Regulation of Inflammatory Response and the Production of Reactive Oxygen Species by a Functional Cooked Ham Reformulated with Natural Antioxidants in a Macrophage Immunity Model
Abstract
:1. Introduction
2. Materials and Methods
2.1. Reagents
2.2. Samples
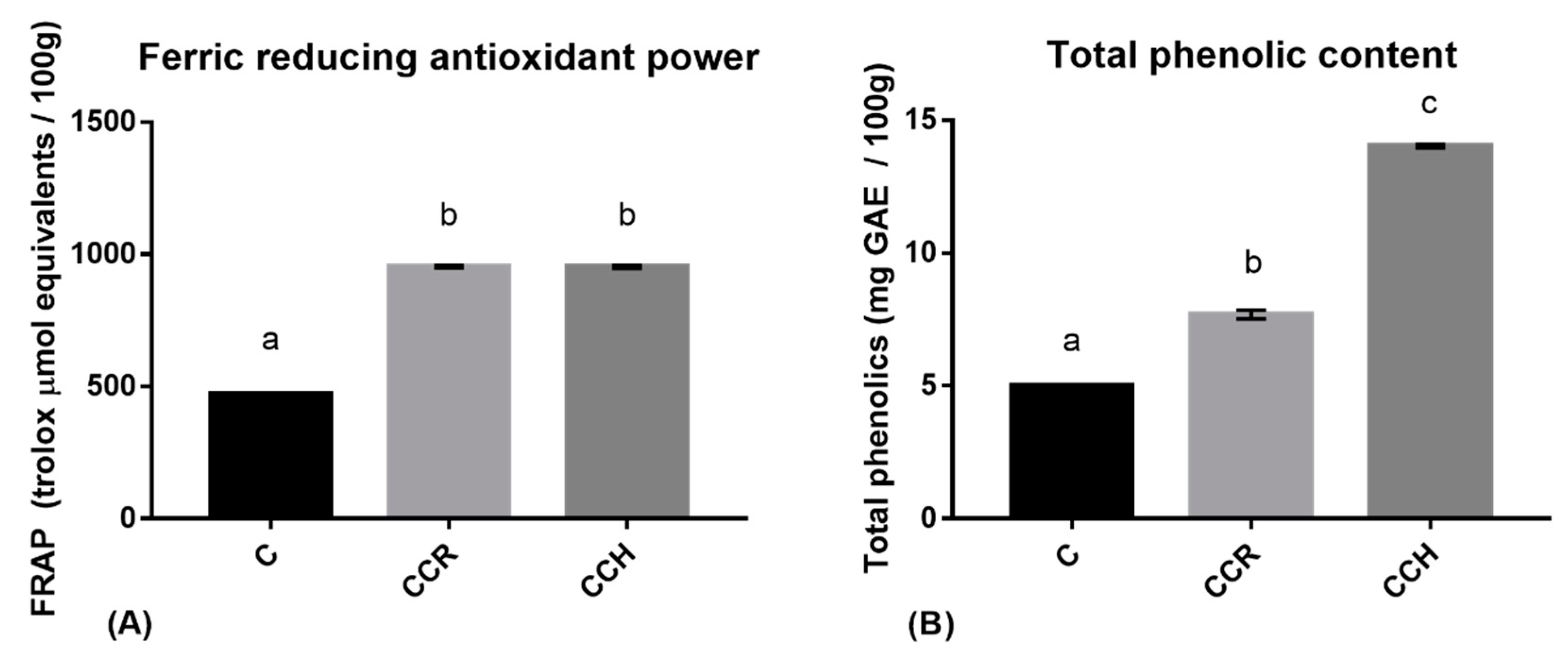
2.3. Ferric-Reducing Antioxidant Power (FRAP) Assay
2.4. Determination of Total Phenolic Content
2.5. Cell Culture
2.5.1. Cell Viability
2.5.2. Lipopolysaccharide-Induced Inflammation Assay
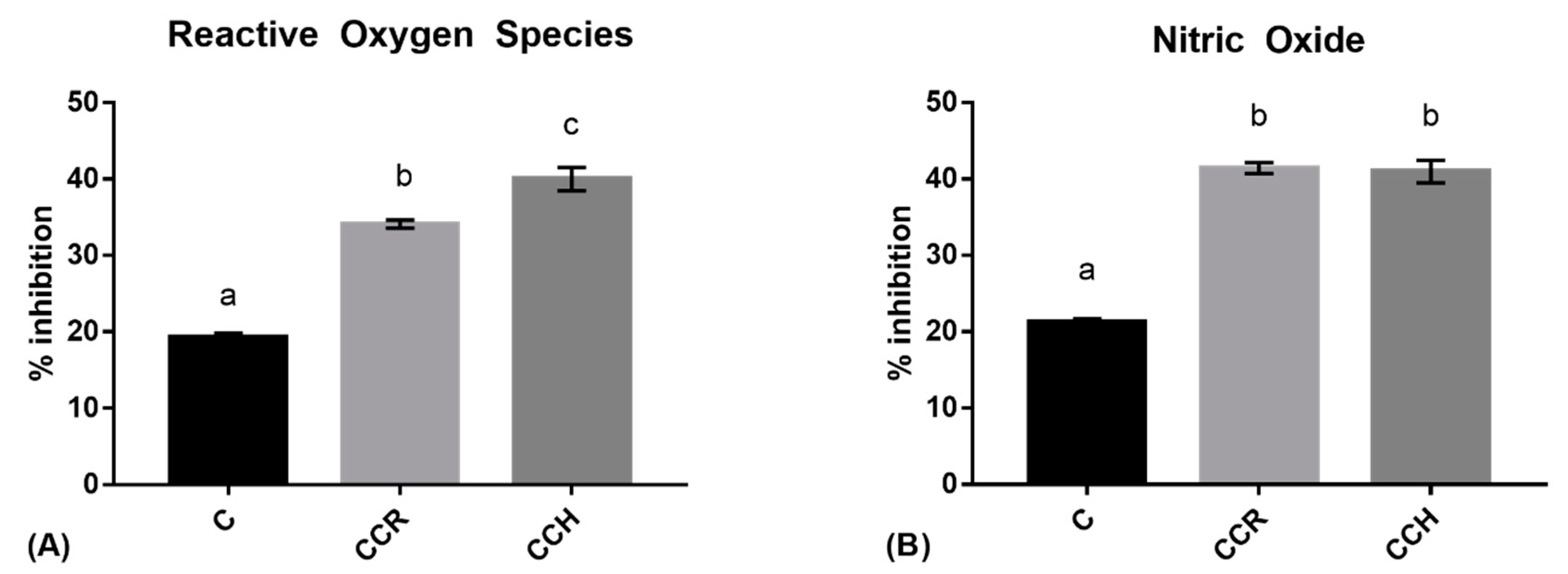
2.5.3. Measurement of Reactive Oxygen Species
2.5.4. Measurement of Nitric Oxide
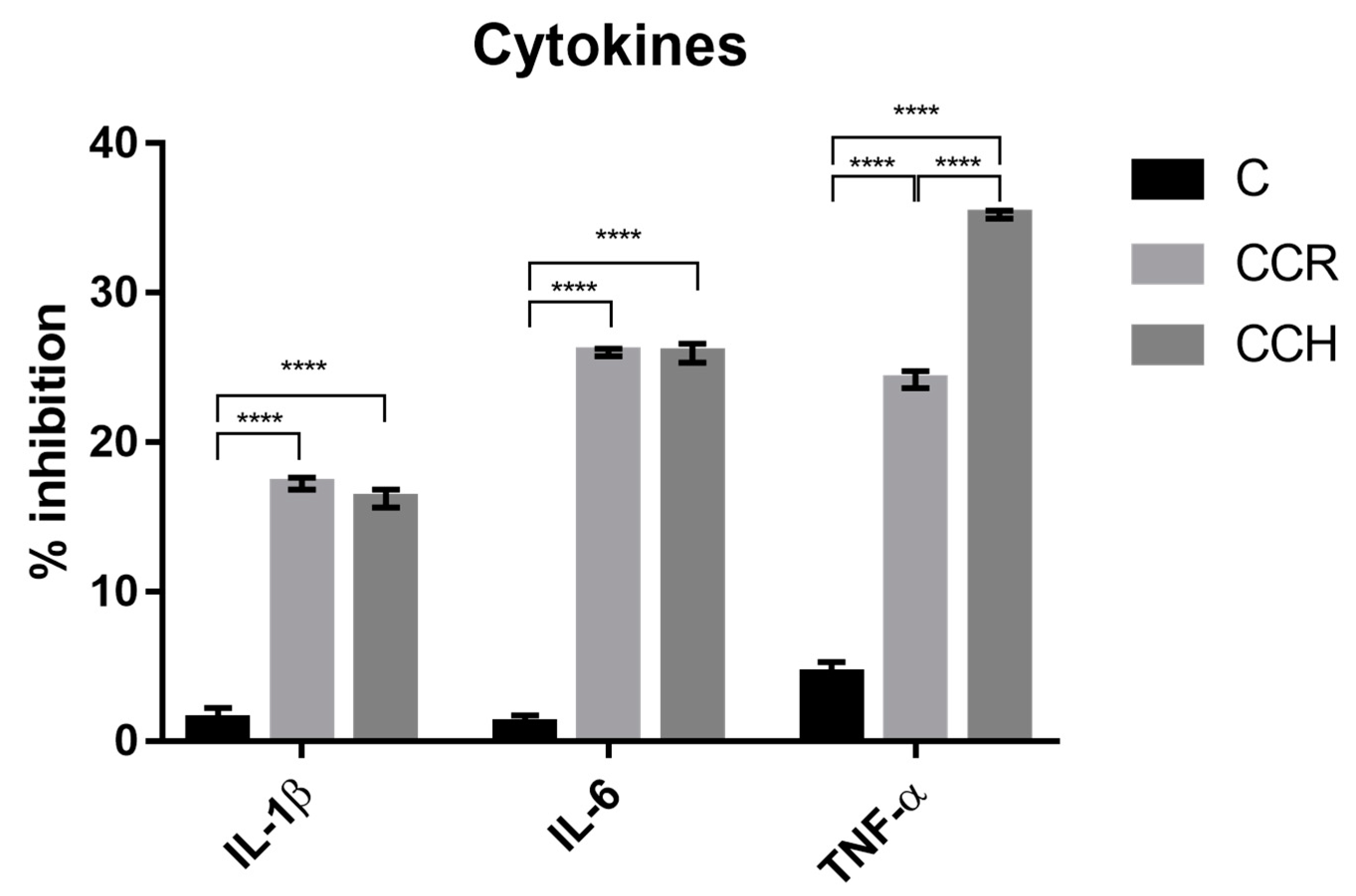
2.5.5. Measurement of Cytokines
2.5.6. Statistics
3. Results
3.1. Preliminary Antioxidant Assays
3.2. Intracellular Reactive Oxygen Species
3.3. Nitric Oxide
3.4. Cytokine Measurement
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Boada, L.; Henríquez-Hernández, L.; Luzardo, O. The impact of red and processed meat consumption on cancer and other health outcomes: Epidemiological evidences. Food Chem. Toxicol. 2016, 92, 236–244. [Google Scholar] [CrossRef] [PubMed]
- Hammerling, U.; Bergman Laurila, J.; Grafström, R.; Ilbäck, N. Consumption of Red/Processed Meat and Colorectal Carcinoma: Possible Mechanisms Underlying the Significant Association. Crit. Rev. Food Sci. Nutr. 2015, 56, 614–634. [Google Scholar] [CrossRef] [PubMed]
- Jeyakumar, A.; Dissabandara, L.; Gopalan, V. A critical overview on the biological and molecular features of red and processed meat in colorectal carcinogenesis. J. Gastroenterol. 2016, 52, 407–418. [Google Scholar] [CrossRef] [PubMed]
- Czerwonka, M.; Tokarz, A. Iron in red meat–friend or foe. Meat Sci. 2017, 123, 157–165. [Google Scholar] [CrossRef] [PubMed]
- Cohn, J. Oxidized fat in the diet, postprandial lipaemia and cardiovascular disease. Curr. Opin. Lipidol. 2002, 13, 19–24. [Google Scholar] [CrossRef]
- O’Keefe, J.H.; Abuannadi, M. Dietary strategies for the prevention & treatment of metabolic syndrome. Mo Med. 2010, 107, 406–409. [Google Scholar]
