Comparison of Electroencephalogram Power Spectrum Characteristics of Left and Right Dragon Boat Athletes after 1 km of Rowing
Abstract
:1. Introduction
2. Materials and Methods
2.1. Participants
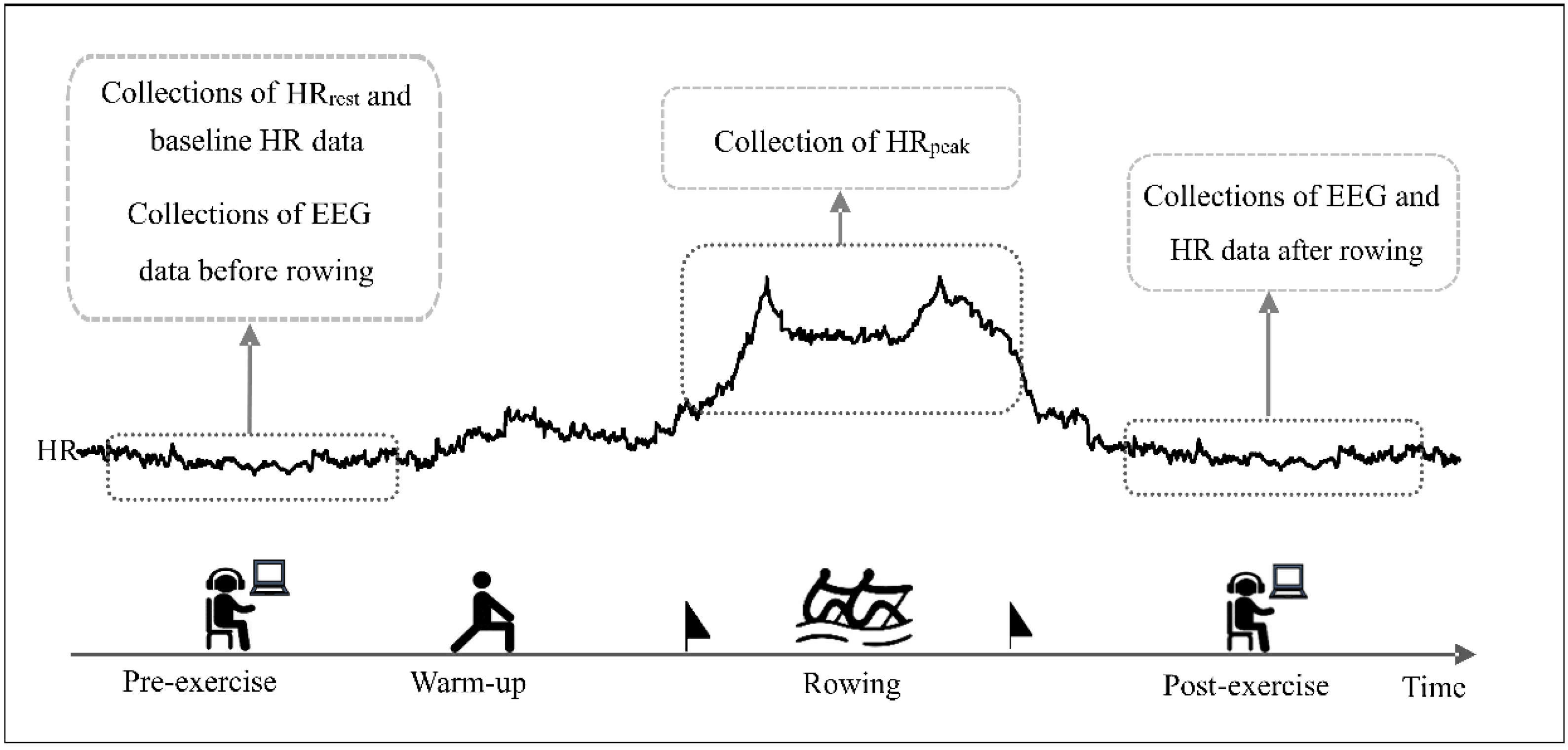
2.2. Experimental Protocol
2.3. Collection and Preprocessing of EEG Data
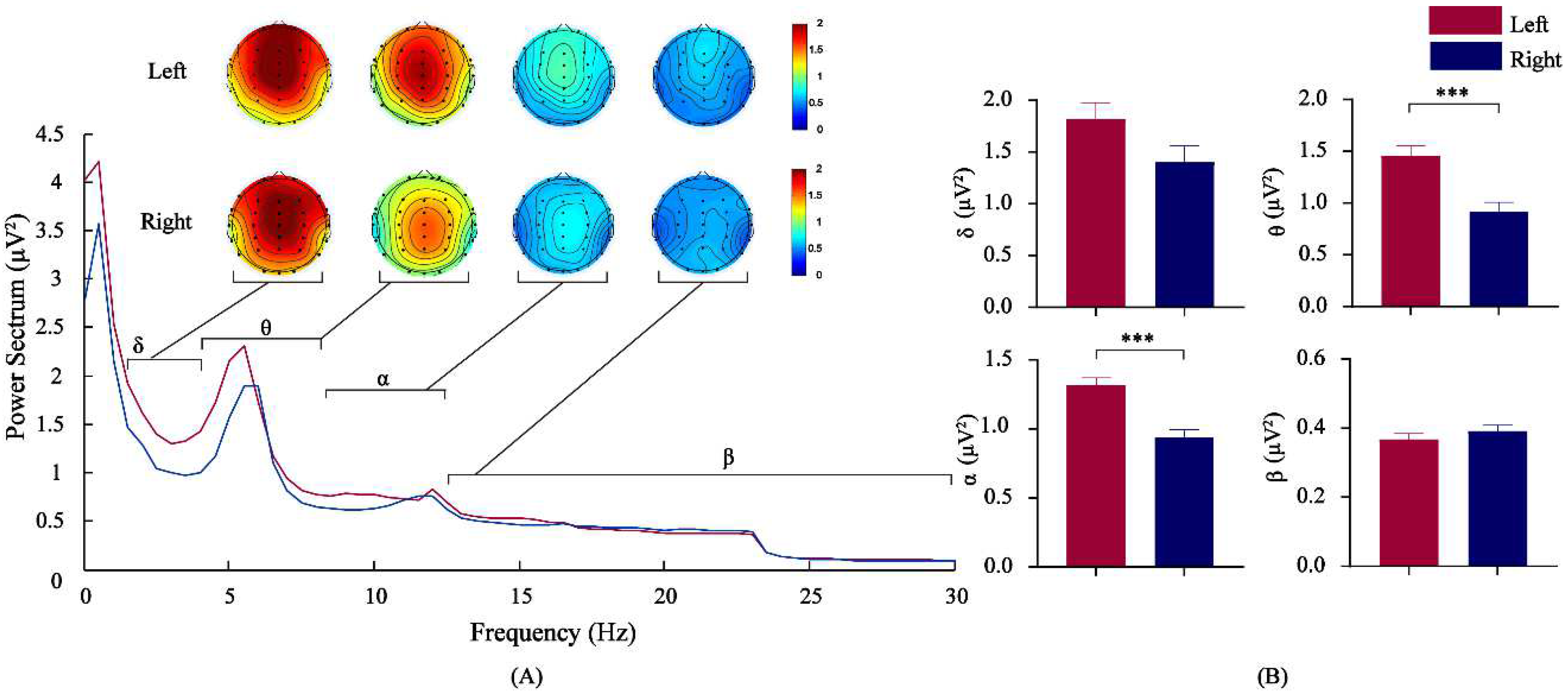
2.4. EEG Power Spectrum Analysis
2.5. Statistical Analysis
3. Results
3.1. Comparison of Athletes’ Post-Exercise Performance
3.2. Comparison of EEG Power Spectrum Values by Frequency Bands for Athletes
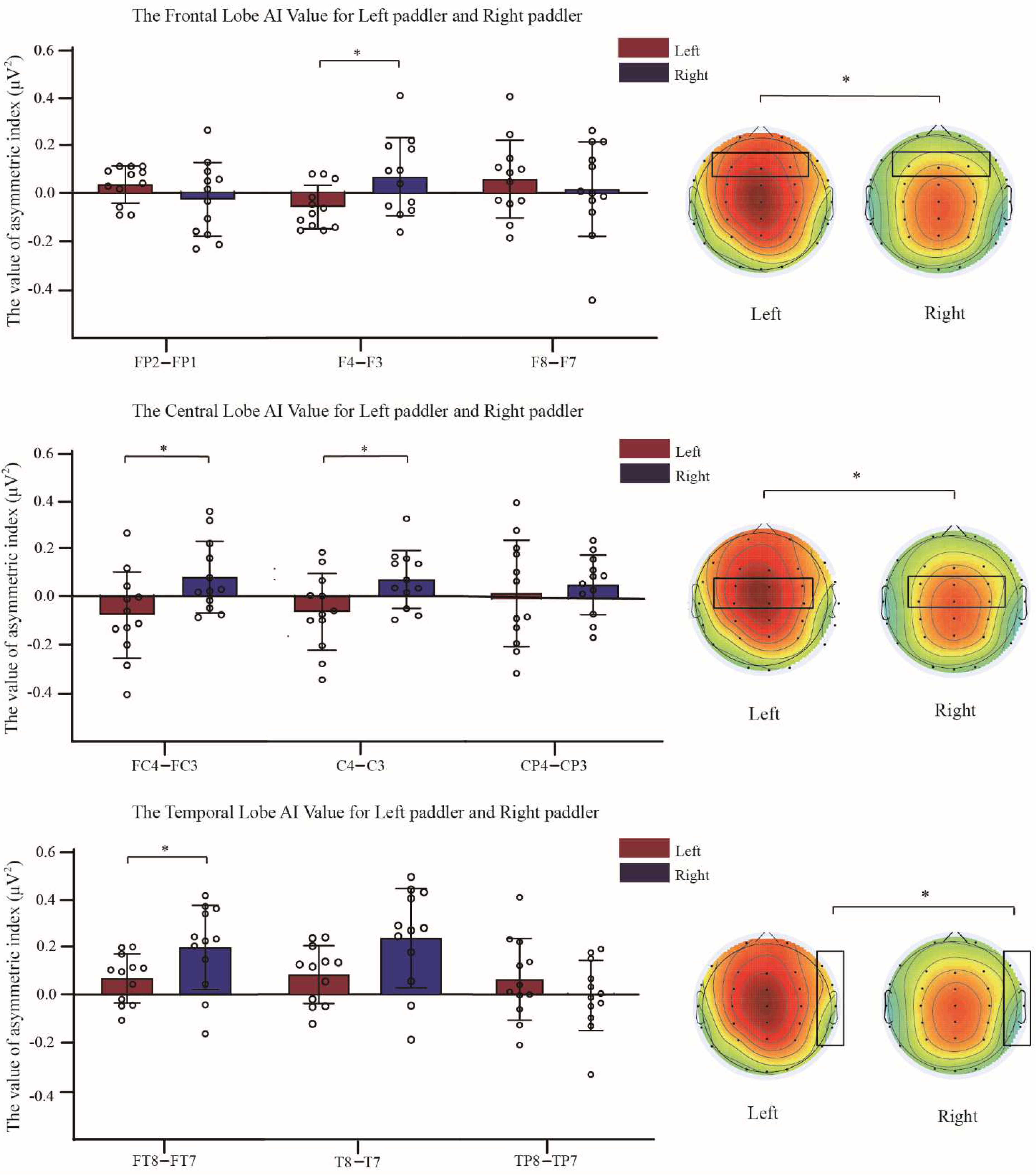
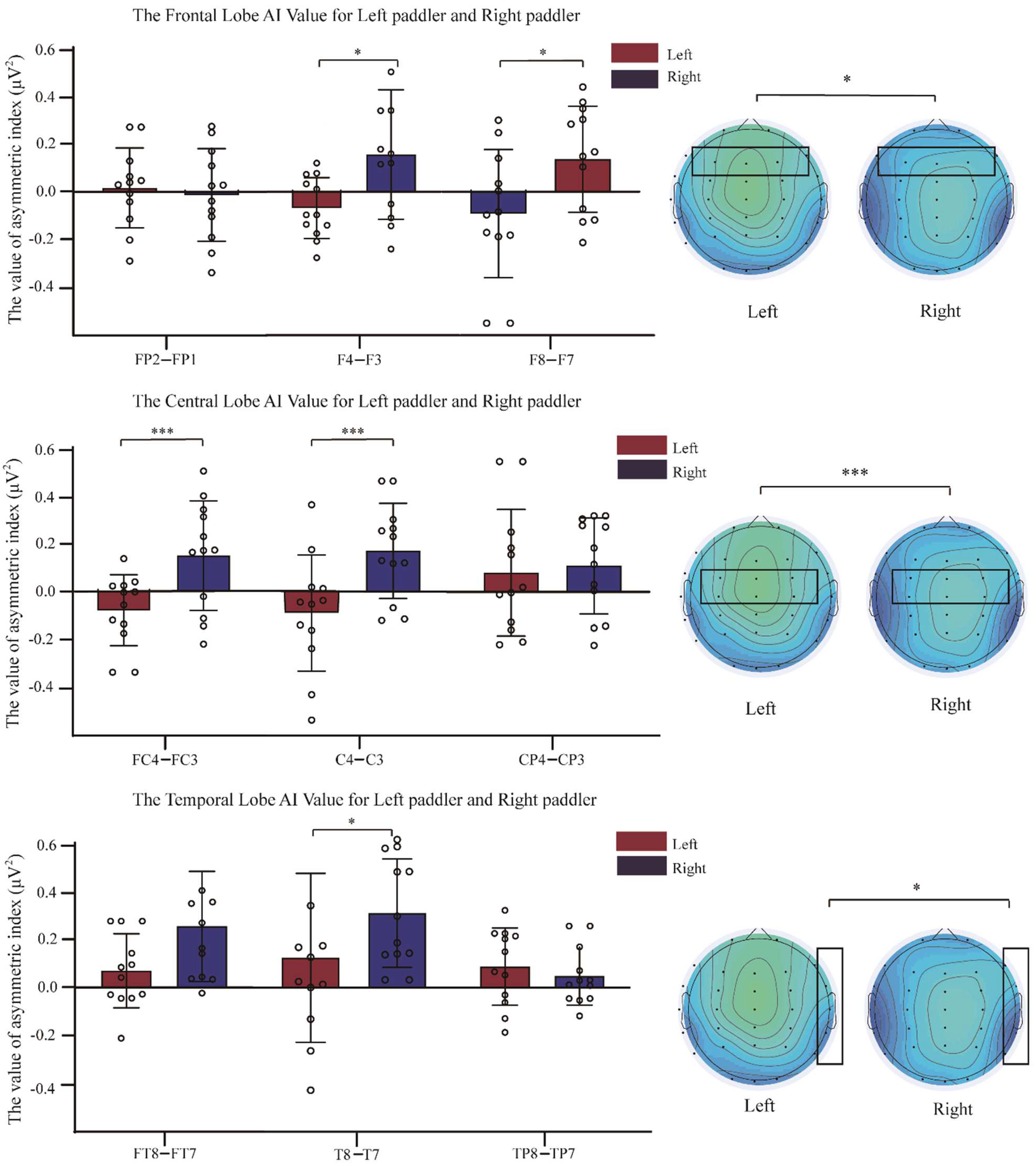
3.3. Comparison of Asymmetry Indices between Two Groups of Dragon Boat Athletes
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Henatsch, H.D.; Langer, H.H. Basic neurophysiology of motor skills in sport: A review. Int. J. Sports Med. 1985, 6, 2–14. [Google Scholar] [CrossRef] [PubMed]
- Riemann, B.L.; Lephart, S.M. The sensorimotor system, part I: The physiologic basis of functional joint stability. J. Athl. Train. 2002, 37, 71–79. [Google Scholar] [PubMed]
- Dieterich, M.; Brandt, T. Global orientation in space and the lateralization of brain functions. Curr. Opin. Neurol. 2018, 31, 96–104. [Google Scholar] [CrossRef] [PubMed]
- Miller, J. Exaggerated redundancy gain in the split brain: A hemispheric coactivation account. Cogn. Psychol. 2004, 49, 118–154. [Google Scholar] [CrossRef] [PubMed]
- Ghacibeh, G.A.; Mirpuri, R.; Drago, V.; Jeong, Y.; Heilman, K.M.; Triggs, W.J. Ipsilateral motor activation during unimanual and bimanual motor tasks. Clin. Neurophysiol. 2007, 118, 325–332. [Google Scholar] [CrossRef] [PubMed]
