Physiological Responses of Tomato Plants with Varied Susceptibility to Multiple Drought Stress
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Materials
2.2. Experimental Design
2.3. Measurements
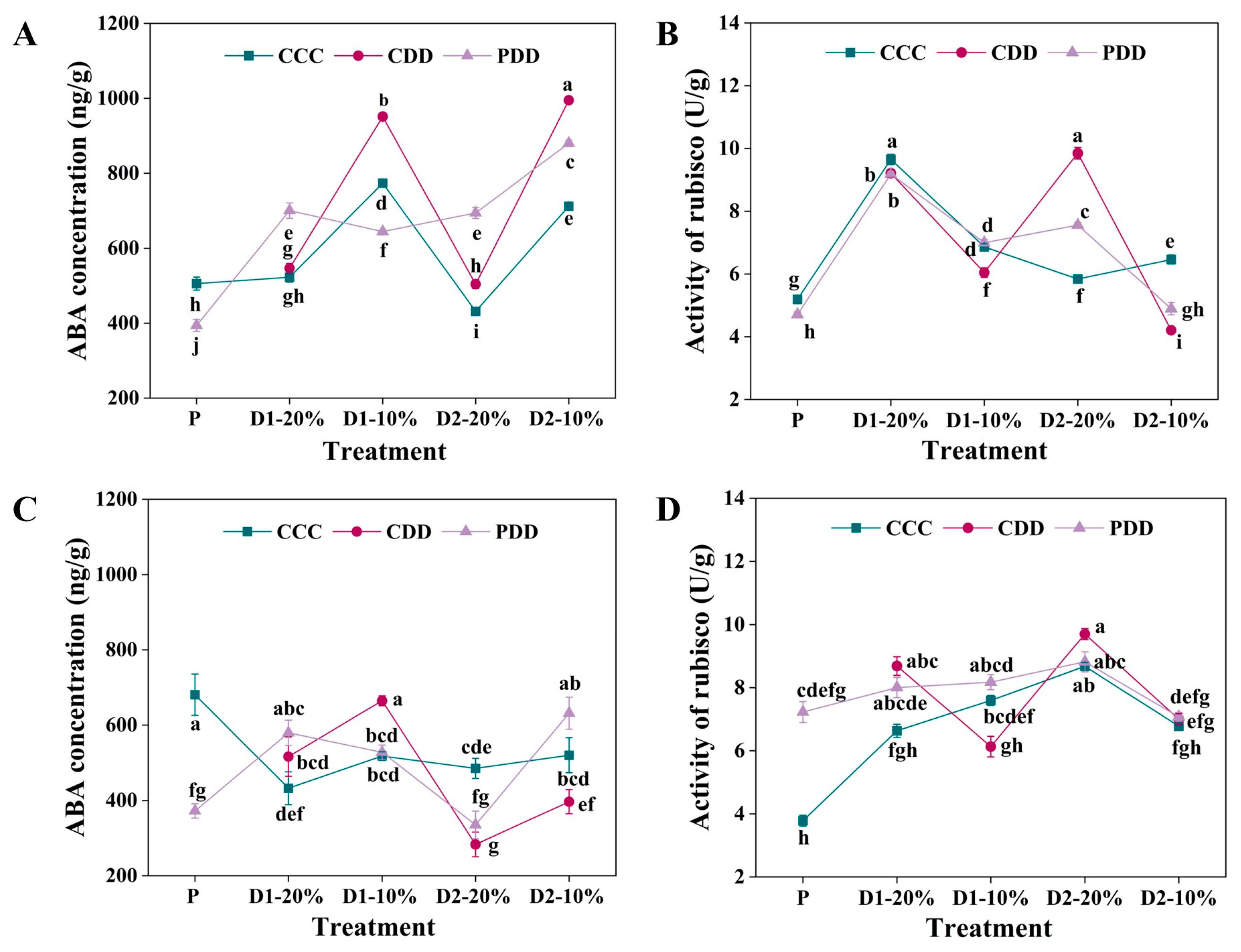
2.3.1. ABA Concentration and Rubisco Activity
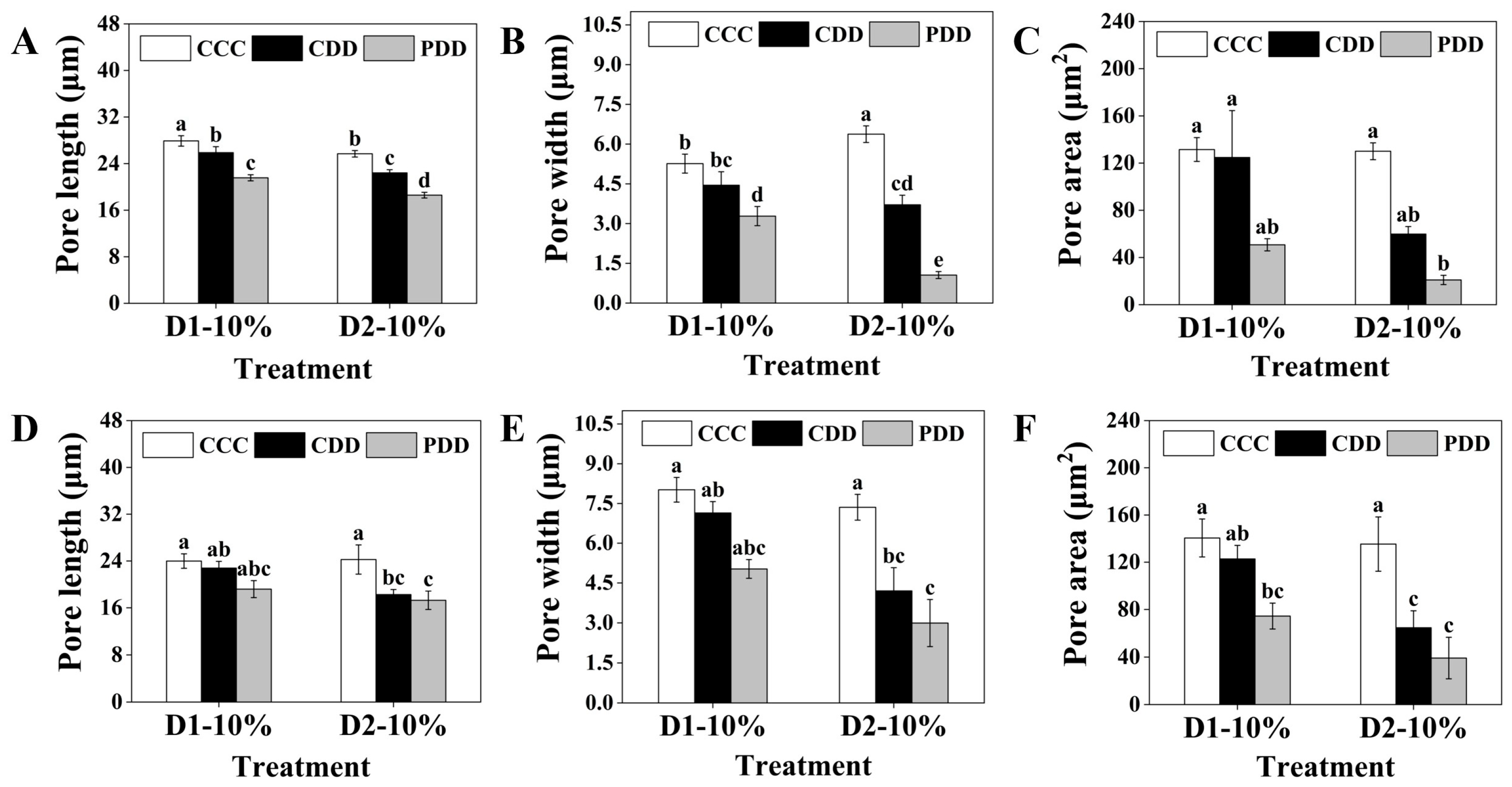
2.3.2. Stomatal Characterization
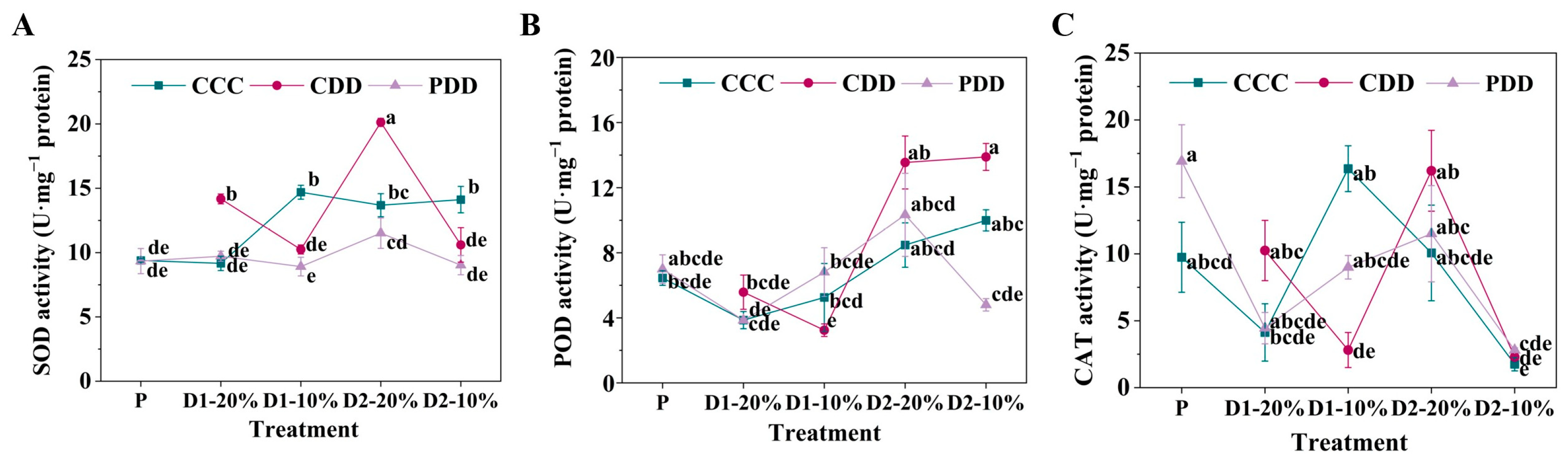
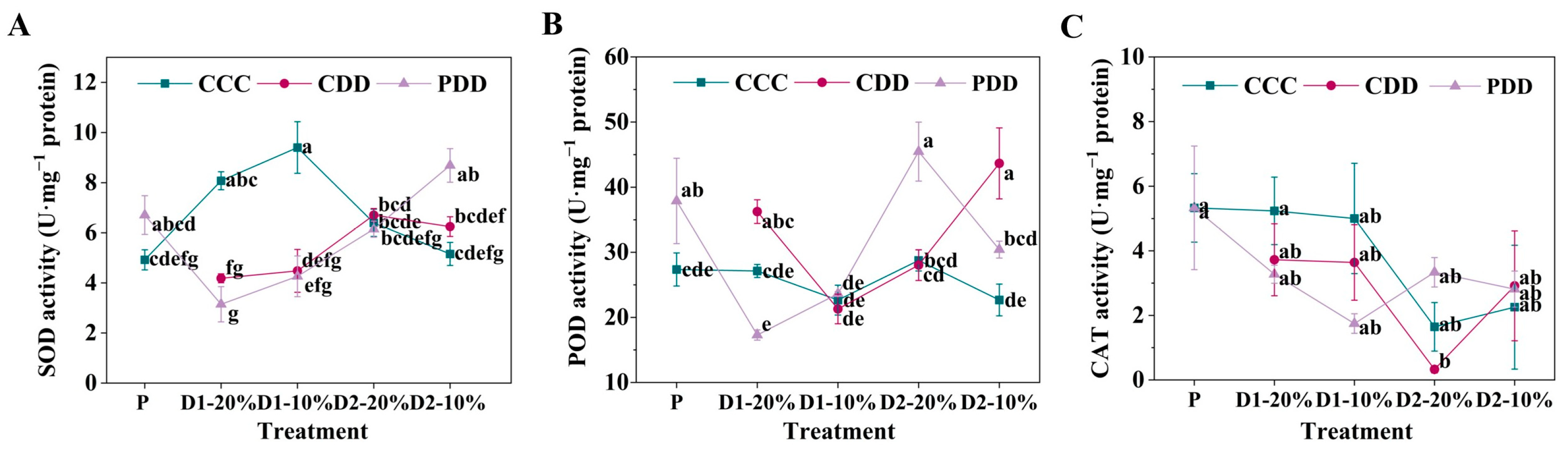
2.3.3. O2·− Production Rate, H2O2 Content, and Antioxidant Enzyme Activities
2.3.4. qRT-PCR
2.3.5. Morphological Indicators
2.4. Data Analysis
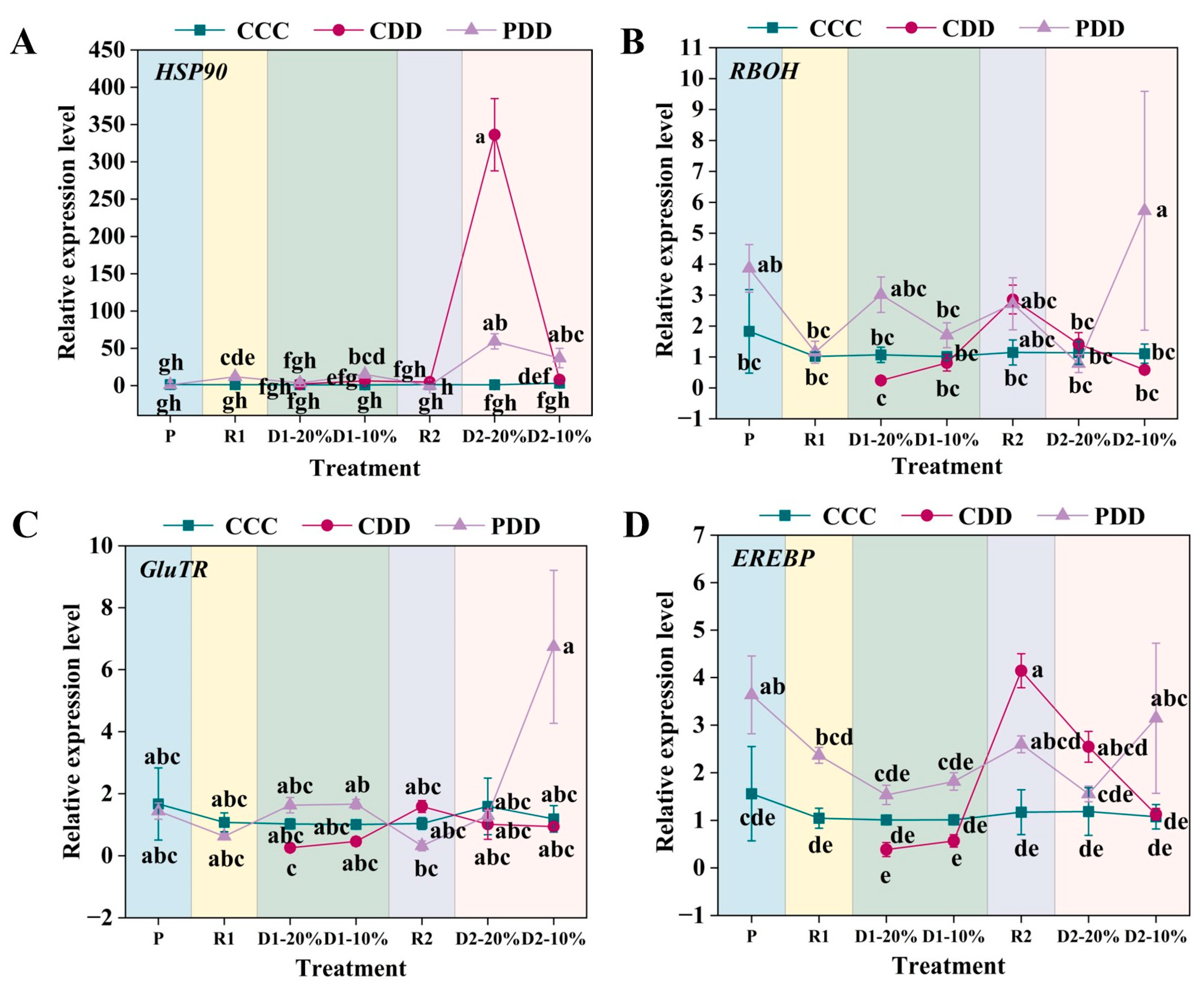
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Muscolo, A.; Junker, A.; Klukas, C.; Weigelt-Fischer, K.; Riewe, D.; Altmann, T. Phenotypic and metabolic responses to drought and salinity of four contrasting lentil accessions. J. Exp. Bot. 2015, 66, 5467–5480. [Google Scholar] [CrossRef]
- Spinonia, J.; Barbosaa, P.; Jagera, A.D.; McCormicka, N.; Naumanna, G.; Vogta, V.J.; Magnib, D.; Masanteb, D.; Mazzeschi, M. A new global database of meteorological drought events from 1951 to 2016. J. Hydrol. 2019, 22, 100593. [Google Scholar]
- Li, Y.P.; Ye, W.; Wang, M.; Yan, X.D. Climate change and drought: A risk assessment of crop-yield impacts. Clim. Res. 2009, 39, 31–46. [Google Scholar] [CrossRef]
- Su, B.; Huang, J.L.; Fischer, T.; Wang, Y.J.; Kundzewicz, Z.W.; Zhai, J.Q.; Sun, H.M.; Wang, A.Q.; Zeng, X.F.; Wang, G.J.; et al. Drought losses in China might double between the 1.5 °C and 2.0 °C warming. Proc. Natl. Acad. Sci. USA 2018, 115, 10600–10605. [Google Scholar] [CrossRef]
- Suter, G. Climate change 2022: Impacts, adaptation and vulnerability. Integr. Environ. Assess. Manag. 2022, 18, 1117–1118. [Google Scholar]
- Xie, Z.T.; Liu, H.X. Evolution characteristics of agricultural drought disasters in China. In Proceedings of the Ninth International Conference on Management Science and Engineering Management, Karlsruhe, Germany, 21–23 July 2015; Advances in Intelligent Systems and Computing; Springer: Berlin/Heidelberg, Germany, 2015; Volume 362, pp. 1407–1417. [Google Scholar]
- Kim, W.; Iizumi, T.; Nishimori, M. Global patterns of crop production losses associated with droughts from 1983 to 2009. J. Appl. Meteorol. Climatol. 2019, 58, 1233–1244. [Google Scholar] [CrossRef]
