Neural Correlates of Amusia in Williams Syndrome
Abstract
:1. Introduction
2. Experimental Section
2.1. Participants
| Variable | Total Sample (n = 17) | Non-amusics (n = 13) | Amusics (n = 4) |
|---|---|---|---|
| Age (years) | 26.1 ± 9.1 (16–48) | 25.1 ± 10.0 (16–48) | 29.3 ± 4.9 (23–34) |
| Gender | 12 males, 5 females | 10 males, 3 females | 2 males, 2 females |
| Full Scale IQ | 70.3 ± 17.4 (43–93) | 74.4 ± 15.2 (46–93) | 57.0 ± 19.8 (43–86) |
| Handedness | 0.6 ± 0.7 (−1–+1) | 0.52 ± 0.75 (−1–+1) | 0.87 ± 0.26 (0.48–1) |
| DTT score | 20.9 ± 4.3 (12–26) | 22.8 ± 2.3 (20–26) | 14.5 ± 2.4 (12–17) |
| Cumulative years of private extra-curricular training | 9.2 ± 11.9 (0–46) | 10.2 ± 12.5 (0–46) | 5.9 ± 10.5 (0–21.5) |
| Number of lesson types | 3.4 ± 1.8 (0–6) | 3.9 ± 1.7 (0–6) | 1.8 ± 1.0 (1–3) |
| Time play music currently (hours) | 1.4 ± 1.7 (0–6) | 1.4 ± 1.6 (0–6) | 1.7 ± 2.2 (0–5) |
| Time listen music currently (hours) | 2.7 ± 2.1 (0.5–8) | 2.7 ± 2.2 (0.5–8) | 2.9 ± 2.3 (1–6) |
| Age began private music lessons (years) | 11.7 ± 3.2 (6–19 years; n = 14) | 11.6 ± 3.3 (6–19 years; n = 12) | 12.5 ± 3.5 (10–15 years; n = 2) |
| Sensitivity to specific (non-musical) sounds | 49.6 ± 21.1 (24–102) | 47.1 ± 21.0 (24–102) | 57.8 ± 22.2 (26–78) |