- Ziemann, U.; Hallett, M. Hemispheric asymmetry of ipsilateral motor cortex activation during unimanual motor tasks: Further evidence for motor dominance. Clin. Neurophysiol. 2001, 112, 107–113. [Google Scholar] [CrossRef] [PubMed]
- Muellbacher, W.; Facchini, S.; Boroojerdi, B.; Hallett, M. Changes in motor cortex excitability during ipsilateral hand muscle activation in humans. Clin. Neurophysiol. 2000, 111, 344–349. [Google Scholar] [CrossRef]
- Rao, S.M.; Binder, J.R.; Bandettini, P.A.; Hammeke, T.A.; Yetkin, F.Z.; Jesmanowicz, A.; Lisk, L.M.; Morris, G.L.; Mueller, W.M.; Estkowski, L.D.; et al. Functional magnetic resonance imaging of complex human movements. Neurology 1993, 43, 2311–2318. [Google Scholar] [CrossRef]
- Suzuki, T.; Higashi, T.; Takagi, M.; Sugawara, K. Hemispheric asymmetry of ipsilateral motor cortex activation in motor skill learning. Neuroreport 2013, 24, 693–697. [Google Scholar] [CrossRef]
- Manzoni, D. The cerebellum and sensorimotor coupling: Looking at the problem from the perspective of vestibular reflexes. Cerebellum 2007, 6, 24–37. [Google Scholar] [CrossRef]
- Wang, Y.; Lu, Y.; Deng, Y.; Gu, N.; Parviainen, T.; Zhou, C. Predicting domain-specific actions in expert table tennis players activates the semantic brain network. Neuroimage 2019, 200, 482–489. [Google Scholar] [CrossRef]
- D’Hondt, J.; Chapelle, L.; Droogenbroeck, L.V.; Aerenhouts, D.; Clarys, P.; D’Hondt, E. Bioelectrical impedance analysis as a means of quantifying upper and lower limb asymmetry in youth elite tennis players: An explorative study. Eur. J. Sport Sci. 2022, 22, 1343–1354. [Google Scholar] [CrossRef]
- Taddei, F.; Viggiano, M.P.; Mecacci, L. Pattern reversal visual evoked potentials in fencers. Int. J. Psychophysiol. 1991, 11, 257–260. [Google Scholar] [CrossRef]