- Wan, L.L.; Bento, V.A.; Qu, Y.P.; Qiu, J.X.; Song, H.Q.; Zhang, R.R.; Wu, X.P.; Xu, F.; Lu, J.K.; Wang, Q.F. Drought characteristics and dominant factors across China: Insights from high-resolution daily SPEI dataset between 1979 and 2018. Sci. Total Environ. 2023, 901, 166362. [Google Scholar] [CrossRef]
- Wang, Z.L.; Wang, J.; Wang, J.S. Risk assessment of agricultural drought disaster in Southern China. Discret. Dyn. Nat. Soc. 2015, 2015, 172919. [Google Scholar] [CrossRef]
- Orlowsky, B.; Seneviratne, S.I. Elusive drought: Uncertainty in observed trends and short- and long-term CMIP5 projections. Hydrol. Earth Syst. Sci. 2013, 17, 1765–1781. [Google Scholar] [CrossRef]
- Abbas, K.; Li, J.R.; Gong, B.B.; Lu, Y.S.; Wu, X.L.; Lü, G.; Gao, H.B. Drought stress tolerance in vegetables: The functional role of structural features, key gene pathways, and exogenous hormones. Int. J. Mol. Sci. 2023, 24, 13876. [Google Scholar] [CrossRef]
- Chen, L.; Shen, Y.R.; Yang, W.J.; Pan, Q.M.; Li, C.; Sun, Q.G.; Zeng, Q.; Li, B.H.; Zhang, L.G. Comparative metabolic study of two contrasting Chinese cabbage genotypes under mild and severe drought stress. Int. J. Mol. Sci. 2022, 23, 5947. [Google Scholar] [CrossRef]
- Junaid, M.D.; Öztürk, Z.N.; Gökçe, A.F. Exploitation of tolerance to drought stress in carrot (Daucus carota L.): An overview. Stress Biol. 2023, 3, 55. [Google Scholar] [CrossRef]
- Yang, X.Y.; Lu, M.Q.; Wang, Y.F.; Wang, Y.R.; Liu, Z.J.; Chen, S. Response mechanism of plants to drought stress. Horticulturae 2021, 7, 50. [Google Scholar] [CrossRef]
- Bhandari, U.; Gajurel, A.; Khadka, B.; Thapa, I.; Chand, I.; Bhatta, D.; Poudel, A.; Pandey, M.; Shrestha, S.; Shrestha, J. Morpho-physiological and biochemical response of rice (Oryza sativa L.) to drought stress: A review. Heliyon 2023, 9, e13744. [Google Scholar] [CrossRef]
- Zafar, S.; Afzala, H.; Ijaza, A.; Mahmoodb, A.; Ayubc, A.; Nayabd, A.; Hussaine, S.; UL-Hussanf, M.; Sabirg, M.A.; Zulfiqarh, U.; et al. Cotton and drought stress: An updated overview for improving stress tolerance. South Afr. J. Bot. 2023, 161, 258–268. [Google Scholar] [CrossRef]
- Sagar, S.; Ramamoorthy, P.; Ramalingam, S.; Muthurajan, R.; Natarajan, S.; Doraiswamy, U.; Subramanian, S. Drought’s physiological footprint: Implications for crop improvement in rice. Mol. Biol. Rep. 2025, 52, 298. [Google Scholar] [CrossRef]