| Sensitivity to sound characteristics | 18.1 ± 6.3 (5–29) | 18.0 ± 7.0 (5–29) | 18.3 ± 4.6 (14–24) |
2.2. Neuroimaging Data Acquisition
2.3. Surface Reconstruction and Volumetric Calculation

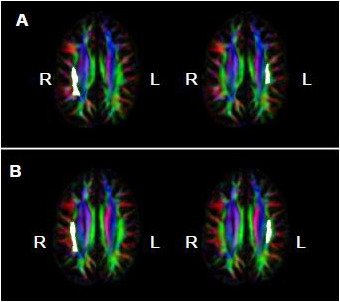
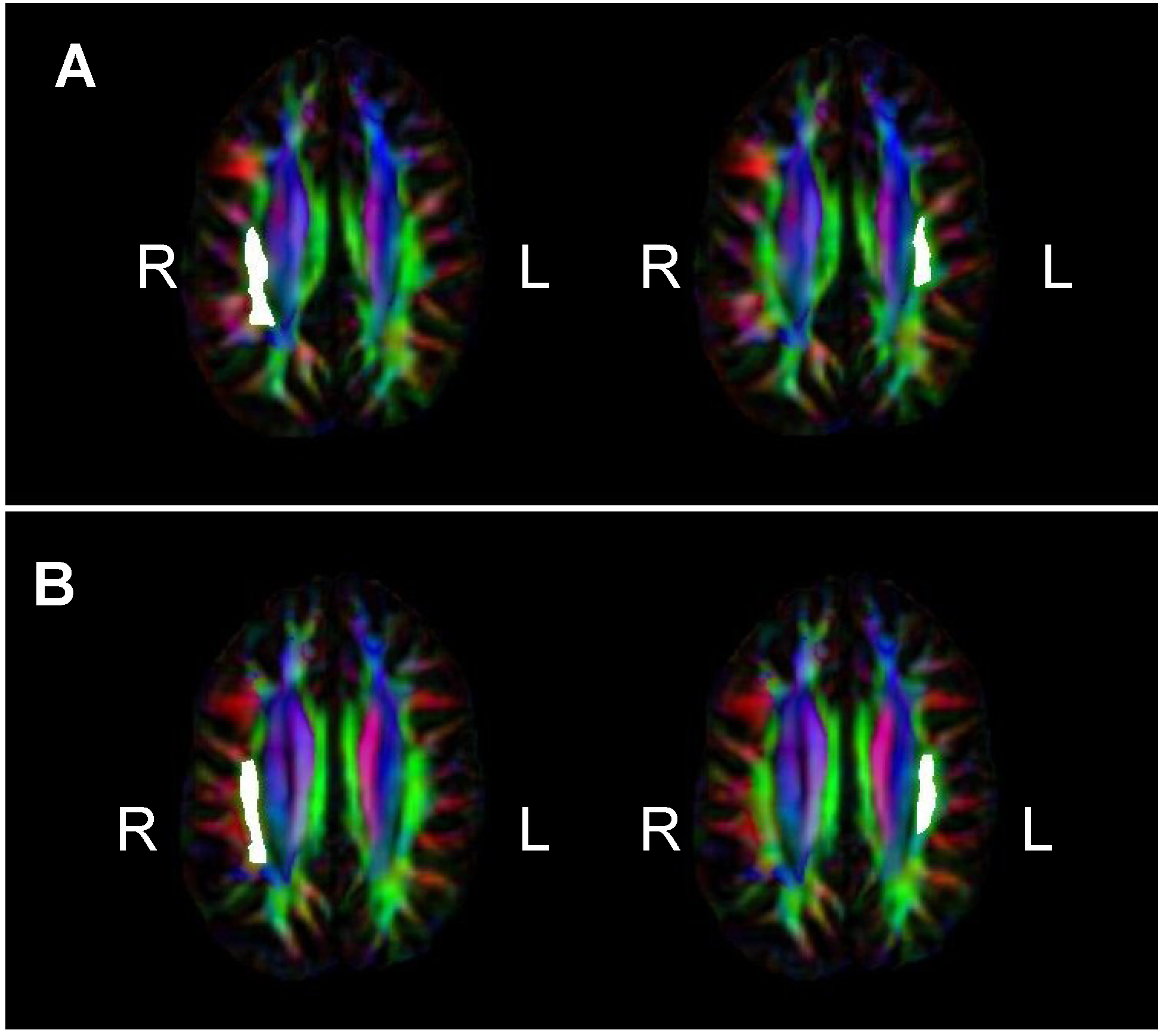
2.4. DTI Image Processing and Analysis
2.5. Statistical Analyses
3. Results
 = Amusia. (A) DTT scores and white matter volume of right pars orbitalis (r = 0.507, p = 0.038); (B) DTT scores and gray matter volume of right pars orbitalis (r = 0.479, p = 0.052); (C) DTT scores and FA of right inferior SLF (r = 0.694, p = 0.002); (D) DTT scores and FA of right superior SLF (r = 0.506, p = 0.038).
= Amusia. (A) DTT scores and white matter volume of right pars orbitalis (r = 0.507, p = 0.038); (B) DTT scores and gray matter volume of right pars orbitalis (r = 0.479, p = 0.052); (C) DTT scores and FA of right inferior SLF (r = 0.694, p = 0.002); (D) DTT scores and FA of right superior SLF (r = 0.506, p = 0.038).
 = Amusia. (A) DTT scores and white matter volume of right pars orbitalis (r = 0.507, p = 0.038); (B) DTT scores and gray matter volume of right pars orbitalis (r = 0.479, p = 0.052); (C) DTT scores and FA of right inferior SLF (r = 0.694, p = 0.002); (D) DTT scores and FA of right superior SLF (r = 0.506, p = 0.038).