- Dubrow, R.; Darefsky, A.S.; Park, Y.; Mayne, S.T.; Moore, S.C.; Kilfoy, B.; Cross, A.J.; Sinha, R.; Hollenbeck, A.R.; Schatzkin, A.; et al. Dietary Components Related to N-Nitroso Compound Formation: A Prospective Study of Adult Glioma Cancer. Epidemiol. Biomark. Prev. 2010, 19, 1709–1722. [Google Scholar] [CrossRef]
- Zheng, W.; Lee, S. Well-Done Meat Intake, Heterocyclic Amine Exposure, and Cancer Risk. Nutr. Cancer 2009, 61, 437–446. [Google Scholar] [CrossRef]
- McAfee, A.; McSorley, E.; Cuskelly, G.; Moss, B.; Wallace, J.; Bonham, M.; Fearon, A. Red meat consumption: An overview of the risks and benefits. Meat Sci. 2010, 84, 1–13. [Google Scholar] [CrossRef]
- Riccioti, E.; FitzGerald, G.A. Prostaglandins and Inflammation. Arterioscler. Thromb. Vasc. Biol. 2011, 31, 986–1000. [Google Scholar] [CrossRef]
- Arya, F.; Egger, S.; Colquhoun, D.; Sullivan, D.; Pal, S.; Egger, G. Differences in postprandial inflammatory responses to a ‘modern’ v. traditional meat meal: A preliminary study. Br. J. Nutr. 2010, 104, 724–728. [Google Scholar] [CrossRef] [PubMed]
- Lin, W.-W.; Karin, M. A cytokine-mediated link between innate immunity, inflammation, and cancer. J. Clin. Investig. 2007, 117, 1175–1183. [Google Scholar] [CrossRef] [PubMed]
- Cheng, A.; Yan, H.; Han, C.; Wang, W.; Tian, Y.; Chen, X. Polyphenols from blueberries modulate inflammation cytokines in LPS-induced RAW264.7 macrophages. Int. J. Biol. Macromol. 2014, 69, 382–387. [Google Scholar] [CrossRef] [PubMed]
- Heinrich, P.; Behrmann, I.; Haan, S.; Hermanns, H.; Müller-Newen, G.; Schaper, F. Principles of interleukin (IL)-6-type cytokine signalling and its regulation. Biochem. J. 2003, 374, 1–20. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chahbouni, M.; López, M.; Molina-Carballo, A.; de Haro, T.; Muñoz-Hoyos, A.; Fernández-Ortiz, M.; Guerra-Librero, A.; Acuña-Castroviejo, D. Melatonin Treatment Reduces Oxidative Damage and Normalizes Plasma Pro-Inflammatory Cytokines in Patients Suffering from Charcot-Marie-Tooth Neuropathy: A Pilot Study in Three Children. Molecules 2017, 22, 1728. [Google Scholar] [CrossRef]
