A Study of the Fruits of Catalpa bignonioides Walt.: Evaluation of the Antioxidant, Anti-Inflammatory, and Anti-Cancer Activities in Colorectal Adenocarcinoma Cells in Relation to Phytochemical Profile
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals and Reagents
2.2. Plant Materials
2.3. Extraction and Fractionation
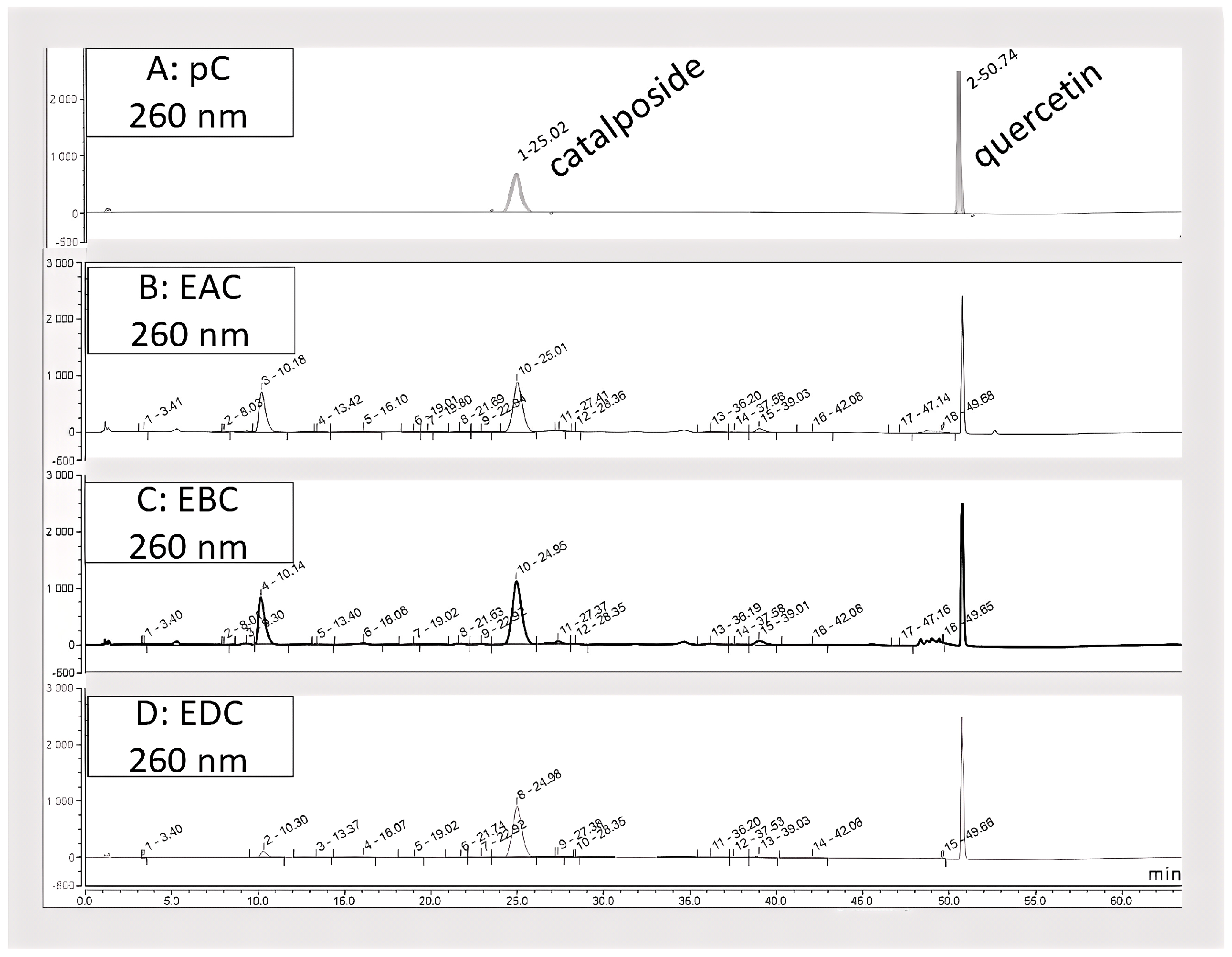
2.4. High Performance Liquid Chromatography (HPLC) Analyses
2.5. Quantification of Catalposide
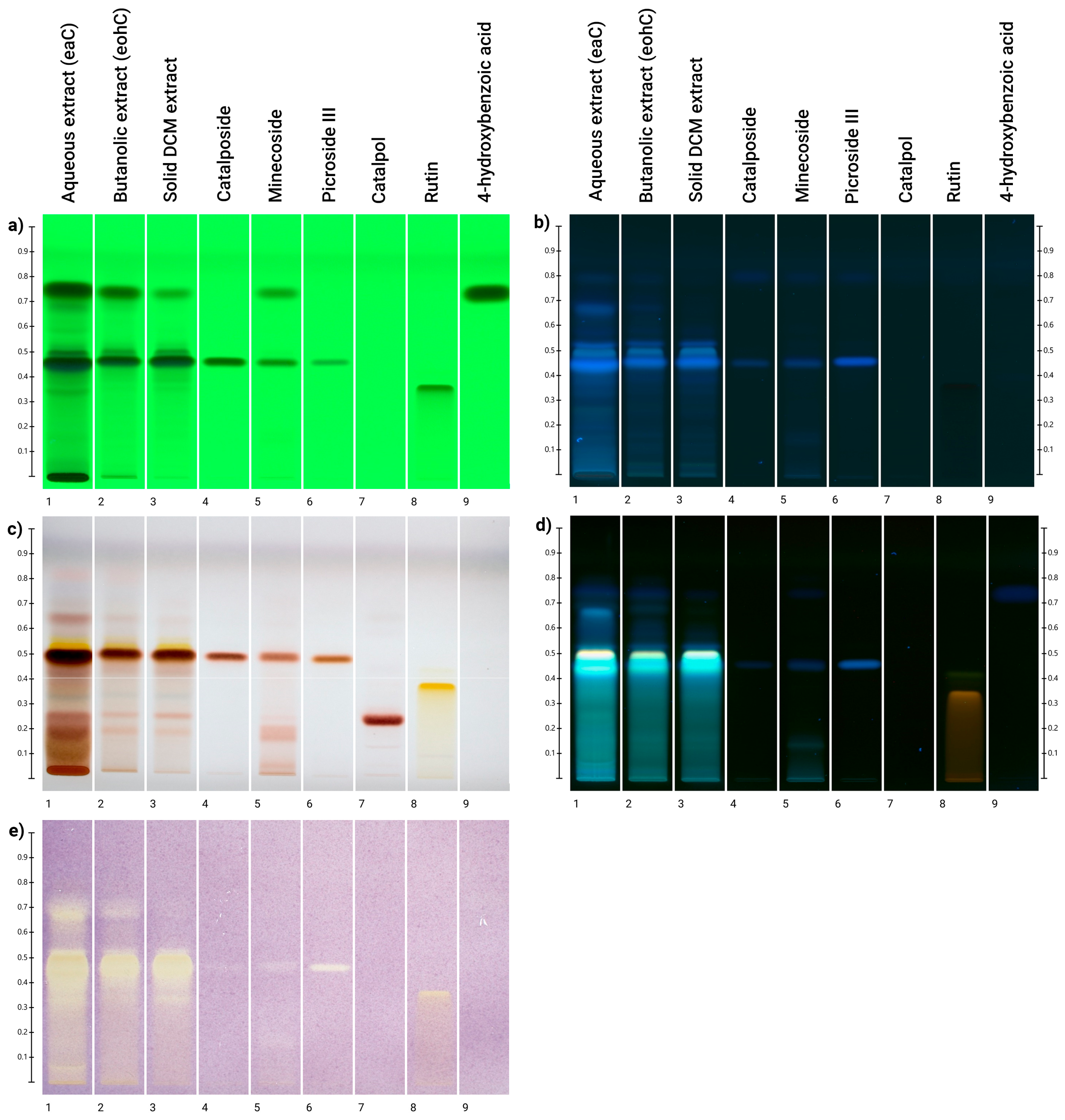
2.6. High-Performance Thin-Layer Chromatography (HPTLC) Analysis
2.7. Cell Culture
2.8. Trypan Blue Assay
2.9. MTT Assay
2.10. Clonogenic Assay
2.11. Western Blotting Analysis
2.12. ROS Measurement
2.13. In Vitro Antioxidant Properties
2.13.1. β-Carotene Bleaching Test
2.13.2. FRAP Test
2.13.3. ABTS Test
2.13.4. DPPH Test
2.14. Statistical Analysis
3. Results and Discussion
3.1. Chemical Composition of C. bignonioides Fruit Extracts
| Entry | Compounds | Molecular Formula | Molecular Weight | Pseudo-Molecular Ion [M+H]+ | err [ppm] | mSigma | Score (%) | RT | Fragments Ions [+H]+ | Samples | Ref. | ||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| EAC | EBC | EDC | |||||||||||
| Flavonoids | |||||||||||||
| F1 | 5,6-Dihydroxy-7,4′- dimethoxyflavone- 6-O-sophoroside | C29H34O16 | 638.1920 | 639.1892 | 4.4 | 2.8 | 100 | 8.6 | 315.0840 | ✓ | ✓ | ✓ | [11] |
| F2 | 6-Hydroxyluteolin-O- coumaroyl glycoside | C30H26O14 | 610.13226 | 611.1374 | 2.8 | 9.7 | 100 | 9.2 | 303.0489 | ✓ | ✓ | [36] | |
| F3 | 6-Hydroxyluteolin- O-feruloyl glycoside | C31H28O15 | 640.14282 | 641.1494 | 1.1 | 8.8 | 100 | 8.9 | 303.0486 | ✓ | ✓ | [35] | |
| F4 | 5,6,3′,4′-Tetrahydroxy- flavon-7-glucoside | C21H20O12 | 464.09548 | 465.1019 | 1.9 | 2.7 | 100 | 7.7 | 303.0484 | ✓ | ✓ | [30] | |
| Iridoids | |||||||||||||
| I1 | Catalpol | C15H22O10 | 363.30340 | 363.1065 | 2.4 | 6.5 | 100 | 7.9 | 163.0389 | ✓ | [37] | ||
| I2 | Catalposide | C22H26O12 | 482.14243 | 483.1485 | 2.5 | 11.5 | 100 | 7.8 | 321.0945 303.0857 | ✓ | ✓ | ✓ | [37] |