= Amusia. (A) DTT scores and white matter volume of right pars orbitalis (r = 0.507, p = 0.038); (B) DTT scores and gray matter volume of right pars orbitalis (r = 0.479, p = 0.052); (C) DTT scores and FA of right inferior SLF (r = 0.694, p = 0.002); (D) DTT scores and FA of right superior SLF (r = 0.506, p = 0.038).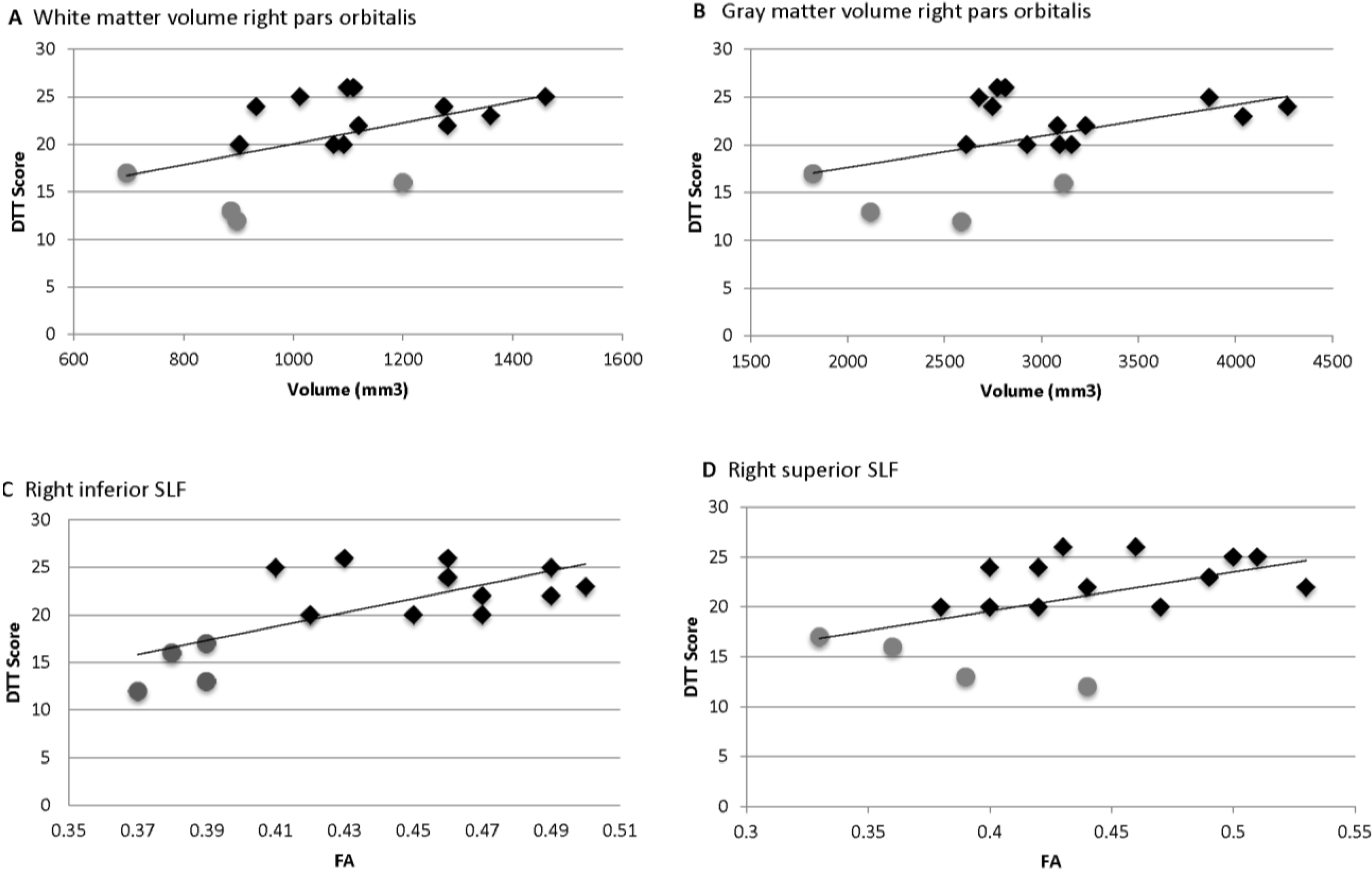
4. Discussion and Conclusions
Supplementary Files
Supplementary File 1Acknowledgments
Author Contributions
Conflicts of Interest
References
- Tramo, M.J. Biology and music. Music of the hemispheres. Science 2001, 291, 54–56. [Google Scholar] [CrossRef]
- Merrett, D.L.; Peretz, I.; Wilson, S.J. Moderating variables of music training-induced neuroplasticity: A review and discussion. Front. Psychol. 2013, 4, 606. [Google Scholar] [CrossRef]
- Peretz, I. The biological foundations of music: Insights from congenital amusia. In The Psychology of Music, 3rd ed.; Deutsch, D., Ed.; Elsevier Inc.: Amsterdam, The Netherlands, 2013; pp. 551–564. [Google Scholar]
- Henry, M.J.; McAuley, J.D. On the prevalence of congenital amusia. Music Percept. 2010, 27, 413–418. [Google Scholar] [CrossRef]
- Foxton, J.M.; Dean, J.L.; Gee, R.; Peretz, I.; Griffiths, T.D. Characterization of deficits in pitch perception underlying “tone deafness”. Brain J. Neurol. 2004, 127, 801–810. [Google Scholar] [CrossRef]
- Drayna, D.; Manichaikul, A.; de Lange, M.; Snieder, H.; Spector, T. Genetic correlates of musical pitch recognition in humans. Science 2001, 291, 1969–1972. [Google Scholar] [CrossRef] [PubMed]
- Hyde, K.L.; Zatorre, R.J.; Griffiths, T.D.; Lerch, J.P.; Peretz, I. Morphometry of the amusic brain: A two-site study. Brain 2006, 129, 2562–2570. [Google Scholar] [CrossRef] [PubMed]
- Loui, P.; Alsop, D.; Schlaug, G. Tone deafness: A new disconnection syndrome? J. Neurosci. 2009, 19, 10215–10220. [Google Scholar] [CrossRef]
- Hyde, K.L.; Zatorre, R.J.; Peretz, I. Functional MRI evidence of an abnormal neural network for pitch processing in congenital amusia. Cereb. Cortex 2011, 21, 292–299. [Google Scholar] [CrossRef] [PubMed]
- Albouy, P.; Mattout, J.; Bouet, R.; Maby, E.; Sanchez, G.; Aquera, P.E.; Daliqault, S.; Delpuech, C.; Bertrand, O.; Caclin, A.; et al. Impaired pitch perception and memory in congenital amusia: The deficit starts in the auditory cortex. Brain 2013, 136, 1639–1661. [Google Scholar] [CrossRef] [PubMed]
- Särkämö, T.; Tervaniemi, M.; Soinila, S.; Autti, T.; Silvennoinen, H.M.; Laine, M.; Hietanen, M. Cognitive deficits associated with acquired amusia after stroke: A neuropsychological follow-up study. Neuropsychologia 2009, 47, 2642–2651. [Google Scholar] [CrossRef] [PubMed]
- Särkämö, T.; Tervaniemi, M.; Soinila, S.; Autti, T.; Silvennoinen, H.M.; Laine, M.; Hietanen, M.; Pihko, E. Auditory and cognitive deficits associated with acquired amusia after stroke: A magnetoencephalography and neuropsychological follow-up study. PLoS One 2010, 5, e15157. [Google Scholar] [CrossRef] [PubMed]
- Mignault Goulet, G.; Moreau, P.; Robitaille, N.; Peretz, I. Congenital amusia persists in the developing brain after daily music listening. PLoS One 2012, 7, e36860. [Google Scholar] [CrossRef] [PubMed]
