Superoxide Production by the Red Tide-Producing Chattonella marina Complex (Raphidophyceae) Correlates with Toxicity to Aquacultured Fishes
Abstract
:1. Introduction
2. Materials and Methods
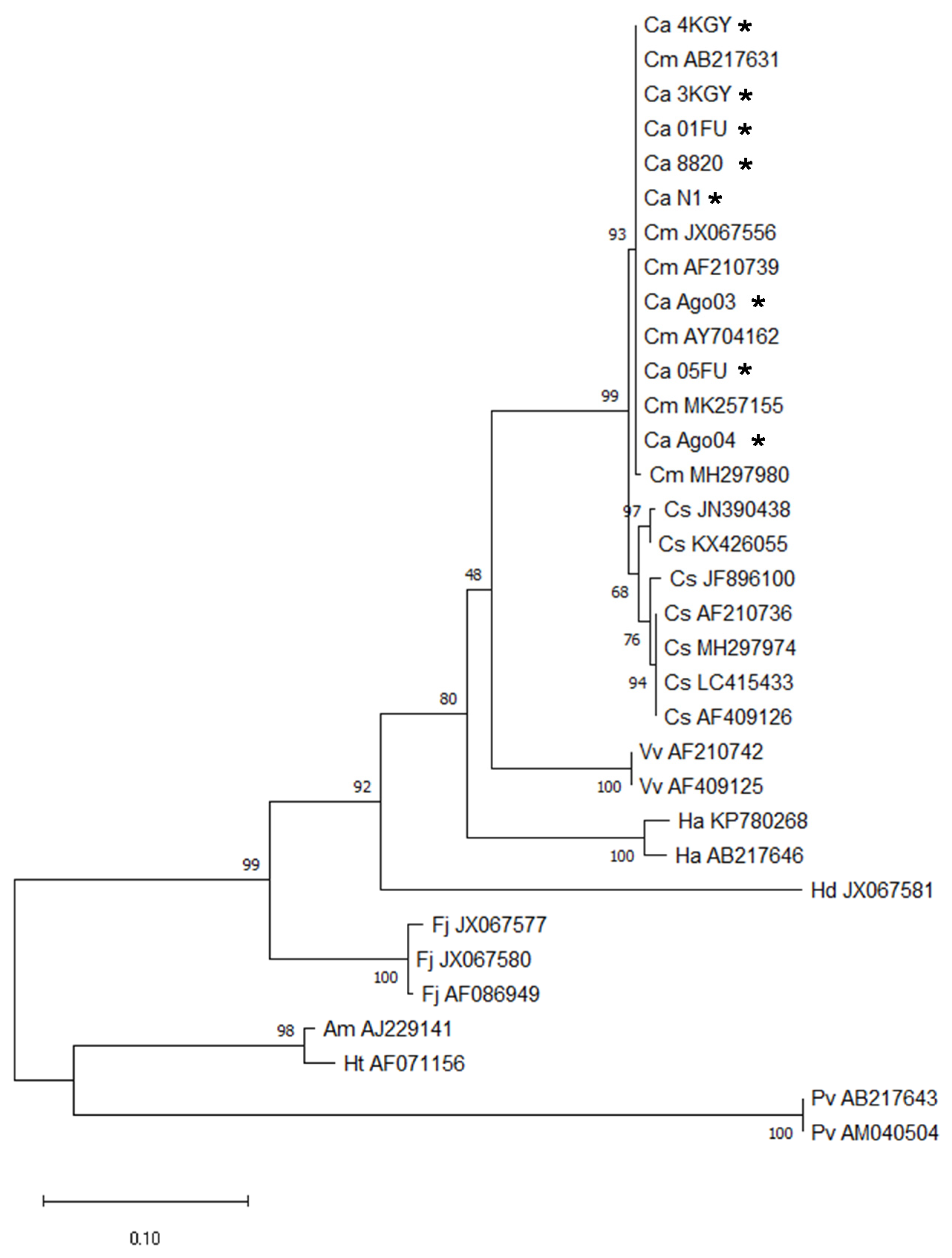
2.1. Chattonella Strains
2.2. Experimental Fish
2.3. Toxicity Bioassays
2.4. Cell Size Measurements
2.5. O2•− Detection
2.6. FA Analysis
2.7. Analysis of Sugar Content and Composition of Monosaccharides
2.8. Statistical Analyses
3. Results
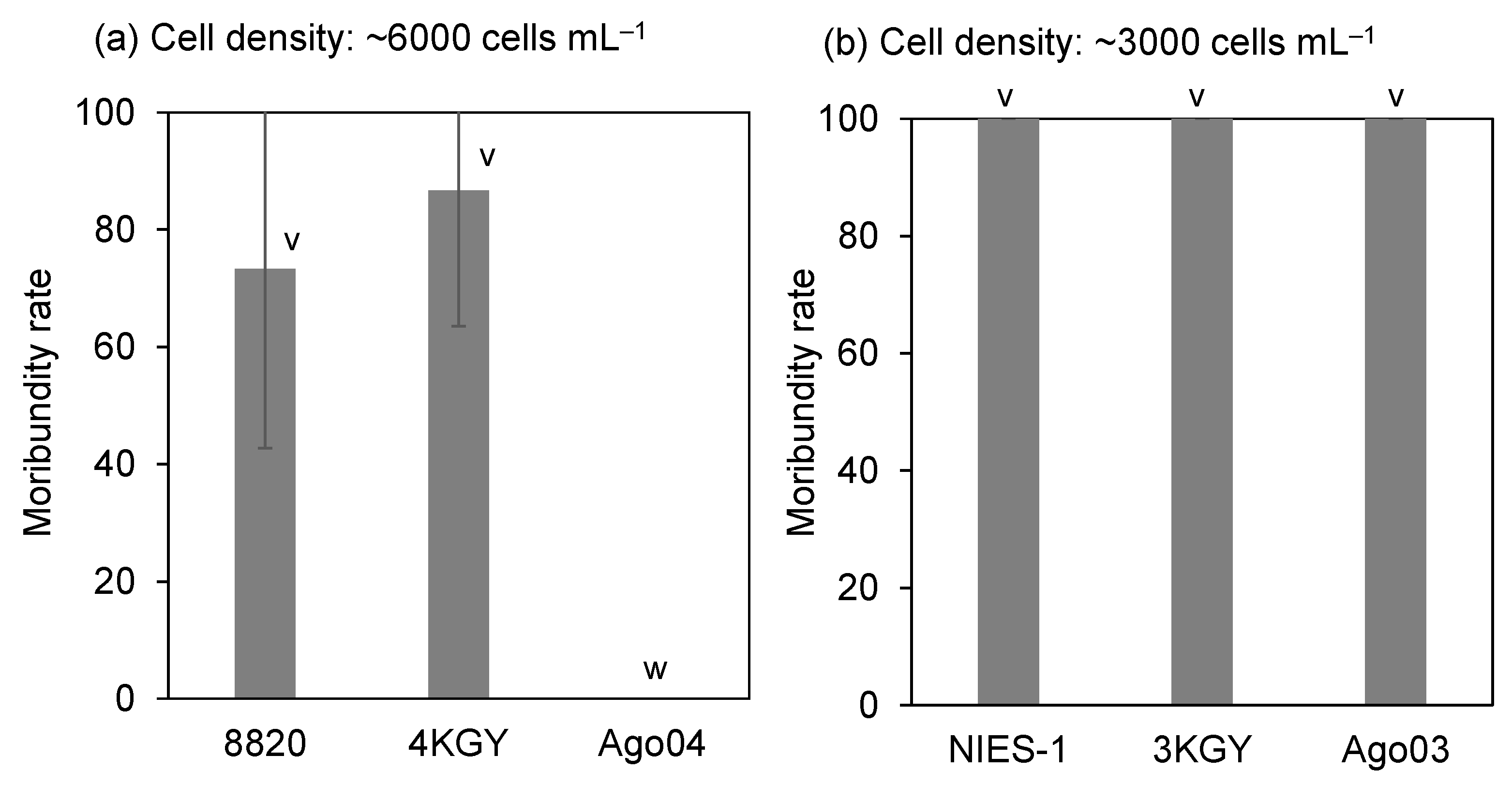
3.1. Ichthyotoxicity
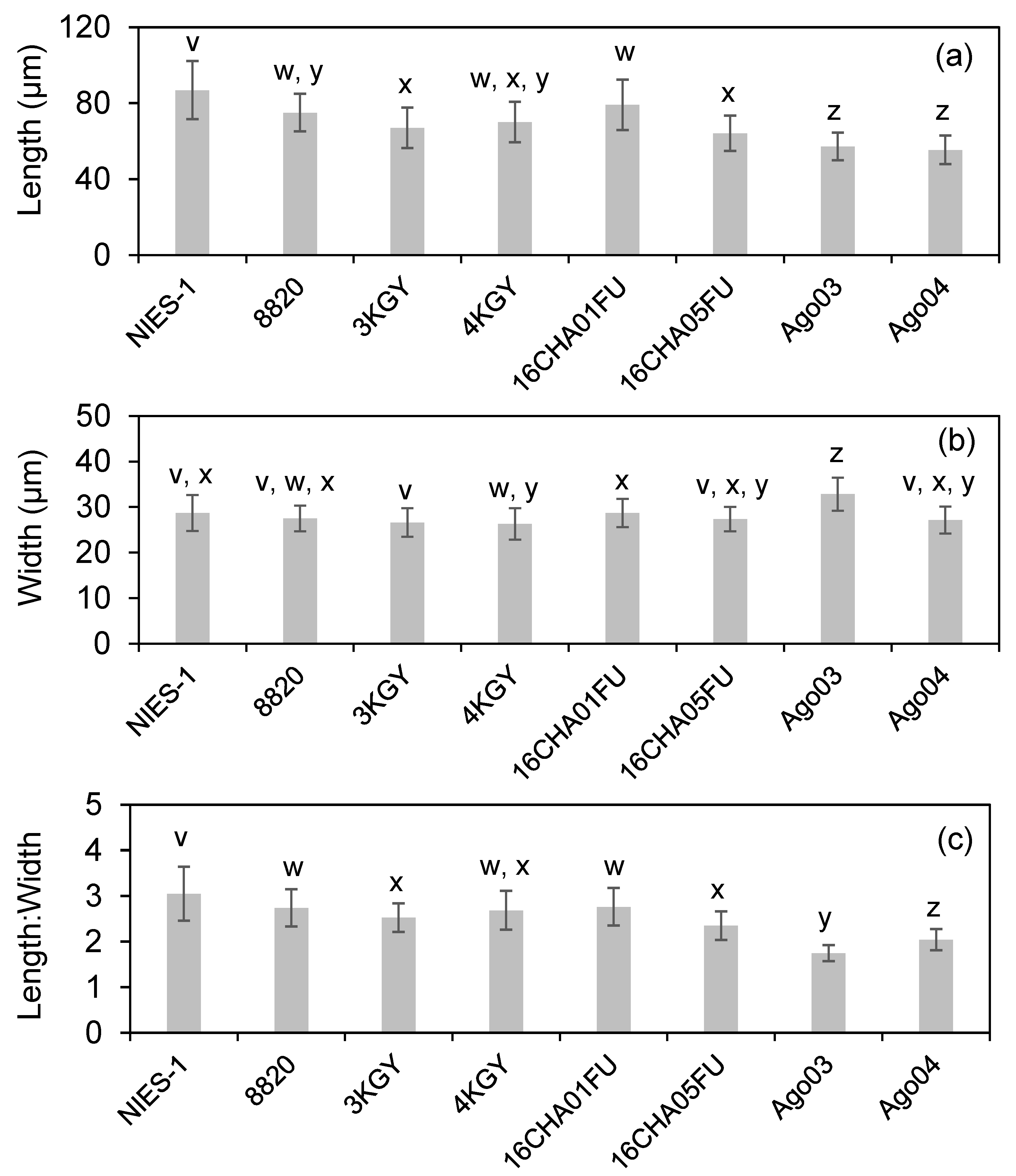
3.2. Cell Size
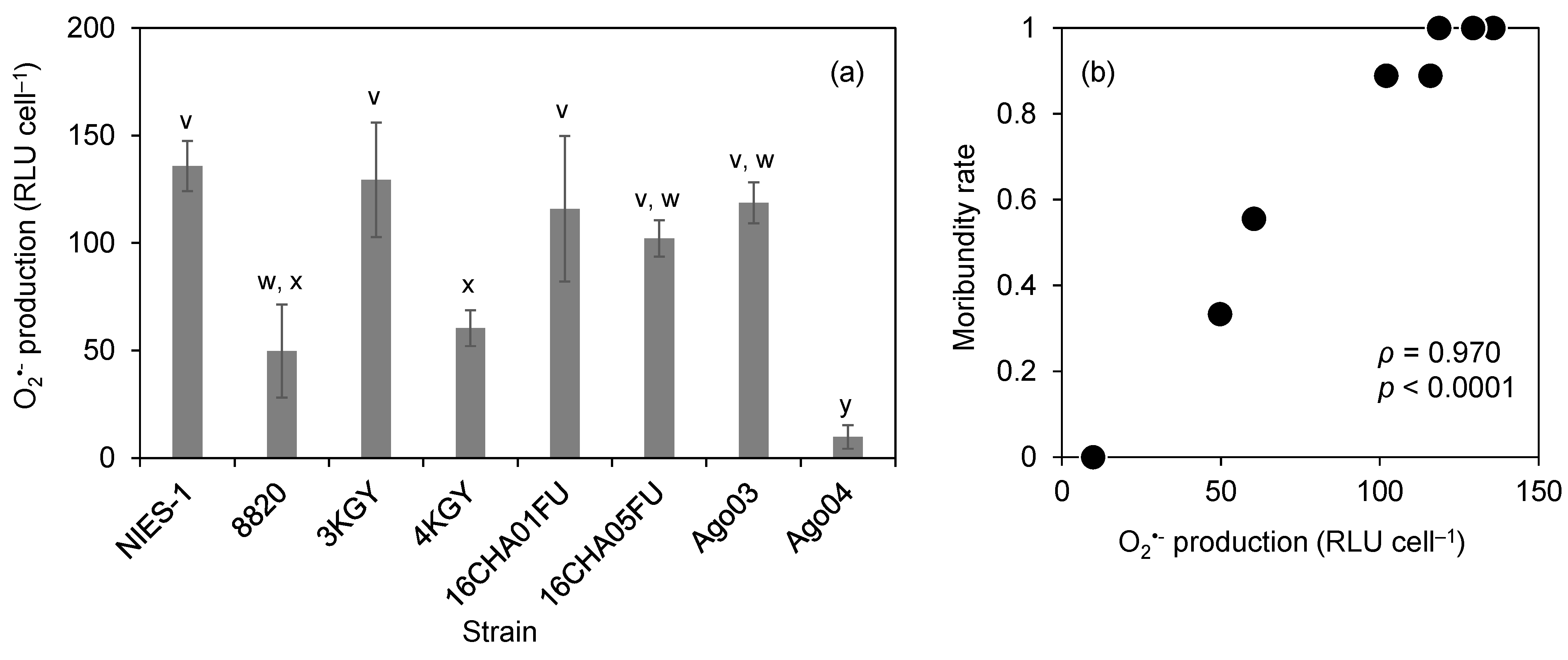
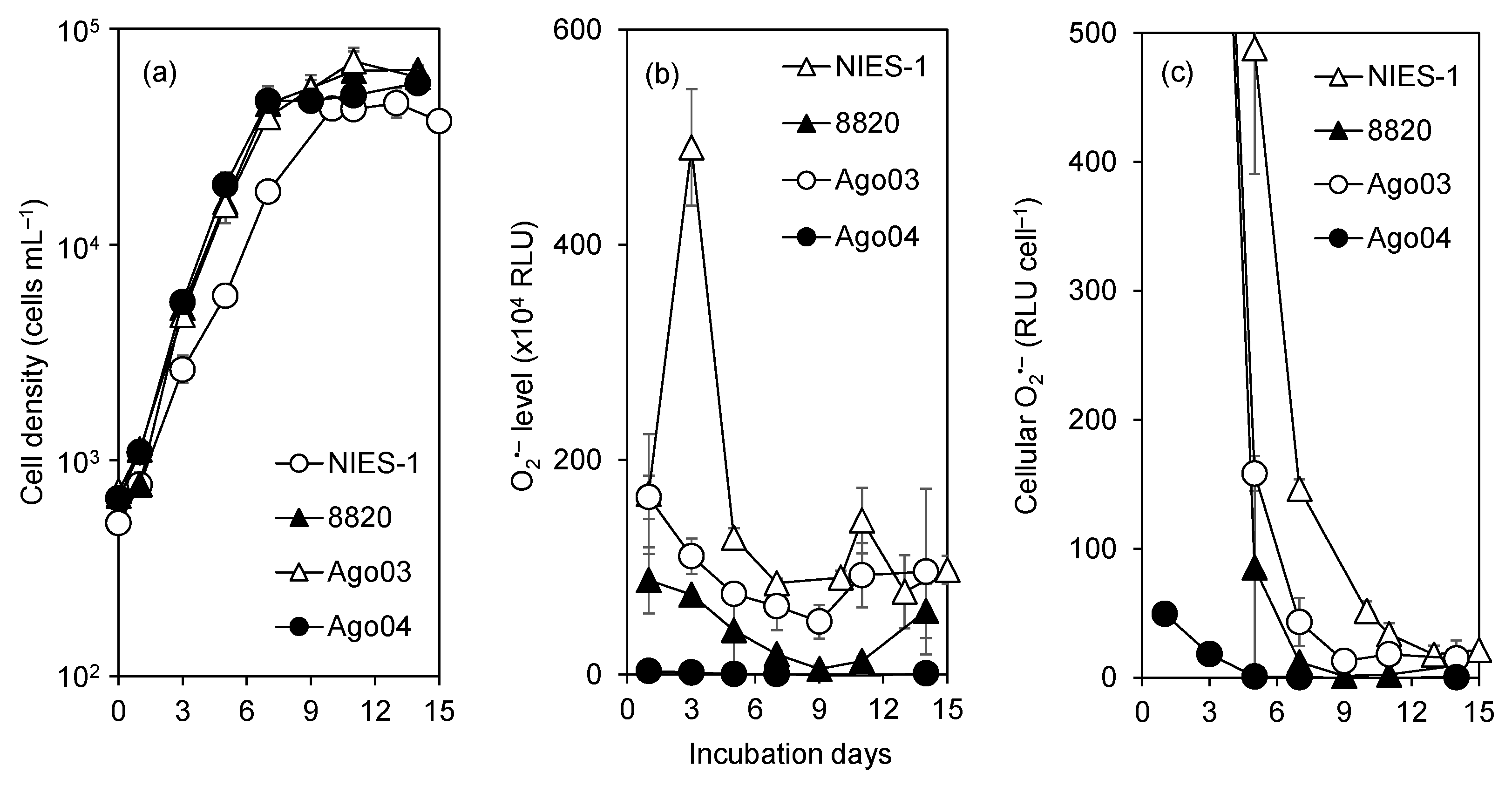
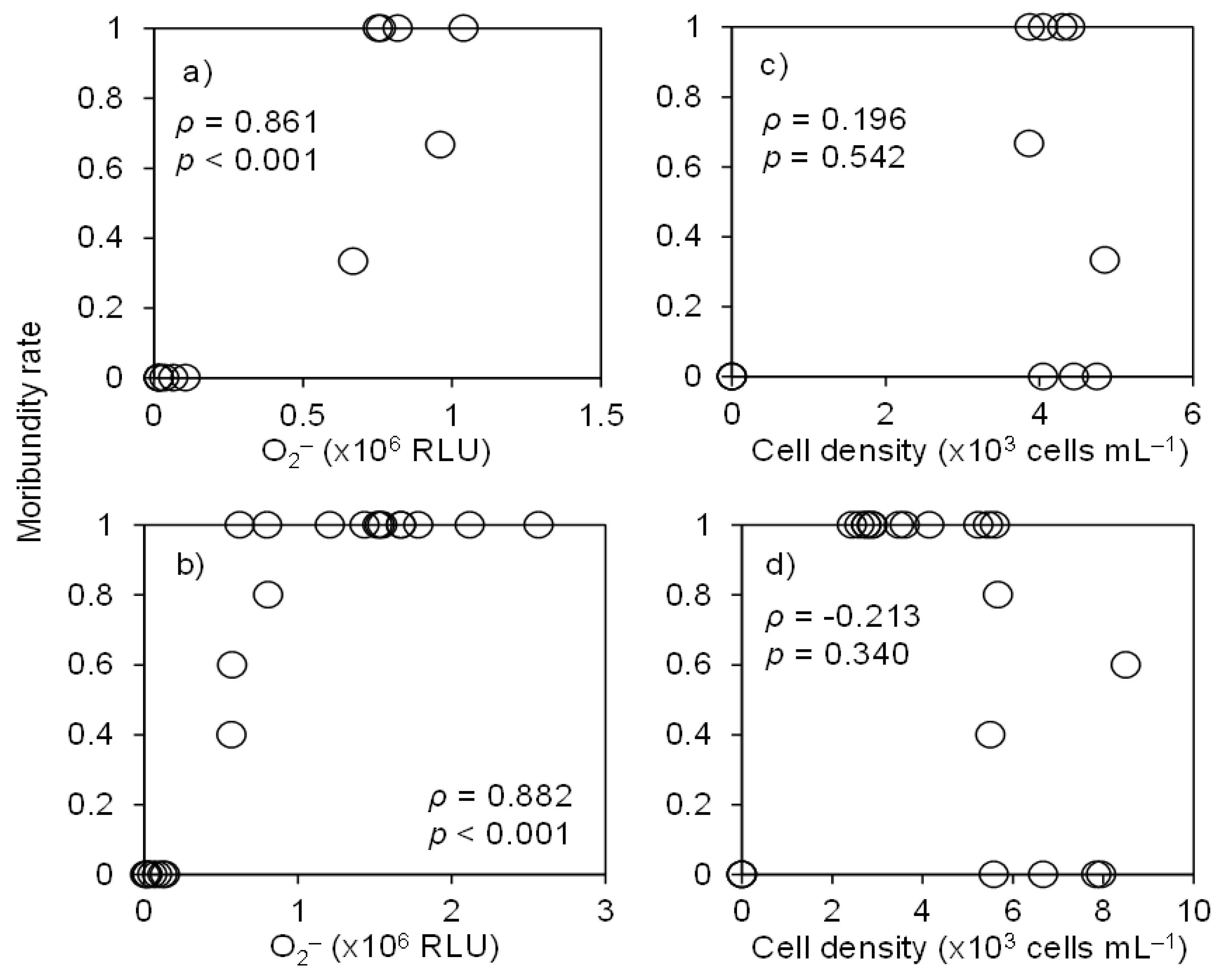
3.3. O2•− Production
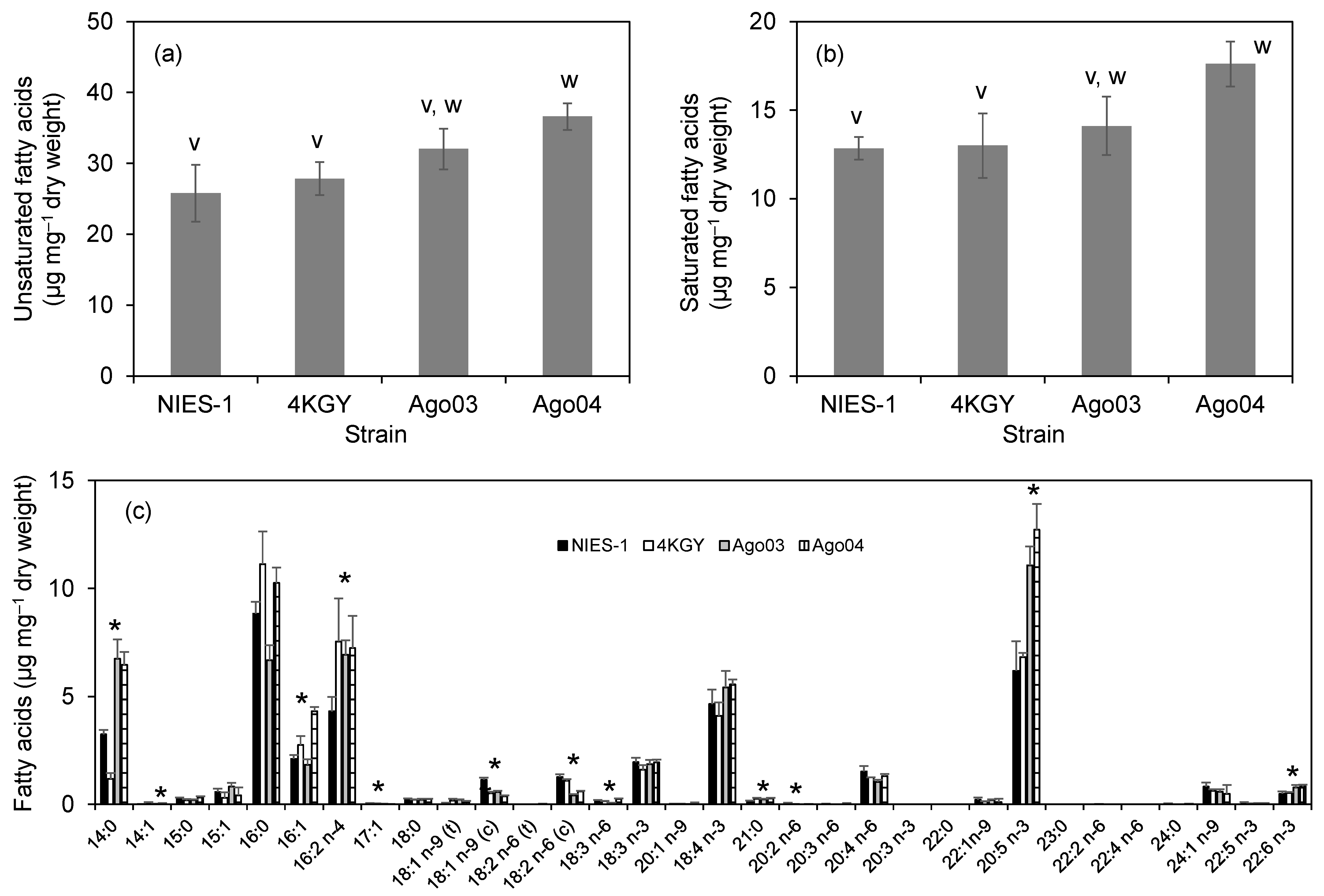
3.4. Fatty Acid Composition
3.5. Sugar Content and Composition of Monosaccharides
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Anderson, D.M. Turning back the harmful red tide. Nature 1997, 388, 513–514. [Google Scholar] [CrossRef]
- Imai, I.; Yamaguchi, M. Life cycle, physiology, ecology and red tide occurrences of the fish-killing raphidophyte Chattonella. Harmful Algae 2012, 14, 46–70. [Google Scholar] [CrossRef]
- Lum, W.M.; Benico, G.; Doan-Nhu, H.; Furio, E.; Leaw, C.P.; Leong, S.C.Y.; Lim, P.T.; Lim, W.A.; Lirdwitayaprasit, T.; Lu, S.; et al. The harmful raphidophyte Chattonella (Raphidophyceae) in Western Pacific: Its red tides and associated fisheries damage over the past 50 years (1969–2019). Harmful Algae 2021, 107, 102070. [Google Scholar] [CrossRef] [PubMed]
- Hara, Y. Raphidophyceae. In Red Tide Organisms in Japan—An Illustrated Taxonomic Guide; Fukuyo, Y., Takano, H., Chihara, M., Matsuoka, K., Eds.; Uchida Rokakuho: Tokyo, Japan, 1995; pp. 332–349. [Google Scholar]
