Intraoperative Neurophysiological Monitoring in Contemporary Spinal Surgery: A Systematic Review of Clinical Outcomes and Cost-Effectiveness
Abstract
1. Introduction
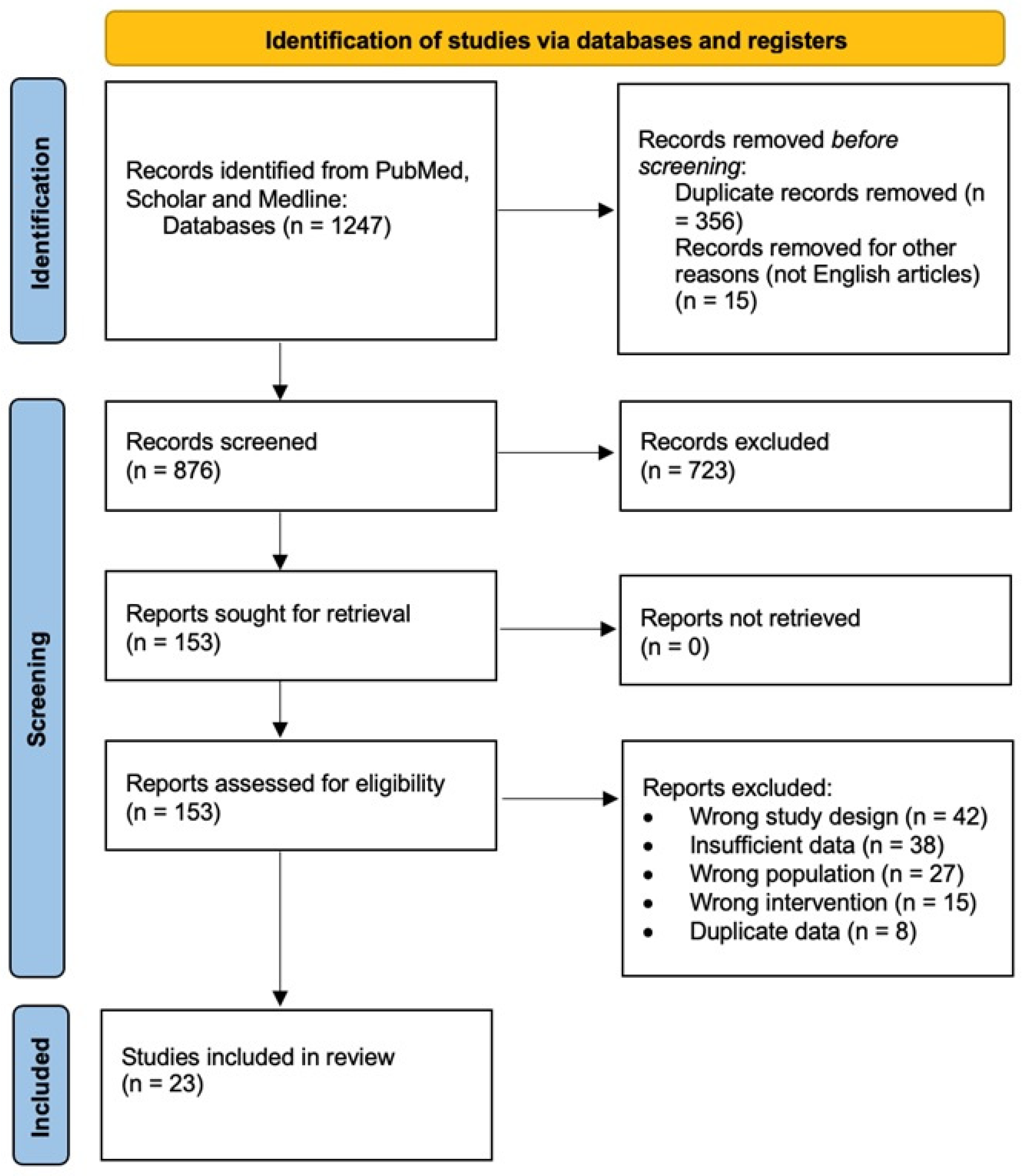
2. Materials and Methods
2.1. Literature Search, Study Selection, and Data Extraction
2.2. Quality Assessment and Statistical Analysis
3. Results: Clinical Outcomes
3.1. Study Characteristics
3.2. Efficacy of IONM in Reducing Neurological Complications
3.2.1. Overall Efficacy
3.2.2. Efficacy by Surgery Type
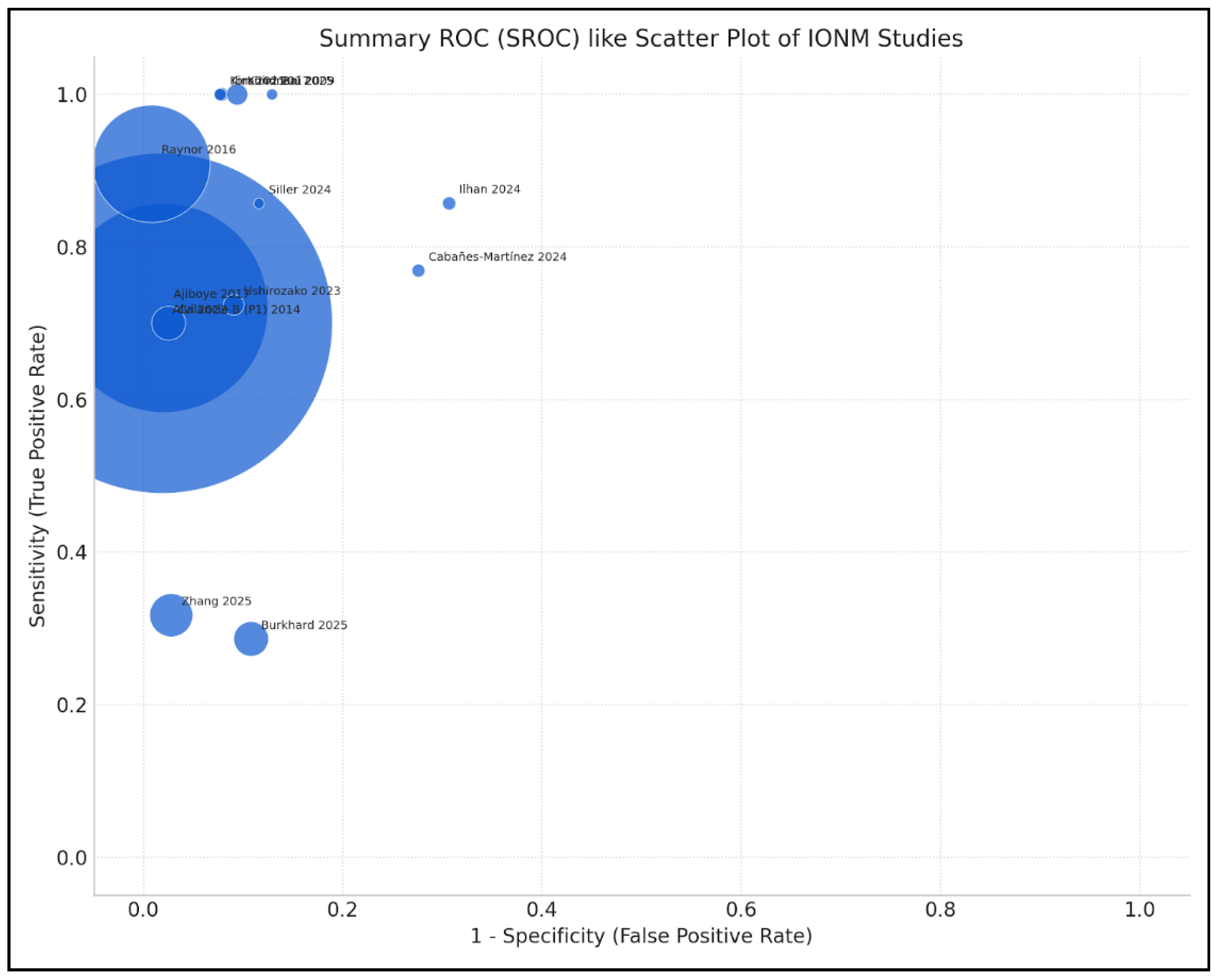
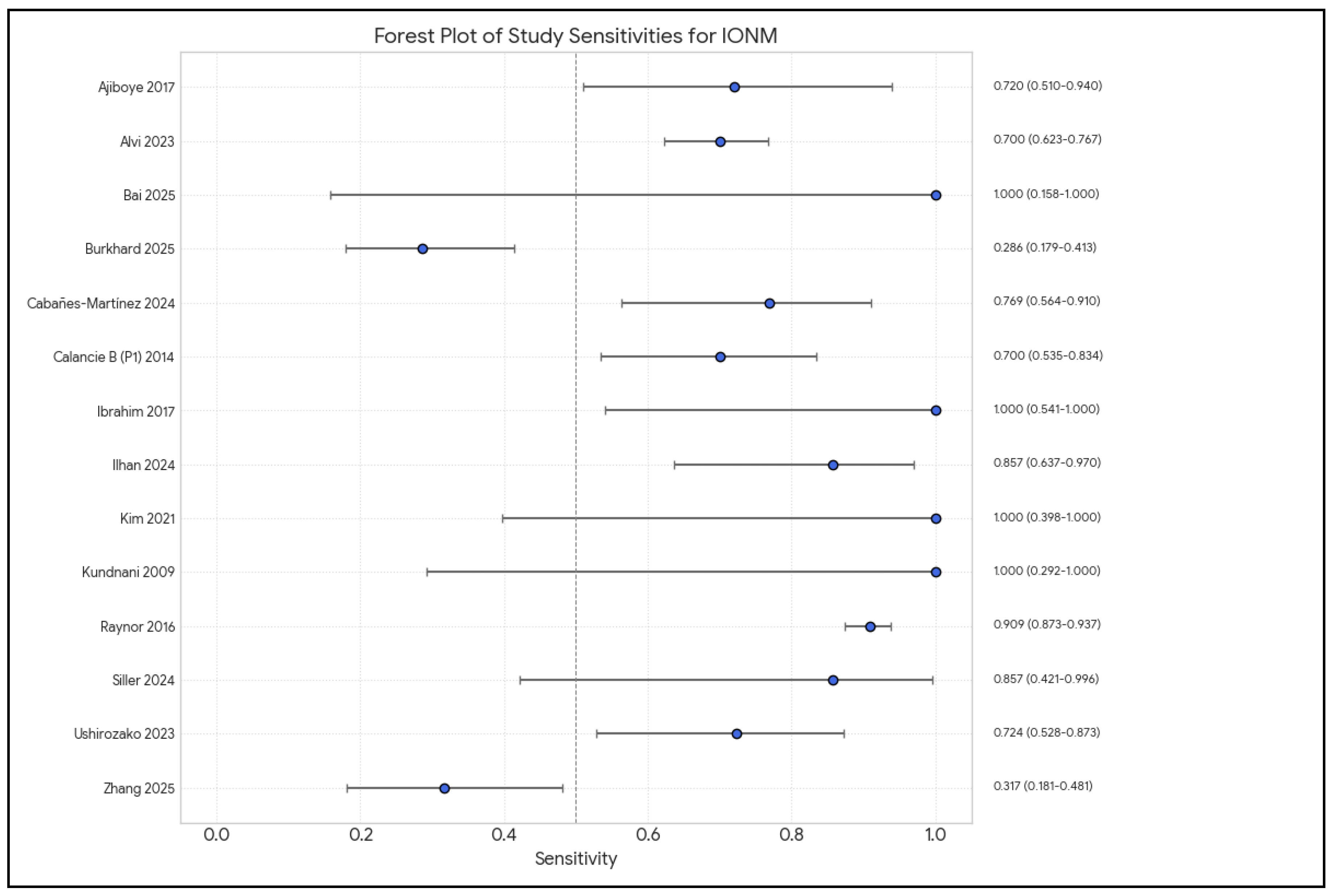
3.2.3. Diagnostic Accuracy of IONM, Multimodal Monitoring
3.2.4. D-Wave Monitoring for Intramedullary Tumors
3.2.5. IONM in Post-Traumatic and Osteoporotic Vertebral Fractures
3.3. Impact on Surgical Decision-Making and Long-Term Outcomes
4. Results: Cost-Effectiveness Analysis
4.1. Study Characteristics
4.2. Economic Outcomes
4.2.1. Direct Costs of IONM
4.2.2. Cost Savings
4.2.3. Factors Influencing Cost-Effectiveness and Cost-Effectiveness Thresholds
4.2.4. Cost-Effectiveness by Surgery Type
4.3. Long-Term Economic Impact and Economic Models
| Author | Year | Study Type | Number of Patients | Type of Pathology | Type of Intervention Performed |
|---|---|---|---|---|---|
| Kundnani [19] | 2010 | Analysis of prospectively collected IONM data | 354 consecutive patients | Adolescent idiopathic scoliosis (AIS) | Corrective surgery for AIS (instrumented fusion) |
| Calancie (Part 1) [16] | 2014 | Prospective, blinded, randomized clinical study (Methods and alarm criteria) | 71 patients (802 screws) | Patients requiring thoracic pedicle screws (T1-L1) for various disorders (e.g., scoliosis, kyphosis, fusion) | Implantation of thoracic pedicle screws (T1-L1) |
| Calancie (Part 2) [27] | 2014 | Prospective, blinded, randomized neuromonitoring study (Role of feedback) | 71 surgical cases (820 pedicle tracks tested) | Patients requiring thoracic pedicle screws | Implantation of thoracic pedicle screws, with/without IONM feedback guiding trajectory |
| Raynor [4] | 2016 | Retrospective review | 12,375 patients | All spinal pathologies (deformity, degenerative, pathologic disease, trauma) | All spinal surgical procedures (cervical, thoracic/thoracolumbar, lumbosacral) |
| Ajiboye [9] | 2017 | Systematic review and meta-analysis | 26,357 patients (from 10 studies included in the meta-analysis) | Patients undergoing anterior cervical spine surgery (ACSS) for conditions such as myelopathy, radiculopathy, infection, tumor, trauma, OPLL | Anterior cervical spine surgery (ACSS), including anterior cervical discectomy and fusions (ACDFs) and corpectomies |
| Ibrahim [14] | 2017 | Retrospective data collection/analysis | 121 patients | Various spinal pathologies requiring surgery (including tumor resection, ACDF, fusion, laminectomy across cervical, thoracic, lumbar regions) | Various spinal procedures (halo adjustment, corpectomy, ACDF, tumor resection, laminectomy, fusion, etc.) |
| Thirumala [12] | 2017 | Systematic review and meta-analysis | 2102 patients with IS (from 12 studies; 8 for the meta-analysis) | Idiopathic scoliosis (IS) | Corrective spinal surgery for IS |
| Charalampidis [24] | 2020 | Narrative review | N/A (Review summarizes multiple studies with varied patient numbers, e.g., Hilibrand et al. 427 cases, Nuwer et al. 51,263 cases) | Various spinal conditions (degenerative cervical, deformity, tumors, degenerative lumbar) | Spine surgery |
| Kim [15] | 2021 | Retrospective review with historical controls | 196 patients (132 IONM, 64 non-IONM) for ACDF only | Ossification of the posterior longitudinal ligament (OPLL), with/without myelopathy | Anterior cervical spine discectomy with fusion (ACDF) |
| Ushirozako [21] | 2023 | Prospective multicenter observational cohort study | 350 consecutive patients (TcMEP derivation rate 94%) | Traumatic spinal injury (cervical, thoracic, lumbar) | Traumatic spinal injury surgery |
| Alvi [10] | 2024 | Systematic review and meta-analysis | 99,937 patients (from 163 studies) in total across different modalities. SSEP: 16,310 (52 studies). MEP: 71,144 (68 studies). EMG: 7888 (16 studies). Multimodal: 17,968 (69 studies). | Various spinal pathologies (deformity, degenerative, tumors, trauma, conus/cauda equina injuries, etc.) | Spine surgery (various types for the included pathologies) |
| Cabañes-Martínez [22] | 2024 | Observational, descriptive, retrospective cohort study | 91 patients (36 monitored, 55 unmonitored) | Intradural spinal tumors (extramedullary and intramedullary) | Surgical resection of intradural spinal tumors |
