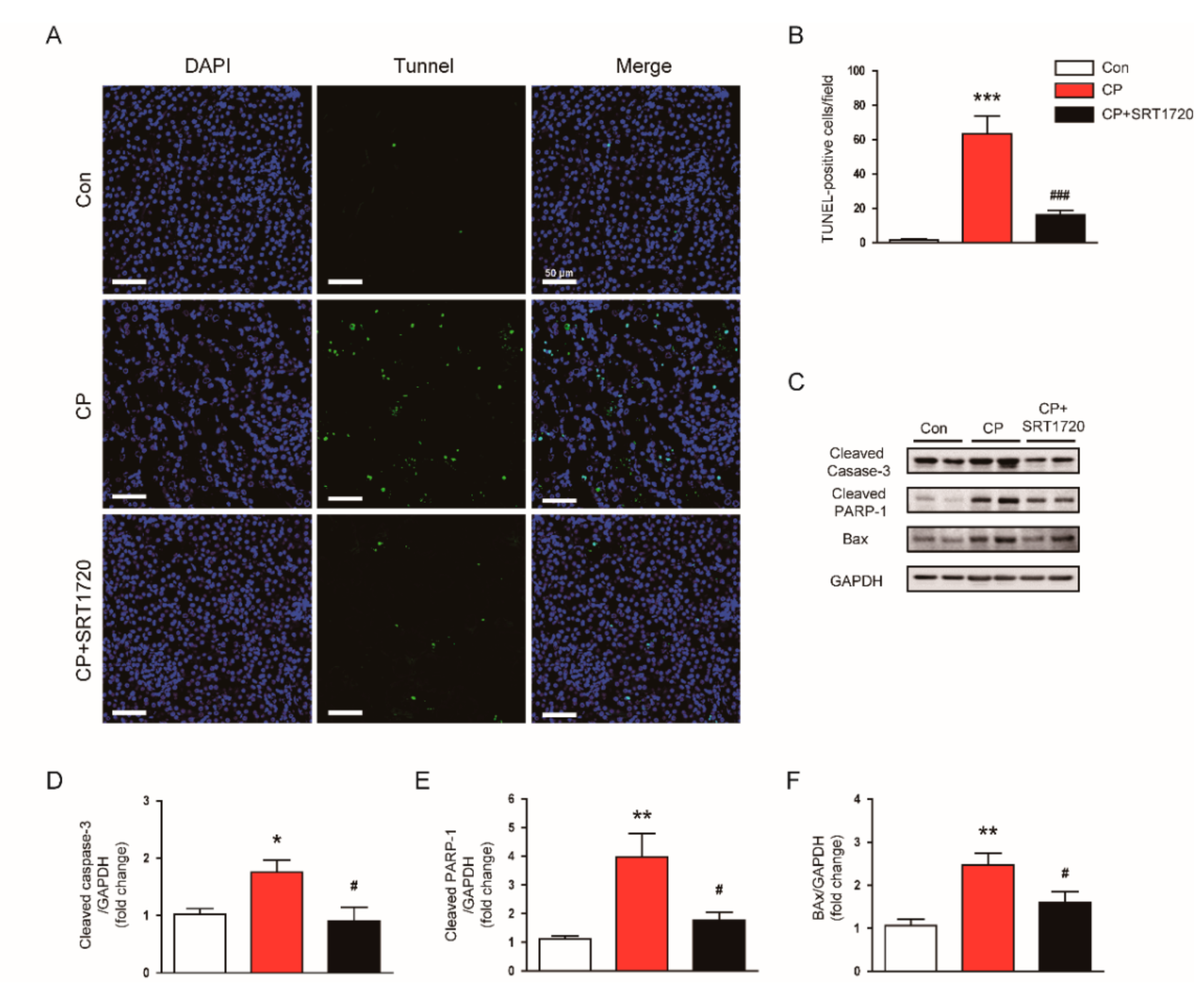
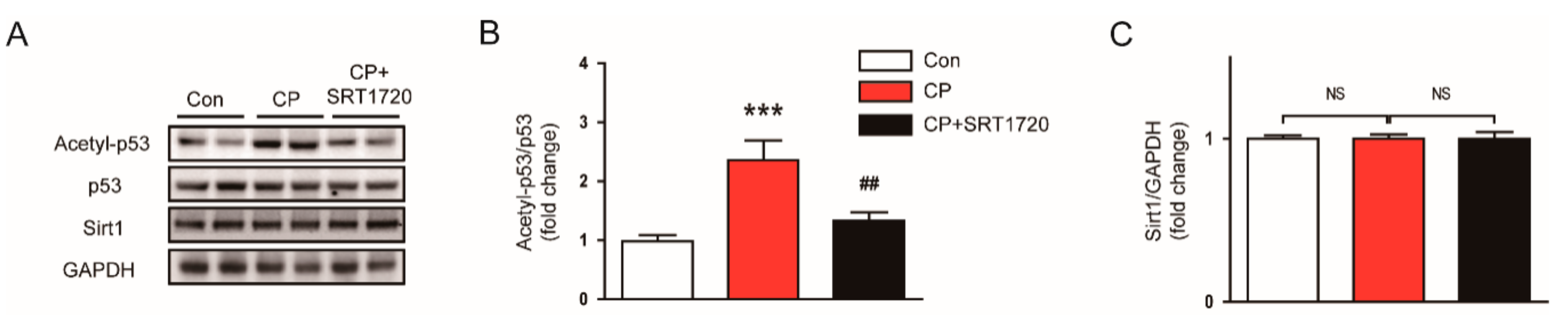
2.4. Western Blot Analysis
2.5. Terminal Deoxynucleotidyl Transferase -Mediated Deoxyuridine Triphosphate Nick End Labeling (TUNEL) Staining
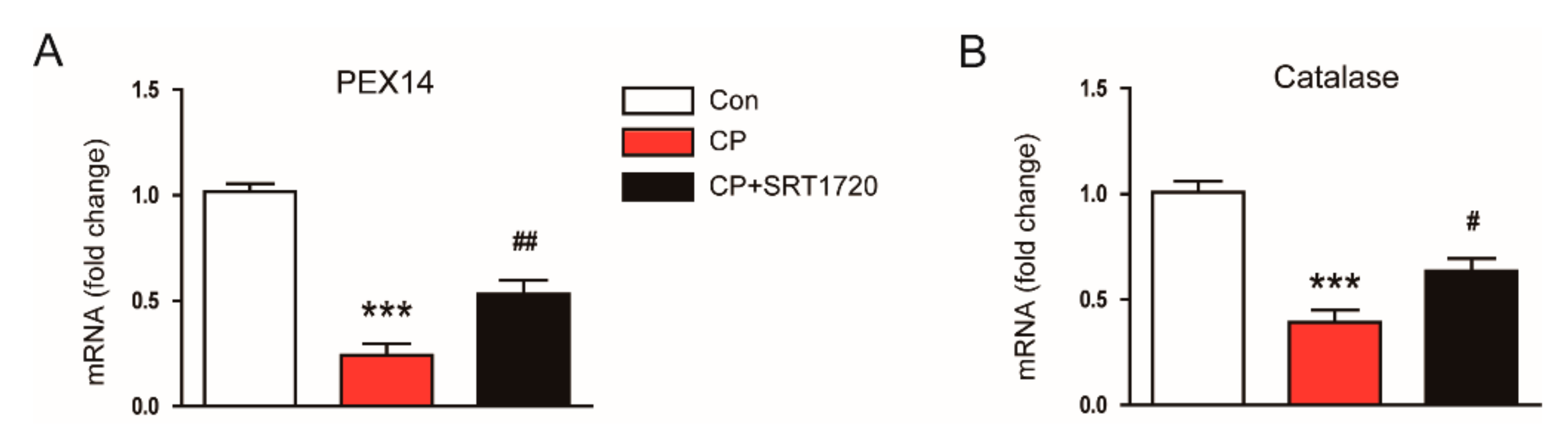
2.6. Gene Expression Analysis
2.7. Statistical Analysis
3. Results
3.1. SRT1720 Ameliorated Cisplatin-Induced AKI
3.2. SRT1720 Suppressed Cisplatin-Induced Cell Apoptosis
3.3. SRT1720 Suppressed p53 Acetylation in Mice Treated With Cisplatin
3.4. SRT1720 Decreased Cisplatin-Induced Oxidative Stress and Preserved Peroxisome Function
3.5. SRT1720 Attenuated Cisplatin-Induced Inflammatory Responses
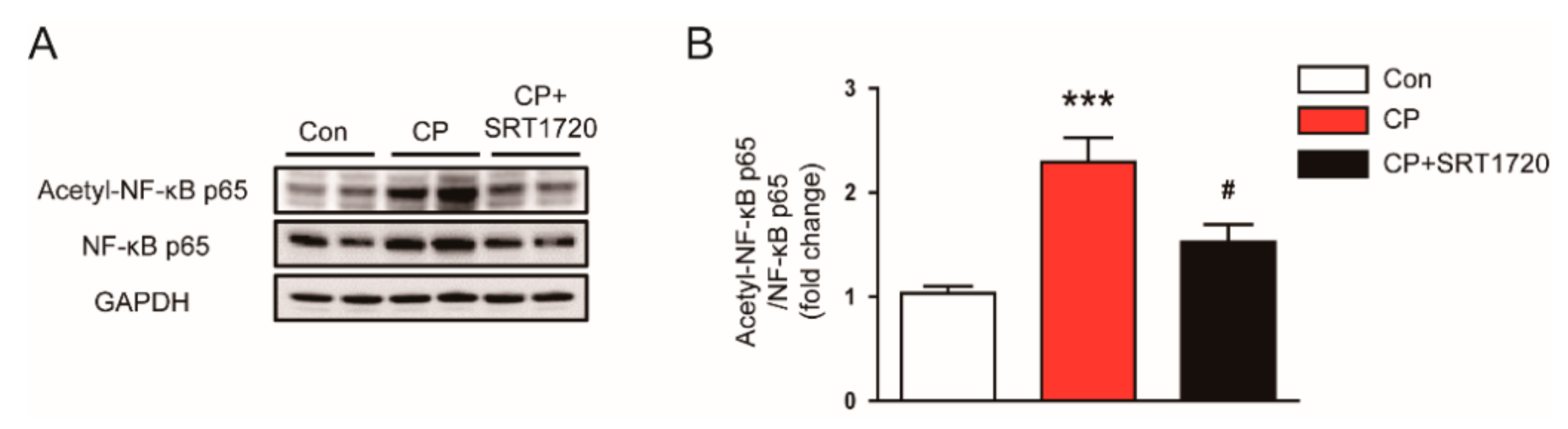
3.6. SRT1720 Reduced Acetylation of NF-κB p65 In Mice Treated With Cisplatin
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Pabla, N.; Dong, Z. Cisplatin nephrotoxicity: Mechanisms and renoprotective strategies. Kidney Int. 2008, 73, 994–1007. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sánchez-González, P.D.; López-Hernández, F.J.; López-Novoa, J.M.; Morales, A.I. An integrative view of the pathophysiological events leading to cisplatin nephrotoxicity. Crit. Rev. Toxicol. 2011, 41, 803–821. [Google Scholar] [CrossRef] [PubMed]
- Perše, M.; Večerić-Haler, Ž. Cisplatin-induced rodent model of kidney injury: Characteristics and challenges. Biomed. Res. Int. 2018, 2018, 1462802. [Google Scholar] [CrossRef] [PubMed]
- Ghosh, H.S. The anti-aging, metabolism potential of SIRT1. Curr. Opin. Investig. Drugs 2008, 9, 1095–1102. [Google Scholar] [PubMed]
- Chang, H.C.; Guarente, L. SIRT1 and other sirtuins in metabolism. Trends Endocrinol. Metab. 2014, 25, 138–145. [Google Scholar] [CrossRef]
- Vaziri, H.; Dessain, S.K.; Ng Eaton, E.; Imai, S.I.; Frye, R.A.; Pandita, T.K.; Guarente, L.; Weinberg, R.A. hSIR2(SIRT1) functions as an NAD-dependent p53 deacetylase. Cell 2001, 107, 149–159. [Google Scholar] [CrossRef]