- Hallegraeff, G.M.; Hara, Y. Taxonomy of harmful marine raphidophytes. In Manual on Harmful Marine Microalgae; Hallegraeff, G.M., Anderson, D.M., Cembella, A.D., Enevoldsen, H.O., Eds.; UNESCO: Paris, France, 2004; pp. 511–522. [Google Scholar]
- Kamikawa, R.; Masuda, I.; Oyama, K.; Yoshimatsu, S.; Sako, Y. Genetic variation in mitochondrial genes and intergenic spacer region in harmful algae Chattonella species. Fish. Sci. 2007, 73, 871–880. [Google Scholar] [CrossRef]
- Oyama, K.; Yoshimatsu, S. The changes in the size and form of Chattonella antiqua and Chattonella marina by investigating cultured strains over a long period. Bull. Akashiwo Res. Inst. Kagawa Pref. 2007, 6, 13–19, (In Japanese with English Abstract). [Google Scholar]
- Demura, M.; Noël, M.H.; Kasai, F.; Watanabe, M.M.; Kawachi, M. Taxonomic revision of Chattonella antiqua, C. marina and C. ovata (Raphidophyceae) based on their morphological characteristics and genetic diversity. Phycologia 2009, 48, 518–535. [Google Scholar] [CrossRef]
- Matsuyama, Y.; Oda, T. Toxic effects of harmful algal blooms on finfish and shellfish. In Handbook of Algal Science, Technology and Medicine; Konur, O., Ed.; Elsevier B.V.: Amsterdam, The Netherlands, 2020; pp. 543–560. [Google Scholar]
- Hishida, Y. Elucidation of Some Factors in Fish Death by Chattonella. Ph.D. Thesis, Nagasaki University, Nagasaki, Japan, 1999. (In Japanese with English Abstract). [Google Scholar]
- Matsusato, T.; Kobayashi, H. Studies on death of fish caused by red tide. Bull. Nansei Reg. Fish. Res. Lab. 1974, 7, 43–67, (In Japanese with English Abstract). [Google Scholar]
- Ishimatsu, A.; Sameshima, M.; Tamura, A.; Oda, T. Histological analysis of the mechanisms of Chattonella-induced hypoxemia in yellowtail. Fish. Sci. 1996, 62, 50–58. [Google Scholar] [CrossRef] [Green Version]