- Fuller, B. Role of PGE-2 and Other Inflammatory Mediators in Skin Aging and Their Inhibition by Topical Natural Anti-Inflammatories. Cosmetics 2019, 6, 6. [Google Scholar] [CrossRef]
- Padoan, A.; Plebani, M.; Basso, D. Inflammation and Pancreatic Cancer: Focus on Metabolism, Cytokines, and Immunity. Int. J. Mol. Sci. 2019, 20, 676. [Google Scholar] [CrossRef]
- Chen, L.; Mo, H.; Zhao, L.; Gao, W.; Wang, S.; Cromie, M.M.; Lu, C.; Wang, J.S.; Shen, C.L. Therapeutic properties of green tea against environmental insults. J. Nutr. Biochem. 2017, 40, 1–13. [Google Scholar] [CrossRef]
- Ullah, N.; Ahmad, M.; Aslam, H.; Tahir, M.A.; Aftab, M.; Bibi, N.; Ahmad, S. Green tea phytocompounds as anticancer: A review. Asian Pac. J. Trop. Dis. 2016, 6, 330–336. [Google Scholar] [CrossRef]
- Cao, W.; Hu, C.; Wu, L.; Xu, L.; Jiang, W. Rosmarinic acid inhibits inflammation and angiogenesis of hepatocellular carcinoma by suppression of NF-κBsignaling in H22 tumor-bearing mice. J. Pharmacol. Sci. 2016, 132, 131–137. [Google Scholar] [CrossRef]
- Rahbardar, M.G.; Amin, B.; Mehri, S.; Mirnajafi-Zadeh, S.J.; Hosseinzadeh, H. Anti-inflammatory effects of ethanolic extract of Rosmarinus officinalis L. and rosmarinic acid in a rat model of neuropathic pain. Biomed. Pharmacother. 2017, 86, 441–449. [Google Scholar] [CrossRef] [PubMed]
- López, S.; Motserrat de la Paz, S.; Lucas, R.; Bermudez, B.; Abia, R.; Morales, J.C.; Muriana, F.J.G. Effect of metabolites of hydroxytyrosol on protection against oxidative stress and inflammation in human endothelial cells. J. Funct. Foods 2017, 29, 238–247. [Google Scholar] [CrossRef] [Green Version]
- Shi, H.S.; Satsu, H.; Bae, M.J.; Zhao, Z.; Ogiwara, H.; Totsuka, M.; Shimuzu, M. Anti-inflammatory effect of chlorogenic acid on the IL-8 production in Caco-2 cells and the dextran sulphate sodium-induced colitis symptoms in C57BL/6 mice. Food Chem. 2015, 168, 167–175. [Google Scholar] [CrossRef]