| CreveCoeur [23] | 2024 | Multicenter, multidisciplinary, retrospective study | 66 pediatric patients (<18 years) whose surgery was aborted due to IONM signal loss | Pediatric spinal deformity (idiopathic, congenital, neuromuscular) | Pediatric spinal deformity surgery (posterior spinal instrumentation and fusion), aborted due to IONM loss |
| Fehlings [26] | 2024 | Clinical Practice Guideline (GRADE process) | Refers to Alvi et al. 2024 [10] meta-analysis data: 99,937 patients (164 studies) | Patients undergoing spine surgery, focus on “high risk” for ISCI (complex deformity, cord compression/myelopathy, intramedullary tumors, unstable fx, OPLL) | Spine surgery |
| Guzzi [25] | 2024 | Narrative review | N/A (Review article) | Various neurosurgical conditions (brain tumors, neurovascular, epilepsy, spinal, peripheral nerve) | Neurosurgery (brain, spinal, peripheral nerve) |
| Ilhan [17] | 2024 | Prospective cohort study (based on methods) | 67 patients | Intradural spinal cord tumors (SCTs), including intramedullary and extramedullary | Surgical resection of intradural spinal cord tumors |
| Siller [20] | 2024 | Prospective assessment/study | 20 patients | High-cervical intramedullary spinal cord tumors (IMSCTs) (above C4/5 level) | Microsurgical resection of high-cervical IMSCTs |
| Yu [2] | 2024 | Prospective, randomized, controlled study | 46 patients (23 IONM, 23 non-IONM) | Cervical spondylosis requiring anterior cervical surgery (≤2 segments) | Anterior cervical spine surgery (ACSS), including ACDF |
| Bai [13] | 2025 | Retrospective analysis | 127 patients (64 IONM group, 63 control group) | Lumbar degenerative diseases (lumbar spinal stenosis, lumbar disc herniation, lumbar degenerative spondylolisthesis) | Unilateral biportal endoscopic (UBE) lumbar spine surgery (decompression, discectomy, ULBD) |
| Burkhard [3] | 2025 | Retrospective, single-center cohort study | 401 patients | Degenerative lumbar conditions requiring primary posterior lumbar interbody fusion (PLIF) | Primary posterior lumbar interbody fusion (PLIF) (open posterior decompression laminectomy and instrumented fusion with pedicle screw fixation) |
| El Choueiri [11] | 2025 | Meta-analysis | 187,162 patients (21,686 with IONM, 165,476 without IONM) from 7 studies | Degenerative cervical spine disorders (spondylosis, OPLL, radiculopathy, myelopathy) | Degenerative cervical spine surgeries (e.g., ACDF, laminoplasty) |
| Kim [18] | 2025 | Prospective observational study | 45 patients (23 EELF, 22 TLIF) completed 12-month follow-up (52 enrolled) | Lumbar foraminal stenosis with dominant unilateral radicular pain | Extended endoscopic lumbar foraminotomy (EELF) vs. transforaminal lumbar interbody fusion (TLIF) |
| Zhang [1] | 2025 | Retrospective study | 1622 patients | Cervical spinal canal stenosis (disc degeneration, ligamentum flavum hypertrophy, OPLL) | Cervical spinal canal decompression surgery (anterior or posterior approach) |
| Author | Year | IONM Sensitivity | IONM Specificity | Monitoring Modalities (SSEP, MEP, D-Wave, EMG, etc.) |
|---|---|---|---|---|
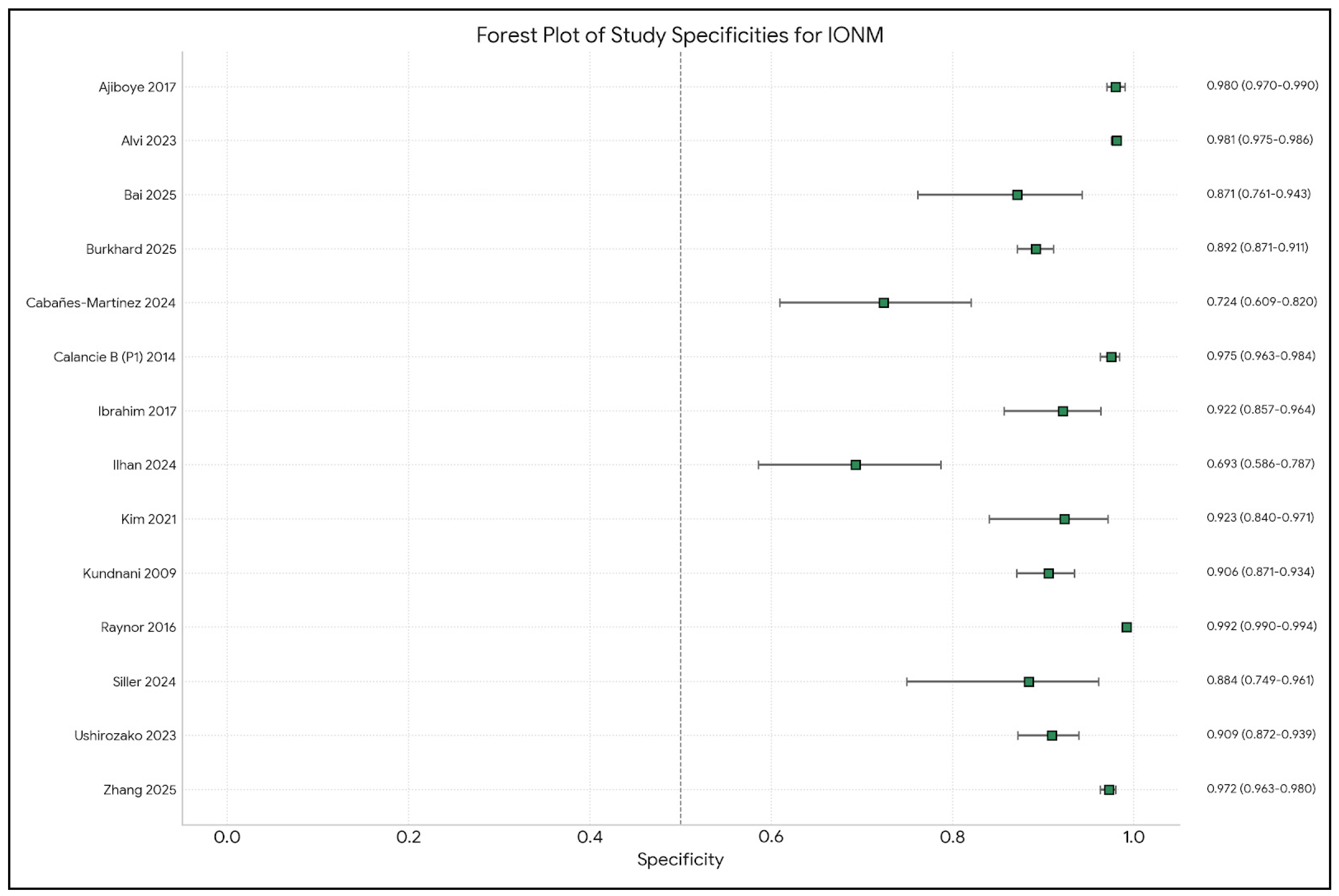
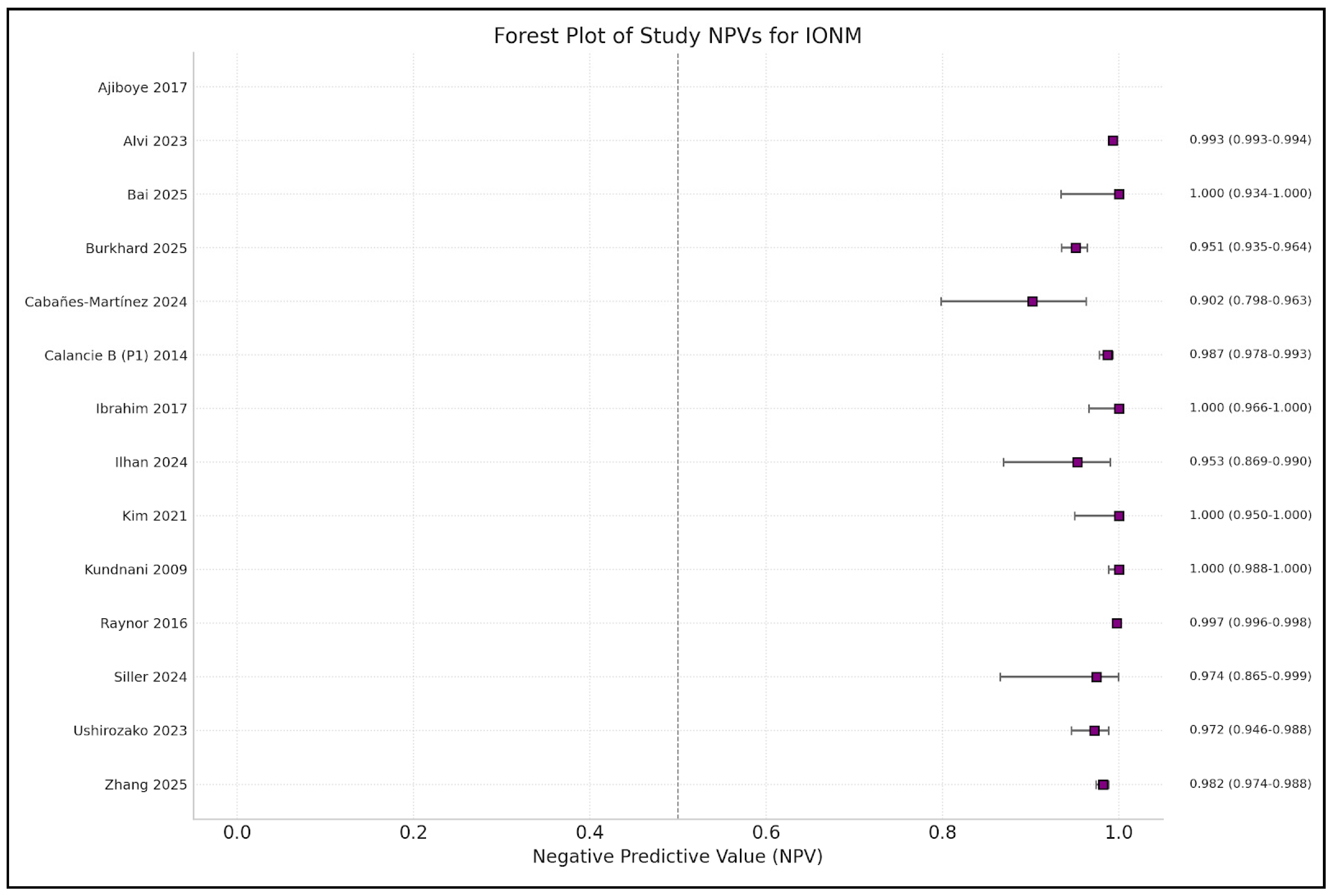
| Ajiboye [9] | 2017 | Pooled: 71% (CI: 48–87%) for ACSS. Unimodal: 68% (CI: 39–88%). Multimodal: 88% (CI: 4–100%). | Pooled: 98% (CI: 92–100%) for ACSS. Unimodal: 99% (CI: 97–100%). Multimodal: 92% (CI: 81–96%). | SSEP, MEP, EMG (Unimodal and Multimodal IONM mentioned; specific modalities from reviewed studies in Table 3 include SSEP, MEP, and EMG) |
| Alvi [10] | 2024 | SSEP: 71.4% (95% CI: 54.8–83.7). MEP: 90.2% (95% CI: 86.2–93.1). EMG: 48.3% (95% CI: 31.4–65.6). Multimodal: 83.5% (95% CI: 81–85.7). (SSEP and multimodal values from the Abstract, the Results section in the paper has slightly different values) | SSEP: 97.1% (95% CI: 95.3–98.3). MEP: 96% (95% CI: 94.3–97.2). EMG: 92.9% (95% CI: 84.4–96.9). Multimodal: 93.8% (95% CI: 90.6–95.9). | SSEP, MEP, EMG, D-waves, Multimodal IONM |
| Bai [13] | 2025 | N/A (Study does not provide TP/FP/FN/TN data for calculating sensitivity for neurological deficits) | N/A (Study does not provide TP/FP/FN/TN data for calculating specificity for neurological deficits) | Somatosensory-evoked potentials (SEPs), motor-evoked potentials (MEPs), free electromyography (freeEMG) |
| Burkhard [3] | 2025 | SSEP events for PMD: 13.6%. EMG events for PMD: 36.4%. | SSEP events for PMD: 96.0%. EMG events for PMD: 71.0%. | Free-run electromyography (EMG) and somatosensory-evoked potentials (SSEPs) |