| I3 | Dihydrocatalposide or 6-O-p-hydroxybenzoyl- 5,7-bisdeoxycynanchoside | C22H28O12 | 484.15808 | 485.1685 | 1.1 | 6.0 | 100 | 7.4 | 287.0909 | ✓ | ✓ | ✓ | [27,28] |
| I4 | Verproside | C22H26O13 | 498.13734 | 499.1438 | 4.7 | 15.8 | 100 | 7.1 | 137.0234 319.0814 | ✓ | ✓ | ✓ | [32] |
| I5 | 6-O-(p-Hydroxybenzoyl)-descinnamoylglobularimin | C22H28O13 | 500.1530 | 501.1583 | 3.5 | 10.9 | 100 | 6.6 | 321.0958 483.1483 | ✓ | ✓ | ✓ | [27] |
| I6 | Verminoside | C24H28O13 | 524.15299 | 525.1587 | 3.1 | 15.9 | 100 | 6.0 | 163.0389 | ✓ | ✓ | [32] | |
| I7 | 6-O-cis(trans)-p-Coumaroyl-5,7-bisdeoxycynanchoside | C24H30O12 | 510.17373 | 511.1794 | 3.2 | 12.7 | 100 | 8.2 | - | ✓ | ✓ | ✓ | [28] |
| I8 | Minecoside * | C25H30O13 | 538.16864 | 539.1745 | 2.6 | 18.6 | 100 | 8.3 | 377.1226 177.0543 | ✓ | ✓ | ✓ | [11] |
| I9 | Picroside III * | C25H30O13 | 538.16864 | 539.1743 | 3.1 | 8.9 | 100 | 8.4 | 377.1252 177.0545 | ✓ | ✓ | ✓ | [11] |
| I10 | Specioside | C24H28O12 | 508.15808 | 509.1645 | 1.8 | 6.8 | 100 | 8.6 | - | ✓ | ✓ | ✓ | [11] |
| Phenolic acid | |||||||||||||
| P1 | p-Hydroxybenzoic acid | C7H6O3 | 138.03169 | 139.0392 | 2.3 | 3.2 | 100 | 6.6 | 121.0399 77.0387 65.0388 | ✓ | ✓ | ✓ | [11] |
| P2 | p-Coumaric acid | C9H8O3 | 164.04734 | 165.0546 | 0.2 | 13.2 | 100 | 1.3 | - | ✓ | ✓ | [11] | |
| Phenolic glycosides | |||||||||||||
| P3 | p-Hydroxybenzoyl-glycoside | C13H16O8 | 300.08452 | 301.0909 | 3.9 | 4.5 | 100 | 4.5 | 139.0389 | ✓ | ✓ | [29] | |
| P4 | Martynoside | C31H40O15 | 652.23672 | 653.2421 | 2.9 | 16.0 | 100 | 8.9 | 177.0544 | ✓ | ✓ | ✓ | [34] |
| P5 | Caffeolyl phenylethanoid glycoside isomer (Verbascoside isomer) | C29H36O15 | 624.20542 | 625.2113 | 2.3 | 8.5 | 100 | 8.1 | 163.0389 | ✓ | ✓ | ✓ | [34] |
| Peaks | RT | Identification | [M-H]−/[M+45]− * | UV λmax (nm) | EAC | EBC | EDC |
|---|---|---|---|---|---|---|---|
| 1 | 3.48 | 6-O-(p-Hydroxybenzoyl)-glucoside | 299/344 | 215, 262 | ✓ | ✓ | ✓ |