- Ong, A.L.C.; Ramasamy, T.S. Role of Sirtuin1-p53 regulatory axis in aging, cancer and cellular reprogramming. Aging Res. Rev. 2018, 43, 64–80. [Google Scholar] [CrossRef]
- Yeung, F.; Hoberg, J.E.; Ramsey, C.S.; Keller, M.D.; Jones, D.R.; Frye, R.A.; Mayo, M.W. Modulation of NF-kappaB-dependent transcription and cell survival by the SIRT1 deacetylase. EMBO J. 2004, 23, 2369–2380. [Google Scholar] [CrossRef]
- Xie, J.; Zhang, X.; Zhang, L. Negative regulation of inflammation by SIRT1. Pharmacol. Res. 2013, 67, 60–67. [Google Scholar] [CrossRef]
- Hasegawa, K.; Wakino, S.; Yoshioka, K.; Tatematsu, S.; Hara, Y.; Minakuchi, H.; Sueyasu, K.; Washida, N.; Tokuyama, H.; Tzukerman, M.; et al. Kidney-specific overexpression of Sirt1 protects against acute kidney injury by retaining peroxisome function. J. Biol. Chem. 2010, 285, 13045–13056. [Google Scholar] [CrossRef]
- Milne, J.C.; Lambert, P.D.; Schenk, S.; Carney, D.P.; Smith, J.J.; Gagne, D.J.; Jin, L.; Boss, O.; Perni, R.B.; Vu, C.B.; et al. Small molecule activators of SIRT1 as therapeutics for the treatment of type 2 diabetes. Nature 2007, 450, 712–716. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mitchell, S.J.; Martin-Montalvo, A.; Mercken, E.M.; Palacios, H.H.; Ward, T.M.; Abulwerdi, G.; Minor, R.K.; Vlasuk, G.P.; Ellis, J.L.; Sinclair, D.A.; et al. The SIRT1 activator SRT1720 extends lifespan and improves health of mice fed a standard diet. Cell Rep. 2014, 6, 836–843. [Google Scholar] [CrossRef] [PubMed]
- Feige, J.N.; Lagouge, M.; Canto, C.; Strehle, A.; Houten, S.M.; Milne, J.C.; Lambert, P.D.; Mataki, C.; Elliott, P.J.; Auwerx, J. Specific SIRT1 activation mimics low energy levels and protects against diet-induced metabolic disorders by enhancing fat oxidation. Cell Metab. 2008, 8, 347–358. [Google Scholar] [CrossRef] [PubMed]