- Haghpanah, M.; Hashemipetroudi, S.; Arzani, A.; Araniti, F. Drought tolerance in plants: Physiological and molecular responses. Plants 2024, 13, 2962. [Google Scholar] [CrossRef] [PubMed]
- Oskuei, B.K.; Bandehagh, A.; Farajzadeh, D.; Lajayer, B.A.; Rajput, V.D.; Astatkie, T. Morphological, biochemical, and physiological responses of canola cultivars to drought stress. Int. J. Environ. Sci. Technol. 2023, 20, 13551–13560. [Google Scholar] [CrossRef]
- Zhou, Q.; Song, S.; Wang, X.; Yan, C.; Ma, C.M.; Dong, S.K. Effects of drought stress on flowering soybean physiology under different soil conditions. Plant Soil Environ. 2022, 68, 487–498. [Google Scholar] [CrossRef]
- Xie, G.R.; Xu, R.; Chong, L.; Zhu, Y.F. Understanding drought stress response mechanisms in tomato. Veg. Res. 2024, 4, e001. [Google Scholar] [CrossRef]
- Wang, Q.; Lu, X.; Sun, Y.; Yu, J.H.; Cao, Q.T.; Xiao, Y.T.; Jiang, N.; Chen, L.F.; Zhou, Y.W. Studies on the physiological response of Hemerocallis middendorffii to two types of drought stresses. Int. J. Mol. Sci. 2024, 25, 13733. [Google Scholar] [CrossRef]
- Jin, X.Y.; Zheng, Y.C.; Wang, J.Y.; Chen, W.; Yang, Z.; Chen, Y.X.; Yang, Y.H.; Lu, G.H.; Sun, B. SbNAC9 improves drought tolerance by enhancing scavenging ability of reactive oxygen species and activating stress-responsive genes of Sorghum. Int. J. Mol. Sci. 2023, 24, 2401. [Google Scholar] [CrossRef]
- Menezes-Silva, P.E.; Sanglard, L.M.V.P.; Ávila, R.T.; Morais, L.E.; Martins, S.C.V.; Nobres, P.; Patreze, C.M.; Ferreira, M.A.; Araújo, W.L.; Fernie, A.R.; et al. Photosynthetic and metabolic acclimation to repeated drought events play key roles in drought tolerance in coffee. J. Exp. Bot. 2017, 68, 4309–4322. [Google Scholar] [CrossRef] [PubMed]
- Xiao, W.; Fu-lai, L.; Dong, J. Priming: A promising strategy for crop production in response to future climate. J. Integr. Agric. 2017, 16, 2709–2716. [Google Scholar] [CrossRef]
- Leuendorf, J.E.; Frank, M.; Schmülling, T. Acclimation, priming and memory in the response of Arabidopsis thaliana seedlings to cold stress. Sci. Rep. 2020, 10, 689. [Google Scholar] [CrossRef]
- Zhu, Z.Y.; Dai, Y.; Yu, G.R.; Zhang, X.; Chen, Q.; Kou, X.B.; Mehareb, E.M.; Raza, G.; Zhang, B.H.; Wang, B.H.; et al. Dynamic physiological and transcriptomic changes reveal memory effects of salt stress in maize. BMC Genom. 2023, 24, 726. [Google Scholar] [CrossRef]