| 2 | 10.83 | p-Hydroxybenzoic acid | 137/183 | 215, 257 | ✓ | ✓ | ✓ |
| 3 | 10.83 | 6-O-(p-Hydroxybenzoyl)- descinnamoylglobularimin | 499/545 | 215, 257 | ✓ | ✓ | ✓ |
| 4 | 13.90 | Dihydrocatalposide or 6-O-(p-hydroxybenzoyl)- 5,7-bisdeoxycynanchoside | 483/529 | 225, 260 | ✓ | ✓ | ✓ |
| 5 | 16.65 | Verproside | 497/543 | 224, 265, 300 | ✓ | ✓ | ✓ |
| 6 | 20.34 | 6-Hydroxyluteolin- O-feruloyl glycoside | 639/685 | 225, 290, 323 | ✓ | ✓ | |
| 7 | 22.33 | p-Coumaric acid | 163/209 | 227, 310 | ✓ | ✓ | |
| 8 | 24.90 | Catalposide | 481/527 | 216, 261 | ✓ | ✓ | ✓ |
| 9 | 27.47 | 5,6,3′,4′-Tetrahydroxy- flavon-7-glucoside | 463/509 | 254, 354 | ✓ | ✓ | |
| 10 | 28.14 | Verbascoside isomer | 623/669 | 224, 300, 330 | ✓ | ✓ | ✓ |
| 11 | 29.09 | Verminoside | 523/569 | 224, 328 | ✓ | ✓ | |
| 12 | 33.41 | 6-O-cis/trans-p-Coumaroyl- 5,7- bisdeoxycynanchoside | 509/555 | 224, 302 | ✓ | ✓ | ✓ |
| 13 | 35.43 | Minecoside ** | 537/583 | 224, 328 | ✓ | ✓ | ✓ |
| 14 | 37.58 | Picroside III ** | 537/583 | 224, 328 | ✓ | ✓ | ✓ |
| 15 | 38.34 | 6-Hydroxyluteolin- O-coumaroyl glycoside | 609/655 | 227, 258, 355 | ✓ | ✓ | ✓ |
| 16 | 39.86 | Specioside | 507/553 | 230, 315 | ✓ | ✓ | ✓ |
| 17 | 48.40 | Martynoside | 651/697 | 224, 323 | ✓ | ✓ | ✓ |
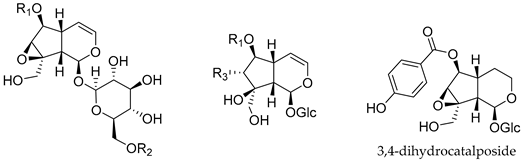 | |||
|---|---|---|---|
| Compound | R1 | R2 | R3 |
| Catalpol | H | H | - |
| Catalposide | 4-hydroxybenzoyl | H | - |
| Verproside | 3,4-dihydroxybenzoyl | H | - |
| Minecoside | 3-hydroxy-4-methoxy cinnamoyl | H | - |
| Specioside | 4-hydroxy cinnamoyl | H | - |
| Verminoside | 3,4-dihydroxy cinnamoyl | H | - |
| Picroside III | H | 3-methoxy-4-hydroxy cinnamoyl | - |
| 6-O-(p-Hydroxybenzoyl)- 5,7-bisdeoxycynanchoside | 4-hydroxybenzoyl | - | H |
| 6-O-(p-Hydroxybenzoyl)- descinnamoylglobularimin | 4-hydroxybenzoyl | - | OH |
| 6-O-cis(trans)-p-Coumaroyl- 5,7-bisdeoxycynanchoside | 4-hydroxy cinnamoyl | - | H |
3.2. Yields of the Major Iridoids of C. bignonioides Fruit Extracts