- Peretz, I.; Cummings, S.; Dubé, M.-P. The genetics of congenital amusia (tone deafness): A family-aggregation study. Am. J. Hum. Genet. 2007, 81, 582–588. [Google Scholar] [CrossRef] [PubMed]
- Peretz, I.; Brattico, E.; Järvenpää, M.; Tervaniemi, M. The amusic brain: In tune, out of key, and unaware. Brain J. Neurol. 2009, 132, 1277–1286. [Google Scholar] [CrossRef]
- Moreau, P.; Jolicœur, P.; Peretz, I. Pitch discrimination without awareness in congenital amusia: Evidence from event-related potentials. Brain Cogn. 2013, 81, 337–344. [Google Scholar] [CrossRef] [PubMed]
- Lense, M.D.; Shivers, C.M.; Dykens, E.M. (A)musicality in Williams syndrome: Examining relationships among auditory perception, musical skill, and emotional responsiveness to music. Front. Psychol. 2013, 4, 525. [Google Scholar] [CrossRef] [PubMed]
- Ewart, A.K.; Morris, C.A.; Atkinson, D.; Jin, W.; Sternes, K.; Spallone, P.; Stock, A.D.; Leppert, M.; Keating, M.T. Hemizygosity at the elastin locus in a developmental disorder, Williams syndrome. Nat. Genet. 1993, 5, 11–16. [Google Scholar] [CrossRef] [PubMed]
- Martens, M.A.; Wilson, S.J.; Reutens, D.C. Research Review: Williams syndrome: A critical review of the cognitive, behavioral, and neuroanatomical phenotype. J. Child Psychol. Psychiatry 2008, 49, 576–608. [Google Scholar] [CrossRef] [PubMed]
- Levitin, D.J.; Cole, K.; Lincoln, A.; Bellugi, U. Aversion, awareness, and attraction: Investigating claims of hyperacusis in the Williams syndrome phenotype. J. Child Psychol. Psychiatry 2005, 46, 514–523. [Google Scholar] [CrossRef] [PubMed]
- Levitin, D.J.; Cole, K.; Chiles, M.; Lai, Z.; Lincoln, A.; Bellugi, U. Characterizing the musical phenotype in individuals with Williams Syndrome. Child Neuropsychol. 2004, 10, 223–247. [Google Scholar] [CrossRef] [PubMed]
- Dykens, E.M.; Rosner, B.A.; Ly, T.; Sagun, J. Music and anxiety in Williams syndrome: A harmonious or discordant relationship? Am. J. Ment. Retard. 2005, 110, 346–358. [Google Scholar] [CrossRef] [PubMed]
- Reiss, A.L.; Eliez, S.; Schmitt, J.E.; Straus, E.; Lai, Z.; Jones, W.; Bellugi, U. IV. Neuroanatomy of Williams syndrome: A high-resolution MRI study. J. Cogn. Neurosci. 2000, 12 (Suppl. 1), 65–73. [Google Scholar] [CrossRef]
- Martens, M.A.; Reutens, D.C.; Wilson, S.J. Auditory cortical volumes and musical ability in Williams syndrome. Neuropsychologia 2010, 48, 2602–2609. [Google Scholar] [CrossRef] [PubMed]
- Wengenroth, M.; Blatow, M.; Bendszus, M.; Schneider, P. Leftward lateralization of auditory cortex underlies holistic sound perception in Williams syndrome. PLoS One 2010, 5, e12326. [Google Scholar] [CrossRef] [PubMed]
- Holinger, D.P.; Bellugi, U.; Mills, D.L.; Korenberg, J.R.; Reiss, A.L.; Jones, W.; Bellugi, U. Relative sparing of primary auditory cortex in Williams Syndrome. Brain Res. 2005, 1037, 35–42. [Google Scholar] [CrossRef] [PubMed]
- Sampaio, A.; Sousa, N.; Férnandez, M.; Vasconcelos, C.; Shenton, M.E.; Goncalves, O.F. MRI assessment of superior temporal gyrus in Williams syndrome. Cogn. Behav. Neurol. 2008, 21, 150–156. [Google Scholar] [CrossRef] [PubMed]