- Ru, C.; Hu, X.T.; Chen, D.Y.; Wang, W.N.; Song, T.Y. Heat and drought priming induce tolerance to subsequent heat and drought stress by regulating leaf photosynthesis, root morphology, and antioxidant defense in maize seedlings. Environ. Exp. Bot. 2022, 202, 105010. [Google Scholar] [CrossRef]
- Bittner, A.; Buer, J.V.; Baier, M. Cold priming uncouples light- and cold-regulation of gene expression in Arabidopsis thaliana. BMC Plant Biol. 2020, 20, 281. [Google Scholar] [CrossRef]
- Ling, Y.; Serrano, N.; Gao, G.; Atia, M.; Mokhtar, M.; Woo, Y.H.; Bazin, J.; Veluchamy, A.; Benhamed, M.; Crespi, M.; et al. Thermopriming triggers splicing memory in Arabidopsis. J. Exp. Bot. 2018, 69, 2659–2675. [Google Scholar] [CrossRef] [PubMed]
- Samantaray, D.; Allu, A.D. Studying thermopriming-mediated short- and long-term acquired thermotolerance in Arabidopsis thaliana. Methods Mol. Biol. 2024, 2832, 223–231. [Google Scholar]
- Li, J.; Yang, P.; Li, J.; Du, H.B.; Yang, R.P.; Li, J. The duration of priming, elimination and maintenance of low temperature stress memory response to periodic chilling risk in pepper (Capsicum annuum L.). Environ. Exp. Bot. 2024, 226, 105914. [Google Scholar] [CrossRef]
- Abid, M.; Tian, Z.W.; Ata-Ul-Karim, S.T.; Liu, Y.; Cui, Y.K.; Zahoor, R.; Jiang, D.; Dai, T.B. Improved tolerance to post-anthesis drought stress by pre-drought priming at vegetative stages in drought-tolerant and -sensitive wheat cultivars. Plant Physiol. Biochem. 2016, 106, 218–227. [Google Scholar] [CrossRef]
- Lv, Z.Y.; Zhang, H.; Huang, Y.; Zhu, L.; Yang, X.; Wu, L.F.; Chen, M.J.; Wang, H.B.; Jing, Q.K.; Shen, J.X.; et al. Drought priming at seedling stage improves photosynthetic performance and yield of potato exposed to a short-term drought stress. J. Plant Physiol. 2024, 292, 154157. [Google Scholar] [CrossRef] [PubMed]
- Sintaha, M.; Man, C.K.; Yung, W.S.; Duan, S.W.; Li, M.W.; Lam, H.M. Drought stress priming improved the drought tolerance of Soybean. Plants 2022, 11, 2954. [Google Scholar] [CrossRef] [PubMed]
- Klunklin, W.; Savage, G. Effect on quality characteristics of tomatoes grown under well-watered and drought stress conditions. Foods 2017, 6, 56. [Google Scholar] [CrossRef] [PubMed]