- Shen, M.; Xu, J.; Tsang, T.Y.; Au, D.W. Toxicity comparison between Chattonella marina and Karenia brevis using marine medaka (Oryzias melastigma): Evidence against the suspected ichthyotoxins of Chattonella marina. Chemosphere 2010, 80, 585–591. [Google Scholar] [CrossRef]
- Dorantes-Aranda, J.J.; Nichols, P.D.; David Waite, T.; Hallegraeff, G.M. Strain variability in fatty acid composition of Chattonella marina (Raphidophyceae) and its relation to differing ichthyotoxicity toward rainbow trout gill cells. J. Phycol. 2013, 49, 427–438. [Google Scholar] [CrossRef]
- Dorantes-Aranda, J.J.; Seger, A.; Mardones, J.I.; Nichols, P.D.; Hallegraeff, G.M. Progress in understanding algal bloom-mediated fish kills: The role of superoxide radicals, phycotoxins and fatty acids. PLoS ONE 2015, 10, e0133549. [Google Scholar] [CrossRef]
- Chang, F.H. Cytotoxic effects of Vicicitus globosus (Class Dictyochophyceae) and Chattonella marina (Class Raphidophyceae) on rotifers and other microalgae. J. Mar. Sci. Eng. 2015, 3, 401–411. [Google Scholar] [CrossRef]
- Kuroda, A.; Nakashima, T.; Yamaguchi, K.; Oda, T. Isolation and characterization of light-dependent hemolytic cytotoxin from harmful red tide phytoplankton Chattonella marina. Comp. Biochem. Physiol. Part C Toxicol. Pharmacol. 2005, 141, 297–305. [Google Scholar] [CrossRef]
- Wu, N.; Tong, M.; Gou, S.; Zeng, W.; Xu, Z.; Jiang, T. Hemolytic activity in relation to the photosynthetic system in Chattonella marina and Chattonella ovata. Mar. Drugs 2021, 19, 336. [Google Scholar] [CrossRef]