- McLean, J.M.; Ciurczak, F.M. Bimanual dexterity in major league baseball players: A statistical study. N. Engl. J. Med. 1982, 307, 1278–1279. [Google Scholar]
- Loffing, F.; Hagemann, N.; Strauss, B. Automated processes in tennis: Do left-handed players benefit from the tactical preferences of their opponents? J. Sports Sci. 2010, 28, 435–443. [Google Scholar] [CrossRef]
- Beaton, A.A. The relation of planum temporale asymmetry and morphology of the corpus callosum to handedness, gender, and dyslexia: A review of the evidence. Brain Lang. 1997, 60, 255–322. [Google Scholar] [CrossRef] [Green Version]
- Gutwinski, S.; Loscher, A.; Mahler, L.; Kalbitzer, J.; Heinz, A.; Bermpohl, F. Understanding left-handedness. Dtsch. Arztebl. Int. 2011, 108, 849–853. [Google Scholar] [CrossRef]
- Nigmatullina, Y.; Siddiqui, S.; Khan, S.; Sander, K.; Lobo, R.; Bronstein, A.M.; Arshad, Q. Lateralisation of the Vestibular Cortex Is More Pronounced in Left-Handers. Brain Stimul. 2016, 9, 942–944. [Google Scholar] [CrossRef]
- Oldfield, R.C. The assessment and analysis of handedness: The Edinburgh inventory. Neuropsychologia 1971, 9, 97–113. [Google Scholar] [CrossRef]
- Jung, T.P.; Makeig, S.; Humphries, C.; Lee, T.W.; McKeown, M.J.; Iragui, V.; Sejnowski, T.J. Removing electroencephalographic artifacts by blind source separation. Psychophysiology 2000, 37, 163–178. [Google Scholar] [CrossRef]
- Davidson, R.J. EEG measures of cerebral asymmetry: Conceptual and methodological issues. Int. J. Neurosci. 1988, 39, 71–89. [Google Scholar] [CrossRef] [PubMed]