- Ke, D.; Sun, G.; Wang, Z. Effects of superoxide radicals on ACC synthase activity in chilling-stressed etiolated mungbean seedlings. Plant Growth Regul. 2007, 51, 83–91. [Google Scholar] [CrossRef]
- Chakrabarty, D.; Datta, S.K. Micropropagation of gerbera: Lipid peroxidation and antioxidant enzyme activities during acclimatization process. Acta Physiol. Plant. 2008, 30, 325–331. [Google Scholar] [CrossRef]
- Zhou, W.; Zhao, D.; Lin, X. Effects of waterlogging on nitrogen accumulation and alleviation of waterlogging damage by application of nitrogen fertilizer and mixtalol in winter rape (Brassica napus L.). J. Plant Growth Regul. 1997, 16, 47–53. [Google Scholar] [CrossRef]
- Muñoz-Muñoz, J.L.; García-Molina, F.; García-Ruiz, P.A.; Arribas, E.; Tudela, J.; García-Cánovas, F.; Rodríguez-López, J.N. Enzymatic and chemical oxidation of trihydroxylated phenols. Food Chem. 2009, 113, 435–444. [Google Scholar] [CrossRef]
- Aebi, H. Catalase in vitro. In Methods in Enzymology; Elsevier: Amsterdam, The Netherlands, 1984; Volume 105, pp. 121–126. [Google Scholar]
- Ribeyre, Z.; Messier, C.; Nolet, P. No stress memory pattern was detected in sugar maple and white spruce seedlings subjected to experimental droughts. Ecosphere 2022, 13, e4332. [Google Scholar] [CrossRef]
- Provera, I.; Martinez, M.; Zenone, A.; Giacalone, V.M.; D’Anna, G.; Badalamenti, F.; Marín-Guirao, L.; Procaccini, G. Exploring priming strategies to improve stress resilience of Posidonia oceanica seedlings. Mar. Pollut. Bull. 2024, 200, 116057. [Google Scholar] [CrossRef] [PubMed]
- Sadder, M.T.; Musallam, A.; Allouzi, M.; Duwayri, M.A. Dehydration stress memory genes in Triticum turgidum L. ssp. durum (Desf.). BioTech 2022, 11, 43. [Google Scholar] [CrossRef] [PubMed]
- Kambona, C.M.; Koua, P.A.; Léon, J.; Ballvora, A. Stress memory and its regulation in plants experiencing recurrent drought conditions. Theor. Appl. Genet. 2023, 136, 26. [Google Scholar] [CrossRef]
- de Freitas Guedes, F.A.; Menezes-Silva, P.E.; DaMatta, F.M.; Alves-Ferreira, M. Using transcriptomics to assess plant stress memory. Plant Physiol. 2019, 31, 47–58. [Google Scholar] [CrossRef]
- Ullah, A.; Manghwar, H.; Shaban, M.; Khan, A.H.; Akbar, A.; Ali1, U.; Ali, E.; Fahad, S. Phytohormones enhanced drought tolerance in plants: A coping strategy. Environ. Sci. Pollut. Res. 2018, 25, 33103–33118. [Google Scholar] [CrossRef]