- Thompson, P.M.; Lee, A.D.; Dutton, R.A.; Geaga, J.A.; Hayashi, K.M.; Eckert, M.A.; Bellugi, U.; Galaburda, A.M.; Korenberg, J.R.; Mills, D.L.; et al. Abnormal cortical complexity and thickness profiles mapped in Williams Syndrome. J. Neurosci. 2005, 25, 4146–4158. [Google Scholar] [CrossRef] [PubMed]
- Marenco, S.; Siuta, M.A.; Kippenhan, J.S.; Grodofsky, S.; Chang, W.-L.; Kohn, P.; Mervis, C.B.; Morris, C.A.; Weinberger, D.R.; Meyer-Linderberg, A.; et al. Genetic contributions to white matter architecture revealed by diffusion tensor imaging in Williams syndrome. Proc. Natl. Acad. Sci. USA 2007, 104, 15117–15122. [Google Scholar] [CrossRef] [PubMed]
- Makris, N.; Kennedy, D.N.; McInerney, S.; Sorensen, A.G.; Wang, R.; Caviness, V.S., Jr.; Pandya, D.N. Segmentation of subcomponents within the superior longitudinal fascicle in humans: A quantitative, in vivo, DT-MRI study. Cereb. Cortex 2005, 5, 854–869. [Google Scholar] [CrossRef]
- Basser, P.J.; Pierpaoli, C. Microstructural and physiological features of tissues elucidated by quantitative-diffusion-tensor MRI. J. Magn. Reson. B 1996, 111, 209–219. [Google Scholar] [CrossRef] [PubMed]
- Muetzel, R.L.; Collins, P.F.; Mueller, B.A.; Schissel, A.; Lim, K.O.; Luciana, M. The development of corpus callosum microstructure and associations with bimanual task performance in healthy adolescents. NeuroImage 2008, 39, 1918–1925. [Google Scholar] [CrossRef] [PubMed]
- Hoeft, F.; Barnea-Goraly, N.; Haas, B.W.; Golarai, G.; Ng, D.; Mills, D.; Korenberg, J.; Bellugi, U.; Galaburda, A.; Reiss, A.L. More is not always better: Increased fractional anisotropy of superior longitudinal fasciculus associated with poor visuospatial abilities in Williams syndrome. J. Neurosci. 2007, 27, 11960–11965. [Google Scholar] [CrossRef] [PubMed]
- Faria, A.V.; Landau, B.; O’Hearn, K.M.; Li, X.; Jiang, H.; Oishi, K.; Zhang, J.; Mori, S. Quantitative analysis of gray and white matter in Williams syndrome. Neuroreport 2012, 23, 283–289. [Google Scholar] [CrossRef] [PubMed]
- Hyde, K.L.; Lerch, J.P.; Zatorre, R.J.; Griffiths, T.D.; Evans, A.C.; Peretz, I. Cortical thickness in congenital amusia: When less is better than more. J. Neurosci. 2007, 27, 13028–13032. [Google Scholar] [CrossRef] [PubMed]
- Mandell, J.; Schulze, K.; Schlaug, G. Congenital amusia: An auditory-motor feedback disorder? Restor. Neurol. Neurosci. 2007, 25, 323–334. [Google Scholar] [PubMed]
- Kaufman, A.; Kaufman, N. Kaufman Brief Intelligence Test, 2nd ed.; American Guidance Service: Circle Pines, MN, USA, 2004. [Google Scholar]
- Oldfield, R.C. The assessment and analysis of handedness: The Edinburgh inventory. Neuropsychologia 1971, 9, 97–113. [Google Scholar] [CrossRef] [PubMed]
- Lense, M.; Dykens, E. Musical learning in children and adults with Williams syndrome. J. Intellect. Disabil. Res. 2013, 57, 850–860. [Google Scholar] [CrossRef] [PubMed]
- Corrigall, K.A.; Schellenberg, E.G.; Misura, N.M. Music training, cognition, and personality. Front. Psychol. 2013, 4, 222. [Google Scholar] [CrossRef] [PubMed]
- Klein, A.J.; Armstrong, B.L.; Greer, M.K.; Brown, F.R., III. Hyperacusis and otitis media in individuals with Williams syndrome. J. Speech Hear. Disord. 1990, 55, 339–344. [Google Scholar] [CrossRef] [PubMed]