3.3. HPTLC Analyses and Direct Bioautography with DPPH
3.4. In Vitro Evaluation of Antioxidant Activity of C. bignonioides
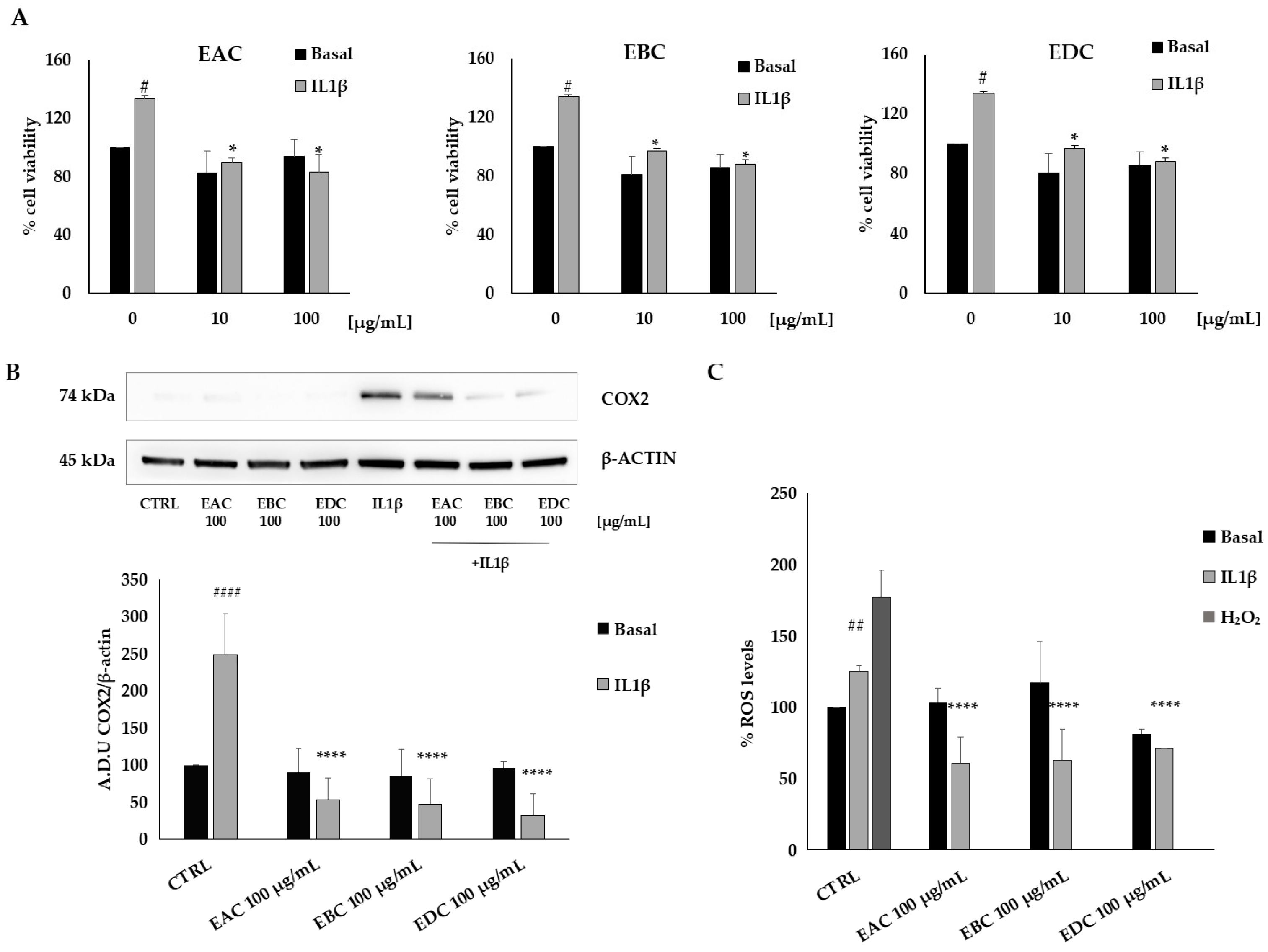
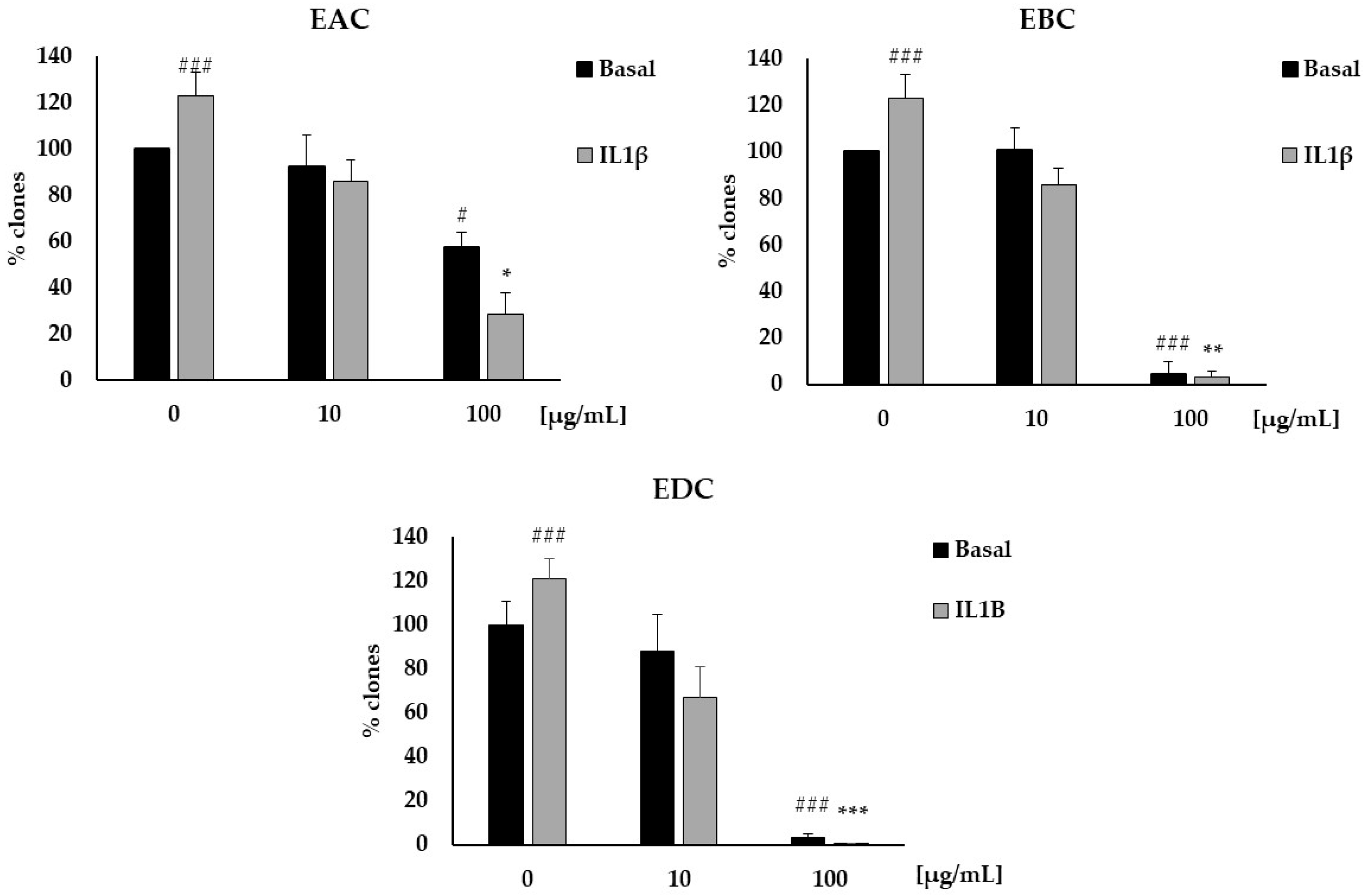
3.5. Biological Activity of C. bignonioides Extracts in Cellular Models of Colorectal Cancer
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Dinda, B. Pharmacology of Iridoids. In Pharmacology and Applications of Naturally Occurring Iridoids. In Pharmacology and Applications of Naturally Occurring Iridoids; Springer: Cham, Switzerland, 2019. [Google Scholar] [CrossRef]
- Benarba, B.; Pandiella, A. Colorectal cancer and medicinal plants: Principle findings from recent studies. Biomed. Pharmacother. 2018, 107, 408–423. [Google Scholar] [CrossRef]
- Han, X.; Zhang, X.; Wang, Q.; Wang, L.; Yu, S. Antitumor potential of Hedyotis diffusa Willd: A systematic review of bioactive constituents and underlying molecular mechanisms. Biomed. Pharmacother. 2020, 130, 110735. [Google Scholar] [CrossRef]
- Dinda, B.; Chowdhury, D.R.; Mohanta, B.C. Naturally occurring iridoids, secoiridoids and their bioactivity. An updated review, Part 3. Chem. Pharm. Bull. 2009, 57, 765–796. [Google Scholar] [CrossRef]
- Dinda, B.; Debnath, S.; Banik, R. Naturally occurring iridoids and secoiridoids. An updated review, Part 4. Chem. Pharm. Bull. 2011, 59, 803–833. [Google Scholar] [CrossRef]
- von Poser, G.L.; Schripsema, J.; Henriques, A.T.; Jensen, S.R. The distribution of iridoids in Bignoniaceae. Biochem. Syst. Ecol. 2000, 28, 351–366. [Google Scholar] [CrossRef] [PubMed]
- Cook, W. Catalpa bignonioides in The Physio-Medical Dispensatory: A Treatise on Therapeutics, Materia Medica, and Pharmacy in Accordance with the Principles of Physiological Medication. WM. H. COOK Edition, NO. 101 West Sixth-Street, Cincinnati, OH, USA. 1869, p. 142. Available online: https://www.amazon.com/PHYSIO-MEDICAL-DISPENSATORY-THERAPEUTICS-PHYSIOLOGICAL-MEDICATION-ebook/dp/B00JRGBN86 (accessed on 25 May 2025).