- Serrano, A.; Ros, G.; Nieto, G. Bioactive Compounds and Extracts from Traditional Herbs and Their Potential Anti-Inflammatory Health Effects. Medicines 2018, 5, 76. [Google Scholar] [CrossRef] [PubMed]
- Minekus, M.; Alminger, P.; Alvito, S.; Ballance, T.; Bohn, C.; Bourlieu, F.; Carrière, R.; Boutrou, M.; Corredig, D.; Dupont, C.; et al. A standardised static in vitro digestion method suitable for food—An international consensus. Food Funct. 2014, 5, 1113–1124. [Google Scholar] [CrossRef] [PubMed]
- Benzie, I.; Strain, J. The Ferric Reducing Ability of Plasma (FRAP) as a Measure of “Antioxidant Power”: The FRAP Assay. Anal. Biochem. 1996, 239, 70–76. [Google Scholar] [CrossRef] [PubMed]
- Kaur, C.; Kapoor, H. Anti-oxidant activity and total phenolic content of some Asian vegetables. Int. J. Food Sci. Technol. 2002, 37, 153–161. [Google Scholar] [CrossRef]
- Mosmann, T. Rapid colorimetric assay for cellular growth and survival: Application to proliferation and cytotoxicity assays. J. Immunol. Methods 1983, 65, 55–63. [Google Scholar] [CrossRef]
- Moon, S.; Lee, S.; Hong, J.; Kim, J.; Kim, D.; Kim, C. Oleamide suppresses inflammatory responses in LPS-induced RAW264.7 murine macrophages and alleviates paw edema in a carrageenan-induced inflammatory rat model. Int. Immunopharmacol. 2018, 56, 179–185. [Google Scholar] [CrossRef]
- Girard-Lalancette, K.; Pichette, A.; Legault, J. Sensitive cell-based assay using DCFH oxidation for the determination of pro- and antioxidant properties of compounds and mixtures: Analysis of fruit and vegetable juices. Food Chem. 2009, 115, 720–726. [Google Scholar] [CrossRef]
- Jiang, J.; Zhang, X.; True, A.; Zhou, L.; Xiong, Y. Inhibition of Lipid Oxidation and Rancidity in Precooked Pork Patties by Radical-Scavenging Licorice (Glycyrrhizaglabra) Extract. J. Food Sci. 2013, 78, C1686–C1694. [Google Scholar] [CrossRef] [PubMed]
- Utrera, M.; Morcuende, D.; Ganhão, R.; Estévez, M. Role of Phenolics Extracting from Rosa canina L. on Meat Protein Oxidation During Frozen Storage and Beef Patties Processing. Food Bioprocess Technol. 2014, 8, 854–864. [Google Scholar] [CrossRef]