- Baik, S.Y.; Kim, C.; Kim, S.; Yook, D.W.; Kim, H.S.; Chang, H.; Lee, S.H. The moderating effect of heart rate variability on the relationship between alpha asymmetry and depressive symptoms. Heliyon 2019, 5, e01290. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Muller-Putz, G.R. Electroencephalography. Handb. Clin. Neurol. 2020, 168, 249–262. [Google Scholar] [PubMed] [Green Version]
- Dressler, O.; Schneider, G.; Stockmanns, G.; Kochs, E.F. Awareness and the EEG power spectrum: Analysis of frequencies. Br. J. Anaesth. 2004, 93, 806–809. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cross, Z.R.; Santamaria, A.; Corcoran, A.W.; Chatburn, A.; Alday, P.M.; Coussens, S.; Kohler, M.J. Individual alpha frequency modulates sleep-related emotional memory consolidation. Neuropsychologia 2020, 148, 107660. [Google Scholar] [CrossRef]
- Klimesch, W. Memory processes, brain oscillations and EEG synchronization. Int. J. Psychophysiol. 1996, 24, 61–100. [Google Scholar] [CrossRef]
- Klimesch, W. EEG alpha and theta oscillations reflect cognitive and memory performance: A review and analysis. Brain Res. Brain Res. Rev. 1999, 29, 169–195. [Google Scholar] [CrossRef]
- Ruimin, W.; Kamezawa, R.; Watanabe, A.; Iramina, K. EEG alpha power change during working memory encoding in adults with different memory performance levels. Annu. Int. Conf. IEEE Eng. Med. Biol. Soc. 2017, 2017, 982–985. [Google Scholar]
- Sauseng, P.; Griesmayr, B.; Freunberger, R.; Klimesch, W. Control mechanisms in working memory: A possible function of EEG theta oscillations. Neurosci. Biobehav. Rev. 2010, 34, 1015–1022. [Google Scholar] [CrossRef]
- Herweg, N.A.; Solomon, E.A.; Kahana, M.J. Theta Oscillations in Human Memory. Trends Cogn. Sci. 2020, 24, 208–227. [Google Scholar] [CrossRef]
- Kahana, M.J.; Seelig, D.; Madsen, J.R. Theta returns. Curr. Opin. Neurobiol. 2001, 11, 739–744. [Google Scholar] [CrossRef]