- Peretz, I.; Champod, A.S.; Hyde, K. Varieties of musical disorders. The Montreal Battery of Evaluation of Amusia. Ann. N. Y. Acad. Sci. 2003, 999, 58–75. [Google Scholar] [CrossRef] [PubMed]
- Ayotte, J.; Peretz, I.; Hyde, K. Congenital amusia: A group study of adults afflicted with a music-specific disorder. Brain 2002, 125, 238–251. [Google Scholar] [CrossRef]
- Don, A.J.; Schellenberg, E.G.; Rourke, B.P. Music and language skills of children with Williams syndrome. Child Neuropsychol. 1999, 5, 154–170. [Google Scholar] [CrossRef]
- Rhodes, S.M.; Riby, D.M.; Matthews, K.; Coghill, D.R. Attention-deficit/hyperactivity disorder and Williams syndrome: Shared behavioral and neuropsychological profiles. J. Clin. Exp. Neuropsychol. 2011, 33, 147–156. [Google Scholar] [CrossRef] [PubMed]
- Peretz, I.; Gosselin, N.; Tillman, B.; Cuddy, L.L.; Gagnon, B.; Trimmer, C.G.; Paquette, S.; Bouchard, B. On-line identification of congenital amusia. Music Percept. 2008, 25, 331–343. [Google Scholar] [CrossRef]
- Wechsler, D. Wechsler Adult Intelligence Scale-Fourth Edition; The Psychological Corporation: San Antonio, TX, USA, 2008. [Google Scholar]
- Lense, M.L.; Dykens, E.M.; Vanderbilt University, Nashville, TN, USA. Neuropsychological correlates of amusia in Williams syndrome. Unpublished work. 2014. [Google Scholar]
- Tuch, D.S.; Reese, T.G.; Wiegell, M.R.; Makris, N.; Belliveau, J.W.; Wedeen, V.J. High angular resolution diffusion imaging reveals intravoxel white matter fiber heterogeneity. Magn. Reson. Med. 2002, 48, 577–582. [Google Scholar] [CrossRef] [PubMed]
- Hosey, T.; Williams, G.; Ansorge, R. Inference of multiple fiber orientations in high angular resolution diffusion imaging. Magn. Reson. Med. 2005, 54, 1480–1489. [Google Scholar] [CrossRef] [PubMed]
- Reuter, M.; Rosas, H.D.; Fischl, B. Highly accurate inverse consistent registration: A robust approach. NeuroImage 2010, 53, 1181–1196. [Google Scholar] [CrossRef] [PubMed]
- Ségonne, F.; Dale, A.M.; Busa, E.; Glessner, M.; Salat, D.; Hahn, H.K.; Fischl, B. A hybrid approach to the skull stripping problem in MRI. NeuroImage 2004, 22, 1060–1075. [Google Scholar] [CrossRef] [PubMed]
- Fischl, B.; Salat, D.H.; Busa, E.; Albert, M.; Dieterich, M.; Haselgrove, C.; van der Kouwe, A.; Killiany, R.; Kennedy, D.; Klaveness, S.; et al. Whole brain segmentation: Automated labeling of neuroanatomical structures in the human brain. Neuron 2002, 33, 341–355. [Google Scholar] [CrossRef] [PubMed]
- Fischl, B.; Salat, D.H.; van der Kouwe, A.J.W.; Makris, N.; Ségonne, F.; Quinn, B.T.; Dale, A.M. Sequence-independent segmentation of magnetic resonance images. NeuroImage 2004, 23, S69–S84. [Google Scholar] [CrossRef] [PubMed]
- Sled, J.G.; Zijdenbos, A.P.; Evans, A.C. A nonparametric method for automatic correction of intensity nonuniformity in MRI data. IEEE Trans. Med. Imaging 1998, 17, 87–97. [Google Scholar] [CrossRef] [PubMed]
- Fischl, B.; Liu, A.; Dale, A.M. Automated manifold surgery: Constructing geometrically accurate and topologically correct models of the human cerebral cortex. IEEE Trans. Med. Imaging 2001, 20, 70–80. [Google Scholar] [CrossRef] [PubMed]