- Muñoz-Mingarro, D.; Acero, N.; Llinares, F.; Pozuelo, J.M.; de Mera, A.G.; Vicenten, J.A.; Perez, C. Biological activity of extracts from Catalpa bignonioides Walt. (Bignoniaceae). J. Ethnopharmacol. 2003, 87, 163–167. [Google Scholar] [CrossRef] [PubMed]
- Dvorská, M.; Zemlicka, M.; Muselík, J.; Karafiátová, J.; Suchý, V. Antioxidant activity of Catalpa bignonioides. Fitoterapia 2007, 78, 437–439. [Google Scholar] [CrossRef]
- Jeong, H.; Lee, D.-j.; Kwon, S.-P.; Park, S.; Kim, S.-R.; Kim, S.H.; Park, J.-I.; Lee, D.-C.; Choi, K.-M.; Lee, W.; et al. Catalpa bignonioides extract improves exercise performance through regulation of growth and metabolism in skeletal muscles. Asian Pac. J. Trop. Biomed. 2024, 14, 47–54. [Google Scholar] [CrossRef]
- Oh, Y.; Lee, D.; Park, S.; Kim, S.H.; Kang, K.S. The chemical constituents from fruits of Catalpa bignonioides Walt. and their α-glucosidase inhibitory activity and insulin secretion effect. Molecules 2021, 26, 362. [Google Scholar] [CrossRef]
- Kimura, K.; Okuda, T.; Takano, T. Studies on the constituents of Catalpa ovata G. Don. I. Active principles of fruit. Yakugaku Zasshi 1963, 83, 635–638. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, H.; Murakami, M.; Yashizaki, M.; Havada, M. Preparations of catalpa fruits and changes in the contents of catalposide, catalpol and p-hydroxybenzoic acid. Iyakuhin Kenkyu 1991, 22, 359–367. [Google Scholar]
- Noro, Y.; Hisato, Y.; Okuda, K.; Kawamura, T.; Higuchi, Y.; Tanaka, T. Pharmacognostical studies of Catalpae fructus. 1. Relation between the growth of fruit and the iridoid glycoside contents. Shoyakugaku Zasshi 1992, 46, 14–18. [Google Scholar]
- Olsen, R.T.; Kirkbride, J.H. Taxonomic revision of the genus Catalpa (Bignoniaceae). Brittonia 2017, 69, 387–421. [Google Scholar] [CrossRef]
- Elansary, H.O.; Szopa, A.; Kubica, P.; Al-Mana, F.A.; Mahmoud, E.A.; El-Abedin, T.K.A.Z.; Mattar, M.A.; Ekiert, H. Phenolic compounds of Catalpa speciosa, Taxus cuspidate, and Magnolia acuminata have antioxidant and anticancer activity. Molecules 2019, 24, 412. [Google Scholar] [CrossRef]
- Fujiwara, A.; Mori, T.; Iida, A.; Ueda, S.; Hano, Y.; Nomura, T.; Tokuda, H.; Nishino, H. Antitumorpromoting naphthoquinones from Catalpa ovata. J. Nat. Prod. 1998, 61, 629–632. [Google Scholar] [CrossRef]
- Kil, Y.S.; So, Y.K.; Choi, M.J.; Han, A.R.; Jin, C.H.; Seo, E.K. Cytoprotective dihydronaphthalenones from the wood of Catalpa ovata. Phytochemistry 2018, 147, 14–20. [Google Scholar] [CrossRef]
- Qin, Y.; Liu, S.; Zou, Q.; Cai, X.; Guo, Z.; Yu, L.; Wang, J.; Deng, Z. Naphthoquinones from Catalpa bungei “Jinsi” as potent antiproliferation agents inducing DNA damage. Fitoterapia 2022, 160, 105196. [Google Scholar] [CrossRef] [PubMed]
- Marković, D.; Barboux, C.; Salle de Chou, Y.; Bettach, J.; Grougnet, R.; Deguin, B. Centrifugal partition chromatography: An efficient tool to access highly polar and unstable synthetic compounds on a large scale. RSC Adv. 2014, 4, 63254–63259. [Google Scholar] [CrossRef]
- Bernardi, C.; Macrì, G.; Biagi, M.; Miraldi, E.; Finetti, F.; Trabalzini, L. In Vitro digestion of Phaseolus vulgaris L. Cooked beans induces autophagy in colon cancer cells. Foods 2023, 12, 839. [Google Scholar] [CrossRef] [PubMed]
- Trabalzini, L.; Ercoli, J.; Trezza, A.; Schiavo, I.; Macrì, G.; Moglia, A.; Spiga, O.; Finetti, F. Pharmacological and in silico analysis of oat Avenanthramides as EGFR inhibitors: Effects on EGF-induced lung cancer cell growth and migration. Int. J. Mol. Sci. 2022, 23, 8534. [Google Scholar] [CrossRef]
- Bernardi, C.; Cappellucci, G.; Baini, G.; Aloisi, A.M.; Finetti, F.; Trabalzini, L. Potential human health benefits of Phaseolus vulgaris L. var Venanzio: Effects on cancer cell growth and inflammation. Nutrients 2024, 16, 2534. [Google Scholar] [CrossRef]
- Tenuta, M.C.; Loizzo, M.R.; Tundis, R.; Dugay, A.; Bouzidi, C.; Marie, A.; Acquaviva, R.; Cappello, A.R.; Deguin, B. Iridoid- and flavonoid-enriched fractions of Cornus sanguinea and Cornus mas exert antioxidant and anti-inflammatory effects and inhibit key enzymes in the treatment of metabolic disorders. Food Funct. 2023, 14, 8838–8853. [Google Scholar] [CrossRef]
- Rau, E.A.; Felter, H.W.; Lloyd, J.U. King’s American Dispensatory, 19th ed.; Third revision; Ohio Valley Co.: Cincinnati, OH, USA, 1905; Volume 1, pp. 460–461. [Google Scholar]
- Bourquelot, E.; Herissey, H. Sur un glucoside nouveau, l’aucubine, retiré des graines d’Aucuba Japonica L. C. R. Acad. Sci. 1902, 134, 1441–1443. [Google Scholar]
- Iwagawa, T.; Hamada, T.; Kurogi, S.; Hase, T.; Okubo, T.; Kim, M. Iridoids from Catalpa bignonioides. Phytochemistry 1991, 30, 4057–4060. [Google Scholar] [CrossRef]
- Dal Piaz, F.; Vassallo, A.; Temraz, A.; Cotugno, R.; Belisario, M.A.; Bifulco, G.; Chini, M.G.; Pisano, C.; De Tommasi, N.; Braca, A. A chemical-biological study reveals C9-type iridoids as novel heat shock protein 90 (Hsp90) inhibitors. J. Med. Chem. 2013, 56, 1583–1595. [Google Scholar] [CrossRef]
- de Abreu, M.B.; Temraz, A.; Vassallo, A.; Braca, A.; De Tommasi, N. Phenolic glycosides from Tabebuia argentea and Catalpa bignonioides. Phytochem. Lett. 2014, 7, 85–88. [Google Scholar] [CrossRef]