- Mun, S.; Whang, M.; Park, S.; Park, M.C. Effects of mental workload on involuntary attention: A somatosensory ERP study. Neuropsychologia 2017, 106, 7–20. [Google Scholar] [CrossRef]
- Weavil, J.C.; Sidhu, S.K.; Mangum, T.S.; Richardson, R.S.; Amann, M. Fatigue diminishes motoneuronal excitability during cycling exercise. J. Neurophysiol. 2016, 116, 1743–1751. [Google Scholar] [CrossRef] [Green Version]
- Ghorbani, M.; Clark, C.C.T. Brain function during central fatigue induced by intermittent high-intensity cycling. Neurol. Sci. 2021, 42, 3655–3661. [Google Scholar] [CrossRef]
- Fournier, L.R.; Wilson, G.F.; Swain, C.R. Electrophysiological, behavioral, and subjective indexes of workload when performing multiple tasks: Manipulations of task difficulty and training. Int. J. Psychophysiol. 1999, 31, 129–145. [Google Scholar] [CrossRef]
- Ghorbani, M.; Ghazalian, F.; Ebrahim, K.; Abednatanzi, H. Altered Neural Response Induced by Central-Fatigue in the Cortical Area During High-Intensity Interval Pedaling. Basic Clin. Neurosci. 2019, 10, 631–639. [Google Scholar] [CrossRef] [Green Version]
- Kelly, R.; Mizelle, J.C.; Wheaton, L.A. Distinctive laterality of neural networks supporting action understanding in left- and right-handed individuals: An EEG coherence study. Neuropsychologia 2015, 75, 20–29. [Google Scholar] [CrossRef]
- Shadli, S.M.; Tewari, V.; Holden, J.; McNaughton, N. Laterality of an EEG anxiety disorder biomarker largely follows handedness. Cortex 2021, 140, 210–221. [Google Scholar] [CrossRef]
- Wilkins, K.B.; Yao, J. Coordination of multiple joints increases bilateral connectivity with ipsilateral sensorimotor cortices. Neuroimage 2020, 207, 116344. [Google Scholar] [CrossRef]