- Ichikawa, T.; Hayashi, R.; Suzuki, K.; Imanishi, S.; Kambara, K.; Okazawa, S.; Inomata, M.; Yamada, T.; Yamazaki, Y.; Koshimizu, Y.; et al. Sirtuin 1 activator SRT1720 suppresses inflammation in an ovalbumin-induced mouse model of asthma. Respirology 2013, 18, 332–339. [Google Scholar] [CrossRef] [PubMed]
- Yao, H.; Sundar, I.K.; Ahmad, T.; Lerner, C.; Gerloff, J.; Friedman, A.E.; Phipps, R.P.; Sime, P.J.; McBurney, M.W.; Guarente, L. SIRT1 protects against cigarette smoke-induced lung oxidative stress via a FOXO3-dependent mechanism. Am. J. Physiol.-Lung Cell. Mol. Physiol. 2014, 306, L816–L828. [Google Scholar] [CrossRef] [PubMed]
- Khader, A.; Yang, W.L.; Godwin, A.; Prince, J.M.; Nicastro, J.M.; Coppa, G.F.; Wang, P. Sirtuin 1 stimulation attenuates ischemic liver injury and enhances mitochondrial recovery and autophagy. Crit. Care Med. 2016, 44, e651–e663. [Google Scholar] [CrossRef] [PubMed]
- Khader, A.; Yang, W.L.; Hanse, L.W.; Rajayer, S.R.; Prince, J.M.; Nicastro, J.M.; Coppa, G.F.; Wang, P. SRT1720, a sirtuin 1 activator, attenuates organ injury and inflammation in sepsis. J. Surg. Res. 2017, 219, 288–295. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.Y.; Park, J.H.; Kim, K.; Jo, J.; Leem, J.; Park, K.K. Pharmacological inhibition of caspase-1 ameliorates cisplatin-induced nephrotoxicity through suppression of apoptosis, oxidative stress, and inflammation in mice. Mediators Inflamm. 2018, 2018, 6571676. [Google Scholar] [CrossRef] [PubMed]
- Negishi, K.; Noiri, E.; Sugaya, T.; Li, S.; Megyesi, J.; Nagothu, K.; Portilla, D. A role of liver fatty acid-binding protein in cisplatin-induced acute renal failure. Kidney Int. 2007, 72, 348–358. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhai, Z.; Tang, M.; Yang, Y.; Lu, M.; Zhu, W.G.; Li, T. Identifying human SIRT1 substrates by integrating heterogeneous information from various sources. Sci. Rep. 2017, 7, 4614. [Google Scholar] [CrossRef] [PubMed]