| Cabañes-Martínez [22] | 2024 | N/A (Study reported predictive outcomes for specific cases rather than overall sensitivity percentages from a 2 × 2 table analysis: 3 deficits were predicted by IONM alerts) | N/A (Similar to sensitivity, specific percentage not calculable from provided data) | mMEPs (muscle motor-evoked potentials via tES), Epidural MEPs (D-wave), SSEPs (lower and upper limb), Bulbocavernous Reflex (BCR), Motor Root Mapping, free-run EMG |
| Calancie [27] (Part 1) | 2014 | 100% for predicting medial screw malpositioning ≥2 mm using 4-pulse pedicle track stimulation and leg EMG (with 15 mA cutoff) | High (AUC = 0.93 for predicting medial malposition ≥2 mm). Specificity depends on cutoff (e.g., 90% for 10 mA cutoff; 74% for 15 mA cutoff based on stated false positive rates of 10% and 26%, respectively). | Novel method: 4-pulse train stimulation in the pedicle track with evoked EMG from leg muscles (Quads, TA, AbH). Comparison methods: screw stimulation with IC/Abs EMG. Standard SSEP and transcranial MEP also performed. |
| Calancie [16] (Part 2) | 2014 | 100% (for the IONM test itself, for ≥2 mm medial malposition, as established in Part 1) | N/A (Refer to Calancie et al. 2014 [27] Part 1) | 4-pulse stimulus trains in the pedicle track, evoked EMG from leg muscles (AbH, TA, Quads), SSEP, MEP |
| Charalampidis [24] | 2020 | Varies by modality and study (Data from Table 2): - MEP (cervical CSM): 71% (Clark et al.). - tcMEP (cervical): 100% (Hilibrand et al.). - SSEP (cervical): 25% (Hilibrand et al.). - MIONM (ACDF): 80% (Kim et al.). - MIONM (deformity): ~100% (Quraishi et al., Bhagat et al.). | Varies by modality and study (Data from Table 2): - MEP (cervical CSM): 94% (Clark et al.). - tcMEP (cervical): 100% (Hilibrand et al.). - SSEP (cervical): 100% (Hilibrand et al.). - MIONM (ACDF): 97% (Kim et al.). - MIONM (deformity): 84–93.3% (Quraishi et al., Bhagat et al.). | SSEP, MEP (tcMEP, D-wave), EMG (spontaneous and triggered), Multimodal IONM (MIONM) |
| CreveCoeur [23] | 2024 | N/A (Study on outcomes after IONM loss, not diagnostic accuracy of IONM itself) | N/A (Study on outcomes after IONM loss, not diagnostic accuracy of IONM itself) | SSEPs, MEPs |
| El Choueiri [11] | 2025 | N/A (Meta-analysis evaluated effect on complication rates, not the pooled diagnostic sensitivity of IONM. One included study, Kim et al. 2021 [15], reported a sensitivity of 84.2% for multimodal IONM) | N/A (Meta-analysis evaluated effect on complication rates, not the pooled diagnostic specificity of IONM. One included study, Kim et al. 2021 [15], reported a specificity of 93.7% for multimodal IONM) | SSEP, MEP (tcMEP), EMG, Multimodal IONM |
| Fehlings [26] | 2024 | Summarizes Alvi et al.: SSEP: 67.5% (or 71.4%). MEP: 90%. EMG: 48.3%. Multimodal: 91% (or 83.5%). | Summarizes Alvi et al.: SSEP: 96.8% (or 97.1%). MEP: 95.6% (or 96%). EMG: 92.9%. Multimodal: 93.8%. | SSEP, MEP, EMG, D-Wave, Multimodal |
| Guzzi [25] | 2024 | N/A (Review of techniques and warning criteria, e.g., SSEP: >50% ampl. reduction or >10% lat. increase; BAEPs: >50% wave V ampl. reduction or >0.5 ms lat. increase; VEPs >50% or >20% decrement) | N/A (Review of techniques and warning criteria) | ECOG, SEEG, EMG, SSEP, MEP, DCS, BAEPs, VEPs |
| Ibrahim [14] | 2017 | 57.14% | 98.20% | Multimodality: SSEPs (98.4%), TCMEPs (86.3%), EMG (90.2%), TOF (34.1%), EEG (19.5%), BAER (1.6%) |
| Ilhan [17] | 2024 | Combined MEPs–DW/SSEPs (any alert, patient level for clinical deterioration at 3 months): 95%. For individual limb deterioration (any alert): MEPs–DW 78%; SSEPs 42%; Combined 81%. | Combined Persistent MEPs–DW/SSEPs (patient level for clinical deterioration at 3 months): 84%. For individual limb deterioration (any alert): MEPs–DW 82%; SSEPs 83%; Combined 72%. | Multimodal IONM: SSEPs, MEPs (transcranial electrical stimulation for muscle MEPs and D-wave) |
| Kim [15] | 2021 | Multimodal IONM: 84.2% (including indeterminate warnings). tcMEP: 71.4%. EMG: 69.2%. SSEP: 0%. | Multimodal IONM: 93.7% (including indeterminate warnings). tcMEP: 94.1%. EMG: 99.1%. SSEP: 100%. | Multimodal IONM: tcMEP, SSEP, continuous EMG |
| Kim [18] | 2025 | N/A (IONM used in the EELF group, but the study is a cost-effectiveness comparison, not the diagnostic accuracy of IONM) | N/A (IONM used in the EELF group, but the study is a cost-effectiveness comparison, not the diagnostic accuracy of IONM) | For EELF: Multimodal IONM (fEMG, MEP, SSEP) |