- Cho, K.; Kasaoka, T.; Ueno, M.; Basti, L.; Yamasaki, Y.; Kim, D.; Oda, T. Haemolytic activity and reactive oxygen species production of four harmful algal bloom species. Eur. J. Phycol. 2017, 52, 311–319. [Google Scholar] [CrossRef]
- Endo, M.; Onoue, Y.; Kuroki, A. Neurotoxin-induced cardiac disorder and its role in the death of fish exposed to Chattonella marina. Mar. Biol. 1992, 112, 371–376. [Google Scholar] [CrossRef]
- Khan, S.; Arakawa, O.; Onoue, Y. A toxicological study of the marine phytoflagellate, Chattonella antiqua (Raphidophyceae). Phycologia 1996, 35, 239–244. [Google Scholar] [CrossRef]
- Haque, S.M.; Onoue, Y. Variation in toxin compositions of two harmful raphidophytes, Chattonella antiqua and Chattonella marina, at different salinities. Environ. Toxicol. 2002, 17, 113–118. [Google Scholar] [CrossRef] [PubMed]
- Marshall, J.A.; Nichols, P.D.; Hamilton, B.; Lewis, R.J.; Hallegraeff, G.M. Ichthyotoxicity of Chattonella marina (Raphidophyceae) to damselfish (Acanthochromis polycanthus): The synergistic role of reactive oxygen species and free fatty acids. Harmful Algae 2003, 2, 273–281. [Google Scholar] [CrossRef]
- Tanaka, K.; Yoshimatsu, S.; Shimada, M. Generation of superoxide anions by Chattonella antiqua: Possible causes for fish death caused by ‘Red Tide’. Experientia 1992, 48, 888–890. [Google Scholar] [CrossRef]
- Oda, T.; Nakamura, A.; Shikayama, M.; Kawano, I.; Ishimatsu, A.; Muramatsu, T. Generation of reactive oxygen species by raphidophycean phytoplankton. Biosci. Biotech. Biochem. 1997, 61, 1658–1662. [Google Scholar] [CrossRef]
- Marshall, J.A.; de Salas, M.; Oda, T.; Hallegraeff, G. Superoxide production by marine microalgae. I. Survey of 37 species from 6 classes. Mar. Biol. 2005, 147, 533–540. [Google Scholar] [CrossRef]