- Ségonne, F.; Pacheco, J.; Fischl, B. Geometrically accurate topology-correction of cortical surfaces using nonseparating loops. IEEE Trans. Med. Imaging 2007, 26, 518–529. [Google Scholar] [PubMed]
- Dale, A.M.; Sereno, M.I. Improved localization of cortical activity by combining EEG and MEG with MRI cortical surface reconstruction: A linear approach. J. Cogn. Neurosci. 1993, 5, 162–176. [Google Scholar] [CrossRef] [PubMed]
- Dale, A.M.; Fischl, B.; Sereno, M.I. Cortical surface-based analysis. I. Segmentation and surface reconstruction. NeuroImage 1999, 9, 179–194. [Google Scholar] [CrossRef] [PubMed]
- Fischl, B.; Dale, A.M. Measuring the thickness of the human cerebral cortex from magnetic resonance images. Proc. Natl. Acad. Sci. USA 2000, 97, 11050–11055. [Google Scholar] [CrossRef] [PubMed]
- Fischl, B.; van der Kouwe, A.; Destrieux, C.; Halgren, E.; Ségonne, F.; Salat, D.H.; Busa, W.; Seidman, L.J.; Goldstein, J.; Kennedy, D.; et al. Automatically parcellating the human cerebral cortex. Cereb. Cortex 2004, 14, 11–22. [Google Scholar] [CrossRef] [PubMed]
- Desikan, R.S.; Ségonne, F.; Fischl, B.; Quinn, B.T.; Dickerson, B.C.; Blacker, D.; Buckner, R.L.; Dale, A.M.; Maguire, R.P.; Hyman, B.T.; et al. An automated labeling system for subdividing the human cerebral cortex on MRI scans into gyral based regions of interest. NeuroImage 2006, 31, 968–980. [Google Scholar] [CrossRef] [PubMed]
- Han, X.; Jovicich, J.; Salat, D.; van der Kouwe, A.; Quinn, B.; Czanner, S.; Busa, E.; Pacheco, J.; Albert, M.; Killiany, R.; et al. Reliability of MRI-derived measurements of human cerebral cortical thickness: The effects of field strength, scanner upgrade and manufacturer. NeuroImage 2006, 32, 180–194. [Google Scholar] [CrossRef] [PubMed]
- Reuter, M.; Schmansky, N.J.; Rosas, H.D.; Fischl, B. Within-subject template estimation for unbiased longitudinal image analysis. NeuroImage 2012, 61, 1402–1418. [Google Scholar] [CrossRef] [PubMed]
- Smith, S.M.; Jenkinson, M.; Woolrich, M.W.; Beckmann, C.F.; Behrens, T.E.J.; Johansen-Berg, H.; Bannister, P.R.; De Luca, M.; Drobnjak, I.; Flitney, D.E.; et al. Advances in functional and structural MR image analysis and implementation as FSL. NeuroImage 2004, 23, S208–S219. [Google Scholar] [CrossRef] [PubMed]
- Jenkinson, M.; Beckmann, C.F.; Behrens, T.E.J.; Woolrich, M.W.; Smith, S.M. FSL. NeuroImage 2012, 62, 782–790. [Google Scholar] [PubMed]
- Niogi, S.N.; Mukherjee, P.; McCandliss, B.D. Diffusion tensor imaging segmentation of white matter structures using a Reproducible Objective Quantification Scheme (ROQS). NeuroImage 2007, 35, 166–174. [Google Scholar] [CrossRef] [PubMed]
- Levitin, D.J.; Menon, V.; Schmitt, J.E.; Eliez, S.; White, C.D.; Glover, G.H.; Kadis, J.; Korenberg, J.R.; Bellugi, U.; Reiss, A.L. Neural correlates of auditory perception in Williams syndrome: An fMRI study. NeuroImage 2003, 18, 74–82. [Google Scholar] [CrossRef] [PubMed]
- Thornton-Wells, T.A.; Cannistraci, C.J.; Anderson, A.W.; Kim, C.-Y.; Eapen, M.; Gore, J.C.; Blake, R.; Dykens, E.M. Auditory attraction: Activation of visual cortex by music and sound in Williams syndrome. Am. J. Intellect. Dev. Disabil. 2010, 115, 172–189. [Google Scholar] [CrossRef] [PubMed]