| Kundnani [19] | 2010 | NMEP: 100%. SSEP: 51%. Combined SSEP + NMEP: 100%. | NMEP: 96%. SSEP: 100%. Combined SSEP + NMEP: 99%. (Abstract reports 98.5% for combined specificity) | Multimodal: SSEP and NMEP (neurogenic motor-evoked potential via transcranial electrical stimulation) |
| Raynor [4] | 2016 | N/A (Study focuses on false negative rates, i.e., failure of IOM to detect deficits, not overall diagnostic sensitivity) | N/A (Study focuses on false negative rates) | Multimodal IOM: SSEPs, DNEPs (descending neurogenic), MEPs (transcranial), DSEPs (dermatomal), spEMG (spontaneous), trgEMG (triggered) |
| Siller [20] | 2024 | D-wave for fine-motor/complex hand function: Low. Multimodal IONM (D-wave/mMEPs/EMG/SSEPs) for complex distal upper extremity function (NHPT): 43% (long-term deterioration). | Multimodal IONM (D-wave/mMEPs/EMG/SSEPs) for complex distal upper extremity function (NHPT): 79% (long-term deterioration). | Multimodal IONM: D-wave, mMEPs (muscular MEPs via TcMEP), free-running EMG (frEMG), SSEPs. Dorsal column mapping (DCM) also used. |
| Thirumala [12] | 2017 | Pooled mean 91% (95% CI: 34–100%) for TcMEP changes | Pooled mean 96% (95% CI: 92–98%) for TcMEP changes | Transcranial motor-evoked potential (TCMEP) monitoring (some studies also used SSEP) |
| Ushirozako [21] | 2023 | 88% (also 87.5%) for TcMEP | 90% (also 90.3%) for TcMEP | Multimodal IONM: TcMEP, SSEP, free-run EMG (study focused on TcMEP) |
| Yu [2] | 2024 | N/A (Study evaluated the IONM effect on dysphagia, not diagnostic accuracy for general neurological deficits) | N/A (Study evaluated the IONM effect on dysphagia, not diagnostic accuracy for general neurological deficits) | Multimode monitoring: motor-evoked potential (MEP), somatosensory-evoked potential (SEP), electromyography (EMG) |
| Zhang [1] | 2025 | High-risk group: 100%. Low-risk group: 91.7%. | High-risk group: 98.84%. Low-risk group: 98.79%. | Multimodal IONM: SSEP, MEP, EMG (TOF also mentioned for anesthesia) |
| Author | Year | Key Findings |
|---|---|---|
| Ajiboy e [9] | 2017 | The risk of neurological injury after ACSS is low (0.64% weighted). Corpectomies may carry higher risk (1.02%) than ACDFs (0.20%). For ACDFs, no difference in neurological injury risk with/without IONM. Unimodal IONM has higher specificity than multimodal IONM. |
| Alvi [10] | 2024 | All neuromonitoring modalities (SSEP, MEP, EMG, multimodal monitoring) have diagnostic utility in detecting impending/incident intraoperative neurologic injuries, though accuracy differs. MEP showed the highest sensitivity (90.2%). SSEP showed high specificity (97.1%). EMG had lower sensitivity (48.3%). Multimodal monitoring showed high sensitivity (83.5%) and specificity (93.8%). |
| Bai [13] | 2025 | In the IONM group, 62.5% showed freeEMG stimulation; 15.6% had an MEP amplitude decrease. No significant SEP changes. Postop 24 h leg VAS lower in the IONM group ($p\<0.001$). IONM provides timely neurological function info, reduces invasiveness, and reduces early postop leg pain. |
| Burkhard [3] | 2025 | SSEP events associated with postoperative motor deficits (PMDs) (p = 0.043), EMG events were not. Persistent SSEP signal loss strongly predicted PMDs (OR 10.41). Overall test accuracy of IONM (SSEP and EMG) limited; IONM should be tailored to individual cases. |
| Cabañes-Martínez [22] | 2024 | IONM use was associated with improvements in Prolo, Brice–McKissock, and McCormick scales. Rate of new/worsened neurological deficits was 8.3% in the monitored group vs. 14.5% in the unmonitored group. IONM predicted deficits in 3 monitored patients with worsened outcomes. Six IONM events were resolved intraoperatively without deficit. |
| Calancie [27] (Part 1) | 2014 | Novel method (4-pulse train in the pedicle track, leg EMG) accurately predicts medially malpositioned thoracic screws (100% sensitivity for ≥2 mm encroachment). This method is superior to direct screw stimulation or using IC/Abs EMG. Optimal alarm criteria: 10 mA (lower) and 15 mA (upper) cutoffs. |
| Calancie [16] (Part 2) | 2014 | Providing intraoperative feedback from the novel IONM test (alarm ≤ 10 mA) significantly reduced clinically relevant (≥2 mm) thoracic pedicle screw medial malpositioning (p = 0.02). All 32 screws with ≥2 mm medial encroachment occurred when feedback was withheld or not acted upon. Traditional SSEP/MEP failed to detect these malpositioned screws. |