- Harborne, J.B. Comparative biochemistry of the flavonoids-VI.: Flavonoid patterns in the bignoniaceae and the gesneriaceae. Phytochemistry 1967, 6, 1643–1651. [Google Scholar] [CrossRef]
- McDaniel, C.A. Major antitermitic components of the heartwood of southern catalpa. J. Chem. Ecol. 1992, 18, 359–369. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Wu, L.; Liu, J.; Chen, K.; Li, Y. Iridoids and derivatives from Catalpa ovata with their antioxidant activities. Fitoterapia 2023, 169, 105599. [Google Scholar] [CrossRef] [PubMed]
- Birkorfer, L.; Kaiser, C.; Becker, F.; Kosmol, H. Bignonoside in acyliertes flavonaus Catalpa bignonioides. Z. Naturforsch. 1965, 20b, 923. [Google Scholar] [CrossRef]
- Tang, H.Y.; Bai, M.M.; Tian, J.M.; Pescitelli, G.; Ivšić, T.; Huang, X.H.; Lee, H.; Nan Son, Y.; Kim, J.H.; Kim, Y.H.; et al. Chemical components from the seeds of Catalpa bungei and their inhibition of soluble epoxide hydrolase, cholinesterase and nuclear factor kappa B activities. RSC Adv. 2016, 6, 40706–40716. [Google Scholar] [CrossRef]
- Saracoglu, I.; Varel, M.; Harput, U.S.; Nagatsu, A. Acylated flavonoids and phenol glycosides from Veronica thymoides subsp. pseudocinerea. Phytochemistry 2004, 65, 2379–2385. [Google Scholar] [CrossRef] [PubMed]
- Meng, Y.; Krzysiak, A.J.; Durako, M.J.; Kunzelman, J.I.; Wright, J.L.C. Flavones and flavone glycosides from Halophila johnsonii. Phytochemistry 2008, 69, 2603–2608. [Google Scholar] [CrossRef] [PubMed]
- Plouvier, V. Sur les hétérosides de Catalpa bignonioides Walt (Bignoniacée) et du Paulownia tomentosa C. Koch (Scrofulariacée). C. R. Acad. Sci. 1947, 224, 670–672. [Google Scholar]
- Do, T.K.T.; De Vaumas, R.; Reich, E. Phytochemical profiling of iridoids by high-performance thin-layer chromatography. JPC-J. Planar. Chromat. 2021, 34, 361–366. [Google Scholar] [CrossRef]
- Islam, M.K.; Sostaric, T.; Lim, L.Y.; Hammer, K.; Locher, C. Antioxidant HPTLC-DPPH fingerprinting of honeys and tracking of antioxidant constituents upon thermal exposure. Foods 2021, 10, 357. [Google Scholar] [CrossRef]
- Kahraman, C.; Tatlı, I.I.; Orhan, I.E.; Akdemir, Z.S. Cholinesterase inhibitory and antioxidant properties of Verbascum mucronatum Lam. and its secondary metabolites Z. Naturforsch 2010, 65, 667–674. [Google Scholar] [CrossRef]
- Gökmen, A.; Kúsz, N.; Karaca, N.; Demirci, F.; Hohmann, J.; Kırmızıbekmez, H. Secondary metabolites from Verbascum bugulifolium Lam. and their bioactivities. Nat. Prod. Res. 2021, 35, 5294–5298. [Google Scholar] [CrossRef]
- Pisoschi, A.M.; Pop, A. The role of antioxidants in the chemistry of oxidative stress: A review. Eur. J. Med. Chem. 2015, 97, 55–74. [Google Scholar] [CrossRef]
- Moon, J.-K.; Shibamoto, T. Antioxidant assays for plant and food components. J. Agric. Food Chem. 2009, 57, 1655–1666. [Google Scholar] [CrossRef]
- Xu, H.; Hu, G.; Dong, J.; Wei, Q.; Shao, H.B.; Lei, M. Antioxidative activities and active compounds of extracts from Catalpa plant leaves. Sci. World J. 2014, 2014, 857982. [Google Scholar] [CrossRef]
- Lu, Q.; Sun, Y.; Shu, Y.; Tan, S.; Yin, L.; Guo, Y.; Tang, L. HSCCC Separation of the two iridoid glycosides and three phenolic compounds from Veronica ciliata and their in vitro antioxidant and anti-hepatocarcinoma activities. Molecules 2016, 21, 1234. [Google Scholar] [CrossRef]
- Moon, M.K.; Choi, B.-M.; Oh, G.-S.; Pae, H.-O.; Kim, J.-D.; Oh, H.; Oh, C.-S.; Kim, D.-H.; Rho, Y.-D.; Shin, M.-K.; et al. Catalposide protects Neuro 2A cells from hydrogen peroxide-induced cytotoxicity via the expression of heme oxygenase-1. Toxicol. Lett. 2003, 145, 46–54. [Google Scholar] [CrossRef] [PubMed]
- Kwak, J.H.; Kim, H.J.; Lee, K.H.; Kang, S.C.; Zee, O.P. Antioxidative iridoid glycosides and phenolic compounds from Veronica peregrine. Arch. Pharm. Res. 2009, 32, 207–213. [Google Scholar] [CrossRef] [PubMed]
- Shen, Y.; Song, X.; Li, L.; Sun, J.; Jaiswal, Y.; Huang, J.; Liu, C.; Yang, W.; Williams, L.; Zhang, H.; et al. Protective effects of p-coumaric acid against oxidant and hyperlipidemia-an in vitro and in vivo evaluation. Biomed. Pharmacother. 2019, 111, 579–587. [Google Scholar] [CrossRef] [PubMed]
- You, L.; Peng, H.; Liu, J.; Cai, M.; Wu, H.; Zhang, Z.; Bai, J.; Yao, Y.; Dong, X.; Yin, X.; et al. Catalpol protects ARPE-19 cells against oxidative stress via activation of the Keap1/Nrf2/ARE Pathway. Cells 2021, 10, 2635. [Google Scholar] [CrossRef]
- Shah, S.C.; Itzkowitz, S.H. Colorectal cancer in inflammatory bowel disease: Mechanisms and management. Gastroenterology 2022, 162, 715–730.e3. [Google Scholar] [CrossRef]
- Grivennikov, S.I.; Greten, F.R.; Karin, M. Immunity, inflammation, and cancer. Cell 2010, 140, 883–899. [Google Scholar] [CrossRef]
- Grivennikov, S.I. Inflammation and colorectal cancer: Colitis-associated neoplasia. Semin. Immunopathol. 2013, 35, 229–244. [Google Scholar] [CrossRef]
- Beaugerie, L.; Itzkowitz, S.H. Cancers complicating inflammatory bowel disease. N. Engl. J. Med. 2015, 372, 1441–1452. [Google Scholar] [CrossRef]
- Liu, L.; Gao, H.; Wang, H.; Zhang, Y.; Xu, W.; Lin, S.; Wang, H.; Wu, Q.; Guo, J. Catalpol promotes cellular apoptosis in human HCT116 colorectal cancer cells via microRNA-200 and the downregulation of PI3K-Akt signaling pathway. Oncol. Lett. 2017, 14, 3741–3747. [Google Scholar] [CrossRef]