- Yuasa, K.; Shikata, T.; Kitatsuji, S.; Yamasaki, Y.; Nishiyama, Y. Extracellular secretion of superoxide is regulated by photosynthetic electron transport in the noxious red-tide-forming raphidophyte Chattonella antiqua. J. Photochem. Photobiol. B 2020, 205, 111839. [Google Scholar] [CrossRef]
- Shimasaki, Y.; Mukai, K.; Takai, Y.; Qiu, X.; Oshima, Y. Recent Progress in the Study of Peroxiredoxin in the Harmful Algal Bloom Species Chattonella marina. Antioxidants 2021, 10, 162. [Google Scholar] [CrossRef]
- Okaichi, T. The cause of fish mortality due to Chattonella antiqua. In Red-Tide Species and the Environmental Conditions; Okaichi, T., Honjo, T., Fukuyo, Y., Eds.; Terra Scientific Publishing Company: Tokyo, Japan; Kluwer Academic Publishers: London, UK; Boston, MA, USA, 2004; pp. 331–332. [Google Scholar]
- Yokote, M.; Honjo, T. Morphological and histochemical demonstration of a glycocalyx on the cell surface of Chattonella antiqua, a ‘naked flagellate’. Experientia 1985, 41, 1143–1145. [Google Scholar] [CrossRef]
- Jenkinson, I.R.; Shikata, T.; Honjo, T. Modified ichthyoviscometer shows high viscosity in Chattonella culture. Harmful Algae News 2007, 35, 2–5. [Google Scholar]
- Kim, D.; Okamoto, T.; Oda, T.; Tachibana, K.; Lee, K.S.; Ishimatsu, A.; Matsuyama, Y.; Honjo, T.; Muramatsu, T. Possible involvement of the glycocalyx in the ichthyotoxicity of Chattonella marina (Raphidophyceae): Immunological approach using antiserum against cell surface structures of the flagellate. Mar. Biol. 2001, 139, 625–632. [Google Scholar]
- Cho, K.; Sakamoto, J.; Noda, T.; Nishiguchi, T.; Ueno, M.; Yamasaki, Y.; Yagi, M.; Kim, D.; Oda, T. Comparative studies on the fish-killing activities of Chattonella marina isolated in 1985 and Chattonella antiqua isolated in 2010, and their possible toxic factors. Biosci. Biotech. Biochem. 