- Do Amaral, C.L.; Francescato, H.D.; Coimbra, T.M.; Coista, R.S.; Darin, J.D.; Antunes, L.M.; Bianchi Mde, L. Resveratrol attenuates cisplatin-induced nephrotoxicity in rats. Arch. Toxicol. 2008, 82, 363–370. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.H.; Jung, Y.J.; Lee, J.E.; Lee, A.S.; Kang, K.P.; Lee, S.; Park, S.K.; Han, M.K.; Lee, S.Y.; Ramkumar, K.M.; et al. SIRT1 activation by resveratrol ameliorates cisplatin-induced renal injury through deacetylation of p53. Am. J. Physiol.-Ren. Physiol. 2011, 301, F427–F435. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kaeberlein, M.; Mcdonagh, T.; Heltweg, B.; Hixon, J.; Westman, E.A.; Caldwell, S.D.; Napper, A.; Curtis, R.; DiStefano, P.S.; Fields, S.; et al. Substrate-specific activation of sirtuins by resveratrol. J. Biol. Chem. 2005, 280, 17038–17045. [Google Scholar] [CrossRef] [PubMed]
- Borra, M.T.; Smith, B.C.; Denu, J.M. Mechanism of human SIRT1 activation by resveratrol. J. Biol. Chem. 2005, 280, 17187–17195. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, S.; Hussain, A.; Hussain, A.; Abdullah, I.; Ali, M.S.; Froeyen, M.; Mirza, M.U. Quantification of beberine in Berberis vulgaris L. root extract and its curative and prophylactic role in cisplatin-induced in vivo toxicity and in vitro cytotoxicity. Antioxidants 2019, 8, 185. [Google Scholar] [CrossRef]
- Salem, N.; Helmi, N.; Assaf, N. Renoprotective effect of platelet-rich plasma on cisplatin-induced nephrotoxicity in rats. Oxid. Med. Cell. Longev. 2018, 2018, 9658230. [Google Scholar] [CrossRef] [PubMed]