- Andres, F.G.; Mima, T.; Schulman, A.E.; Dichgans, J.; Hallett, M.; Gerloff, C. Functional coupling of human cortical sensorimotor areas during bimanual skill acquisition. Brain 1999, 122 (Pt 5), 855–870. [Google Scholar] [CrossRef] [Green Version]
- Caulo, M.; Briganti, C.; Mattei, P.A.; Perfetti, B.; Ferretti, A.; Romani, G.L.; Tartaro, A.; Colosimo, C. New morphologic variants of the hand motor cortex as seen with MR imaging in a large study population. AJNR Am. J. Neuroradiol. 2007, 28, 1480–1485. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Auer, T.; Dewiputri, W.I.; Frahm, J.; Schweizer, R. Higher-order Brain Areas Associated with Real-time Functional MRI Neurofeedback Training of the Somato-motor Cortex. Neuroscience 2018, 378, 22–33. [Google Scholar] [CrossRef] [PubMed]
- Gazzaniga, M.S. Principles of human brain organization derived from split-brain studies. Neuron 1995, 14, 217–228. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vallortigara, G.; Rogers, L.J. Survival with an asymmetrical brain: Advantages and disadvantages of cerebral lateralization. Behav. Brain Sci. 2005, 28, 575–589; discussion 589–633. [Google Scholar] [CrossRef]
- Amunts, K.; Schlaug, G.; Jancke, L.; Steinmetz, H.; Schleicher, A.; Dabringhaus, A.; Zilles, K. Motor cortex and hand motor skills: Structural compliance in the human brain. Hum. Brain Mapp. 1997, 5, 206–215. [Google Scholar] [CrossRef]
- Foundas, A.L.; Hong, K.; Leonard, C.M.; Heilman, K.M. Hand preference and magnetic resonance imaging asymmetries of the central sulcus. Neuropsychiatry Neuropsychol. Behav. Neurol. 1998, 11, 65–71. [Google Scholar]