| Charalampidis [24] | 2020 | MIONM is generally effective for detecting perioperative neurological injury. SSEP can be delayed; MEPs are sensitive to anesthesia; EMG has high a false positive rate. Growing evidence supports IONM, but protocols for managing alerts are lacking. Controversy exists for routine use in noncomplex cervical procedures. MIONM well-supported for spinal tumor resection. |
| CreveCoeur [23] | 2024 | Nearly 75% of children with aborted spinal deformity surgery (due to IONM loss) had monitorable IONM signals at repeat OR within 2 weeks. Bilateral SSEP loss, combined bilateral SSEP/MEP loss, and delayed neurologic recovery (>72 h) linked to unmonitorable IONM at repeat surgery. All patients had full neurologic recovery. |
| El Choueiri [11] | 2025 | Pooled analysis showed no statistically significant protective effect of IONM in degenerative cervical spine surgery (OR = 0.90). Subgroup analysis suggested potential benefits when EMG is used with SSEP and MEP (OR = 0.39). Sample size significantly explained heterogeneity. |
| Fehlings [26] | 2024 | Recommends IONM for high-risk spine surgery patients (Strong recommendation, Low-quality evidence). Suggests proactive identification of “high-risk” patients, multidisciplinary team discussions, and intraop protocol with IONM (Weak recommendation, Very-Low-quality evidence). Developed AO Spine-PRAXIS Care Pathway for ISCI. |
| Guzzi [25] | 2024 | IONM is crucial for enhancing safety and precision in neurosurgery. Requires a detailed anesthetic plan (TIVA preferred). Discusses IONM applications in intracranial tumor resection, neurovascular surgery, epilepsy surgery, spinal surgery, and peripheral nerve surgery. Acknowledges limitations like false positives. |
| Ibrahim [14] | 2017 | IONM had low sensitivity (57.14%) in this cohort, potentially producing excessive false negatives. Specificity was high (98.20%). Authors suggest surgeons rely on clinical/surgical judgment. |
| Ilhan [17] | 2024 | Pre- and intraoperative neurophysiological alterations (MEPs, SSEPs) are strongly associated with risk of neurological deterioration 3 months after surgery. Transient intraoperative MEP/SSEP alterations did not pose a risk of neurological deterioration. Machine learning model predicted clinical outcome with 84% accuracy. |
| Kim [15] | 2021 | Postoperative neurological deficit rates significantly lower in the IONM group (3.79%) vs. the non-IONM group (14.06%). Use of IONM (OR: 0.139) associated with postoperative neurological complications. Multimodal IONM may be useful in ACDF for high-risk OPLL. No SSEP warnings observed. |
| Kim [18] | 2025 | EELF was significantly more cost-effective than TLIF. EELF had shorter operating times, less blood loss, and shorter hospital stays. No significant difference in clinical outcomes at 12 months. fEMG events in 52.2% of EELFs. MEP decline in 21.7%. No SSEP declines. |
| Kundnani [19] | 2010 | NMEP is superior to SSEP for identifying evolving spinal cord injury (sensitivity 100% vs. 51%). Specificity of SSEP (100%) is higher than that of NMEP (96%). Combined (SSEP + NMEP) monitoring achieved 100% sensitivity and 99% specificity. Multimodality monitoring should be standard of care. |
| Raynor [4] | 2016 | In total, 45 of 12,375 patients (0.36%) had false negative IOM outcomes. Most false negatives involved spEMG (48.8%). Eight patients (0.064%) had permanent neurologic deficits not detected by IOM. Undetected deficits have a higher risk of being permanent. Nerve root monitoring (spEMG) failures were most common. |
| Siller [20] | 2024 | Unimpaired D-wave reliably predicts preserved gross-motor function but fails for distal upper extremity fine-motor/complex function in high-cervical IMSCT surgery. Permanent deterioration in complex distal upper extremity function (15%) best predicted by multimodal IONM (sens, 43%; spec, 79%). Underlines the importance of multimodal IONM for fine-motor/complex hand function. |
| Thirumala [12] | 2017 | TcMEP monitoring is a highly sensitive (91%) and specific (96%) test for detecting new spinal cord injuries in IS surgery. A patient with a new neurological deficit from IS surgery was 250 times more likely to have TcMEP changes. AUC for TcMEP was 0.98. |
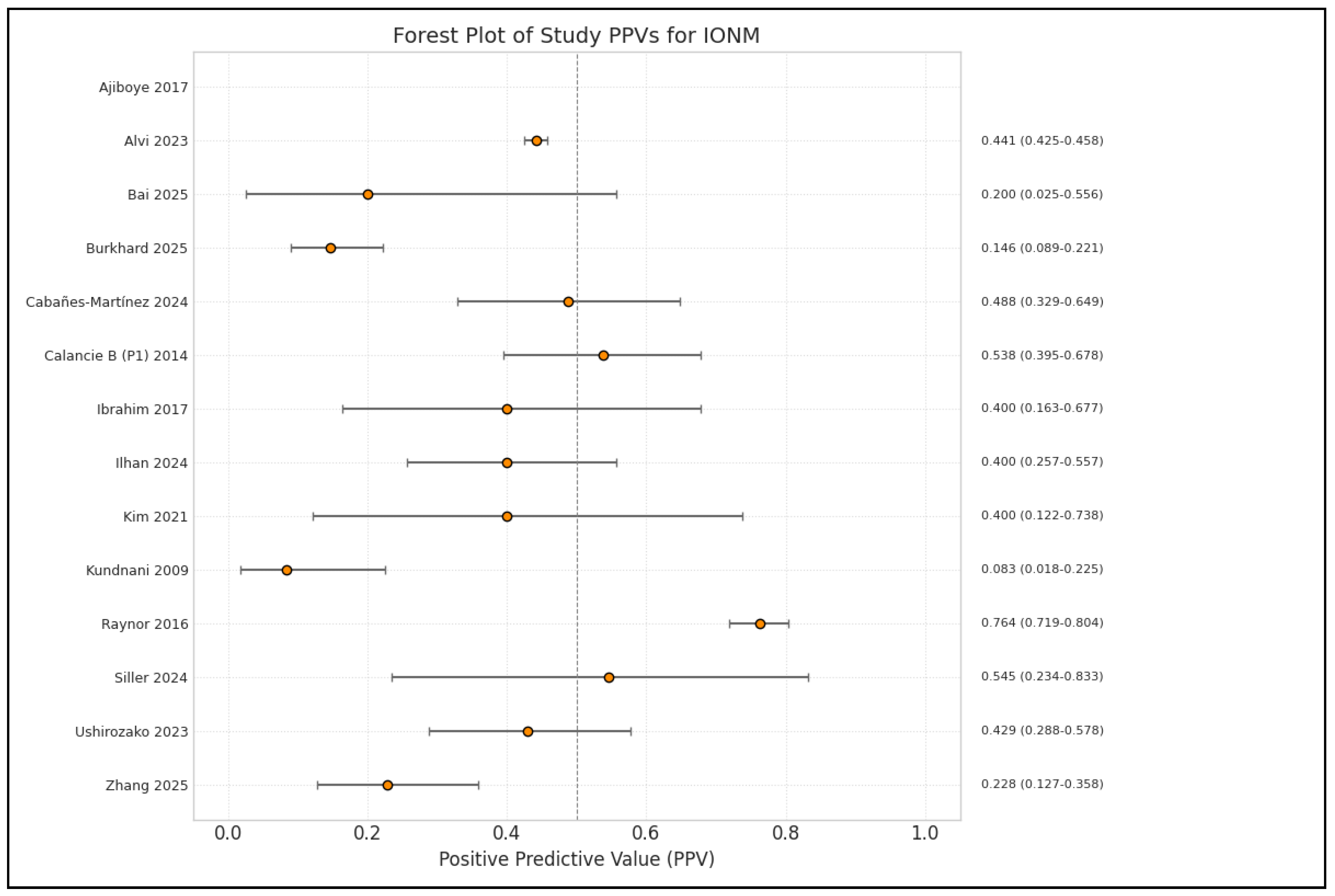
| Ushirozako [21] | 2023 | TcMEP monitoring showed 88% sensitivity and 90% specificity. Low PPV (18.4%). Neurological complication rate was 2.3%. Most common TcMEP alert timing: during decompression (40%). Single usage of TcMEP not recommended due to a low PPV. |
| Yu [2] | 2024 | IONM significantly reduced dysphagia 12 weeks post-ACSS (13.0% vs. 43.5%). No significant difference at 3rd day or 6th week post-surgery. Significantly less delayed swallowing trigger and residue with IONM at 12 weeks. |