2016, 80, 811–817. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shikata, T.; Taniguchi, E.; Sakamoto, S.; Kitatsuji, S.; Yamasaki, Y.; Yoshida, M.; Oikawa, H. Phylogeny, growth and toxicity of the noxious red-tide dinoflagellate Alexandrium leei in Japan. Reg. Stud. Mar. Sci. 2020, 36, 101265. [Google Scholar] [CrossRef]
- Shikata, T.; Sakurada, K.; Jomoto, Y.; Oyama, N.; Onji, M.; Yoshida, M.; Ohwada, K. Growth dynamics of Chattonella antiqua in relation to nutrients in the Yatsushiro Sea. Nippon Suisan Gakk. 2011, 77, 40–52, (In Japanese with English Abstract). [Google Scholar] [CrossRef]
- Thomas, W.H.; Gibson, C.H. Quantified small-scale turbulence inhibits a red tide dinoflagellate, Gonyaulax polyedra Stein. Deep Sea Res. Part A 1990, 37, 1583–1593. [Google Scholar] [CrossRef]
- Takayama, H. Gyorui ni oyobosu akashio no eikyou ni tsuite. Aquiculture 1976, 23, 115–118. (In Japanese) [Google Scholar]
- Yuasa, K.; Shikata, T.; Ichikawa, T.; Tamura, Y.; Nishiyama, Y. Nutrient deficiency stimulates the production of superoxide in the noxious red-tide-forming raphidophyte Chattonella antiqua. Harmful Algae 2020, 99, 101938. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, R.; Uchida, K.; Ishimaru, M.; Ito, M.; Matsumoto, N.; Taoka, Y.; Hatate, H. Effect of seawater reared on the nutritional composition and antioxidant activity of edible muscle in smoltified landlocked masu salmon (Oncorhynchus masou masou). J. Food Meas. Charact. 2018, 12, 200–208. [Google Scholar] [CrossRef]
- Mckelvy, J.; Lee, Y.C. Microheterogeneity of the carbohydrate group of Aspergillus oryzae α-amylase. Arch. Biochem. Biophys. 1969, 132, 99–110. [Google Scholar] [CrossRef]