- Aslam, M.M.; Waseem, M.; Jakada, B.H.; Okal, E.J.; Lei, Z.L.; Saqib, H.A.S.; Yuan, W.; Xu, W.F.; Zhang, Q. Mechanisms of abscisic acid-mediated drought stress responses in plants. Int. J. Mol. Sci. 2022, 23, 1084. [Google Scholar] [CrossRef]
- Wang, X.; Vignjevic, M.; Liu, F.; Jacobsen, S.; Jiang, D.; Wollenweber, B. Drought priming at vegetative growth stages improves tolerance to drought and heat stresses occurring during grain filling in spring wheat. Plant Growth Regul. 2015, 75, 677–687. [Google Scholar] [CrossRef]
- Neves, D.M.; Aragão da HoraAlmeida, L.; Souza Santana-Vieira, D.D.; Freschi, L.; Ferreira, C.F.; dos Santos Soares Filho, W.; Cardoso Costa, M.G.; Micheli, F.; Coelho Filho, M.A.; da Silva Gesteira, A. Recurrent water deficit causes epigenetic and hormonal changes in citrus plants. Sci. Rep. 2017, 7, 13684. [Google Scholar] [CrossRef]
- Takahashi, Y.; Kinoshita, T. Stomatal function has an element of hysteresis. New Phytol. 2015, 205, 455–457. [Google Scholar] [CrossRef]
- Zhou, R.; Yu, X.; Ottosen, C.O.; Rosenqvist, E.; Zhao, L.; Wang, Y.; Yu, W.; Zhao, T.; Wu, Z. Drought stress had a predominant effect over heat stress on three tomato cultivars subjected to combined stress. BMC Plant Biol. 2017, 17, 24. [Google Scholar] [CrossRef]
- Wang, X.; Vignjevic, M.; Jiang, D.; Jacobsen, S.; Wollenweber, B. Improved tolerance to drought stress after anthesis due to priming before anthesis in wheat (Triticum aestivum L.) var. Vinjett. J. Exp. Bot. 2014, 65, 6441–6456. [Google Scholar] [CrossRef] [PubMed]
- Mittler, R.; Zandalinas, S.I.; Fichman, Y.; Van Breusegem, F. Reactive oxygen species signalling in plant stress responses. Nat. Rev. Mol. Cell Biol. 2022, 23, 663–679. [Google Scholar] [CrossRef] [PubMed]
- Mohanta, T.K.; Bashir, T.; Hashem, A.; Abd_Allah, E.F.; Khan, A.L.; Al-Harrasi, A.S. Early events in plant abiotic stress signaling: Interplay between calcium, reactive oxygen species and phytohormones. J. Plant Growth Regul. 2018, 37, 1033–1049. [Google Scholar] [CrossRef]
- Saglam, A.; Saruhan, N.; Terzi, R.; Kadioglu, A. The relations between antioxidant enzymes and chlorophyll fluorescence parameters in common bean cultivars differing in sensitivity to drought stress. Russ. J. Plant Physiol. 2011, 58, 60–68. [Google Scholar] [CrossRef]