- Meng, H.; Fu, G.; Shen, J.; Shen, K.; Xu, Z.; Wang, Y.; Jin, B.; Pan, H. Ameliorative effect of daidzein on cisplatin-induced nephrotoxicity in mice via modulation of inflammation, oxidative stress, and cell death. Oxid. Med. Cell. Longev. 2017, 2017, 3140680. [Google Scholar] [CrossRef]
- Luo, J.; Li, M.; Tang, Y.; Laszkowska, M.; Roeder, R.G.; Gu, W. Acetylation of p53 augments its site-specific DNA binding both in vitro and in vivo. Proc. Natl. Acad. Sci. USA 2004, 101, 2259–2264. [Google Scholar] [CrossRef]
- Jiang, M.; Yi, X.; Hsu, S.; Wang, C.Y.; Dong, Z. Role of p53 in cisplatin-induced tubular cell apoptosis: Dependence on p53 transcriptional activity. Am. J. Physiol.-Ren. Physiol. 2004, 287, F1140–F1147. [Google Scholar] [CrossRef]
- Wei, Q.; Dong, G.; Yang, T.; Megyesi, J.; Price, P.M.; Dong, Z. Activation and involvement of p53 in cisplatin-induced nephrotoxicity. Am. J. Physiol.-Ren. Physiol. 2007, 293, F1282–F1291. [Google Scholar] [CrossRef] [Green Version]
- Dong, G.; Luo, J.; Kumar, V.; Dong, Z. Inhibitors of histone deacetylases suppress cisplatin-induced p53 activation and apoptosis in renal tubular cells. Am. J. Physiol.-Ren. Physiol. 2010, 298, F293–F300. [Google Scholar] [CrossRef] [Green Version]
- Molitoris, B.A.; Dagher, P.C.; Sandoval, R.M.; Campos, S.B.; Ashush, H.; Fridman, E.; Brafman, A.; Faerman, A.; Atkinson, S.J.; Thompson, J.D.; et al. siRNA targeted to p53 attenuates ischemic and cisplatin-induced acute kidney injury. J. Am. Soc. Nephrol. 2009, 20, 1754–1764. [Google Scholar] [CrossRef]
- Schrader, M.; Fahimi, H.D. Peroxisomes and oxidative stress. Biochim. Biophys. Acta 2006, 1763, 1755–1766. [Google Scholar] [CrossRef] [Green Version]
- Rjeibi, I.; Feriani, A.; Ben Saad, A.; Sdayria, J.; Saidi, I.; Ncib, S.; Souid, S.; Allagui, M.S.; Hfaiedh, N. Lycium europaeum extract: A new potential antioxidant source against cisplatin-induced liver and kidney injuries in mice. Oxid. Med. Cell. Longev. 2018, 2018, 1630751. [Google Scholar] [CrossRef]