- Sebranek, J.; Sewalt, V.; Robbins, K.; Houser, T. Comparison of a natural rosemary extract and BHA/BHT for relative antioxidant effectiveness in pork sausage. Meat Sci. 2005, 69, 289–296. [Google Scholar] [CrossRef] [PubMed]
- Nieto, G.; Díaz, P.; Bañón, S.; Garrido, M.D. Dietary administration of ewe diets with a distillate fromrosemary leaves (Rosmarinus officinalis L.): Influence on lamb meat quality. Meat Sci. 2010, 84, 23–29. [Google Scholar] [CrossRef] [PubMed]
- Nieto, G.; Estrada, M.; Jordán, M.J.; Garrido, M.D.; Bañon, S. Effects in ewe diet of rosemary by-producton lipid oxidation and the eating of cooked lamb under retail display conditions. Food Chem. 2011, 124, 1423–1429. [Google Scholar] [CrossRef]
- Serpen, A.; Gökmen, V.; Fogliano, V. Total antioxidant capacities of raw and cooked meats. Meat Sci. 2012, 90, 60–65. [Google Scholar] [CrossRef] [PubMed]
- Brenes, A.; Viveros, A.; Chamorro, S.; Arija, I. Use of polyphenol-rich grape by-products in monogastric nutrition. A review. Anim. Feed Sci. Technol. 2016, 211, 1–17. [Google Scholar] [CrossRef]
- Martínez, J.; Nieto, G.; Ros, G. Total antioxidant capacity of meat and meat products consumed in a reference ‘Spanish standard diet’. Int. J. Food Sci. Technol. 2014, 49, 2610–2618. [Google Scholar] [CrossRef]
- Kongkatitham, V.; Muangnoi, C.; Kyokong, N.; Thaweesest, W.; Likhitwitayawuid, K.; Rojsitthisak, P.; Sritularak, B. Anti-oxidant and anti-inflammatory effects of new bibenzyl derivatives from Dendrobium parishii in hydrogen peroxide and lipopolysaccharide treated RAW264.7 cells. Phytochem. Lett. 2018, 24, 31–38. [Google Scholar] [CrossRef]
- Robles-Almazan, M.; Pulido-Moran, M.; Moreno-Fernandez, J.; Ramirez-Tortosa, C.; Rodriguez-Garcia, C.; Quiles, J.; Ramirez-Tortosa, M. Hydroxytyrosol: Bioavailability, toxicity, and clinical applications. Food Res. Int. 2018, 105, 654–667. [Google Scholar] [CrossRef]
- Karakaya, S. Bioavailability of Phenolic Compounds. Crit. Rev. Food Sci. Nutr. 2004, 44, 453–464. [Google Scholar] [CrossRef] [PubMed]