- Zhu, P.; Wu, Y.; Yang, A.; Fu, X.; Mao, M.; Liu, Z. Catalpol suppressed proliferation, growth and invasion of CT26 colon cancer by inhibiting inflammation and tumor angiogenesis. Biomed. Pharmacother. 2017, 95, 68–76. [Google Scholar] [CrossRef] [PubMed]
- Bhattamisra, S.K.; Yap, K.H.; Rao, V.; Choudhury, H. Multiple biological effects of an iridoid glucoside, catalpol and its underlying molecular mechanisms. Biomolecules 2020, 10, 32. [Google Scholar] [CrossRef]
- Qiao, P.-F.; Yao, L.; Zeng, Z.-L. Catapol-mediated microRNA-34a suppresses autophagy and malignancy by regulating SIRT1 in colorectal cancer. Oncol. Rep. 2020, 43, 1053–1066. [Google Scholar] [CrossRef] [PubMed]
- An, S.J.; Pae, H.O.; Oh, G.S.; Choi, B.M.; Jeong, S.; Jang, S.I.; Oh, H.; Kwon, T.O.; Song, C.E.; Chung, H.T. Inhibition of TNF-alpha, IL-1beta, and IL-6 productions and NF-kappa B activation in lipopolysaccharide-activated RAW 264.7 macrophages by catalposide, an iridoid glycoside isolated from Catalpa ovata G. Don (Bignoniaceae). Int. Immunopharmacol. 2002, 2, 1173–1181. [Google Scholar] [CrossRef]
- Kim, S.W.; Choi, S.C.; Choi, E.Y.; Kim, K.S.; Oh, J.M.; Lee, H.J.; Oh, H.M.; Kim, S.; Oh, B.S.; Kimm, K.C.; et al. Catalposide, a compound isolated from Catalpa ovata, attenuates induction of intestinal epithelial proinflammatory gene expression and reduces the severity of trinitrobenzene sulfonic acid-induced colitis in mice. Inflamm. Bowel Dis. 2004, 10, 564–572. [Google Scholar] [CrossRef] [PubMed]
- Lu, Q.; Tan, S.; Gu, W.; Li, F.; Hua, W.; Zhang, S.; Chen, F.; Tang, L. Phytochemical composition, isolation and hepatoprotective activity of active fraction from Veronica ciliata against acetaminophen-induced acute liver injury via p62-Keap1-Nrf2 signaling pathway. J. Ethnopharmacol. 2019, 243, 112089. [Google Scholar] [CrossRef]
- Lee, J.A.; Ngo, T.H.; Shin, M.R.; Choi, J.W.; Choi, H.; Nam, J.W.; Roh, S.S. Efficacy of Veronica incana for treating osteoarthritis induced by monosodium iodoacetate in rats. J. Med. Food 2023, 26, 379–389. [Google Scholar] [CrossRef]
- Guerrero-Pepinosa, N.Y.; Veloza, L.A.; Sepúlveda-Arias, J.C. The n-butanol extract obtained from the inner bark of Tabebuia rosea (bertol.) DC, specioside, and catalposide induce leukemia cell apoptosis in the presence of apicidin. Molecules 2024, 29, 3986. [Google Scholar] [CrossRef]
- Kim, B.; Min, Y.H.; Park, B. Minecoside modulates cell invasion via regulation of CXCR4 expression in breast and colon cancer cells. Planta Med. 2020, 86, 331–337. [Google Scholar] [CrossRef]
- Kim, B.; Lee, K.Y.; Park, B. Minecoside promotes apoptotic progression through STAT3 inactivation in breast cancer cells. Oncol. Lett. 2022, 23, 94. [Google Scholar] [CrossRef]
- Sun, Y.; Zhang, Y.; Wang, T.; Wen, L.; Xing, T.; Peng, J.; Liang, Y. Picroside III ameliorates colitis in mice: A study based on colon transcriptome and fecal 16S amplicon profiling. Chem. Biodivers. 2023, 20, e202301806. [Google Scholar] [CrossRef]
- Huan, Q.; Gao, Z.; Wu, P.; Zhang, Y.; Xiao, H.; Peng, J. Picroside III, an active ingredient of Picrorhiza scrophulariiflora, ameliorates experimental colitis by protecting intestinal barrier integrity. Chem. Biodivers. 2023, 20, e202300572. [Google Scholar] [CrossRef]
- Küpeli, E.; Harput, U.S.; Varel, M.; Yesilada, E.; Saracoglu, I. Bioassay-guided isolation of iridoid glucosides with antinociceptive and anti-inflammatory activities from Veronica anagallis-aquatica L. J. Ethnopharmacol. 2005, 102, 170–176. [Google Scholar] [CrossRef]
- Picerno, P.; Autore, G.; Marzocco, S.; Meloni, M.; Sanogo, R.; Aquino, R.P. Anti-inflammatory activity of verminoside from Kigelia africana and evaluation of cutaneous irritation in cell cultures and reconstituted human epidermis. J. Nat. Prod. 2005, 68, 1610–1614. [Google Scholar] [CrossRef]
- Oh, S.R.; Lee, M.Y.; Ahn, K.; Park, B.Y.; Kwon, O.K.; Joung, H.; Lee, J.; Kim, D.Y.; Lee, S.; Kim, J.H.; et al. Suppressive effect of verproside isolated from Pseudolysimachion longifolium on airway inflammation in a mouse model of allergic asthma Int. Immunopharmacol. 2006, 6, 978–986. [Google Scholar] [CrossRef] [PubMed]
- Gründemann, C.; Garcia-Käufer, M.; Sauer, B.; Stangenberg, E.; Könczöl, M.; Merfort, I.; Zehl, M.; Huber, R. Traditionally used Veronica officinalis inhibits proinflammatory mediators via the NF-κB signalling pathway in a human lung cell line. J. Ethnopharmacol. 2013, 145, 118–126. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.U.; Sung, M.H.; Ryu, H.W.; Lee, J.; Kim, H.S.; In, H.J.; Ahn, K.S.; Lee, H.J.; Lee, H.K.; Shin, D.H.; et al. Verproside inhibits TNF-α-induced MUC5AC expression through suppression of the TNF-α/NF-κB pathway in human airway epithelial cells. Cytokine 2016, 77, 168–175. [Google Scholar] [CrossRef] [PubMed]
- Oh, E.S.; Ryu, H.W.; Kim, M.O.; Lee, J.W.; Song, Y.N.; Park, J.Y.; Kim, D.Y.; Ro, H.; Lee, J.; Kim, T.D.; et al. Verproside, the most active ingredient in YPL-001 isolated from Pseudolysimachion rotundum var. subintegrum, decreases inflammatory response by inhibiting PKCδ activation in human lung epithelial cells. Int. J. Mol. Sci. 2023, 24, 7229. [Google Scholar] [CrossRef]