| Zhang [1] | 2025 | Ligamentum flavum hypertrophy, OPLL, and preoperative mJOA < 12 are high-risk factors for intraoperative alarms. Sensitivity and PPV higher in the high-risk group. Irreversible IONM alarms correlated with poorer mJOA recovery. Reversibility of alarms is a principal predictor of outcomes. |
5. Discussion
5.1. Clinical Implications
5.2. Economic Implications
5.3. Medico-Legal Issue of Intraoperative Neurophysiological Monitoring
5.4. Future Directions
5.5. Limitations of the Study
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Conflicts of Interest
Abbreviations
| IONM | Intraoperative Neurophysiological Monitoring |
| SSEP | Somatosensory-Evoked Potential |
| MEP | Motor Evoked Potential |
| EMG | Electromyography |
| MIONM | Multimodal Intraoperative Neurophysiological Monitoring |
| NMEP | Neurogenic Motor-Evoked Potential |
| TcMEP | Transcranial Motor-Evoked Potential |
| ACSS | Anterior Cervical Spine Surgery |
| ACDF | Anterior Cervical Discectomy and Fusion |
| PLIF | Posterior Lumbar Interbody Fusion |
| IMSCT | Intramedullary Spinal Cord Tumor |
| OPLL | Ossification of the Posterior Longitudinal Ligament |
| PMD | Postoperative Motor Deficit |
References
- Zhang, Y.; Li, J.; Yuan, Y.; Wang, Y.; Huang, D.; Qi, H. The Application Value of Intraoperative Neurophysiological Monitoring in Cervical Spinal Canal Stenosis Decompression Surgery. Spine J. 2025, 4, S1529943025001755. [Google Scholar] [CrossRef] [PubMed]
- Yu, C.; Chunmei, L.; Qin, L.; Caiping, S. Application of Intraoperative Neurophysiological Monitoring (IONM) for Preventing Dysphagia After Anterior Cervical Surgery: A Prospective Study. World Neurosurg. 2024, 184, e390–e396. [Google Scholar] [CrossRef] [PubMed]
- Burkhard, M.D.; Evangelisti, G.; Altorfer, F.C.S.; Paschal, P.K.; Achebe, C.C.; Gorgy, G.; Kelly, M.J.; Zelenty, W.D.; Girardi, F.P.; Lebl, D.R.; et al. Is Intraoperative Neuromonitoring with SSEPs and EMG Predictable for Postoperative Neurologic Deficit in Posterior Lumbar Fusion Surgery? A Retrospective Cohort Analysis. Glob. Spine J. 2025, 4, 21925682251341820. [Google Scholar] [CrossRef] [PubMed]
- Raynor, B.L.; Padberg, A.M.; Lenke, L.G.; Bridwell, K.H.; Riew, K.D.; Buchowski, J.M.; Luhmann, S.J. Failure of Intraoperative Monitoring to Detect Postoperative Neurologic Deficits: A 25-Year Experience in 12,375 Spinal Surgeries. Spine 2016, 41, 1387–1393. [Google Scholar] [CrossRef] [PubMed]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 Statement: An Updated Guideline for Reporting Systematic Reviews. Br. Med. J. 2021, 372, n71. [Google Scholar] [CrossRef] [PubMed]
- Margulis, A.V.; Pladevall, M.; Riera-Guardia, N.; Varas-Lorenzo, C.; Hazell, L.; Berkman, N.D.; Viswanathan, M.; Perez-Gutthann, S. Quality Assessment of Observational Studies in a Drug-Safety Systematic Review, Comparison of Two Tools: The Newcastle-Ottawa Scale and the RTI Item Bank. Clin. Epidemiol. 2014, 6, 359–368. [Google Scholar] [CrossRef] [PubMed]
- Risk of Bias Tools-RoB 2 Tool. Available online: https://sites.google.com/site/riskofbiastool/welcome/rob-2-0-tool (accessed on 29 May 2025).
- Husereau, D.; Drummond, M.; Augustovski, F.; de Bekker-Grob, E.; Briggs, A.H.; Carswell, C.; Caulley, L.; Chaiyakunapruk, N.; Greenberg, D.; Loder, E.; et al. Consolidated Health Economic Evaluation Reporting Standards 2022 (CHEERS 2022) Statement: Updated Reporting Guidance for Health Economic Evaluations. BMC Med. 2022, 20, 23. [Google Scholar] [CrossRef] [PubMed]
- Ajiboye, R.M.; Zoller, S.D.; Sharma, A.; Mosich, G.M.; Drysch, A.; Li, J.; Reza, T.; Pourtaheri, S. Intraoperative Neuromonitoring for Anterior Cervical Spine Surgery: What Is the Evidence? Spine 2017, 42, 385–393. [Google Scholar] [CrossRef] [PubMed]
- Alvi, M.A.; Kwon, B.K.; Hejrati, N.; Tetreault, L.A.; Evaniew, N.; Skelly, A.C.; Fehlings, M.G. Accuracy of Intraoperative Neuromonitoring in the Diagnosis of Intraoperative Neurological Decline in the Setting of Spinal Surgery—A Systemat-ic Review and Meta-Analysis. Glob. Spine J. 2024, 14, 105S–149S. [Google Scholar] [CrossRef] [PubMed]
- El Choueiri, J.; Pellicanò, F.; Caimi, E.; Laurelli, F.; Colella, F.; Cossa, C.; Colonna, V.; Sicuri, M.; Stefini, R.; Cannizzaro, D. Intraoperative Neuromonitoring in Cervical Degenerative Spine Surgery: A Meta-Analysis of Its Impact on Neurological Outcomes. Neurosurg. Rev. 2025, 48, 360. [Google Scholar] [CrossRef] [PubMed]