- Ames, B.; Shigenaga, M.; Hagen, T. Oxidants, antioxidants, and the degenerative diseases of aging. Proc. Natl. Acad. Sci. USA 1993, 90, 7915–7922. [Google Scholar] [CrossRef] [PubMed]
- Liochev, S. Reactive oxygen species and the free radical theory of aging. Free Radic. Biol. Med. 2013, 60, 1–4. [Google Scholar] [CrossRef] [PubMed]
- Coleman, J. Nitric oxide in immunity and inflammation. Int. Immunopharmacol. 2001, 1, 1397–1406. [Google Scholar] [CrossRef]
- Klop, B.; Proctor, S.; Mamo, J.; Botham, K.; Castro Cabezas, M. Understanding Postprandial Inflammation and Its Relationship to Lifestyle Behaviour and Metabolic Diseases. Int. J. Vasc. Med. 2012, 2012. [Google Scholar] [CrossRef] [PubMed]
- Terzić, J.; Grivennikov, S.; Karin, E.; Karin, M. Inflammation and Colon Cancer. Gastroenterology 2010, 138, 2101–2114. [Google Scholar] [CrossRef] [PubMed]
- Lin, H.; Juan, S.; Shen, S.; Hsu, F.; Chen, Y. Inhibition of lipopolysaccharide-induced nitric oxide production by flavonoids in RAW264.7 macrophages involves heme oxygenase-1. Biochem. Pharmacol. 2003, 66, 1821–1832. [Google Scholar] [CrossRef]
- Song, J.; Li, T.; Cheng, X.; Ji, X.; Gao, D.; Du, M.; Jiang, N.; Liu, X.; Mao, X. Sea cucumber peptides exert anti-inflammatory activity through suppressing NF-κB and MAPK and inducing HO-1 in RAW264.7 macrophages. Food Funct. 2016, 7, 2773–2779. [Google Scholar] [CrossRef]
- O’Shea, J.; Murray, P. Cytokine Signaling Modules in Inflammatory Responses. Immunity 2008, 28, 477–487. [Google Scholar] [CrossRef] [Green Version]
- Hartman, J.; Frishman, W. Inflammation and atherosclerosis: A review of the role of interleukin-6 in the development of atherosclerosis and the potential for targeted drug therapy. Cardiol. Rev. 2014, 22, 147–151. [Google Scholar] [CrossRef]
- Arranz, E.; Mes, J.; Wichers, H.; Jaime, L.; Mendiola, J.; Reglero, G.; Santoyo, S. Anti-inflammatory activity of the basolateral fraction of Caco-2 cells exposed to a rosemary supercritical extract. J. Funct. Food 2015, 13, 384–390. [Google Scholar] [CrossRef] [Green Version]