- Wang, P.T.; Liu, W.C.; Han, C.; Wang, S.T.; Bai, M.Y.; Song, C.P. Reactive oxygen species: Multidimensional regulators of plant adaptation to abiotic stress and development. J. Integr. Plant Biol. 2024, 66, 330–367. [Google Scholar] [CrossRef]
- Zhou, R.; Kong, L.P.; Yu, X.Q.; Ottosen, C.; Zhao, T.M.; Jiang, F.L.; Wu, Z. Oxidative damage and antioxidant mechanism in tomatoes responding to drought and heat stress. Acta Physiol. Plant. 2019, 41, 20. [Google Scholar] [CrossRef]
- di Donato, M.; Geisler, M. HSP90 and co-chaperones: A multitaskers’ view on plant hormone biology. FEBS Lett. 2019, 593, 1415–1430. [Google Scholar] [CrossRef]
- Xiao, D.Y.; Jiang, Y.J.; Wang, Z.F.; Li, X.Y.; Li, H.; Tang, S.H.; Zhang, J.L.; Xia, M.Q.; Zhang, M.X.; Deng, X.F.; et al. Genome-wide identification and expression analysis of the HSP90 gene family in relation to developmental and abiotic stress in Ginger (Zingiber officinale Roscoe). Plants 2025, 14, 1660. [Google Scholar] [CrossRef]
- Xu, J.; Liu, S.W.; Ren, Y.M.; You, Y.; Wang, Z.F.; Zhang, Y.Q.; Zhu, X.J.; Hu, P. Genome-wide identification of HSP90 gene family in Rosa chinensis and its response to salt and drought stresses. Biotech 2024, 14, 204. [Google Scholar] [CrossRef]
- Zhang, J.W.; Xie, Y.D.; Ali, B.; Ahmed, W.; Tang, Y.; Li, H.X. Genome-wide identification, classification, evolutionary expansion and expression of Rboh family genes in pepper (Capsicum annuum L.). Trop. Plant Biol. 2021, 14, 251–266. [Google Scholar] [CrossRef]
- Wang, J.; Liu, X.J.; Kang, Y.D.; Liu, A.Z.; Li, P. Functional analysis and interaction networks of Rboh in poplar under abiotic stress. Front. Plant Sci. 2025, 16, 1553057. [Google Scholar] [CrossRef]
- Jiang, Q.X.; Zhou, X.Y.; Tang, J.; Yi, D.X.; Ma, L.; Wang, X.M. Genome-wide identification and expression profile analysis of the NADPH oxidase gene family in Avena sativa L. Int. J. Mol. Sci. 2025, 26, 2576. [Google Scholar] [CrossRef] [PubMed]
- Dietz, K.-J.; Vogel, M.O.; Viehhauser, A. AP2/EREBP transcription factors are part of gene regulatory networks and integrate metabolic, hormonal and environmental signals in stress acclimation and retrograde signalling. Protoplasma 2010, 245, 3–14. [Google Scholar] [CrossRef] [PubMed]