- Wang, Z.; Li, Y.F.; Han, X.Y.; Sun, Y.S.; Zhang, L.X.; Liu, W.; Liu, X.X.; Li, W.; Liu, Y.Y. Kidney protection effect of ginsenoside Re and its underlying mechanisms on cisplatin-induced kidney injury. Cell. Physiol. Biochem. 2018, 48, 2219–2229. [Google Scholar] [CrossRef]
- Hasegawa, K.; Wakino, S.; Yoshioka, K.; Tatematsu, S.; Hara, Y.; Minakuchi, H.; Washida, N.; Tokuyama, H.; Hayashi, K.; Itoh, H. Sirt1 protects against oxidative stress-induced renal tubular cell apoptosis by the bidirectional regulation of catalase expression. Biochem. Biophys. Res. Commun. 2008, 372, 51–56. [Google Scholar] [CrossRef]
- Brezniceanu, M.L.; Liu, F.; Wei, C.C.; Chénier, I.; Godin, N.; Zhang, S.L.; Filep, J.G.; Ingelfinger, J.R.; Chan, J.S. Attenuation of interstitial fibrosis and tubular apoptosis in db/db transgenic mice overexpressing catalase in renal proximal tubular cells. Diabetes 2008, 57, 451–459. [Google Scholar] [CrossRef]
- Ramesh, G.; Reeves, W.B. TNF-alpha mediates chemokine and cytokine expression and renal injury in cisplatin nephrotoxicity. J. Clin. Investig. 2002, 110, 835–842. [Google Scholar] [CrossRef]
- Ramesh, G.; Zhang, B.; Uematus, S.; Akira, S.; Reeves, W.B. Endotoxin and cisplatin synergistically induce renal dysfunction and cytokine production in mice. Am. J. Physiol.-Ren. Physiol. 2007, 293, F325–F332. [Google Scholar] [CrossRef]
- Zhang, B.; Ramesh, G.; Norbury, C.C.; Reeves, W.B. Cisplatin-induced nephrotoxicity is mediated by tumor necrosis factor-alpha produced by renal parenchymal cells. Kidney Int. 2007, 72, 37–44. [Google Scholar] [CrossRef]
- Cao, Q.; Wang, Y.; Harris, D.C. Pathogenic and protective role of macrophages in kidney disease. Am. J. Physiol.-Ren. Physiol. 2013, 305, F3–F11. [Google Scholar] [CrossRef] [Green Version]