- Thirumala, P.D.; Crammond, D.J.; Loke, Y.K.; Cheng, H.L.; Huang, J.; Balzer, J.R. Diagnostic Accuracy of Motor Evoked Potentials to Detect Neurological Deficit during Idiopathic Scoliosis Correction: A Systematic Review. J. Neurosurg. Spine 2017, 26, 374–383. [Google Scholar] [CrossRef] [PubMed]
- Bai, T.-Y.; Meng, H.; Lin, J.-S.; Fan, Z.-H.; Fei, Q. Application of Intraoperative Neurophysiological Monitoring in Unilat-eral Biportal Endoscopic Lumbar Spine Surgery. J. Orthop. Surg. Res. 2025, 20, 334. [Google Scholar] [CrossRef] [PubMed]
- Ibrahim, T.; Mrowczynski, O.; Zalatimo, O.; Chinchilli, V.; Sheehan, J.; Harbaugh, R.; Rizk, E. The Impact of Neurophysiological Intraoperative Monitoring during Spinal Cord and Spine Surgery: A Critical Analysis of 121 Cases. Cureus 2017, 9, e1861. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.-E.; Kim, J.-S.; Yang, S.; Choi, J.; Hyun, S.-J.; Kim, K.-J.; Park, K.S. Neurophysiological Monitoring during Anterior Cervical Discectomy and Fusion for Ossification of the Posterior Longitudinal Ligament. Clin. Neurophysiol. Pract. 2021, 6, 56–62. [Google Scholar] [CrossRef] [PubMed]
- Calancie, B.; Donohue, M.L.; Moquin, R.R. Neuromonitoring with Pulse-Train Stimulation for Implantation of Thoracic Pedicle Screws: A Blinded and Randomized Clinical Study. Part 2. The Role of Feedback: Clinical Article. J. Neurosurg. Spine 2014, 20, 692–704. [Google Scholar] [CrossRef] [PubMed]
- Ilhan, F.; Boulogne, S.; Morgado, A.; Dauleac, C.; André-Obadia, N.; Jung, J. The Impact of Neurophysiological Monitoring during Intradural Spinal Tumor Surgery. Cancers 2024, 16, 2192. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.-H.; Park, H.; Lee, C.-H.; Kim, C.H. Cost-Effectiveness Analysis of Extended Endoscopic Lumbar Foraminotomy (EELF) and Transforaminal Lumbar Interbody Fusion (TLIF): A Prospective Observational Study. Sci. Rep. 2025, 15, 3602. [Google Scholar] [CrossRef] [PubMed]
- Kundnani, V.K.; Zhu, L.; Tak, H.H.; Wong, H.K. Multimodal Intraoperative Neuromonitoring in Corrective Surgery for Adolescent Idiopathic Scoliosis: Evaluation of 354 Consecutive Cases. Indian J. Orthop. 2010, 44, 64–72. [Google Scholar] [CrossRef] [PubMed]
- Siller, S.; Duell, S.; Tonn, J.-C.; Szelenyi, A. Multimodal Intraoperative Neurophysiological Monitoring May Better Pre-dict Postoperative Distal Upper Extremities’ Complex-Functional Outcome than Spinal and Muscular Motor Evoked Po-tentials Alone in High-Cervical Intramedullary Spinal Cord Tumor Surgery. Clin. Neurophysiol. 2024, 168, 52–60. [Google Scholar] [CrossRef] [PubMed]
- Ushirozako, H.; Yoshida, G.; Imagama, S.; Machino, M.; Ando, M.; Kawabata, S.; Yamada, K.; Kanchiku, T.; Fujiwara, Y.; Taniguchi, S.; et al. Role of Transcranial Motor Evoked Potential Monitoring During Traumatic Spinal Injury Surgery: A Prospective Multicenter Study of the Monitoring Committee of the Japanese Society for Spine Surgery and Related Research. Spine 2023, 48, 1388–1396. [Google Scholar] [CrossRef] [PubMed]
- Cabañes-Martínez, L.; Fedirchyk-Tymchuk, O.; Viñas, L.L.; Abreu-Calderón, F.; Moro, R.C.; Álamo, M.D.; Regidor, I. Usefulness of Intraoperative Neurophysiological Monitoring in Intradural Spinal Tumor Surgeries. J. Clin. Med. 2024, 13, 7588. [Google Scholar] [CrossRef] [PubMed]
- CreveCoeur, T.S.; Iyer, R.R.; Goldstein, H.E.; Delgardo, M.W.; Hankinson, T.C.; Erickson, M.A.; Garg, S.; Skaggs, D.L.; Andras, L.; Kennedy, B.C.; et al. Timing of Intraoperative Neurophysiological Monitoring (IONM) Recovery and Clinical Recovery after Termination of Pediatric Spinal Deformity Surgery Due to Loss of IONM Signals. Spine J. 2024, 24, 1740–1749. [Google Scholar] [CrossRef] [PubMed]
- Charalampidis, A.; Jiang, F.; Wilson, J.R.F.; Badhiwala, J.H.; Brodke, D.S.; Fehlings, M.G. The Use of Intraoperative Neurophysiological Monitoring in Spine Surgery. Glob. Spine J. 2020, 10, 104S–114S. [Google Scholar] [CrossRef] [PubMed]
- Guzzi, G.; Ricciuti, R.A.; Torre, A.D.; Turco, E.L.; Lavano, A.; Longhini, F.; Torre, D.L. Intraoperative Neurophysiological Monitoring in Neurosurgery. J. Clin. Med. 2024, 13, 2966. [Google Scholar] [CrossRef] [PubMed]
- Fehlings, M.G.; Alvi, M.A.; Evaniew, N.; Tetreault, L.A.; Martin, A.R.; McKenna, S.L.; Rahimi-Movaghar, V.; Ha, Y.; Kirshblum, S.; Hejrati, N.; et al. A Clinical Practice Guideline for Prevention, Diagnosis and Management of Intraoperative Spinal Cord Injury: Recommendations for Use of Intraoperative Neuromonitoring and for the Use of Preoperative and Intraoperative Protocols for Patients Undergoing Spine Surgery. Glob. Spine J. 2024, 14, 212S–222S. [Google Scholar] [CrossRef] [PubMed]