- Wu, S.; Xu, H.; Peng, J.; Wang, C.; Jin, Y.; Liu, K.; Sun, H.; Qin, J. Potent anti-inflammatory effect of dioscin mediated by suppression of TNFα-induced VCAM-1, ICAM-1and EL expression via the NF-κB pathway. Biochimie 2015, 110, 62–72. [Google Scholar] [CrossRef] [PubMed]
- Antognoni, F.; Mandrioli, R.; Bordoni, A.; Di Nunzio, M.; Viadel, B.; Gallego, E.; Villalba, M.; Tomás-Cobos, L.; TaneyoSaa, D.; Gianotti, A. Integrated Evaluation of the Potential Health Benefits of Einkorn-Based Breads. Nutrients 2017, 9, 1232. [Google Scholar] [CrossRef] [PubMed]
- Valli, V.; Taccari, A.; Di Nunzio, M.; Danesi, F.; Bordoni, A. Health benefits of ancient grains. Comparison among bread made with ancient, heritage and modern grain flours in human cultured cells. Food Res. Int. 2018, 107, 206–215. [Google Scholar] [CrossRef] [PubMed]
- Ward, J.B.J.; Lajczak, K.; Kelly, O.B.; O’Dwyer, A.M.; Giddam, A.K.; Gabhann, J.N.; Franco, P.; Tumbuwala, M.M.; Jefferies, C.A.; Keely, S.; et al. Ursodeoxycholic acid and lithocholic acid exert anti-inflammatory actions in the colon. Am. J. Physiol. Gastrointest. Liver Physiol. 2017, 312, G550–G558. [Google Scholar] [CrossRef] [Green Version]
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Serrano, A.; Ros, G.; Nieto, G. Regulation of Inflammatory Response and the Production of Reactive Oxygen Species by a Functional Cooked Ham Reformulated with Natural Antioxidants in a Macrophage Immunity Model. Antioxidants 2019, 8, 286. https://doi.org/10.3390/antiox8080286
Serrano A, Ros G, Nieto G. Regulation of Inflammatory Response and the Production of Reactive Oxygen Species by a Functional Cooked Ham Reformulated with Natural Antioxidants in a Macrophage Immunity Model. Antioxidants. 2019; 8(8):286. https://doi.org/10.3390/antiox8080286
Chicago/Turabian StyleSerrano, Antonio, Gaspar Ros, and Gema Nieto. 2019. "Regulation of Inflammatory Response and the Production of Reactive Oxygen Species by a Functional Cooked Ham Reformulated with Natural Antioxidants in a Macrophage Immunity Model" Antioxidants 8, no. 8: 286. https://doi.org/10.3390/antiox8080286