- Jung, Y.J.; Lee, J.E.; Lee, A.S.; Kang, K.P.; Lee, S.; Park, S.K.; Lee, S.Y.; Han, M.K.; Kim, D.H.; Kim, W. SIRT1 overexpression decreases cisplatin-induced acetylation of NF-κB p65 subunit and cytotoxicity in renal proximal tubule cells. Biochem. Biophys. Res. Commun. 2012, 419, 206–210. [Google Scholar] [CrossRef]




| Gene | Primer Sequence (5′→3′) | Product Size (bp) |
|---|---|---|
| PEX14 1 | Forward: GCCACCACATCAACCAACTG Reverse: GTCTCCGATTCAAAAGAAGTCCT | 97 |
| catalase | Forward: CAAGTACAACGCTGAGAAGCCTAAG Reverse: CCCTTCGCAGCCATGTG | 74 |
| GAPDH 2 | Forward: ACTCCACTCACGGCAAATTC Reverse: TCTCCATGGTGGTGAAGACA | 170 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, J.-Y.; Jo, J.; Kim, K.; An, H.-J.; Gwon, M.-G.; Gu, H.; Kim, H.-J.; Yang, A.Y.; Kim, S.-W.; Jeon, E.J.; et al. Pharmacological Activation of Sirt1 Ameliorates Cisplatin-Induced Acute Kidney Injury by Suppressing Apoptosis, Oxidative Stress, and Inflammation in Mice. Antioxidants 2019, 8, 322. https://doi.org/10.3390/antiox8080322
Kim J-Y, Jo J, Kim K, An H-J, Gwon M-G, Gu H, Kim H-J, Yang AY, Kim S-W, Jeon EJ, et al. Pharmacological Activation of Sirt1 Ameliorates Cisplatin-Induced Acute Kidney Injury by Suppressing Apoptosis, Oxidative Stress, and Inflammation in Mice. Antioxidants. 2019; 8(8):322. https://doi.org/10.3390/antiox8080322
Chicago/Turabian StyleKim, Jung-Yeon, Jungmin Jo, Kiryeong Kim, Hyun-Jin An, Mi-Gyeong Gwon, Hyemin Gu, Hyun-Ju Kim, A Young Yang, Sung-Woo Kim, Eon Ju Jeon, and et al. 2019. "Pharmacological Activation of Sirt1 Ameliorates Cisplatin-Induced Acute Kidney Injury by Suppressing Apoptosis, Oxidative Stress, and Inflammation in Mice" Antioxidants 8, no. 8: 322. https://doi.org/10.3390/antiox8080322