- Ishikawa, M.; Kuroyama, H.; Takeuchi, Y.; Tsumuraya, Y. Characterization of pectin methyltransferase from soybean hypocotyls. Planta 2000, 210, 782–791. [Google Scholar] [CrossRef]
- Tang, J.Y.; Anderson, D.M.; Au, D.W. Hydrogen peroxide is not the cause of fish kills associated with Chattonella marina: Cytological and physiological evidence. Aquat. Toxicol. 2005, 72, 351–360. [Google Scholar] [CrossRef]
- Kim, D.; Nakamura, A.; Okamoto, T.; Komatsu, N.; Oda, T.; Iida, T.; Ishimatsu, A.; Muramatsu, T. Mechanism of superoxide anion generation in the toxic red tide phytoplankton Chattonella marina: Possible involvement of NAD(P)H oxidase. Biochim. Biophys. Acta (BBA)-Gen. Subj. 2000, 1524, 220–227. [Google Scholar] [CrossRef]
- Nakamura, A.; Okamoto, T.; Komatsu, N.; Ooka, S.; Oda, T.; Ishimatsu, A.; Muramatsu, T. Fish mucus stimulates the generation of superoxide anion by Chattonella marina and Heterosigma akashiwo. Fish. Sci. 1998, 64, 866–869. [Google Scholar] [CrossRef] [Green Version]
- Marshall, J.A.; Nichols, P.D.; Hallegraeff, G.M. Chemotaxonomic survey of sterols and fatty acids in six marine raphidophyte algae. J. Appl. Phycol. 2002, 14, 255–265. [Google Scholar] [CrossRef]
- Kaczmarzyk, D.; Fulda, M. Fatty acid activation in cyanobacteria mediated by acyl-acyl carrier protein synthetase enables fatty acid recycling. Plant Physiol. 2010, 152, 1598–1610. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Terasaki, M.; Itabashi, Y. Free fatty acid level and galactolipase activity in a red tide flagellate Chattonella marina (Raphidophyceae). J. Oleo Sci. 2002, 51, 213–218. [Google Scholar] [CrossRef]
- Aquino-Cruz, A.; Band-Schmidt, C.J.; Zenteno-Savín, T. Superoxide production rates and hemolytic activity linked to cellular growth phases in Chattonella species (Raphidophyceae) and Margalefidinium polykrikoides (Dinophyceae). J. Appl. Phycol. 2020, 32, 4029–4046. [Google Scholar] [CrossRef]