- Maghraby, A.; Alzalaty, M. Genome-wide identification and evolutionary analysis of the AP2/EREBP, COX and LTP genes in Zea mays L. under drought stress. Sci. Rep. 2024, 14, 7610. [Google Scholar] [CrossRef]
- Friedrich, T.; Oberkofler, V.; Trindade, I.; Altmann, S.; Brzezinka, K.; Lämke, J.; Gorka, M.; Kappel, C.; Sokolowska, E.; Skirycz, A.; et al. Heteromeric HSFA2/HSFA3 complexes drive transcriptional memory after heat stress in Arabidopsis. Nat. Commun. 2021, 12, 3426. [Google Scholar] [CrossRef] [PubMed]
- Godwin, J.; Farrona, S. Plant epigenetic stress memory induced by drought: A physiological and molecular perspective. Plant Epigenet. Epigenom. 2020, 2093, 243–259. [Google Scholar]




| Gene Name | Gene ID | Forward Primer Sequence (5′-3′) | Reverse Primer Sequence (5′-3′) |
|---|---|---|---|
| HSP90 | Solyc06g036290 | GTACCTAAGAGGGCTCCATTTG | GTTCCTCACAGTTGTCCATGA |
| RBOH | Solyc03g117980 | GTACGTCAGAAACTCGGTATGG | GACGCACTAAGGCCGATAAT |
| GluTR | Solyc01g106390 | TCTCCAGCGGATCAGTATCA | CACACGAGCCATAGAAGAAGAA |
| EREBP | Solyc03g093550 | GGAGATCCGTGACCCAAATAG | TTAAACGCTGCCCTGTCATA |
| ACTIN | LOC101264601 | CTCTACATACTTGAGAGGTGCC | AGACGAGGAGAAAACATCACAA |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, H.; Liu, Y.; Ding, F.; Li, Y.; Ottosen, C.-O.; Song, X.; Jiang, F.; Wu, Z.; Yu, X.; Zhou, R. Physiological Responses of Tomato Plants with Varied Susceptibility to Multiple Drought Stress. Antioxidants 2025, 14, 1448. https://doi.org/10.3390/antiox14121448
Chen H, Liu Y, Ding F, Li Y, Ottosen C-O, Song X, Jiang F, Wu Z, Yu X, Zhou R. Physiological Responses of Tomato Plants with Varied Susceptibility to Multiple Drought Stress. Antioxidants. 2025; 14(12):1448. https://doi.org/10.3390/antiox14121448
Chicago/Turabian StyleChen, Hong, Yi Liu, Fei Ding, Yankai Li, Carl-Otto Ottosen, Xiaoming Song, Fangling Jiang, Zhen Wu, Xiaqing Yu, and Rong Zhou. 2025. "Physiological Responses of Tomato Plants with Varied Susceptibility to Multiple Drought Stress" Antioxidants 14, no. 12: 1448. https://doi.org/10.3390/antiox14121448
APA StyleChen, H., Liu, Y., Ding, F., Li, Y., Ottosen, C.-O., Song, X., Jiang, F., Wu, Z., Yu, X., & Zhou, R. (2025). Physiological Responses of Tomato Plants with Varied Susceptibility to Multiple Drought Stress. Antioxidants, 14(12), 1448. https://doi.org/10.3390/antiox14121448