- Karolis, V.R.; Corbetta, M.; Thiebaut de Schotten, M. The architecture of functional lateralisation and its relationship to callosal connectivity in the human brain. Nat. Commun. 2019, 10, 1417. [Google Scholar] [CrossRef]
| Variable | Left-Paddlers (n = 21) | Right-Paddlers (n = 22) |
|---|---|---|
| Age (years) | 20.08 ± 1.12 | 20.62 ± 0.65 |
| Height (cm) | 183.54 ± 4.74 | 182.62 ± 6.49 |
| Weight (kg) | 83.23 ± 8.25 | 84.77 ± 10.32 |
| BMI (kg/m2) | 25.33 ± 1.65 | 24.67 ± 1.69 |
| Training age (years) | 4.92 ± 2.49 | 4.31 ± 1.98 |
| Physiological Indexes | Left-Paddles (n = 21) | Right-Paddles (n = 22) | p-Value |
|---|---|---|---|
| HR peak (b/min) | 190.77 ± 2.83 | 190.92 ± 2.92 | 0.99 |
| Percentage of HR max (%) | 95 ± 0.01 | 0.96 ± 0.01 | 1.00 |
| Physiological load index | 2.15 ± 0.05 | 2.17 ± 0.06 | 0.35 |
| RPE > 18 | 20 | 20 | 1.00 |
| Exercise duration (min) | 4.81 ± 0.44 | 5.02 ± 0.51 | 0.41 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, Y.; Jiang, H.; Zhou, W.; Cao, Y.; Shao, C.; Song, J.; Chi, A. Comparison of Electroencephalogram Power Spectrum Characteristics of Left and Right Dragon Boat Athletes after 1 km of Rowing. Brain Sci. 2022, 12, 1621. https://doi.org/10.3390/brainsci12121621
Zhang Y, Jiang H, Zhou W, Cao Y, Shao C, Song J, Chi A. Comparison of Electroencephalogram Power Spectrum Characteristics of Left and Right Dragon Boat Athletes after 1 km of Rowing. Brain Sciences. 2022; 12(12):1621. https://doi.org/10.3390/brainsci12121621
Chicago/Turabian StyleZhang, Yan, Hongke Jiang, Wu Zhou, Yingying Cao, Changzhuan Shao, Jing Song, and Aiping Chi. 2022. "Comparison of Electroencephalogram Power Spectrum Characteristics of Left and Right Dragon Boat Athletes after 1 km of Rowing" Brain Sciences 12, no. 12: 1621. https://doi.org/10.3390/brainsci12121621