| Strain Name | Date Collected | Location | Contamination Status |
|---|---|---|---|
| NIES-1 | September 1978 | Harima-Nada | Axenic |
| 8820 | 20 August 2008 | Yatsushiro Sea | Xenic |
| 3KGY | 3 June 2010 | Yatsushiro Sea | Xenic |
| 4KGY | 3 June 2010 | Yatsushiro Sea | Axenic |
| 16CHA01FU | 6 July 2016 | Seto Inland Sea | Xenic |
| 16CHA05FU | 6 July 2016 | Seto Inland Sea | Xenic |
| Ago03 | 9 July 2013 | Ago Bay | Xenic |
| Ago04 | 9 July 2013 | Ago Bay | Xenic |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shikata, T.; Yuasa, K.; Kitatsuji, S.; Sakamoto, S.; Akita, K.; Fujinami, Y.; Nishiyama, Y.; Kotake, T.; Tanaka, R.; Yamasaki, Y. Superoxide Production by the Red Tide-Producing Chattonella marina Complex (Raphidophyceae) Correlates with Toxicity to Aquacultured Fishes. Antioxidants 2021, 10, 1635. https://doi.org/10.3390/antiox10101635
Shikata T, Yuasa K, Kitatsuji S, Sakamoto S, Akita K, Fujinami Y, Nishiyama Y, Kotake T, Tanaka R, Yamasaki Y. Superoxide Production by the Red Tide-Producing Chattonella marina Complex (Raphidophyceae) Correlates with Toxicity to Aquacultured Fishes. Antioxidants. 2021; 10(10):1635. https://doi.org/10.3390/antiox10101635
Chicago/Turabian StyleShikata, Tomoyuki, Koki Yuasa, Saho Kitatsuji, Setsuko Sakamoto, Kazuki Akita, Yuichiro Fujinami, Yoshitaka Nishiyama, Toshihisa Kotake, Ryusuke Tanaka, and Yasuhiro Yamasaki. 2021. "Superoxide Production by the Red Tide-Producing Chattonella marina Complex (Raphidophyceae) Correlates with Toxicity to Aquacultured Fishes" Antioxidants 10, no. 10: 1635. https://doi.org/10.3390/antiox10101635