- Meda, S.A.; Pryweller, J.R.; Thornton-Wells, T.A. Regional brain differences in cortical thickness, surface area and subcortical volume in individuals with williams syndrome. PLoS One 2012, 7, e31913. [Google Scholar] [CrossRef] [PubMed]
- McDonald, C.; Stewart, L. Uses and functions of music in congenital amusia. Music Percept. 2008, 25, 345–355. [Google Scholar] [CrossRef]
- Omigie, D.; Mullensiefen, D.; Stewart, L. The experience of music in congenital amusia. Music Percept. 2012, 30, 1–18. [Google Scholar] [CrossRef]
- Douglas, K.M.; Bilkey, D.K. Amusia is associated with deficits in spatial processing. Nat. Neurosci. 2007, 10, 915–921. [Google Scholar] [CrossRef] [PubMed]
- Tillmann, B.; Jolicoeur, P.; Ishihara, M.; Gosselin, N.; Bertrand, O.; Rossetti, Y.; Peretz, I. The amusic brain: Lost in music, but not in space. PLoS One 2010, 5, e10173. [Google Scholar] [CrossRef] [PubMed]
- Farran, E.K.; Jarrold, C. Visuospatial cognition in Williams syndrome: Reviewing and accounting for the strengths and weaknesses in performance. Dev. Neuropsychol. 2003, 23, 173–200. [Google Scholar] [CrossRef] [PubMed]
- Loui, P.; Kroog, K.; Zuk, J.; Winner, E.; Schlaug, G. Relating pitch awareness to phonemic awareness in children: Implications for tone-deafness and dyslexia. Front. Psychol. 2011, 2, 111. [Google Scholar] [CrossRef] [PubMed]
- Jones, J.L.; Lucker, J.; Zalewski, C.; Brewer, C.; Drayna, D. Phonological processing in adults with deficits in musical pitch recognition. J. Commun. Disord. 2009, 42, 226–234. [Google Scholar] [CrossRef] [PubMed]
- Laing, E.; Hulme, C.; Grant, J.; Karmiloff-Smith, A. Learning to read in Williams syndrome: Looking beneath the surface of atypical reading development. J. Child Psychol. Psychiatry 2001, 42, 729–739. [Google Scholar] [CrossRef] [PubMed]
- Menghini, D.; Verucci, L.; Vicari, S. Reading and phonological awareness in Williams syndrome. Neuropsychology 2004, 18, 29–37. [Google Scholar] [CrossRef] [PubMed]
- Steele, A.; Scerif, G.; Cornish, K.; Karmiloff-Smith, A. Learning to read in Williams syndrome and Down syndrome: Syndrome-specific precursors and developmental trajectories. J. Child Psychol. Psychiatry 2013, 54, 754–762. [Google Scholar] [CrossRef] [PubMed]
- Williamson, V.J.; Stewart, L. Memory for pitch in congenital amusia: Beyond a fine-grained pitch discrimination problem. Memory 2010, 18, 657–669. [Google Scholar] [CrossRef] [PubMed]
© 2014 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lense, M.D.; Dankner, N.; Pryweller, J.R.; Thornton-Wells, T.A.; Dykens, E.M. Neural Correlates of Amusia in Williams Syndrome. Brain Sci. 2014, 4, 594-612. https://doi.org/10.3390/brainsci4040594
Lense MD, Dankner N, Pryweller JR, Thornton-Wells TA, Dykens EM. Neural Correlates of Amusia in Williams Syndrome. Brain Sciences. 2014; 4(4):594-612. https://doi.org/10.3390/brainsci4040594
Chicago/Turabian StyleLense, Miriam D., Nathan Dankner, Jennifer R. Pryweller, Tricia A. Thornton-Wells, and Elisabeth M. Dykens. 2014. "Neural Correlates of Amusia in Williams Syndrome" Brain Sciences 4, no. 4: 594-612. https://doi.org/10.3390/brainsci4040594