- Calancie, B.; Donohue, M.L.; Harris, C.B.; Canute, G.W.; Singla, A.; Wilcoxen, K.G.; Moquin, R.R. Neuromonitoring with Pulse-Train Stimulation for Implantation of Thoracic Pedicle Screws: A Blinded and Randomized Clinical Study. Part 1. Methods and Alarm Criteria: Clinical Article. J. Neurosurg. Spine 2014, 20, 675–691. [Google Scholar] [CrossRef] [PubMed]
- Ney, J.P.; Van Der Goes, D.N.; Watanabe, J.H. Cost–Benefit Analysis: Intraoperative Neurophysiological Monitoring in Spinal Surgeries. J. Clin. Neurophysiol. 2013, 30, 280–286. [Google Scholar] [CrossRef] [PubMed]
- Ney, J.P.; Van Der Goes, D.N.; Nuwer, M.R. Does Intraoperative Neurophysiologic Monitoring Matter in Noncomplex Spine Surgeries? Neurology 2015, 85, 2151–2158. [Google Scholar] [CrossRef] [PubMed]
- Traynelis, V.C.; Abode-Iyamah, K.O.; Leick, K.M.; Bender, S.M.; Greenlee, J.D.W. Cervical Decompression and Reconstruction without Intraoperative Neurophysiological Monitoring. J. Neurosurg. Spine 2012, 16, 107–113. [Google Scholar] [CrossRef] [PubMed]
- Sala, F.; Bricolo, A.; Faccioli, F.; Lanteri, P.; Gerosa, M. Surgery for Intramedullary Spinal Cord Tumors: The Role of Intraoperative (Neurophysiological) Monitoring. Eur. Spine J. 2007, 16, S130–S139. [Google Scholar] [CrossRef] [PubMed]
- Montemurro, N.; Ius, T.; Ortenzi, V.; Pasqualetti, F.; Acerbi, F. Cervical intramedullary spinal cord metastasis from colon cancer: A systematic review and report of an illustrative case. Neurol Sci. 2025, 46, 3053–3060. [Google Scholar] [CrossRef] [PubMed]
- Hatef, J.; Katzir, M.; Toop, N.; Islam, M.; Clark, T.; Roscoe, C.; Khan, S.; Mendel, E. Damned If You Monitor, Damned If You Don’t: Medical Malpractice and Intraoperative Neuromonitoring for Spinal Surgery. Neurosurg. Focus 2020, 49, E19. [Google Scholar] [CrossRef] [PubMed]
- Montemurro, N.; Zotti, N.; Guercini, J.; Carolis, G.D.; Leoni, C.; Marotta, R.; Tomei, R.; Baggiani, R.; Paolicchi, A.; Lazzini, S.; et al. Value-based healthcare in management of chronic back pain: A multidisciplinary- and lean-based approach. Surg. Neurol. Int. 2024, 15, 348. [Google Scholar] [CrossRef] [PubMed]
- Taylor, J.C.; Rossington, H.; George, R.; Alderson, S.L.; Quirke, P.; Thomas, C.; Howell, S. YCR BCIP Study Group Variation in Perioperative Practice in Elective Colorectal Cancer Surgery: Opportunities for Quality Improvement. Discov. Oncol. 2025, 16, 473. [Google Scholar] [CrossRef] [PubMed]
- Ament, J.D.; Leon, A.; Kim, K.D.; Johnson, J.P.; Vokshoor, A. Intraoperative Neuromonitoring in Spine Surgery: Large Database Analysis of Cost-Effectiveness. N. Am. Spine Soc. J. 2023, 14, 100206. [Google Scholar] [CrossRef] [PubMed]
- Krause, K.L.; Cheaney Ii, B.; Obayashi, J.T.; Kawamoto, A.; Than, K.D. Intraoperative Neuromonitoring for One-Level Lumbar Discectomies Is Low Yield and Cost-Ineffective. J. Clin. Neurosci. 2020, 71, 97–100. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zanin, L.; Broglio, L.; Panciani, P.P.; Bergomi, R.; De Rosa, G.; Ricciardi, L.; Guzzi, G.; Fiorindi, A.; Brembilla, C.; Restelli, F.; et al. Intraoperative Neurophysiological Monitoring in Contemporary Spinal Surgery: A Systematic Review of Clinical Outcomes and Cost-Effectiveness. Brain Sci. 2025, 15, 768. https://doi.org/10.3390/brainsci15070768
Zanin L, Broglio L, Panciani PP, Bergomi R, De Rosa G, Ricciardi L, Guzzi G, Fiorindi A, Brembilla C, Restelli F, et al. Intraoperative Neurophysiological Monitoring in Contemporary Spinal Surgery: A Systematic Review of Clinical Outcomes and Cost-Effectiveness. Brain Sciences. 2025; 15(7):768. https://doi.org/10.3390/brainsci15070768
Chicago/Turabian StyleZanin, Luca, Laura Broglio, Pier Paolo Panciani, Riccardo Bergomi, Giorgia De Rosa, Luca Ricciardi, Giusy Guzzi, Alessandro Fiorindi, Carlo Brembilla, Francesco Restelli, and et al. 2025. "Intraoperative Neurophysiological Monitoring in Contemporary Spinal Surgery: A Systematic Review of Clinical Outcomes and Cost-Effectiveness" Brain Sciences 15, no. 7: 768. https://doi.org/10.3390/brainsci15070768
APA StyleZanin, L., Broglio, L., Panciani, P. P., Bergomi, R., De Rosa, G., Ricciardi, L., Guzzi, G., Fiorindi, A., Brembilla, C., Restelli, F., Costa, F., Montemurro, N., & Fontanella, M. M. (2025). Intraoperative Neurophysiological Monitoring in Contemporary Spinal Surgery: A Systematic Review of Clinical Outcomes and Cost-Effectiveness. Brain Sciences, 15(7), 768. https://doi.org/10.3390/brainsci15070768