- Lee, C.; Ryu, H.W.; Kim, S.; Kim, M.; Oh, S.R.; Ahn, K.S.; Park, J. Verminoside from Pseudolysimachion rotundum var. subintegrum sensitizes cisplatin-resistant cancer cells and suppresses metastatic growth of human breast cancer. Sci Rep. 2020, 10, 20337. [Google Scholar] [CrossRef]
- Frezza, C.; De Vita, D.; Venditti, A.; Baldani, C.; Giampaoli, O.; Sciubba, F.; Bosco, C.D.; Franceschin, M.; Beccaccioli, M.; Reverberi, M.; et al. Phytochemical analysis and biological activities of the aerial parts of Odontites Vulgaris Moench. Fitoterapia 2024, 175, 105936. [Google Scholar] [CrossRef] [PubMed]
- Ramírez-Cisneros, M.Á.; Rios, M.Y.; Aguilar-Guadarrama, A.B.; Rao, P.P.N.; Aburto-Amar, R.; Rodríguez-López, V. In Vitro COX-1 and COX-2 Enzyme inhibitory activities of iridoids from Penstemon Barbatus, Castilleja Tenuiflora, Cresentia Alata and Vitex Mollis. Bioorg. Med. Chem. Lett. 2015, 25, 4505–4508. [Google Scholar] [CrossRef]
- Saracoglu, I.; Suleimanov, T.; Pashaeva, N.; Dogan, Z.; Inoue, M.; Nakashima, K. Iridoids from Veronica Crista-Galli from the Flora of Azerbaijan. Chem. Nat. Compd. 2020, 56, 751–753. [Google Scholar] [CrossRef]
- Phuong Thao, T.T.; Bui, T.Q.; Quy, P.T.; Bao, N.C.; Van Loc, T.; Van Chien, T.; Chi, N.L.; Van Tuan, N.; Van Sung, T.; Ai Nhung, N.T. Isolation, semi-synthesis, docking-based prediction, and bioassay-based activity of Dolichandrone spathacea iridoids: New catalpol derivatives as glucosidase inhibitors. RSC Adv. 2021, 11, 11959–11975. [Google Scholar] [CrossRef] [PubMed]
| Sample | DPPH Test IC50 (μg/mL) | ABTS Test IC50 (μg/mL) | β-Carotene Bleaching Test # IC50 (μg/mL) | FRAP Test * μM Fe(II)/g |
|---|---|---|---|---|
| EAC | 19.7 ± 1.9 ** | 1.2 ± 0.3 ns | 7.1 ± 0.8 *** | 102.7 ± 7.8 *** |
| EBC | 16.9 ± 1.6 * | 0.5 ± 0.04 *** | NA | 102.2 ± 7.5 *** |
| EDC | 15.7 ± 1.7 * | 1.1 ± 0.1 * | NA | 102.6 ± 7.7 *** |
| Catalposide | 60.5 ± 5.3 *** | 0.4 ± 0.03 *** | 27.4 ± 4.6 *** | 14.8 ± 1.8 *** |
| Positive control | ||||
| Ascorbic acid | 5.3 ± 0.6 | 1.7 ± 0.5 | ||
| BHT | 63.3 ± 3.9 | |||
| Propyl gallate | 1.2 ± 0.4 | |||
| HT29 | HCT116 | |||||||
|---|---|---|---|---|---|---|---|---|
| Concentration (µg/mL) | CTRL | EAC | EBC | EDC | CTRL | EAC | EBC | EDC |
| 0.01 | 7.1% ± 1.7 | 3.6% ± 0.8 | 1.6% ± 0.5 | 6.9% ± 4.6 | 7.6% ± 1.7 | 12% ± 2.9 | 16.3% ± 1.8 | 5.1% ± 2.6 |
| 0.1 | 18.9% ± 1.2 | 1.8% ± 0.4 | 12.7% ± 2.3 | 17% ± 2.1 | 10.6% ± 3.7 | 11.4% ± 5.3 | ||
| 1 | 4.8% ± 0.5 | 5.3%± 0.5 | 8.3% ± 3.0 | 11.5% ± 2.4 | 17.6% ± 7.6 | 9.9% ± 6.0 | ||
| 10 | 15.9% ± 1.3 | 4.8% ± 0.8 | 12.8% ± 3.4 | 7.6% ± 2.1 | 6.7% ± 1.67 | 14.3% ± 1.4 | ||
| 100 | 5.9% ± 0.8 | 2.6% ± 0.8 | 15.0% ± 4.5 | 7% ± 1.3 | 16.4% ± 5.9 | 13.0% ± 5.5 | ||
| HT29 | HCT116 | ||||||
|---|---|---|---|---|---|---|---|
| Concentration (µg/mL) | CTRL | EAC | EBC | EDC | EAC | EBC | EDC |
| 0.01 | 100% | 98.5 ± 11.7 | 112.6 ± 10.8 | 111.1 ± 4.5 | 99.5 ± 11.8 | 106.0 ± 7.2 | 101.0 ± 3.2 |
| 0.1 | 88.7 ± 12.6 | 101.6 ± 8.7 | 99.4 ± 4.4 | 80.3 ± 3.0 | 80.5 ± 9.0 | 82.2 ± 4.1 | |
| 1 | 100.3 ± 2.5 | 88.8 ± 1.7 | 105.5 ± 2.9 | 100.2 ± 11.9 | 104.9 ± 6.3 | 100.3 ± 10.4 | |
| 10 | 94.7 ± 14.2 | 94.3 ± 15.2 | 101.1 ± 9.0 | 87.5 ± 1.8 | 82.6 ± 1.5 | 85.4 ± 1.9 | |
| 100 | 90.7 ± 7.5 | 84.3 ± 13.4 | 103.3 ± 2.5 | 94.0 ± 11.8 | 56.5 ± 12.2 * | 66.5 ± 10.2 * | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bernardi, C.; Gaslonde, T.; Finetti, F.; Benmaouche, S.; Macrì, G.; Dugay, A.; Cuyamendous, C.; Bouzidi, C.; Loizzo, M.R.; Belmont, P.; et al. A Study of the Fruits of Catalpa bignonioides Walt.: Evaluation of the Antioxidant, Anti-Inflammatory, and Anti-Cancer Activities in Colorectal Adenocarcinoma Cells in Relation to Phytochemical Profile. Antioxidants 2025, 14, 1116. https://doi.org/10.3390/antiox14091116
Bernardi C, Gaslonde T, Finetti F, Benmaouche S, Macrì G, Dugay A, Cuyamendous C, Bouzidi C, Loizzo MR, Belmont P, et al. A Study of the Fruits of Catalpa bignonioides Walt.: Evaluation of the Antioxidant, Anti-Inflammatory, and Anti-Cancer Activities in Colorectal Adenocarcinoma Cells in Relation to Phytochemical Profile. Antioxidants. 2025; 14(9):1116. https://doi.org/10.3390/antiox14091116
Chicago/Turabian StyleBernardi, Clizia, Thomas Gaslonde, Federica Finetti, Salim Benmaouche, Giulia Macrì, Annabelle Dugay, Claire Cuyamendous, Chouaha Bouzidi, Monica Rosa Loizzo, Philippe Belmont, and et al. 2025. "A Study of the Fruits of Catalpa bignonioides Walt.: Evaluation of the Antioxidant, Anti-Inflammatory, and Anti-Cancer Activities in Colorectal Adenocarcinoma Cells in Relation to Phytochemical Profile" Antioxidants 14, no. 9: 1116. https://doi.org/10.3390/antiox14091116
APA StyleBernardi, C., Gaslonde, T., Finetti, F., Benmaouche, S., Macrì, G., Dugay, A., Cuyamendous, C., Bouzidi, C., Loizzo, M. R., Belmont, P., Tundis, R., Trabalzini, L., & Deguin, B. (2025). A Study of the Fruits of Catalpa bignonioides Walt.: Evaluation of the Antioxidant, Anti-Inflammatory, and Anti-Cancer Activities in Colorectal Adenocarcinoma Cells in Relation to Phytochemical Profile. Antioxidants, 14(9), 1116. https://doi.org/10.3390/antiox14091116









